Abstract
Despite few studies on intracellular heat shock protein70, the clinical association between insulin resistance and extracellular heat shock protein70 (eHSP70) is not well studied. In the current study, we examined the association between homeostatic model assessment-insulin resistance (HOMA-IR) and eHSP70 in patients with type 2 diabetes (T2DM) and healthy controls. A total of 145 patients with T2DM and 41 matched healthy controls were selected. Patients and controls were divided based on waist circumference (WC) to two groups, and eHSP70 was compared between them. The association between HOMA-IR and eHSP70 was examined using regression models adjusted for age, high-sensitive C-reactive protein (hs-CRP), and central obesity as confounding factors. While eHSP70 and hs-CRP were significantly correlated with HOMA-IR in patients with T2DM (p = 0.032, 0.025, respectively), there was no correlation between eHSP70 and HOMA-IR in the control group. Extracellular HSP70 and hs-CRP were not correlated in healthy controls. But a significant association appeared between eHSP70 and hs-CRP in patients with T2DM (p = 0.05). Both BMI and WC were not correlated with eHSP70 in both groups. Extracellular HSP70 was positively associated with HOMA-IR in patients with T2DM, independent from hs-CRP and obesity. We also showed eHSP70 levels remained unchanged through increase in BMI or WC in patients with T2D and in healthy controls. Our findings suggest that eHSP70 may contribute to the pathogenesis of T2DM by increasing insulin resistance.
Keywords: Heat shock protein, Insulin resistance, HOMA-IR, Obesity, hs-CRP, Extracellular HSP70, C-Reactive protein
Introduction
Insulin resistance is one of the major components of type 2 diabetes (T2DM) characterized by decreased tissue response to serum insulin (Gerich 2000). Insulin resistance and inflammation are correlated, as has been already documented by showing correlation of several inflammatory markers, such as CRP, TNF-alpha, and IL-6 with insulin resistance (Chen and Chen 2015; Chen et al. 2017; Fontana et al. 2007; Bruun et al. 2005). Actually, inflammation and insulin resistance could form a vicious cycle in which the body inflammatory media provokes insulin resistance, and insulin resistance itself could lead to increase in inflammation, especially in obese people (Chen and Chen 2015; Chen et al. 2017). In fact, it has been postulated that adipocytes, especially in visceral fat contribute to insulin resistance by producing cytokines, which provide an inflammatory media in the body (Mayer and Bukau 2005; Nakhjavani et al. 2010). It is believed that the abdominal obesity is the fundamental culprit for insulin resistance and its associated abnormalities (Fontana et al. 2007; Bruun et al. 2005).
HSP70 is a family of a larger group of proteins collectively called as heat shock proteins, which their expression is induced by chemical and physical stress. In cells, they are believed to maintain the native folding of proteins in stress conditions (Mayer and Bukau 2005). Increased extracellular HSP70 (eHSP70) has been reported in chronic inflammatory diseases, such as T2DM and rheumatoid arthritis (Nakhjavani et al. 2010; Najafizadeh et al. 2015). Data concerning the role of HSP70 in insulin resistance is mostly limited to works on intracellular HSP70 (iHSP70) (Ohno et al. 2010; Sawa et al. 1996; Yaglom et al. 1999). While iHSP70 is believed to protect against insulin resistance by blocking c-Jun N-terminal kinase (JNKs) and thus reverse the cycle that leads to T2DM (Ohno et al. 2010; Park et al. 2001; Chung et al. 2008; Hooper and Hooper 2009), the role of eHSP70 in insulin resistance is not well-studied.
There are studies on patients with T2DM and elderly patients indicating that serum HSP70 levels are positively associated with inflammatory parameters, such as CRP (Njemini et al. 2004; Nakhjavani et al. 2013). Therefore, it might be hypothesized that inflammatory conditions, such as T2DM and insulin resistance, could trigger a rise in eHSP70. In this study first, we investigated the relationship between eHSP70 and insulin resistance in patients with T2DM and healthy controls stratified into obese and non-obese groups. Then, we questioned whether the correlation between eHSP70 and insulin resistance is independent or it is just a consequence of the inflammatory state in T2DM by adjusting for hs-CRP and obesity.
Material and methods
Study population
This is a cross-sectional study conducted in diabetes clinic of Vali-asr Hospital affiliated with Tehran University of Medical Sciences. We selected 145 patients with T2DM. The diagnosis of diabetes was made based on American Diabetes Association 2015 (Diabetes Care 2015). Exclusion criteria were smoking, pregnancy, proteinuria, renal involvement (creatinine > 1.5 mg/dl or GFR < 70 cc/min), glomerulonephritis, congestive heart failure, insulin therapy, and hospital admission in recent months. None of the participants had any overt diabetic complication. Forty-one healthy participants were selected among the volunteered healthy staff of the hospital as the control group. Before enrollment, written informed consents were taken from all participants. The ethics committee of the Tehran University of Medical Sciences approved the study protocol.
Clinical and laboratory measurements
Demographic and anthropometric characteristics, including age, medication, weight, height, and the waist circumference (WC), were obtained from the participants through the interview and baseline measurements. Body mass index (BMI) was computed as weight in kilograms divided by height per square meter (kg/m2).
After 12 h of fasting, venous blood samples were collected for the biochemical analysis. Fasting blood glucose and 1 h post-prandial glucose were measured by the glucose oxidase method. Measurement of serum creatinine was performed by Jaffe method. Plasma total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were determined using direct enzymatic method (Parsazmun, Karaj, Iran). Serum HSP70 was measured using a quantitative sandwich ELISA immunoassay (EKS-715, Stressgen, USA). The intra- and inter-assay CVs ranged between 4.5 and 7%. Insulin was measured by radioimmunoassay (Immunotech, Prague, Czech Republic). Sensitivity was 0.5 μU/mL, and the upper limits of intra- and inter-assay coefficients of variation were 4.3 and 3.4, respectively. hs-CRP was assessed using a two-site ELISA (CAN-CRP-4360, Diagnostic Biochem). Intra- and inter-assay CV were 5–15.2% and 7.8–9.9%, respectively.
Outcome definitions
The primary outcome of the current study was insulin resistance. Insulin resistance was determined using HOMA-IR. HOMA-IR was calculated by following equation: fasting insulin (U/l) × fasting glucose (mg/dl) / 405.
Central obesity was defined as (WC > 102 for men and WC > 88 for women).
Statistical analysis
Continuous variables were summarized as mean (SD) if were normally distributed and median (interquartile range) otherwise. Also, categorical variables were reported as number (percentage). Before comparing variables, the best transformation for each non-normally distributed parameter assessed by Shapiro–Wilk test. Continuous variables among groups were examined using a one-way analysis of variance (ANOVA) and independent sample t test. Comparisons of HSP70, hs-CRP (both logarithmically transformed), and HOMA-IR (transformed by root squaring) among four groups of subjects based on T2DM (control and patients with T2DM) and BMI (categorized into obese (BMI > 30) and none-obese (BMI < 30)) were done using regression models adjusted for age. The association between eHSP70 and HOMA-IR was examined in four different regression models. The model 1 was adjusted for age and metformin therapy; the model 2 was further adjusted for central obesity; in model 3, we selected age and hs-CRP as covariates, and the model 4 was adjusted for age, central obesity, and hs-CRP. Correlations among eHSP70, BMI, WC, HOMA-IR, and hs-CRP were examined using Pearson correlation.
Results
Baseline characteristics
We had 145 patients with T2DM (70 obese, 85 non-obese patients) (mean age 54.5) and 41 healthy controls (19 obese, 22 non-obese patients) (mean age 52.7). Fourteen of them were on metformin monotherapy, 43 on glibenclamide, and 74 were on combination therapy of metformin and glibenclamide. Percentage of females, WC, hs-CRP, and BMI were significantly higher in obese patients with T2DM compared to non-obese patients. FHDL-C was less in obese controls compared to non-obese subjects, while TG, FBS, BMI, WC, weight, and percentage of female were higher. FBS, TG, hs-CRP, HOMA-IR, insulin, and eHSP70 were significantly higher in patients with diabetes compared to healthy control (Table 1).
Table 1.
Baseline characteristics of control and T2DM groups based on obesity
| Control | T2DM | |||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| Female (%) | 6 (27.3%) | 13 (68.4%)* | 18 (24%) | 61 (87.1%)* |
| Age (year) | 50.4 ± 9.9 | 55.5 ± 12 | 54.9 ± 10.9 | 54.2 ± 10.9 |
| BMI (kg/m2) | 25 ± 2.7 | 29.1 ± 2.3* | 25.6 ± 3.3 | 29.8 ± 4.1* |
| WC (cm) | 88.7 ± 7.7 | 101.8 ± 6.8* | 88.4 ± 8.5 | 101 ± 8.4* |
| Weight (kg) | 67.3 ± 10.7 | 81.6 ± 9.9* | 69.5 ± 9.1 | 80 ± 12.3* |
| T2DM duration (years) | – | – | 6.3 ± 7.6 | 4.5 ± 4.9 |
| Metformin | – | – | 7 (50%) | 7 (50%) |
| Glibenclamide | – | – | 23 (53.5%) | 20 (46.5%) |
| Met + Glb | – | – | 37 (50%) | 37 (50%) |
| FBS (mg/dL) | 83.2 ± 6 | 89.1 ± 9.2* | 190.5 ± 85.7# | 189.6 ± 72.9# |
| Triglyceride (mg/dL) | 110.5 ± 54.2 | 163.4 ± 61.3* | 175.8 ± 86.2# | 187.4 ± 88.8 |
| Cholesterol (mg/dL) | 186.1 ± 24.1 | 179.7 ± 40.7 | 197.6 ± 46.5 | 199.1 ± 43.4 |
| LDL-C (mg/dL) | 93.2 ± 13.7 | 92.3 ± 21.5 | 95.3 ± 38.8 | 92 ± 39 |
| HDL-C (mg/dL) | 48.3 ± 16.2* | 37.4 ± 8.7 | 43 ± 10.3 | 41.1 ± 9.6 |
| Creatinine (mg/dL) | 0.9 ± 0.1 | 1 ± 0.2 | 0.9 ± 0.2 | 1 ± 0.4 |
| HOMA-IR | 3 ± 1 | 5.9 ± 7.4 | 8.7 ± 8.5# | 7.4 ± 5.3 |
| hs-CRP (mg/L) | 1.6 ± 1.2 | 2.5 ± 1.9 | 9.5 ± 16.2# | 16.7 ± 26.4#* |
| Insulin (mIU/L) | 14.6 ± 4.7 | 26.4 ± 31.7 | 17.5 ± 13.4 | 16.4 ± 11.3# |
| HSP70 (ng/mL) | 0.3(0.1–2) | 0.5(0.1–2) | 0.6(0.3–1)# | 0.6(0.2–1) |
*Show a significant difference between obese and lean subjects in control and T2DM groups separately (p < 0.05)
#Shows a significant difference between T2DM patients and healthy subjects in lean and obese group separately
Central obesity is defined by WC > 102 cm or 88 cm for men and women, respectively. FBS: fasting blood sugar, LDL-C: low-density lipoprotein-cholesterol, HDL-C: high-density lipoprotein-cholesterol, HOMA-IR, homeostatic model assessment-insulin resistance, hs-CRP: high sensitivity C-reactive protein, HSP70: heat shock protein 70, T2DM: type 2 diabetes
Extracellular HSP70, hs-CRP, and HOMA-IR were significantly different between patients with diabetes and healthy control groups after adjustment for age (p = 0.001, p < 0.001, and p = 0.001, respectively).
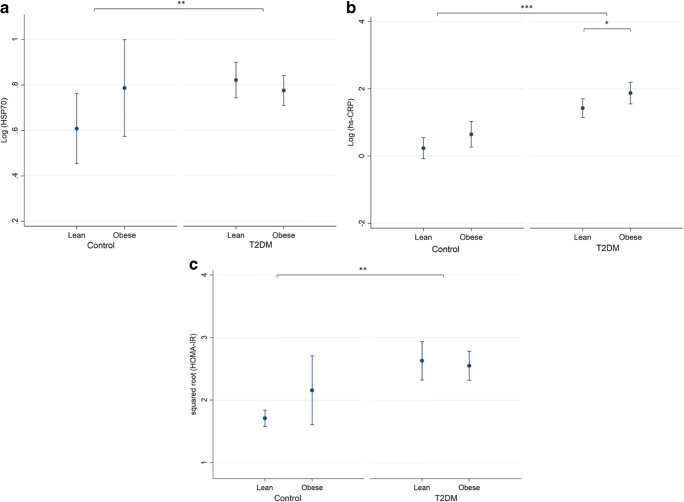
There was no significant difference in eHSP70 and HOMA-IR between obese and non-obese participants in both patients and control groups after adjustment for age (all p > 0.05). However, hs-CRP differed in obese and non-obese people in patients with T2DM (Fig. 1).
Fig. 1.
Comparison of HSP70, hs-CRP (both logarithmically transformed), and HOMA-IR (transformed by squared root) among the control group and patients with T2DM, categorized into central obese (WC > 102 cm or 88 cm for men and women, respectively) and lean are illustrated in b and c, respectively. Although patients with T2DM generally had a significant difference with the control group in HSP70 and HOMA-IR, obesity did not make any change in these two groups. However, hs-CRP differed both between control and T2DM groups and also in obese and non-obese people in patients with T2DM. Comparison was done using regression models adjusted for age. * and ** show significance difference at the level of p < 0.05 and p < 0.01, respectively
Correlations
We studied the correlations between eHSP70, hs-CRP, and HOMA-IR (transformed by squared root). While eHSP70 and hs-CRP were significantly correlated with HOMA-IR in patients with T2DM (p = 0.032, 0.025, respectively), there was no correlation between eHSP70 and HOMA-IR in the control group (Fig. 2). Extracellular HSP70 and hs-CRP were not correlated in healthy controls. But a significant association appeared between eHSP70 and hs-CRP in patients with T2DM (p = 0.05). BMI and WC were not correlated with eHSP70 in both groups, but they were both associated with hs-CRP (p < 0.05) (Table 2).
Fig. 2.
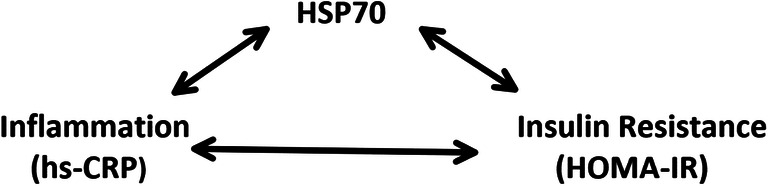
Relationship between HSP70, hs-CRP, and HOMA-IR. Studies have already shown a correlation between HSP70 and inflammation along with a correlation between inflammation and insulin resistance, using hs-CRP and HOMA-IR as markers for inflammation and insulin resistance, respectively. Although rationally an association of HSP70 and insulin resistance might also be deduced, here, we have demonstrated an association between extracellular HSP70 with HOMA-IR even by adjustment for hs-CRP. This fact suggests a non-inflammatory role for extracellular HSP70 in driving insulin resistance
Table 2.
Correlations among HOMA-IR, HSP70, and hs-CRP
| T2DM | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HOMA-IR* | HSP70 | hs-CRP | BMI | WC | HOMA-IR* | HSP70 | hs-CRP | BMI | WC | |
| HOMA-IR* | 1 | – | – | – | – | 1 | – | – | – | – |
| HSP70 | 0.175† | 1 | – | – | – | 0.023 | 1 | – | – | – |
| hs-CRP | 0.183† | − 0.158† | 1 | – | – | 0.282 | 0.268 | 1 | – | – |
| BMI | 0.025 | 0.059 | 0.181† | 1 | – | 0.287 | 0.023 | 0.434† | 1 | – |
| WC | − 0.023 | − 0.066 | 0.173† | 0.725$ | 1 | 0.231 | 0. 170 | 0.322† | 0.722$ | 1 |
HOMA-IR, homeostatic model assessment-insulin resistance, hs-CRP: high sensitivity C-reactive protein, HSP70: heat shock protein 70, WC: waist circumference, T2DM: type 2 diabetes
*To attain normal distribution, squared root of HOMA-IR was used as its transformed variable
†Shows significance at the level p < 0.05
$Shows significance at the level p < 0.001
We studied the association between eHSP70 and HOMA-IR in four models. According to all models, this association was significant after adjustment for age, metformin therapy, central obesity, and hs-CRP (p = 0.026) (Table 3).
Table 3.
Different regression models using HSP70, hs-CRP, and obesity and metformin as dependent variables for predicting HOMA-IR
| Models | R2 | Variable | Coefficient (95% CI) | p |
|---|---|---|---|---|
| HSP70 & Met | 0.079 | HSP70 | − 0.018, 0.544 | 0.066 |
| Metformin | − 1.217, − 0.191 | 0.008 | ||
| HSP70 & obesity & Met | 0.079 | HSP70 | − 0.021, 0.545 | 0.069 |
| Obesity | − 0.403, 0.361 | 0.912 | ||
| Metformin | − 1.219, − 0.188 | 0.008 | ||
| HSP70 & hs-CRP & Met | 0.112 | HSP70 | 0.044, 0.604 | 0.024 |
| hs-CRP | 0.002, 0.019 | 0.015 | ||
| Metformin | − 1.118, − 0.099 | 0.020 | ||
| HSP70 & hs-CRP & obesity & Met | 0.121 | HSP70 | 0.039, 0.602 | 0.026 |
| hs-CRP | 0.002, 0.020 | 0.013 | ||
| Obesity | − 0.476, 0.283 | 0.615 | ||
| Metformin | − 1.11, − 0.089 | 0.022 |
All models also were adjusted for the age. HOMA-IR is transformed by root squaring. HOMA-IR, homeostatic model assessment-insulin resistance, hs-CRP: high sensitivity
Discussion
Despite several experimental studies on iHSP70, there are very few data on serum eHSP70 and its clinical association with insulin resistance. In the current study, we examined this association in patients with T2DM and also in healthy controls. We demonstrated that elevated serum HSP70 levels in patients with T2DM are associated with increased HOMA-IR. Interestingly, this association remained significant after adjustment for hs-CRP and obesity. The results did not show any association between eHSP70 and HOMA-IR in the control group. We also found out that eHSP70 was higher in patients with T2DM compared to healthy subjects.
A number of studies show a dual behavior of HSP70 based on its location. It is reported that iHSP70 expressed in muscle tissues are protective against insulin resistance development (Chichester et al. 2015). On the other hand increased serum eHSP70 (eHSP70) has been reported in chronic diseases such as rheumatoid arthritis and T2DM (Najafizadeh et al. 2015; Dulin et al. 2010). As we have shown earlier, eHSP70 is positively associated with T2DM and also its duration (Nakhjavani et al. 2010). There are two studies examining the association between insulin resistance and eHSP70 only in healthy subjects. To the best of our knowledge, there are no studies focusing on the association of insulin resistance and eHSP70 in patients with T2DM. Krause et al. have indicated that eHSP70 may mediate β cell failure, and it is positively associated with insulin resistance in non-diabetic elderly people (Krause et al. 2014). In another study, an inverse relation between HOMA-IR and serum HSP70 has been reported in 50 healthy African American subjects with no major known disease (Islam et al. 2014). Here, we have demonstrated the appearance of an independent positive association between serum HSP70 and insulin resistance in patients with T2DM.
In order to find out if HSP70 has an independent role in incident insulin resistance or it only reflects the inflammatory state, we adjusted the association between HOMA-IR and eHSP70 for hs-CRP and central obesity, which still remained significant. It is believed that visceral adiposity is the more important contributing factor to the insulin resistance rather than general obesity (Bruun et al. 2005). This is why we decided to use WC instead of BMI for defining obesity.
The impact of medication especially metformin on insulin resistance is well known (Diabetes Care 2013). Metformin increases peripheral insulin sensitivity by the activation of AMP-activated protein kinase alpha2 (Musi et al. 2002). Therefore, it can be postulated that metformin therapy can biased the results. For this reason, we considered medication in all the models and interestingly, the association between eHSP70 and insulin resistance remained significant. It is assumed that eHSP70 mediates activation of JNKs by Toll-like receptors, which leads to phosphorylation of Insulin receptor substrate-1 (IRS-1) at Ser307 in rodents or Ser312 in human and consequently reduced glucose uptake in sensitive tissues or simply put insulin resistance (Krause et al. 2014; Lee et al. 2003; Aguirre et al. 2002).
In this study, there was no significant difference in eHSP70 between obese and non-obese participants in both diabetic and control groups. Moreover, we did not observe any significant association between BMI or WC and eHSP70. Though obese patients are characterized by systematic and adipose low-grade inflammation (Hotamisligil et al. 1995), even lean patients with T2DM have shown increased inflammatory markers (Prattichizzo et al. 2018). It suggests other sources of inflammation and cell stress exist beyond adipose tissues. In our setting, the absence of correlation between BMI and eHSP70 implies that origin of increased circulating HSP70 is not only the fat tissue. Present literature concerning the association between obesity and eHSP70 is controversial. Mardan-Nika et al. have already shown the significant association of HSP70 gene polymorphism with obesity in Iranian population (Mardan-Nik et al. 2016). Also, Rodrigues-Krause et al. have demonstrated that eHSP70 levels are higher in obese patients with T2DM compared to non-obese patients by measuring eHSP70 in seven patients with T2DM and eight healthy controls (Rodrigues-Krause et al. 2012). On the other hand, Islam et al. study reported that HSP70 levels decrease as BMI and WC increase in healthy African American subjects (Islam et al. 2014).
We found an adjusted positive association between eHSP70 and hs-CRP in the patient with T2DM. Interestingly, we did not observe any correlation between hs-CRP and eHSP70 in healthy control subjects. It is believed that the effect of eHSP70 on cells is mainly due to pro-inflammatory pathways. It has been shown that in vitro stimulation of rat splenocytes and macrophages with eHSP70 results in production and release of nitric oxide (NO) and pro-inflammatory cytokines, such as tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β)(Campisi et al. 2003). The appearance of pathological correlations between leptin and eHSP70 and also between asymmetric dimethylarginine and eHSP70 in inflammatory conditions, such as T2DM (Nakhjavani et al. 2013; Nakhjavani et al. 2012), has been reported in previous studies. Extracellular HSP70 could activate pro-inflammatory pathways by binding to TLR-2 and TLR-4 (Vabulas et al. 2002). We postulate that T2DM may provide an inflammatory environment (Wang et al. 2013) and upregulate this reaction.
There are some limitations in our study to note. First, this is a cross-sectional study, and prospective studies are needed to confirm and improve the present results. Second, we did not have data on nutrition habits and physical activity of the participants, which may influence insulin resistance and obesity. Third, we did not have facilities to assess body fat or body composition, so the definite association between adiposity and eHSP70 needs further research. Forth, obese and lean diabetics showed no statistically significant difference in HOMA in our study. This finding may cause some misleading in our study and needs clarification. We have postulated that longer duration of diabetes in lean T2DM patients (with a trend to significance p = 0.08) might propel their HOMA-IR toward obese subjects. It is established that longer duration of disease is associated with decrease in beta cell function and insulin secretion (Rahier et al. 2008). Therefore, it can result in lower HOMA-IR due the same FBS measures in our study participants. Nevertheless, the strength of our study was the relatively large sample size and the similarity in groups in most of the confounding clinical and laboratory parameters.
In conclusion, we have demonstrated that there is an independent positive association between eHSP70 and HOMA-IR in patients with T2DM. We also have shown that eHSP70 is not associated with BMI in patients with T2DM and also in healthy subjects. Our findings suggest that eHSP70 may contribute to the pathogenesis of T2DM by increasing insulin resistance. Further studies are recommended to assess the potential use of HSP70 in prediction and treatment of T2DM.
Acknowledgements
The authors wish to thank patients for their participation and kind cooperation.
Author contribution
The authors’ contributions are listed below:
• Conception or design of the work: Hamid Alemi, Pegah Khaloo, Manouchehr Nakhjavani
• Data analysis and interpretation: Mohammad Ali Mansournia, Hamid Alemi
• Drafting the article: Pegah Khaloo, Hamid Alemi, SoghraRabizadeh
• Critical revision of the article: ManouchehrNakhjavani, Hossein Mirmiranpour, Salome Sadat Salehi, Alireza Esteghamati
All the authors approved the final version and have the agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Hamid Alemi and Pegah Khaloo contributed equally to this work.
Contributor Information
Hamid Alemi, Email: h-alemi@student.tums.ac.ir.
Pegah Khaloo, Email: pegah.khaloo@yahoo.com.
Soghra Rabizadeh, Email: rabizadeh@razi.tums.ac.ir.
Mohammad Ali Mansournia, Email: mansournia_ma@yahoo.com.
Hossein Mirmiranpour, Email: h_mirmiranpour@yahoo.com.
Salome Sadat Salehi, Email: salome.ssalehi@gmail.com.
Alireza Esteghamati, Email: esteghamati@tums.ac.ir.
Manouchehr Nakhjavani, Email: nakhjavanim@tums.ac.ir.
References
- (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8–s16 [DOI] [PubMed]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277(2):1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8(3):272–286. doi: 10.1379/1466-1268(2003)008<0272:SEHIAF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen R (2015) Mechanisms linking inflammation to insulin resistance. 2015:508409 [DOI] [PMC free article] [PubMed]
- Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16(1):203. doi: 10.1186/s12944-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci. 2015;70(2):155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2013) Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care 36(6):1749–1757 [DOI] [PMC free article] [PubMed]
- Dulin E, Garcia-Barreno P, Guisasola MC. Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones. 2010;15(6):929–937. doi: 10.1007/s12192-010-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Gerich JE. Insulin resistance is not necessarily an essential component of type 2 diabetes. J Clin Endocrinol Metab. 2000;85(6):2113–2115. doi: 10.1210/jcem.85.6.6646. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14(2):113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A, Hait SH, Andrews-Shigaki B, Carus S, Deuster PA. Plasma HSP70 levels correlate with health risk factors and insulin resistance in African American subjects. Exp Clin Endocrinol Diabetes. 2014;122(8):496–501. doi: 10.1055/s-0034-1374636. [DOI] [PubMed] [Google Scholar]
- Krause M, Keane K, Rodrigues-Krause J, Crognale D, Egan B, De Vito G, et al. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro. Clin Sci (Lond) 2014;126(10):739–752. doi: 10.1042/CS20130678. [DOI] [PubMed] [Google Scholar]
- Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278(5):2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- Mardan-Nik M, Pasdar A, Jamialahmadi K, Avan A, Mohebati M, Esmaily H, Biabangard-Zak A, Afzal Javan F, Rivandi M, Ferns GA, Ghayour-Mobarhan M. Association of heat shock protein70-2 (HSP70-2) gene polymorphism with obesity. Ann Hum Biol. 2016;43(6):542–546. doi: 10.3109/03014460.2015.1119309. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Najafizadeh SR, Ghazizadeh Z, Nargesi AA, Mahdavi M, Abtahi S, Mirmiranpour H, Nakhjavani M. Analysis of serum heat shock protein 70 (HSPA1A) concentrations for diagnosis and disease activity monitoring in patients with rheumatoid arthritis. Cell Stress Chaperones. 2015;20(3):537–543. doi: 10.1007/s12192-015-0578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, Outeiro TF. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15(6):959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Asgarani F, Khalilzadeh O, Ghazizadeh Z, Bathaie SZ, Esteghamati A. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene. 2012;498(1):107–111. doi: 10.1016/j.gene.2012.01.085. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Nargesi AA, Mostafavi E, Esteghamati A. Appearance of leptin-HSP70 correlation, in type 2 diabetes. Meta Gene. 2013;1:1–7. doi: 10.1016/j.mgene.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2004;5(1):31–38. doi: 10.1023/B:BGEN.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Yamada S, Sugiura T, Ohira Y, Yoshioka T, Goto K. A possible role of NF-kappaB and HSP72 in skeletal muscle hypertrophy induced by heat stress in rats. Gen Physiol Biophys. 2010;29(3):234–242. doi: 10.4149/gpb_2010_03_234. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20(3):446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F, De Nigris V, Spiga R, Mancuso E, La Sala L, Antonicelli R, et al. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev. 2018;41:1–17. doi: 10.1016/j.arr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Krause J, Krause M, O'Hagan C, De Vito G, Boreham C, Murphy C, et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17(3):293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa T, Imamura T, Haruta T, Sasaoka T, Ishiki M, Takata Y, Takada Y, Morioka H, Ishihara H, Usui I, Kobayashi M. Hsp70 family molecular chaperones and mutant insulin receptor: differential binding specificities of BiP and Hsp70/Hsc70 determines accumulation or degradation of insulin receptor. Biochem Biophys Res Commun. 1996;218(2):449–453. doi: 10.1006/bbrc.1996.0080. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277(17):15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem. 1999;274(29):20223–20228. doi: 10.1074/jbc.274.29.20223. [DOI] [PubMed] [Google Scholar]