Abstract
Chondrocyte apoptosis is closely related to the development and progression of osteoarthritis (OA); however, the underlying mechanisms remain enigmatic. Previous studies have confirmed that cell apoptosis is one of the main pathological alterations during oxidative stress, and chondrocyte apoptosis induced by oxidative stress plays an important role in the development of OA. Rat chondrocytes exposed to hydrogen peroxide (H2O2) were used as the experimental oxidative stress model. We assessed cell viability, cell apoptosis, levels of intracellular reactive oxygen species (ROS), nitric oxide (NO) production, gene relative expression level of inducible nitric oxide synthase (iNOS), and expressions of iNOS, PI3K, phospho-Akt, caspase-9, and caspase-3. With the rising of intracellular ROS and increasing iNOS synthesis, producing a large amount of NO in chondrocytes, H2O2 decreased the cell viability and induced cell apoptosis of chondrocytes. Furthermore, the levels of caspase-9 and caspase-3 protein expression were significantly elevated as well as the level of p-Akt protein expression when induced by oxidative stress. These findings suggest that oxidative stress-induced chondrocyte apoptosis occurred via activating both PI3K/Akt and caspase pathways in the early stage in these processes.
Keywords: PI3K/Akt, Caspases, Oxidative stress, Apoptosis
Introduction
Osteoarthritis (OA), known as a degenerative joint disease, is characterized by the loss of chondrocyte function and extracellular matrix (ECM) destruction (LaPrade and Swiontkowski 1999; Oliviero et al. 2010). Previous literature has suggested that chondrocyte apoptosis and cartilage tissue degeneration induced by oxidative stress are among the development and progression processes of OA (Berenbaum 2008; Sekar et al. 2017).
Oxidative stress, which reflects the balance between the systemic manifestation of reactive oxygen species (ROS) and detoxification of reactive intermediates, has been found in a wide range of human diseases, including cell apoptosis in OA (Brandl et al. 2011). Apoptosis is an autonomous programmed cell death process regulated by multiple signaling pathways (Matheny and Adamo 2010). A better understanding of molecular mechanisms concerning oxidative stress-initiated chondrocyte apoptosis could lay a framework for optimal development of innovative therapies for OA.
Akt is a serine/threonine protein kinase that can be phosphorylated and activated by extracellular factors in a phosphoinositide 3-kinase (PI3K)-dependent fashion (Yang et al. 2017). As a potent inhibitory signal for apoptosis, Akt plays an important role in regulating chondrocyte apoptosis and survival and potentially preventing OA. The caspases are believed to function in the effector stage of cell apoptosis following an initiating signal (Carmody and Cotter 2000). Such signals may derive from the direct activation of death receptors such as Fas or from the release of cytochrome c from mitochondria (Dai et al. 2017). When activated, caspase-9 may initiate the activation of caspase-3, and then induces hydrolysis of nucleic acids and cytoskeletal proteins, which ultimately leads to cell apoptosis (Carvour et al. 2008; Huang et al. 2013).
The present study was undertaken to evaluate the molecular mechanism of apoptosis in H2O2-induced chondrocyte.
Materials and methods
Reagents
Collagenase II was obtained from Sigma-Aldrich (St Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) with 100 U penicillin and 100 μg/streptomycin was from Nanjing KeyGEN Biotech. Co., Ltd. (Nanjing, China). Fetal bovine serum (FBS) was from Tianjin Haoyang Biotech. Co., Ltd. (Tianjin, China). FITC Annexin V Apoptosis Detection Kit was from BD Pharmingen™ (San Diego, CA, USA). Cell Counting Kit-8 (CCK-8), 0.25% trypsin, Total Nitric Oxide (NO) Assay Kit, ROS Assay Kit, and BCA Protein Assay Kit were purchased from Beyotime Biotechnology (Shanghai, China). TRIzol was from Invitrogen (Carlsbad, CA, USA). High Capacity cDNA Reverse Transcription Kit was obtained from Applied Biosystems (Foster City, CA, USA). SYBR® Select Master Mix was obtained from Applied Biosystems (Austin, TX, USA). Rabbit anti-rat PI3K antibody, rabbit anti-rat phosphate-Akt antibody, rabbit anti-rat caspase-3 and caspase-9 antibody, rabbit anti-rat iNOS antibody, and rabbit anti-rat GAPDH antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). Collagenase II was dissolved in DMEM and diluted to 2 mg/mL to digest articular cartilage.
Cell harvest and culture
A method for harvesting chondrocytes was conducted as described previously. In brief, chondrocytes were isolated from the articular cartilage of 4-week-old male Sprague Dawley rats from Cavens Lab Animal (Changzhou, China). SD rats were euthanized via an overdose of anesthesia and then the cartilage was removed from animals. The cartilage was cut into thin slices, washed with sterile phosphate-buffered saline (PBS), and then digested with 2 mg/mL collagenase type II in DMEM overnight in the incubator. The digested cartilage was collected and centrifuged. The pellet was re-suspended in DMEM and filtered through a 70-μm nylon mesh. The resulting chondrocytes were cultured in DMEM supplemented with 10% FBS in a 5% CO2 incubator at 37 °C. All of the experiments were conducted when the cells reached confluence within the first passage. This study was reviewed and approved by the ethics committee of the Second People’s Hospital of Changzhou, Jiangsu, China.
Cell treatment
Cultured chondrocytes were grown in 96-well plates (5 × 103 cells/well) for cell viability assay, in 6-well plates (2 × 105 cells/well) for cell apoptosis detection and protein experiments, and in 6-cm dishes (5 × 105 cells/dish) for mRNA extraction. When chondrocytes reached confluence, cells were treated with H2O2 (0, 0.1 mM, 0.3 mM, and 0.5 mM) for 30 min.
Cell viability assay
To assess the dose-response relationship of H2O2in the experiment, the cell viability was evaluated by CCK-8. Chondrocytes were plated in 96-well plates at a density of 5 × 103 cells/well to adhere overnight and treated with four concentrations of H2O2 for 30 min. After the incubation time, 10 μL of CCK-8 solution was added to each well and the cells were further incubated for 1 h at 37 °C in 5% CO2. Absorbance at 450 nm was measured using a microplate reader (Elx808 ™ Bio-Tek Instruments, Winooski, VT, USA).
Cell apoptosis detection
To quantify the percentage of cells undergoing apoptosis, the FITC Annexin V Apoptosis Detection Kit was used according to the manufacturer’s instructions. Briefly, after treatment, chondrocytes were harvested and washed twice with cold PBS, then re-suspended in 100-μL binding buffer into which 5 μL of FITC Annexin V and 5 μL propidium iodide (PI) were added for 15 min at 25 °C in the dark. After incubation, 400-μL binding buffer was added, and the cells were analyzed with a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA).
The measurement of ROS production in chondrocytes
The ROS level in chondrocyte was measured by using a commercialized kit according to the manufacturer’s optimized instructions. Briefly, the number of washed chondrocytes was counted using a cell-count board. They were suspended by diluted DCFH-DA (10 mM) with PBS, and incubated at 37 °C for 20 min. After washing twice with the PBS, ROS production in the re-suspended solution was measured by using a FACScan flow cytometer.
The measurements of NO concentration
The concentrations of NO in cell supernatants were measured according to the instruction of Total NO Assay Kit with the use of the modified Griess reaction method, measuring the concentration of both nitrate and nitrite. The molar concentration of NO in chondrocyte was determined from standard curves generated using standard preparation (n = 6).
Quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from treated samples using TRIzol. High Capacity cDNA Reverse Transcription Kit was used to reverse transcribe total RNA (1 μg) according to the manufacturer’s protocol. Rat-iNOS and Rat-Actin were amplified using SYBR® Select Master Mix in a Bio-Rad iQ5. The specific primer sequences (designed by Sangon Biotech. Co., Ltd., Shanghai, China) are presented in Table 1. The data were calculated by comparative threshold cycle method.
Table 1.
Primer sequences for RT-qPCR
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| iNOS | TCCCAGCCTGCCCCTTCA | CTCCTGCCCACTTCCTCC |
| Actin | CCCATCTATGAGGGTTACGC | TTTAATGTCACGCACGATTTC |
Western blot
Chondrocytes were harvested and lysed in RIPA buffer for total protein extraction. The protein concentration of each sample was determined by the BCA Protein Assay Kit. Equal amounts of protein (10 μg) were boiled and subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to a polyvinylidene fluoride (PVDF) membrane. After being blocked for 1 h in Tris-buffered saline with Tween-20 with 5% nonfat milk, the PVDF membrane was then probed with primary antibodies overnight at 4 °C and then with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The blots were detected with the enhanced Chemiluminescence Assay Kit. Rabbit anti-rat GAPDH antibody was used to detect GAPDH signal as an internal loading control and relative expression levels were quantified by running the Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data were analyzed using Prism (GraphPad Software, San Diego, CA, USA). Two-tailed t test was used for the statistical analysis and P < 0.05 was considered statistically significant.
Results
Cell viability of chondrocytes
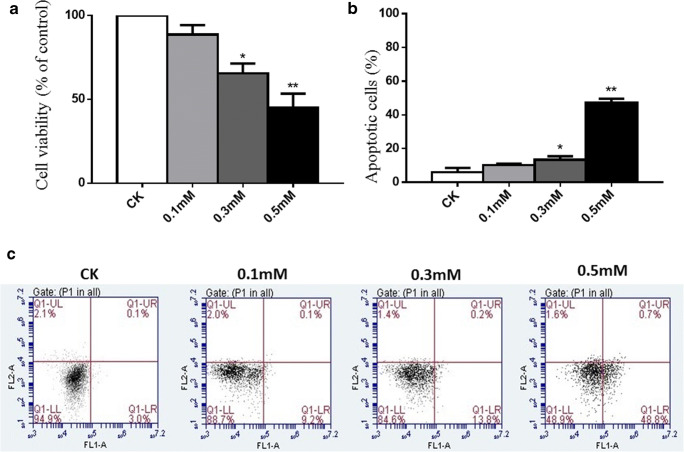
The influence of H2O2 on normal chondrocyte viability was detected by incubating cells with increasing concentrations of H2O2 (0, 0.1 mM, 0.3 mM, and 0.5 mM) for 30 min for subsequent CCK-8 assay. The result showed that 0.1 mM H2O2 did not exhibit significant cytotoxicity for normal chondrocytes; it reduced cell activity by 10%. When treated with 0.3 mM H2O2 for 30 min, the viability of cells decreased by 30% (P < 0.05). When concentration of H2O2reached to 0.5 mM, the viability of cells decreased by 55% (P < 0.01), suggesting the dosage influence of H2O2 on normal chondrocyte viability (Fig. 1a).
Fig. 1.
Cell viability and cell apoptosis in chondrocytes treated with different concentrations of H2O2. a Normal chondrocytes (CK) were treated with different concentrations of H2O2 (0.1 mM, 0.3 mM, and 0.5 mM) for 30 min. Cell viability was detected by CCK-8. b Results of cell apoptosis in different groups. c Normal chondrocytes (CK) were treated with different concentrations of H2O2 (0.1 mM, 0.3 mM, and 0.5 mM) for 30 min. Normal chondrocytes cultured in DMEM for 30 min were used as a negative control. FITC annexin V/PI staining and flow cytometry assays were used to detect cell apoptosis. Results are presented as means ± standard deviation of three independent experiments. Untreated chondrocytes were used as control and considered 100% viable. Asterisk indicates P < 0.05, double asterisks indicate P < 0.01 versus normal control
Cell apoptosis of chondrocytes
The apoptosis of chondrocytes was detected by flow cytometry assay (Fig. 1c). Chondrocytes were treated with increasing concentrations of H2O2 (0.1 mM,0.3 mM, and 0.5 mM) for 30 min. Chondrocytes cultured in DMEM for 30 min was used as a negative control. The percentage of apoptotic cells in quadrant Q1-UR and Q1-LR was calculated and shown in Fig. 1b. The average percentage of apoptotic cells in the 0.1 mM group was 10% and did not show a significant difference from negative control. However, addition of H2O2 (0.3 mM and 0.5 mM) into the cell culture significantly increased the fraction of apoptotic cells, respectively (average 18% P < 0.05 and average 50% P < 0.01). These results suggest that H2O2 can induce the apoptosis of chondrocytes in vitro in a dose-dependent manner.
ROS production and NO concentration in chondrocytes
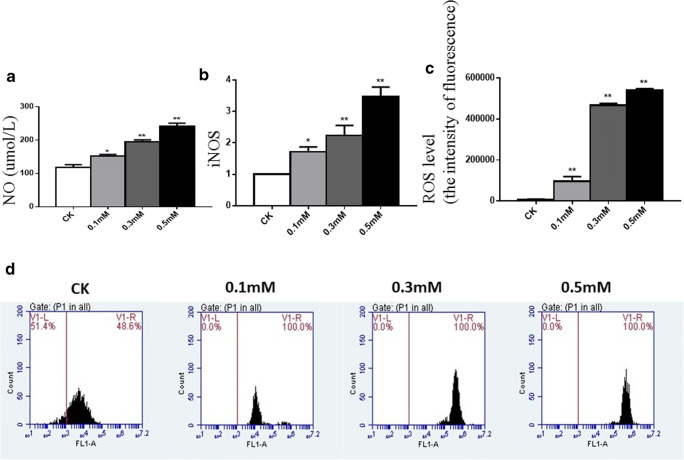
We chose ROS as a marker of oxidative stress. Treatment with H2O2 (0.1 mM, 0.3 mM, and 0.5 mM) for 30 min significantly increased the intensity of fluorescence of ROS by 11-fold, 54-fold, and 62-fold, respectively, when analyzing with a FACScan flow cytometer (Fig. 2d), which suggested the increasing of the level of intracellular ROS compared with vehicle control (Fig. 2c). NO is a highly reactive cytotoxic free radical, which can be induced by oxidative stress. As shown in Fig. 2a, the secretion of NO was increased significantly by treatment with H2O2 (0.1 mM, 0.3 mM, and 0.5 mM) for 30 min by 1.2-fold, 1.5-fold, and 1.8-fold, respectively.
Fig. 2.
Oxidative stress in chondrocytes treated with different concentrations of H2O2. a The levels of NO in cell supernatants in different groups were measured by assay kit. b Cultured and treated chondrocytes were harvested and total RNA was extracted, followed by RT-qPCR for detection of gene expression levels of iNOS. c Results of the levels of intracellular ROS in different groups. d Cultured and treated chondrocytes were harvested and the levels of intracellular ROS in different groups were analyzed with a FACScan flow cytometer. Results are presented as means ± standard deviation of three independent experiments. Untreated chondrocytes were used as normal control. Asterisk indicates P < 0.05, double asterisks indicate P < 0.01 versus normal control
Measurement of iNOS gene expression
ROS induced human chondrocytes to produce iNOS, which is the key enzyme for the synthesis of NO. In our experiment, the expression of iNOS increased by 1.7-fold, 2.2-fold, and 3.4-fold, respectively, with increasing concentrations of H2O2, compared with normal chondrocytes, which was consistent with the secretion of NO (Fig. 2b).
PI3K/Akt and caspase-9 and caspase-3 mediate H2O2-induced chondrocyte apoptosis
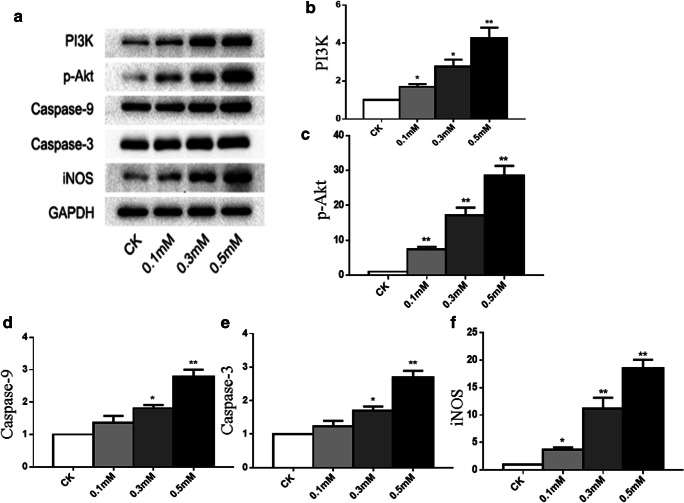
To determine the mechanisms by which H2O2 induced chondrocyte apoptosis, the protein expression of the apoptosis-related markers was studied by western blotting (Fig. 3a). In contrast, protein level of iNOS was found to be upregulated (Fig. 3f), which was consistent with the gene expression of iNOS. The levels of caspase-9 (Fig. 3d) and caspase-3 (Fig. 3e) protein expression were significantly elevated when treated with increasing concentrations of H2O2 compared with vehicle control, suggesting that it acted as a pro-apoptotic pathway. Surprisingly, known as an anti-apoptotic pathway, the level of p-Akt protein expression was also found to be significantly elevated when induced by oxidative stress in our experiment (Fig. 3c).
Fig. 3.
Oxidative stress activates PI3K/Akt and caspase pathways in chondrocyte apoptosis. Following treatment, the levels of iNOS, PI3K, p-Akt, caspase-9, and caspase-3 in total cell lysates were determined by western blot analysis. Representative western blot (a) and quantification data (b–f) are shown respectively. The relative protein levels were normalized to the level of the internal control, GAPDH, and presented as fold changes relative to the control group (the level of the control group was set as 1). Results are presented as mean ± standard deviation of three independent experiments. Untreated chondrocytes were used as normal control. Asterisk indicates P < 0.05, double asterisks indicate P < 0.01 versus normal control
Discussion
OA is a degenerative joint disease with multiple underlying pathogenic mechanisms caused by various risk factors (Betteridge 2003; Palferman 2003). Cytoreduction is a characteristic feature of progressive degradation and destruction of articular cartilage in OA (Zhuang et al. 2016). Cell apoptosis plays a key role in the degeneration and degradation of articular cartilage of OA and increased chondrocyte apoptosis is considered a key pathological feature of OA (Li et al. 2016). The increased production of ROS has been found to suppress the rate of chondrocyte proliferation and induce oxidative stress. Oxidative stress elicited by ROS has been found to be one of the main causative factors involved in the development of OA, which contributes to cartilage degradation by inhibiting matrix synthesis, activating latent matrix-degrading enzymes (Hosseinzadeh et al. 2017; Wang et al. 2017). However, the relationship between oxidative stress and apoptosis in OA has not been well elaborated.
ROS are generated during metabolic processes and perform several biological functions. However, excessive ROS induce oxidative stress and cause cellular damage to DNA, lipids, and proteins (Du et al. 2015; Xu et al. 2012). H2O2, one of the common forms of ROS, can easily penetrate the plasma membrane and affect neighboring cells. Therefore, in our experiments, we chose H2O2 to stimulate normal chondrocytes and mimic the oxidative stress microenvironment. After treatment, with the increasing concentrations of H2O2, the viability of chondrocytes was significantly lower than that of the normal group and the percentage of apoptotic cells was significantly higher than that of the normal group.
As a source of ROS, H2O2 promoted the expression of iNOS in chondrocytes, as we observed in our experiment. iNOS is the key enzyme for the synthesis of NO. Once induced, NO can be synthesized at a constant rate for quite a long time. NO is a highly reactive cytotoxic free radical, which can inhibit the synthesis and secreting of ECM in chondrocytes, affecting the nutritional exchange of cartilage and making cartilage under a bad microenvironment, which eventually leads to the decline and progressive degeneration of cartilage quality (Zhuang et al. 2018). Our results confirmed that in H2O2-stimulated chondrocytes, with the increasing of ROS level, there are marked expression of iNOS and release of NO.
The PI3K/Akt signaling is an important pathway involved in cell differentiation (Faghiri and Bazan 2010; Xu et al. 2012). PI3K activation leads to Akt phosphorylation, which is supposed to promote cell survival against oxidative stress (Kang et al. 2010). Earlier studies have indicated that the PI3K/Akt pathway promoted chondrocyte survival and ECM synthesis. Conversely, inhibition of PI3K/Akt signaling blocks proteoglycan synthesis in chondrocytes and reduces chondrocyte survival (Grishko et al. 2009; Yan et al. 2017). In our experiments, within 30 min, we found the level of p-Akt protein expression was significantly elevated when induced by oxidative stress. The PI3K/Akt pathway participates in multiple apoptotic signaling pathways and we believe activation of the PI3K/Akt pathway in the early stage of cell apoptosis is understandable. With the progression of cell apoptosis, cells can be protected against apoptosis by a transient insult, a preconditioning property of apoptosis, and demonstrate self-sustained apoptosis following withdrawal from a transient apoptotic insult, a remodeling process of apoptosis, in which PI3K/Akt signaling could be turned off through negative feedback mechanism and exhibits protection of cell survival (Facchini et al. 2011).
Caspases, a family of specific cysteine proteases, are critical mediators of apoptosis, which can be activated by oxidative stress. The family of caspases is divided into two classes: initiator caspases, such as caspases-9, activated through the apoptosis-signaling pathways, and effector caspases, such as caspases-3, which carry out apoptosis (Maheshwari et al. 2011). Activation of caspase-9 leads to the mitochondrial release of cytochrome c which in turn results in the activation of caspase-3. Caspase-3 is a member of the caspase signaling pathway and the most important executor of cell apoptosis, which can be activated both by the intrinsic and the extrinsic apoptotic pathways (Mendivil-Perez et al. 2012). In the intrinsic pathway, cytochrome c enters the cytoplasm and activates a chain reaction of caspase family and the cell apoptosis (Hu et al. 2011). Therefore, inhibition of caspase-3 activation may be the key to decrease chondrocyte apoptosis (Yao et al. 2007). Although apoptosis can occur independently of caspase involvement in some cell types (Carmody and Cotter 2000), all of the existing data indicate that caspase activation is a prerequisite for chondrocyte apoptosis. In our experiments, the caspase-9 and caspase-3 expression in cultured chondrocytes was increased after H2O2 exposure within 30 min, which clearly demonstrates that caspase-9 and caspase-3 activation is a necessary step in the cascade of cellular events that leads to chondrocyte apoptosis after exposure to H2O2.
Conclusion
The present study demonstrated that H2O2 caused oxidative damage in the chondrocyte, and ROS acting as mediators of cell apoptosis in vitro was driven by oxidative stress, which was consistent with the increased percentages of chondrocyte apoptosis and ROS-induced oxidative stress. Thus, the results in the current study revealed the H2O2-induced oxidative stress in the chondrocyte caused alterations of iNOS, PI3K/Akt, caspase-9, and caspase-3 protein expression level, and finally resulted in chondrocyte apoptosis via the intrinsic apoptosis pathway.
Compliance with ethical standards
This study was reviewed and approved by the ethics committee of the Second People’s Hospital of Changzhou, Jiangsu, China.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Berenbaum F. New horizons and perspectives in the treatment of osteoarthritis. Arthritis Res Ther. 2008;10(Suppl 2):S1. doi: 10.1186/ar2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge N. Bone and joint diseases around the world. Arthritis--the greatest health, disability, and civil rights challenge: a UK and international perspective. J Rheumatol Suppl. 2003;67:36–37. [PubMed] [Google Scholar]
- Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P. Oxidative stress induces senescence in chondrocytes. J Orthop Res. 2011;29:1114–1120. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- Carmody RJ, Cotter TG. Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ. 2000;7:282–291. doi: 10.1038/sj.cdd.4400646. [DOI] [PubMed] [Google Scholar]
- Carvour M, Song C, Kaul S, Anantharam V, Kanthasamy A, Kanthasamy A. Chronic low-dose oxidative stress induces caspase-3-dependent PKCdelta proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann N Y Acad Sci. 2008;1139:197–205. doi: 10.1196/annals.1432.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Mao Y, Sun X, Li X, Muhammad I, Gu W, Zhang D, Zhou Y, Ma J, Ni Z, Huang S. Attenuation of oxidative stress-induced osteoblast apoptosis by curcumin is associated with preservation of mitochondrial functions and increased Akt-GSK3beta signaling. Cell Physiol Biochem. 2017;41:661–677. doi: 10.1159/000457945. [DOI] [PubMed] [Google Scholar]
- Du Z, Li S, Liu L, Yang Q, Zhang H, Gao C. NADPH oxidase 3-associated oxidative stress and caspase 3-dependent apoptosis in the cochleae of D-galactose-induced aged rats. Mol Med Rep. 2015;12:7883–7890. doi: 10.3892/mmr.2015.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini A, Stanic I, Cetrullo S, Borzi RM, Filardo G, Flamigni F. Sulforaphane protects human chondrocytes against cell death induced by various stimuli. J Cell Physiol. 2011;226:1771–1779. doi: 10.1002/jcp.22506. [DOI] [PubMed] [Google Scholar]
- Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishko V, Xu M, Ho R, Mates A, Watson S, Kim JT, Wilson GL, Pearsall AWt. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem. 2009;284:9132–9139. doi: 10.1074/jbc.M804178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh A, Bahrampour Juybari K, Fatemi MJ, Kamarul T, Bagheri A, Tekiyehmaroof N, Sharifi AM. Protective effect of ginger (Zingiber officinale roscoe) extract against oxidative stress and mitochondrial apoptosis induced by interleukin-1beta in cultured chondrocytes. Cells Tissues Organs. 2017;204:241–250. doi: 10.1159/000479789. [DOI] [PubMed] [Google Scholar]
- Hu L, Chen L, Yang G, Li L, Sun H, Chang Y, Tu Q, Wu M, Wang H. HBx sensitizes cells to oxidative stress-induced apoptosis by accelerating the loss of Mcl-1 protein via caspase-3 cascade. Mol Cancer. 2011;10:43. doi: 10.1186/1476-4598-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wu B. The association between splenocyte apoptosis and alterations of Bax, Bcl-2 and caspase-3 mRNA expression, and oxidative stress induced by dietary nickel chloride in broilers. Int J Environ Res Public Health. 2013;10:7310–7326. doi: 10.3390/ijerph10127310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KA, Wang ZH, Zhang R, Piao MJ, Kim KC, Kang SS, Kim YW, Lee J, Park D, Hyun JW. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 2010;11:4348–4360. doi: 10.3390/ijms11114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrade RF, Swiontkowski MF. New horizons in the treatment of osteoarthritis of the knee. JAMA. 1999;281:876–878. doi: 10.1001/jama.281.10.876. [DOI] [PubMed] [Google Scholar]
- Li H, Song F, Duan LR, Sheng JJ, Xie YH, Yang Q, Chen Y, Dong QQ, Zhang BL, Wang SW. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep. 2016;6:23693. doi: 10.1038/srep23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Misro MM, Aggarwal A, Sharma RK, Nandan D. N-acetyl-L-cysteine counteracts oxidative stress and prevents H2O2 induced germ cell apoptosis through down-regulation of caspase-9 and JNK/c-Jun. Mol Reprod Dev. 2011;78:69–79. doi: 10.1002/mrd.21268. [DOI] [PubMed] [Google Scholar]
- Matheny RW, Jr, Adamo ML. PI3K p110 alpha and p110 beta have differential effects on Akt activation and protection against oxidative stress-induced apoptosis in myoblasts. Cell Death Differ. 2010;17:677–688. doi: 10.1038/cdd.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendivil-Perez M, Velez-Pardo C, Jimenez-Del-Rio M. TPEN induces apoptosis independently of zinc chelator activity in a model of acute lymphoblastic leukemia and ex vivo acute leukemia cells through oxidative stress and mitochondria caspase-3- and AIF-dependent pathways. Oxidative Med Cell Longev. 2012;2012(3):1–14. doi: 10.1155/2012/313275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero F, Ramonda R, Punzi L. New horizons in osteoarthritis. Swiss Med Wkly. 2010;140:w13098. doi: 10.4414/smw.2010.13098. [DOI] [PubMed] [Google Scholar]
- Palferman TG. Bone and joint diseases around the world. The UK perspective. J Rheumatol Suppl. 2003;67:33–35. [PubMed] [Google Scholar]
- Sekar S, Crawford R, Xiao Y, Prasadam I. Dietary fats and osteoarthritis: insights, evidences, and new horizons. J Cell Biochem. 2017;118:453–463. doi: 10.1002/jcb.25758. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang W, Qiu E. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J Biol Sci. 2017;24:837–842. doi: 10.1016/j.sjbs.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qian J, Xie X, Lin L, Zou Y, Fu M, Huang Z, Zhang G, Su Y, Ge J. High density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via activation of the PI3K/Akt pathway and suppression of reactive oxygen species. Int J Mol Sci. 2012;13:17104–17120. doi: 10.3390/ijms131217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Wu H, Wu Z, Hua F, Liang D, Sun H, Yang Y, Huang D, Bian JS. The new synthetic H2S-releasing SDSS protects MC3T3-E1 osteoblasts against H2O2-induced apoptosis by suppressing oxidative stress, inhibiting MAPKs, and activating the PI3K/Akt pathway. Front Pharmacol. 2017;8:07. doi: 10.3389/fphar.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Zhang H, Luo Y, Zhang J, Wang M, Liao P, Cao L, Guo P, Sun G, Sun X (2017) Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: insight into the ERalpha-mediated PI3K/Akt pathway. Int J Mol Sci 18 [DOI] [PMC free article] [PubMed]
- Yao H, Tang X, Shao X, Feng L, Wu N, Yao K. Parthenolide protects human lens epithelial cells from oxidative stress-induced apoptosis via inhibition of activation of caspase-3 and caspase-9. Cell Res. 2007;17:565–571. doi: 10.1038/cr.2007.6. [DOI] [PubMed] [Google Scholar]
- Zhuang C, Wang Y, Zhang Y, Xu N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int J Biol Macromol. 2018;115:281–286. doi: 10.1016/j.ijbiomac.2018.04.083. [DOI] [PubMed] [Google Scholar]
- Zhuang C, Xu NW, Gao GM, Ni S, Miao KS, Li CK, Wang LM, Xie HG. Polysaccharide from Angelica sinensis protects chondrocytes from H2O2-induced apoptosis through its antioxidant effects in vitro. Int J Biol Macromol. 2016;87:322–328. doi: 10.1016/j.ijbiomac.2016.02.031. [DOI] [PubMed] [Google Scholar]