Abstract
Background:
Granulomas caused by infectious lung diseases present as indeterminate pulmonary nodules (IPNs) on radiography. Newly available serum enzyme immunoassay (EIA) for histoplasmosis has not been studied for the evaluation of IPNs. We investigated serum biomarkers of histoplasmosis antibodies as an indication of benign disease in IPNs from a highly endemic region.
Methods:
152 serum samples from patients presenting with pulmonary nodules ≤30mm in maximum diameter were analyzed for histoplasmosis antibodies by immunodiffusion and EIA IgG and IgM tests. Serology and FDG-PET/CT scan diagnostic test characteristics were estimated and compared.
Results:
Cancer prevalence was 55% (n=83). Thirty-nine (26%) individuals were positive for IgG histoplasmosis antibodies. Twelve samples were IgM antibody positive. Immunodiffusion serology was similar to IgM antibody results with thirteen positive tests. Diagnostic likelihood ratios for benign disease were 0.62, 0.33 to 0.28 for FDG-PET/CT, IgG and IgM antibodies, respectively. When both IgG and IgM were positive (n=8), no nodules were cancerous and six were FDG-PET/CT avid.
Conclusions:
A positive EIA test for both IgM and IgG strongly suggested histoplasmosis etiology and benign granuloma for 12% of benign nodules arising from a highly endemic region. Presence of either IgG or IgM histoplasma antibodies was associated with benign disease. The EIA test was more sensitive in assessing histoplasma exposure than immunodiffusion serology.
Impact:
A new CLIA-certified histoplasmosis antibody EIA test measures histoplasmosis exposure, offers a possible alternative clinical diagnosis for benign IPNs and may improve IPN evaluation while avoiding harmful invasive biopsies.
Keywords: Lung cancer, Biomarkers of DNA damage, exposure, phenotype
Introduction
Evaluation and diagnosis of incidental pulmonary nodules (IPNs) is a growing burden for clinicians as chest imaging proliferates and the use of low dose CT screening for lung cancer increases.(1,2) Evaluation of IPNs is further complicated in regions where endemic mycotic diseases (histoplasmosis, blastomycosis, and coccidioidomycosis) induce lung granulomas. A study of CT scans among patients from the histoplasmosis endemic Ohio and Mississippi River Valleys demonstrated three times the false positive rate compared to non-endemic areas.(3) In such endemic regions, granulomatous disease is the most common benign etiology, occurring in 50 to 75% of pathologically determined benign diagnoses.(4,5)
After discovery of an IPN, guidelines suggest 18F-fluorodeoxyglucose positron emission tomography with computed tomography (FDG-PET/CT) may be indicated for moderate risk nodules(6), but we have shown that FDG-PET/CT specificity is markedly reduced in areas of endemic mycotic diseases due to benign granulomas(4) that generate false positive results.(7,8) Granulomas, masquerading as insipient lung cancer, confound diagnostic imaging, including guideline recommended FDG-PET/CT scans.(8) In this environment aggressive pursuit of pathological diagnosis is often warranted, putting patients at higher risk due to complications from a lung biopsy.(9)
A non-invasive biomarker of fungal exposure which indicates possible benign disease and not malignancy would aid clinicians evaluating IPNs arising from endemic areas. By classifying a group of nodules as having serologically detectable histoplasmosis exposure, clinicians would have an alternative approach to differentiating benign from malignant disease and, in combination, improve nodule evaluation over imaging alone. Biomarkers of infectious fungal exposure, measured by serologic tests, are not well studied in the diagnosis of granulomatous lung nodules,(10,11) and current evaluation guidelines for IPNs suspicious for lung cancer do not recommend serologic testing to indicate infectious etiologies due to their poor sensitivity.(6,12)
A newly available serum enzyme immunoassay (EIA) test which is considered more sensitive than existing immunodiffusion or complement fixation for histoplasmosis diagnosis has not been evaluated in patients with nodules.(13) In this pilot study we systematically investigated the performance of standard immunodiffusion and a new EIA assay for histoplasmosis exposure measurement and compared them to FDG-PET/CT scans for the diagnosis of lung cancer among IPNs arising in a region where histoplasmosis is highly endemic (>80%).(14)
METHODS
Population and outcome diagnosis:
Serum was selected from 162 patients with an IPN (Figure 1) whose maximum diameter by CT scan was ≤30mm and who had a FDG-PET/CT scan prior to diagnosis. Nodules were discovered from routine practice or referred to Vanderbilt University Medical Center, a tertiary referral research hospital in Nashville, Tennessee. All samples were prospectively collected between 2006 and 2015 and were frozen and stored in the Vanderbilt Thoracic Biorepository. Patient informed consent was collected under Vanderbilt University Medical Center’s IRB (#000616). This study was approved by the Vanderbilt Institutional Review Board and conducted in accordance with the U.S. Common Rule. Individuals with metastatic lung cancer, lack of original FDG-PET/CT scan, or indeterminate nodule size were excluded. Final diagnosis was determined by either tissue pathology or radiographic evidence of benign disease (absence of growth over two years or development of clearly benign characteristics, such as dense calcification, or spontaneous resolution).
Figure 1: Consort diagram with FDG-PET/CT scan and serological test results.
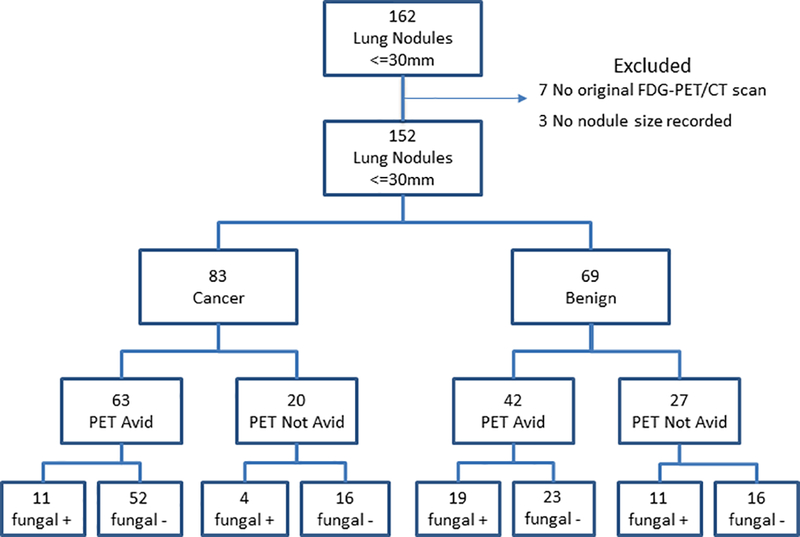
PET Avid: defined as either SUV greater than 2.5 or clinical judgement based on appearance of the CT portion of the PET/CT
Fungal +: Positive EIA test for either IgG or IgM antibodies
Serological testing:
Frozen serum was shipped to MiraVista Diagnostics (Indianapolis, IN, USA) who performed all serological tests. Each participant was tested for histoplasmosis separately by immunodiffusion and by MiraVista’s proprietary EIA for immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies. Methodology for performing EIA testing has been reported elsewhere.(13,15,16) Results for EIA derived IgG and IgM antibodies and immunodiffusion antibodies were reported each separately and for EIA in combination as criteria for positive antibody results. EIA results were standardized to enzyme immunoassay antibody units (EU). EU values between 10 and 80 indicated a positive EIA test for the respective histoplasmosis antibody. These cutoffs were based on the manufacturer’s recommended test’s indication for acute infectious disease detection. Positive results above the quantification limit of 80 EU were analyzed as 80 EU for purposes of this study. Results less than 9.9 EU, including indeterminate results between 8 and 9.9, were interpreted as negative for histoplasmosis. Immunodiffusion was performed according to the manufacturer’s instructions using commercially available reagents (Meridian Biosciences, Cincinnati, OH, USA). Immunodiffusion results were reported as either positive, negative or non-diagnostic and as a continuous antibody measurement. The analytical laboratory was blinded to all patient data.
18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Scans:
FDG-PET/CT scans were performed as standard of practice in the clinical setting at both Vanderbilt University Medical Center and in the community. Original FDG-PET/CT scans were reviewed by a physician (RW) board certified in diagnostic radiology and in nuclear medicine who was blinded to diagnosis and clinical history. Standard Uptake Value (SUV) was measured on a commercial PET/CT workstation (Advanced Workstation 3.2, GE Medical System, Waukesha, WI, USA). Avidity and likelihood for malignancy were determined by either SUV greater than 2.5 or clinical judgment based on appearance on the CT portion of the PET/CT.
Statistical Analysis:
Sensitivity, specificity, positive (PPV) and negative predictive values (NPV), negative diagnostic likelihood ratios and their 95% confidence intervals (CI) were estimated. EIA serologies, both for individual antibodies and as a combined antibody test indicating a positive histoplasmosis result, were compared to FDG-PET/CT scans and the Mayo Clinic Model estimated baseline malignancy risk for each participant.(17) To determine the impact of the histoplasmosis serologic tests on the diagnosis of cancer, diagnostic likelihood ratios were estimated. A positive serological result was converted to a negative diagnostic likelihood ratio, because a positive histoplasmosis serology test indicates benign disease and not cancer. When comparing likelihood ratios with 0 values in their respective 2 by 2 table, 0.5 was added to each cell to calculate a ratio estimate. Comparative differences in demographics by cancer outcome were estimated by the appropriate statistical test given variable type (continuous, dichotomous, or categorical), for example t-test for the normally distributed continuous variable of age. All analyses were performed with Stata v 14.2 (College Station, TX, USA), and p-values <.05 were considered statistically significant.
RESULTS
We selected serum from 162 individuals with lung nodules ≤ 30mm. Ten nodules were excluded in the analysis, as three had no documented size and seven were missing the original FDG-PET/CT scan for review, resulting in 152 nodules available for comparison across all test modalities (Figure 1). Excluded nodules were equally distributed between malignancy (n=5) and benign (n=5) disease. Cancer prevalence among the 152 individuals was 55% (n=83). Benign nodules occurred more often (Table 1) in younger individuals (p=0.002) who were never smokers (p<0.001), with non-spiculated nodule edge (p<0.001), and with lower maximum SUV (p<0.001). The mean Mayo Clinic Model estimated cancer risk was significantly higher (p<0.001) among malignant nodules (50%) compared to benign nodules (33%). FDG-PET/CT scans had a sensitivity of 76% (95%CI: 65, 85), specificity of 39% (95%CI: 28, 52) with a negative diagnostic likelihood ratio of 0.62 (95%CI: 0.38, 1.00) (Figure 2) and NPV of 57% (95%CI: 42, 72). The majority of patients with cancer had adenocarcinoma (n=45; 54% of cancers). Granuloma (n=40, 58% of benign disease) was the most common benign disease, comprising 75% of the 53 nodules with pathologically determined benign disease in this population primarily from middle Tennessee.
Table 1.
Patient Demographics and Nodule Characteristics
| Cancer (n=83) | Benign (n=69) | Overall (n=152) | p-value | |
|---|---|---|---|---|
| Mean Age (SD) | 65 (8.8) | 60 (12) | 63 (10.7) | 0.002 |
| Gender – Male (%) | 61 (73) | 27 (39) | 88 (42) | <0.001 |
| Race (%) Caucasian | 71 (86) | 65 (94) | 136 (89) | |
| African American | 12 (14) | 2 (3) | 14 (9) | |
| Other | 0 (0) | 2 (3) | 2 (1) | 0.01 |
| Ever Smoker (%) | 95 | 74 | 86 | <0.001 |
| Mean Nodule size, mm (SD) | 17.5 (6.4) | 16.1 (5.5) | 16.8 (6.1) | 0.15 |
| Spiculated nodule edge (%) | 28 (34) | 14 (20) | 42 (28) | 0.07 |
| Mean Mayo risk probability (SD)* | 50 (26) | 33 (23) | 42 (26) | <0.001 |
| Mean FDG-PET/CT SUV (SD) | 5.8 (4.3) | 3.2 (1.9) | 4.6 (3.7) | <0.001 |
| Avid nodules by FDG-PET/CT** | 63 | 42 | 105 | 0.05 |
SD=standard deviation, FDG-PET/CT = 18F-fluorodeoxyglucose positron emission
tomography and computed tomography scan, SUV=standard uptake value
Mayo risk probability estimated according to Swensen et al,199714
Avid FDG=PET/CT scan based on either SUV>=2.5 or radiologist’s report of avidity.
Figure 2: Tissue acquisition test-treatment decision diagram.
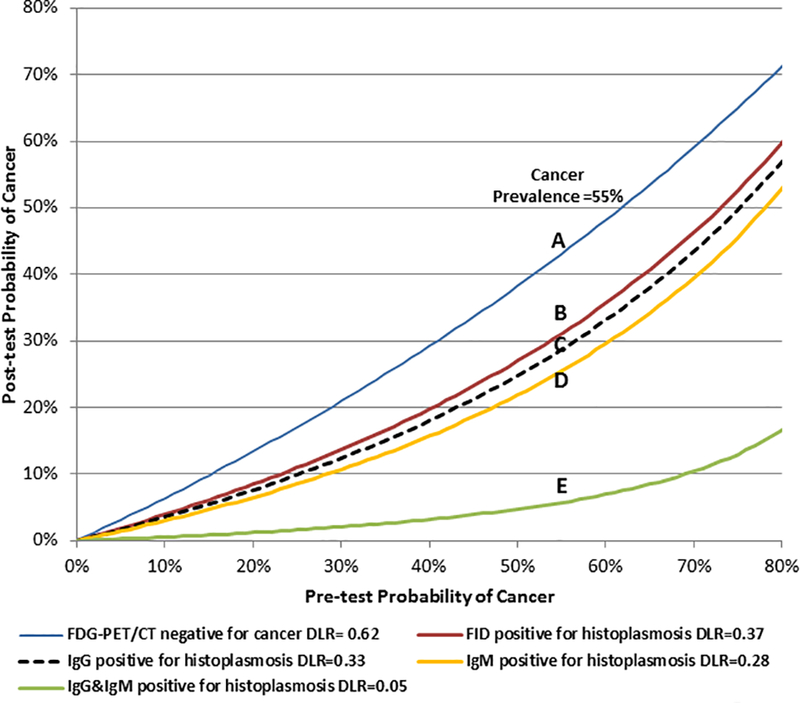
Lines are the calculated diagnostic likelihood ratios (DLR) for 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET/CT) (A), Immunodiffusion (FID) (B), EIA IgG (C), EIA IgM (D) and combined EIA IgG and IgM serologies (E). The prevalence of cancer in the study was 55% with post-test probabilities estimated at those points. To determine the impact of fungal testing to rule out cancer and provide a comparison to FDG-PET scans, a positive histoplasmosis test was considered a negative test for cancer. Example: In a patient with a 55% pre-FDG-PET/CT probability of cancer, a negative FDG-PET scan gives a 43% post-test probability of cancer in our population (Point A). A serology test positive for only IgG antibodies (Point C) decreases the probability of cancer from 55% to 29%, a 26% point reduction in cancer risk. A positive serology for both IgG and IgM antibodies yields a 6% post-test probability of cancer (Point E). In this example using our data, a non-avid FDG-PET result does not change recommendation for resection. However, an enzyme immunoassay positive for both IgG and IgM antibodies would decrease even a 70% pre-test probability of cancer to 11% and to a surveillance strategy.
Histoplasma Serologies
A positive EIA antibody test for the diagnosis of histoplasmosis by either IgM or IgG positivity occurred in 43 (28%) participants. Thirty-nine (26%) individuals were IgG positive and 12 (8%) were IgM positive. Of those with a positive EIA histoplasmosis result, 14 nodules were malignant and 29 benign. Among 40 pathologically ascertained granulomas, 17 (42.5%) tested positive for either IgG or IgM antibodies. A positive EIA test for histoplasmosis by either IgG or IgM was more sensitive (42%; 95%CI: 30, 55) but less specific (83%; 95%CI: 73, 91) than that of either IgG (Sensitivity 41%, Specificity 87%) or IgM (Sensitivity 13%, Specificity 96%) separately (Table 2). Serological results were obtained for the 10 excluded nodules. One was IgG positive (a cancer), one was both IgG and IgM positive (a benign nodule) and the remaining eight were negative for histoplasmosis antibodies.
Table 2.
Test characteristics for immunodiffusion and EIA serology to diagnose histoplasmosis
| Immunodiffusion (95% CI) | IgG Antibody (95% CI) | IgM Antibody (95% CI) | IgG or IgM (95% CI) | IgG + IgM (95% CI) | |
|---|---|---|---|---|---|
| Sensitivity | 13 (6,23) | 41 (29,53) | 13 (6,23) | 42 (30,55) | 12 (5,22) |
| Specificity | 95 (88,99) | 87 (78,93) | 96 (90,99) | 83 (73,91) | 100 (96,100) |
| PPV | 69 (39,91) | 72 (55,85) | 75 (43,95) | 67 (52,81) | 100 (63,100) |
| NPV | 57 (48,65) | 64 (54,73) | 57 (49,66) | 63 (54,72) | 58 (49,66) |
| Positive test | 13 | 39 | 12 | 43 | 8 |
EIA=Enzyme Immunoassay, PPV=positive predictive value, NPV=negative predictive value, IgG= immunoglobulin G antibodies, IgM=immunoglobulin M antibodies
All patients with both IgG and IgM histoplasmosis antibody positivity had benign disease. Of the eight benign nodules with both IgG and IgM antibodies detected by EIA, four were ascertained as granulomatous disease by pathology, and four were ascertained as benign by radiographic surveillance. Six of the eight (75%) were FDG-PET/CT avid (false positive for malignancy), while a ninth individual (granuloma) did not have an available FDG-PET/CT scan to include for comparison (see Supplemental data). Thirteen samples (9%) were positive for histoplasmosis by immunodiffusion (four cancer, nine benign; Sensitivity=13%; 95%CI: 6, 23 and Specificity 95%; 95%CI: 88, 99).
Comparison of FDG-PET and Histoplasmosis serology testing to rule out cancer
The negative predictive value (NPV) for FDG-PET/CT scans to exclude malignancy was 57% (95%CI: 42, 72) in this population of smaller diameter IPNs. The positive predictive value for EIA antibodies to indicate benign disease separately and thus find negative for malignancy was 72% (95%CI: 55, 85) for IgG and 75% (95%CI: 43, 95) for IgM. A positive immunodiffusion test indicating exposure to histoplasma had a predictive value of 69% (95%CI: 39, 91) for histoplasmosis with wide confidence intervals (Table 2) due to the relative rarity of a positive test (9%). A negative serology test for histoplasma antibodies offered little diagnostic information (NPV for no histoplasma exposure IgG=64%, IgM=57%, either antibody=63% and Immunodiffusion=57%).
To determine the clinical impact of each test, we estimated the potential post-test reduction in cancer risk using the negative diagnostic likelihood ratio and converting it into a post-test probability given the pre-test baseline cancer risk (e.g. prevalence of cancer). We directly compared the results for each of the tests. FDG-PET/CT had a negative diagnostic likelihood ratio of 0.62 and was weaker than any individual or combination of serological tests at differentiating benign disease (Point A - Figure 2, Table 3). A negative PET scan in this population reduced the post-test cancer risk by 12 percentage points from 55% to 43% (Point A – Figure 2). The diagnostic likelihood ratio for the EIA test was 0.40 (95%CI: 0.23, 0.70) when either IgG or IgM antibodies were detected for histoplasmosis (negative for cancer). The negative diagnostic likelihood ratio was 0.33 (95%CI: 0.18, 0.61) for IgG antibodies and 0.28 (95% CI: 0.08, 0.98) for IgM antibodies each separately. When a positive IgG test occurred, the post-test probability of cancer fell from 55% to 29% (Point C - Figure 2). The negative diagnostic likelihood ratio for immunodiffusion was 0.37 (95%CI: 0.12, 1.15) which offered little diagnostic information as the 95% confidence interval included 1. The negative likelihood ratio for the EIA test result when both IgG and IgM were positive was 0.05 (95%CI: 0.003, 0.83) which occurred in 8 individuals. Therefore, when both IgG and IgM were positive for histoplasmosis, the post-test probability of cancer dropped from 55% to 6% (Point E – Figure 2).
Table 3.
Diagnostic likelihood ratios (DLR) for histoplasmosis serology and FDG-PET/CT imaging
| Test | DLR negative for cancer | Number of positive tests | Post-test cancer probability reduction** |
|---|---|---|---|
| FDG-PET/CT* | 0.62 (0.38,1.00) | 47 | 12% |
| Immunodiffusion | 0.37 (0.12,1.15) | 13 | 24% |
| EIA IgG | 0.33 (0.18,0.61) | 39 | 26% |
| EIA IgM | 0.28 (0.08,0.98) | 12 | 25% |
| EIA IgG and IgM | 0.05 (0.003,0.83) | 8 | 49% |
Non-avid FDG-PET/CT scans are negative DLR and serology results are positive for histoplasmosis but reported as inverted to a negative DLR, negative for cancer.
Change in post-test probability of cancer is the baseline population prevalence of cancer (55%), minus the post-test probability of cancer given a negative FDG-PET/CT scan or serological test positive for histoplasmosis and expressed as percentage points.
EIA= enzyme immunoassay, IgG= immunoglobulin G antibodies, IgM=immunoglobulin M antibodies
Discussion
In this pilot study population of both known and suspected lung cancer with serum available from our Thoracic Biorepository, we compared a new, more sensitive EIA histoplasmosis test to the long available immunodiffusion test and FDG-PET/CT’s metabolic diagnostic imaging. FDG-PET/CT performed poorly in this cohort of smaller lesions with a sensitivity of 76% and specificity of 39%. The lower sensitivity observed was likely related to the majority (n=111) of nodules being 20mm or less in maximum diameter, as smaller nodule size is a known factor in PET’s reduced sensitivity.(8) Some of the reduction in FDG-PET/CT specificity could be attributable to verification or “work-up” bias in this high cancer prevalence population. Here, verification bias for FDG-PET/CT may occur in those nodules with a pathological diagnosis as the pursuit of tissue pathology is dependent, in part, on the PET results. Therefore, we are more likely to observe higher sensitivity and more false positive results.(18) Cohorts with lower prevalence of cancer or less pathologically determined diagnosis may have a higher specificity than observed here.(8) However, radiographic surveillance, which was the diagnostic method for 16 (11%) nodules, was an option for all nodules. A negative PET scan, with its diagnostic likelihood ratio of 0.62 reduced the post-test diagnostic cancer risk estimate by only 12 percentage points from 55% to 43% (Figure 2). This poor PET performance replicates previous work published from the same region and illustrates the difficulty clinicians face in evaluating lesions arising from endemic areas.(5,19)
A positive serology test by any of IgG, IgM or immunodiffusion tests had similar negative diagnostic likelihood ratios, between 0.28 and 0.37. The best combination of sensitivity to histoplasmosis fungal exposure and PPV (72%) occurred when IgG antibody was positive. IgG antibodies were detected in 43% of benign nodules. If we considered a baseline pre-test risk estimate equal to the study’s 55% prevalence of malignancy, then a positive IgG serology test for histoplasmosis antibodies, with its negative diagnostic likelihood for cancer of 0.33, reduced the post-test risk estimate for malignancy to 29% (Figure 2). A positive immunodiffusion test occurred in 13% of benign diagnoses, making it an inferior diagnostic test compared to EIA. The lack of detectable antibodies for immunodiffusion and low sensitivity of immunodiffusion tests when evaluating lung nodules has been stated in histoplasmosis infectious disease literature as a prime reason to not use the immunodiffusion test for the diagnosis of lung nodules.(20)
Testing of IPNs with histoplasmosis serology by complement fixation or immunodiffusion is not currently recommended. First, due to small burden of residual disease, antigen testing is not useful to discriminate histoplasmosis-related granulomas from malignancy, as the test is universally negative and not performed in clinical practice.(20) The immunodiffusion assay detects both short-lived H antibodies and longer-lived M antibodies for fungal species. Immunodiffusion is indicated for both acute and chronic or past fungal infections.(21,22) Granulomas from fungal exposure may arise from minor exposures years or decades prior to radiographic nodule detection and well outside the time period when M antibodies are detectable, and thus is not recommended for IPN evaluation.(23)
Few studies have examined serologic tests for the diagnosis of benign lung disease. Dall Bello found that 5% of their confirmed histoplasmosis cases originally presented as lung nodules suspicious for cancer.(24) Richmond and colleagues,(11) in a case series of large (>3cm) granulomas, found little evidence of histoplasmosis by immunodiffusion or complement fixation serology. In stark contrast to Richmond’s results, Naeem and colleagues(10) reported high accuracy for serology to diagnose benign disease of hilar adenopathy for malignancy. They found that a positive histoplasmosis complement fixation test had a specificity of 100% and a sensitivity of 61% for benign disease in a pediatric population (n=131) with lymphadenopathy, whose primary cancer histology was lymphoma and benign disease was granuloma. Due to limitations in serum availability in this study, we were unable to perform complement fixation with its larger sample volume requirement and contraindication of sample stability in frozen, stored serum, a limitation of our study.
In our study, 40% of granulomas were positive for IgG antibodies, a 2.5-fold increase in disease detection compared to immunodiffusion in the same population. The strength of EIA to detect histoplasmosis exposures in IPNs, as illustrated by a positive result in over a quarter of all lung nodules and in 43% of benign IPNs, may be unique to the Middle Tennessee population. Edwards et al found that Nashville and its immediately surrounding counties were among the highest histoplasmosis skin test positivity rates (91%) in the United States.(14) However, a positive result for one of the histoplasmosis antibodies, indicating exposure to histoplasmosis, did not exclude the diagnosis of malignancy. We based our cutoff for malignancy on the current level of 10EU for acute disease. Other cutoffs specific to lung nodules may improve the EIA test’s characteristics as we found an improvement in area under the receiver operator curve when EIA results were modeled as a continuous variable (Supplemental Figure). As this is a pilot study of existing samples from our biorepository, more samples will be necessary from both endemic and non-endemic areas to better assess test cutoffs and validate our observations. We will be pursuing this line of investigation in a larger cohort of nodules as this study’s small number of participants limits the generalizability and strength of the results.
Clinically, the EIA test, though intended for infectious histoplasmosis diagnosis, may be adapted to evaluate lung nodules. A positive IgG result likely indicates histoplasmosis fungal exposure and 72% of positive EIA tests with IgG antibodies are benign, which may suggest the clinician obtain additional CT scans at an appropriate three or six month interval instead of pursuing a tissue diagnosis. However, because 11 patients with cancer had EIA IgG antibody positive tests (13% of cancers and 7% of all nodules), reliance on the more sensitive IgG antibody indication alone to exclude cancer in an IPN may not be clinically defensible with its positive predictive value of 72%. Conversely, in the small set of patients where both IgG and IgM antibodies were found in serum, the addition of IgM antibodies precluded cancer and always indicated a nodule with benign etiology and possibly an active histoplasmosis infection. A positive EIA test for both antibodies, found in 5% of the entire nodule population and in 12% of benign nodules, greatly increased the specificity of the test and strongly supported an alternative diagnosis of active histoplasmosis. Conversely, a negative test offered little clinical information.
A positive histoplasmosis EIA test may preclude the need for the more expensive PET imaging as 75% of nodules that had both IgG and IgM detected antibodies were also FDG-PET/CT avid false positive scans. The complex interplay of an EIA histoplasmosis test with the patient’s underlying cancer epidemiologic risks, likely endemic exposures and imaging offers the possibility of improving the non-invasive evaluation of a particularly difficult population with IPNs arising from endemic mycotic areas of the US. The pursuit of an alternative diagnostic pathway with serology begs further evaluation of the histoplasmosis EIA test beyond this small study, and how it may be positioned in the diagnosis of lung nodules.
Conclusion
A positive EIA serological test for histoplasmosis provides clinicians with useful information of histoplasmosis exposure and possible benign disease in IPNs. Serologically significant histoplasmosis exposures may be a new biomarker for benign granuloma. When both IgG and IgM antibodies are present, active histoplasmosis, and not lung cancer, should be considered. The histoplasmosis EIA serum test offers an alternative, non-invasive diagnosis of possibly active infection in 12% of benign nodules arising from a highly endemic area of the country. Further investigations of incorporating EIA serology with existing imaging and other cancer biomarkers for IPNs that may have a histoplasmosis etiology is warranted.
Supplementary Material
Acknowledgments
This work was funded by EDRN U01 CA152662 to P. Massion and E. Grogan. E. Grogan’s research time was also supported by the Veterans Affairs Hospital and he is a past recipient of a VA career development award (10–024).
Funded by EDRN U01 CA152662 to PPM and ELG. Dr. Grogan’s research time was also supported by the Veterans Affairs Hospital and he is a past recipient of a VA career development award (10–024)
Footnotes
LJW is the President of MiraVista Diagnostics. MMD is employed by MiraVista Diagnostics. The other authors declare no potential conflicts of interest.
REFERENCES
- 1.Gould MK, Tang T, Liu I-LA, Lee J, Zheng C, Danforth KN, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. American Journal of Respiratory and Critical Care Medicine 2015;192(10):1208–14 doi 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Annals of Internal Medicine 2013;E-pub ahead of print, July. doi 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 3.Starnes SL, Reed MF, Meyer CA, Shipley RT, Jazieh A-R, Pina EM, et al. Can lung cancer screening by computed tomography be effective in areas with endemic histoplasmosis? The Journal of Thoracic and Cardiovascular Surgery 2011;141(3):688–93 doi DOI: 10.1016/j.jtcvs.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 4.Grogan E, Deppen SA, Ballman K, Andrade GM, Verdail FC, Aldrich MC, et al. Accuracy of FDG-PET to diagnose lung cancer in the ACOSOG Z4031 trial. 2012. June 29-July1, 2012; Chicago, IL [Google Scholar]
- 5.Deppen S, Putnam JB, Andrade G, Speroff T, Nesbitt JC, Lambright ES, et al. Accuracy of FDG-PET to Diagnose Lung Cancer in a Region of Endemic Granulomatous Disease. The Annals of Thoracic Surgery 2011;92(2):428–33 doi 10.1016/j.athoracsur.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer?: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. CHEST Journal 2013;143(5_suppl):e93S–e120S doi 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason R, Broaddus V, Martin T, King T, Schraufnagel D, Murray J, et al. , editors. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Volume 2: Saunders; 2010. [Google Scholar]
- 8.Deppen SA, Blume JD, Kensinger CD, et al. Accuracy of fdg-pet to diagnose lung cancer in areas with infectious lung disease: A meta-analysis. JAMA 2014;312(12):1227–36 doi 10.1001/jama.2014.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deppen SA, Davis WT, Green EA, Rickman O, Aldrich MC, Fletcher S, et al. Cost-Effectiveness of Initial Diagnostic Strategies for Pulmonary Nodules Presenting to Thoracic Surgeons. The Annals of Thoracic Surgery 2014;98(4):1214–22 doi 10.1016/j.athoracsur.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naeem F, Metzger ML, Arnold SR, Adderson EE. Distinguishing Benign Mediastinal Masses from Malignancy in a Histoplasmosis-Endemic Region. The Journal of Pediatrics 2015;167(2):409–15 doi 10.1016/j.jpeds.2015.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richmond BW, Worrell JA, Bastarache JA, Gervich DH, Slattery WR, Loyd JE. Histoplasmomas of Uncommon Size. Chest 2013;143(6):1795–8 doi 10.1378/chest.12-2071. [DOI] [PubMed] [Google Scholar]
- 12.Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. Histoplasmosis: Up-to-Date Evidence-Based Approach to Diagnosis and Management. Semin Respir Crit Care Med 2015;36(05):729–45 doi 10.1055/s-0035-1562899. [DOI] [PubMed] [Google Scholar]
- 13.Richer SM, Smedema ML, Durkin MM, Herman KM, Hage CA, Fuller D, et al. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clinical Infectious Diseases 2016;62(7):896–902 doi 10.1093/cid/ciw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards L, Acquaviva F, Livesay V, Cross F, Palmer C. An altas of sensitivity to tuberculin, PPD-B and histoplasmin in the United States. Am Rev Resp Dis 1969;99(4):S1–132. [PubMed] [Google Scholar]
- 15.Richer SM, Smedema ML, Durkin MM, Brandhorst TT, Hage CA, Connolly PA, et al. Development of a Highly Sensitive and Specific Blastomycosis Antibody Enzyme Immunoassay Using Blastomyces dermatitidis Surface Protein BAD-1. Clinical and Vaccine Immunology 2014;21(2):143–6 doi 10.1128/cvi.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly PA, Durkin MM, LeMonte AM, Hackett EJ, Wheat LJ. Detection of Histoplasma Antigen by a Quantitative Enzyme Immunoassay. Clinical and Vaccine Immunology 2007;14(12):1587–91 doi 10.1128/cvi.00071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157(8):849–55. [PubMed] [Google Scholar]
- 18.Feinstein AR. Clinical Epidemiology: The architecture of clinical research. W. B Saurers Co; 1985. [Google Scholar]
- 19.Croft DR, Trapp J, Kernstine K, Kirchner P, Mullan B, Galvin J, et al. FDG-PET imaging and the diagnosis of non-small cell lung cancer in a region of high histoplasmosis prevalence. Lung Cancer 2002;36(3):297–301. [DOI] [PubMed] [Google Scholar]
- 20.Knox KS, Hage CA. Histoplasmosis. Proc Am Thorac Soc 2010;7(3):169–72 doi 10.1513/pats.200907-069AL. [DOI] [PubMed] [Google Scholar]
- 21.Wheat LJ. Histoplasmosis: Experience during outbreaks in Indianapolis and review of the literature. Medicine 1997;76(5):339–54. [DOI] [PubMed] [Google Scholar]
- 22.Wheat J, French MV, Kohler RB, Zimmerman SE, Smith WR, Norton J, et al. The diagnostic laboratory tests for histoplasmosis: Analysis of experience in a large urban outbreak. Annals of Internal Medicine 1982;97(5):680–5 doi 10.7326/0003-4819-97-5-680. [DOI] [PubMed] [Google Scholar]
- 23.Hage CA, Wheat LJ, Loyd J, Allen SD, Blue D, Knox KS. Pulmonary Histoplasmosis. Semin Respir Crit Care Med 2008;29(02):151,65 doi 10.1055/s-2008-1063854. [DOI] [PubMed] [Google Scholar]
- 24.Dall Bello AG, Severo CB, Guazzelli LS, Oliveira FM, Hochhegger B, Severo LC. Histoplasmose simulando neoplasia primária de pulmão ou metástases pulmonares. Jornal Brasileiro de Pneumologia 2013;39:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


