Abstract
Background.
Metabolism and excretion of the phytoestrogen enterolactone (ENL), which has been associated with breast cancer risk, may be affected by variation in steroid hormone and xenobiotic metabolizing genes.
Methods.
We conducted a randomized, crossover flaxseed intervention study in 252 healthy, postmenopausal women (137 EA and 115 African Americans AA) from western New York. Participants were randomly assigned to maintain usual diet or consume 10 g/d ground flaxseed for 6 weeks. After a 2-month washout period, participants crossed over to the other diet condition for an additional 6 weeks. Urinary ENL excretion was measured by GC-MS and 70 polymorphisms in 29 genes related to steroid hormone and xenobiotic metabolism were genotyped. Mixed additive genetic models were constructed to examine association of genetic variation with urinary ENL excretion at baseline and after the flaxseed intervention.
Results.
SNPs in several genes were nominally (p<0.05) associated with ENL excretion at baseline and/or after intervention: ESR1, CYP1B1, COMT, CYP3A5, ARPC1A, BCL2L11, SHBG, SLCO1B1, and ZKSCAN5. A greater number of SNPs were associated among AA women than among EA women, and no SNPs were associated in both races. No SNPs-ENL associations were statistically significant after correction for multiple comparisons.
Conclusions.
Variation in several genes related to steroid hormone metabolism was associated with lignan excretion at baseline and/or after flaxseed intervention among postmenopausal women.
Impact.
These findings may contribute to our understanding of the differences observed in urinary ENL excretion among AA and EA women and thus hormone related breast cancer risk.
Introduction
Higher circulating estrogen levels are associated with increased risk of breast cancer in postmenopausal women (1–4). Modification of estrogen metabolism through dietary and supplemental intervention thus provides a potential approach for breast cancer prevention. Lignans, phytoestrogens found in plant foods, are structurally similar to endogenous steroid hormones (5) and have received considerable research attention for breast cancer prevention. Flaxseed is the richest source of lignans known to date and the use of flaxseed to increase lignan intake has been widely studied (6). In flaxseed, the predominant lignan is secoisolariciresinol diglucoside (SDG), which has low bioavailability (7, 8). As a result, SDG reaches the lower part of the GI tract where it is deglycosylated to secoisolariciresinol (SECO) and converted by the intestinal microbiota to the enterolignans enterodiol (END) and enterolactone (ENL). END is rapidly further converted to ENL, the predominant circulating lignan (8, 9).
Enterolignans have both estrogenic and anti-estrogenic effects, depending on the presence of stronger estrogens such as estradiol (10). When the concentrations of endogenous estrogens are high during reproductive ages, enterolignans can modify steroid hormone availability through competitive inhibition of estrogen receptors (ERs) and by increasing production of serum hormone-binding globulin (SHBG), thus blocking the actions of endogenous estrogens (11). Enterolignans produced from flaxseed consumption have been shown to increase urinary excretion of 2-hydroxyestrone (2OHE) to 16α- hydroxyestrone (16αOHE) ratio in postmenopausal women (12, 13), which reflects the removal of estrogen from endogenous circulation. After menopause when the levels of endogenous estrogens decrease significantly, lignans can also act as weak estrogens (14). Experimental and epidemiological studies have demonstrated that dietary phytoestrogens may be protective against menopausal symptoms and certain chronic diseases (15–18). There is also a growing body of evidence to support the health benefits of lignans in reducing breast cancer risk and improving survival among postmenopausal women (19–21). It has also been shown that lignan-based dietary intervention can alleviate treatment complications and improve the quality of life of cancer patients (22–27).
Nevertheless, not all studies support a positive association between lignan exposure and optimal health effects, which may be a result of individual variation in metabolism (28). Inconsistent associations have been reported between dietary lignan intakes and breast cancer risk which may be attributable, in part, to genetic variation (24, 29–32), Lignans are structurally similar to estrogen and undergo metabolism by sulfation and/or glucuronidation, similar to estrogens. Therefore, variation in steroid hormone metabolizing genes may also impact the effects of dietary lignan exposure. Limited research describing associations between lignans and other phytoestrogens and genetic variation support that phytoestrogen biomarker levels may be dependent upon variation in genes related to steroid hormone metabolism, e.g., CYP19, COMT, ESR1, SHBG, and CYP1B1 (13, 33–36). These findings highlight the importance of considering genetic variation in the assessment of the effects of flaxseed intervention or lignan exposure from diet. Moreover, most of the previous genetic studies have been conducted in populations of European ancestry (EA) and those from populations of African ancestry (AA) are lacking. Compared to EA women, AA women may have distinct genetic backgrounds (37), tend to have higher circulating levels of estrogens (38), and are at higher risk of aggressive breast cancer (39).
Therefore, we conducted a dietary flaxseed intervention study in healthy postmenopausal women of both African and European descent which aimed to examine associations of single nucleotide polymorphisms (SNPs) in genes important in steroid hormone metabolism with enterolignan excretion at baseline and in response to the flaxseed intervention.
Materials and Methods
Study population
Data and biospecimens for this study were obtained from a randomized, crossover flaxseed intervention study conducted between March 2012 and June 2017. All study procedures were conducted in accordance with ethical standards on human experimentation and the Helsinki Declaration of 1975 (revised 1983). The study protocol was approved by Roswell Park Comprehensive Cancer Center Institutional Review Board and all participants provided written, informed consent. The trial is registered at clinicaltrials.gov as NCT01698294.
Healthy, postmenopausal women aged 45 to 75 years, without a menstrual cycle in the past 12 months and who were from the western New York region were eligible to participate in the study. Women were excluded from the study if they had used any of the following in the 2 months prior to baseline: oral antibiotics, hormone replacement therapy, nonprescription hormones or herbal supplements for menopausal symptoms, black-cohosh, or flaxseed supplements. Participants were block randomized equally on the basis of self-reported race to either 10 g/d ground flaxseed for 6 wk, or maintenance of usual diet. After a 2-month washout period, each participant crossed over to the other diet condition for an additional 6 wk (see study design schema in Supplemental Figure 1). The study statistician generated the random allocation schedule and study coordinators assigned subjects to groups as indicated by the randomization schedule. Blinding was not possible due to the nature of the intervention and each woman served as her own control (pre-post design). Each participant was provided with one 500-g bag of ground brown flaxseed (Heartland Flax, Valley City, ND; 32.62 mg SDG/g) for the 6 wk intervention period and a standardized scoop for measuring daily flaxseed doses. Participants were instructed to consume one level scoop (approximately 10 g) of ground flaxseed per day mixed into water or juice and store the flaxseed in the refrigerator for the duration of the study. Any uneaten flaxseed was returned to monitor compliance. During the 5-month study period, participants were instructed to avoid other high dietary sources of flaxseed such as seeded bakery products and cereals. Fasting blood draws, overnight urine collections, and fecal samples were collected in the morning at baseline and after 6 wk of each crossover period, for a total of 4 collections per subject over the study period. By the end of the funding period, a total of 115 AA women and 137 EA women were recruited and randomized (Figure 1 Consort Diagram). Adverse events were generally mild and primarily related to increased gas and bloating.
Figure 1.
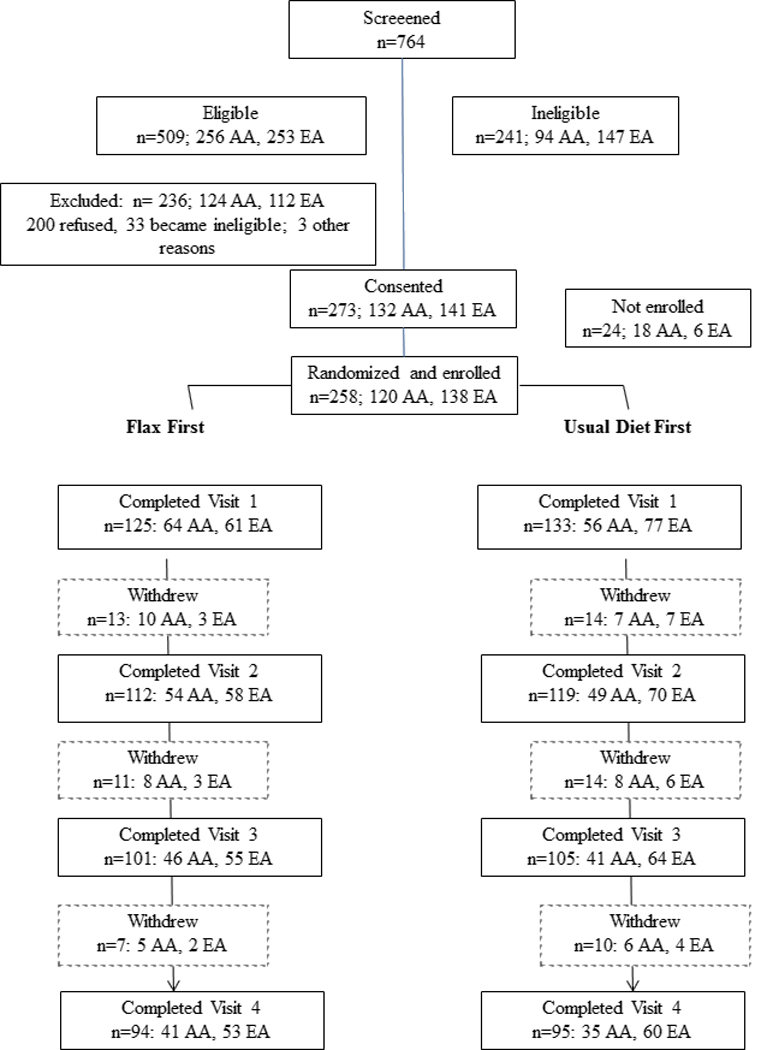
CONSORT Diagram: Recruitment, enrollment, and study completion flow diagram for a flaxseed intervention study in African American and European American women.
Data collection
During the first study visit, participants completed interviewer-administered questionnaires including demographic information, medical and reproductive histories, smoking history, medication and dietary supplement use, chronic disease history, physical activity, and other epidemiologic data relevant to diet and cancer. Anthropometric measures were obtained by study personnel using a standardized protocol. Height was obtained at visit 1 only and weight at visits 1–4. Body composition (lean and fat mass) was assessed by a bioimpedance analysis (BIA) system which has 8-contact electrodes (two on each hand and foot) allowing for rapid evaluation of whole-body and regional body composition (50 kHz BC-418, Tanita Corporation, Tokyo, Japan). Dietary data was collected via 12 telephone-administered 24-hour dietary recalls assigned randomly throughout the 5-month active intervention period using the University of Minnesota Nutrient Data System for Research (NDSR; Cary, NC) dietary analysis program.
Single nucleotide polymorphism (SNP) selection and genotyping
Genomic DNA was extracted from peripheral blood using QIAamp DNA Blood Mini-kit and stored at −80 C until analysis. Putatively functional SNPs in genes with known roles in steroid hormone metabolism pathways that have been reported in the literature were selected by surveying the HuGE Navigator Database, supplemented with top hits from several published genome-wide association studies (GWAS) of circulating steroid hormone levels, as well as SNPs in hormone metabolizing genes that were identified in GWAS of any phenotypes (40–68). A total of 70 SNPs in 29 genes (Supplemental Table 1) (ARPC1A, BAIAP2L1, BMF, COMT, CYP17A1, CYP19A1, CYP1A1, CYP1B1, CYP3A4, CYP3A5, ESR1, GCKR, HHEX, HSD17B1, JMJD1C, LHCGR, NR2F2, PRMT6, SHBG, SLCO1B1, SULT1A1, TDGF3, TSPYL5, UGT1A1, ZBTB10, ZKSCAN5, ZNF652) with a minor allele frequency (MAF) >10% in populations of either African or European ancestry were identified and multiplexed with another 60 SNPs selected as ancestry informative markers (AIMs) to assess genetic admixture. SNP genotyping was performed using the MassARRAY technology and iPLEX Gold assay (Agena BioSciences) performed by the Roswell Park Comprehensive Cancer Center Genomics Shared Resource. Blind duplicates (5%) and HapMap trio samples were included for QC purposes. The overall genotyping success call rate was 98.0%. SNPs with low call rate (<95%), poor clustering, Mendelian error, or Hardy Weinberg error were excluded. As a result, data of 116 SNPs (including 60 putatively functional SNPs and 56 as AIMs) from 252 women were included in the final analysis.
Lignan excretion
Overnight urine collections were obtained for determination of SECO, END, and ENL excretion. SECO, END, and ENL were assayed in the laboratory of Dr. Johanna Lampe (Fred Hutch, Seattle, WA) by isotope dilution gas chromatography-mass spectrometry in the SIM mode (HP 6890 GC, HP 5973 MSD). The methods are based on Adlercreutz et al. (69) and adapted in Dr. Lampe’s lab (22). To adjust for variation in urine output, all urinary lignan variable concentrations were normalized to creatinine concentrations. All samples from each participant were analyzed together in a batch, and participants were randomly assigned to batches by race and treatment group. As ENL is the primary circulating enterolignan, our analyses here focus on this metabolite.
Statistical analysis
Baseline ENL levels were log transformed for analysis and reported after back transformation from the logarithmic scale to the original scale of measurement. Paired t-tests were utilized to examine the intervention effect, which measures the differences in urinary ENL levels between before (visit 1 for group 1 and visit 3 for group 2) and after (visit 2 for group 1 and visit 4 for group 2) flaxseed intervention. Carry-over effect was also assessed using a paired t-test for treatment group 1 (flaxseed intervention during visit 1–2 and usual diet during visit 3–4). The difference in pre- to post-intervention change in ENL values between the two treatment groups was examined using t-test. A repeated measures ANOVA was performed to test the stability of non-intervention measurements of urinary ENL (visit 1, 3, and 4 for group 1 and visit 1, 2, and 3 for group 2).
Proportion of genetic ancestry was estimated using STRUCTURE v2.3.4 (70–72), with HapMap data from ASW (Southwest USA), YRI (Yoruba in Ibadan, Nigeria) and CEU (Utah residents with Northern and Western European ancestry from the CEPH collection) included in the analysis as reference populations. The proportion of European ancestry among the AA women in the study was relatively low, with an average of 2.8%. There were a few AA cases (> 10%, n=8) with high European admixture, as high as 54%, but, in general, the subjects were genetically homogenous within self-reported race with little evidence of admixture. The estimated proportion of European ancestry was related to baseline ENL levels and changes upon intervention, and was used a covariate in genetic association tests.
To investigate the impact of genetic variation on baseline ENL excretion, we used linear regression models. Change in ENL levels across the four visits were assessed by repeated measures mixed models. By design, only one of the four visits was post-intervention, while the other three were visits with usual diet. Analysis of variance revealed similar levels of ENL excretion across non-intervention visits; hence, these visits were all coded as 0 in the mixed models whereas the post intervention visits (visit 2 in group 1 and visit 4 in group 2) were coded as 1. Separate models were fit for each SNP, assuming an additive genetic model. Covariates including baseline age and body mass index (BMI) were adjusted in all models and adjustment for multiple testing was accomplished by using a Bonferroni adjusted p-value threshold of 8.3e-4 after correcting for a total of 60 SNPs. Before fitting the final mixed models, the interaction of treatment visit and group was examined and showed no significant interaction effects. All analyses were performed in R3.4.4 and 2-sided p-value of 0.05 was considered significant, if not otherwise stated.
Results
The baseline demographic characteristics of the participants are shown in Table 1. Compared to EA women, AA women were less likely to achieve higher education, be married, or have a healthier BMI, and more likely to be current smokers (p<0.0001). Due to the differences between these two populations, we stratified subsequent analyses by race. Of the 258 enrolled (120 AA and 138 EA), 190 (74%; 63% AA and 83% EA) completed all 4 visits and 252 had complete data for the present analyses. The most prevalent reason for not completing the study was that the participant became ineligible (antibiotics or other medical reason) and were removed from the study (40.2%) followed by a failure to enroll, i.e., the participant did not return after the consent visit (23.5%), or the participant did not return after enrollment (17.7%). With the exception of race, no significant differences were found in baseline characteristics between those who completed the study and those who did not (Table 1).
Table 1.
Baseline characteristics of participants in the flaxseed intervention study
| Overall (n=252) |
AA (n=115) |
EA (n=137) |
Completed study (n=188) |
AA (n=76) |
EA (n=112) |
|
|---|---|---|---|---|---|---|
| Mean±SD | ||||||
| Age, y | 59.4±6.2 | 59.2±6.0 | 50.7±6.3 | 59.7±6.2 | 59.3±6.2 | 60.0±6.2 |
| BMI†; kg·m−2§ | 30.5±7.6 | 33.7±7.8) | 27.8±6.3 | 30.1±7.6 | 33.2±8.0 | 27.9±6.6 |
| n (%) | ||||||
| Education§ | ||||||
| High school* | 64 (25.4) | 40 (34.8) | 24 (17.5) | 45 (23.9) | 26(34.2) | 19 (17.0) |
| Some college | 85 (33.7) | 51 (44.4) | 34 (24.8) | 60 (31.9) | 33 (43.4) | 27 (24.1) |
| Bachelor degree | 56 (22.2) | 14 (12.2) | 42 (30.7) | 41 (21.8) | 10 (13.2) | 31 (27.7) |
| Graduate degree | 47 (18.7) | 10 (8.7) | 37 (27.0) | 42 (22.3) | 7 (9.2) | 35 (31.3) |
| Marital status§ | ||||||
| Never married | 45 (17.9) | 33 (28.7) | 12 (8.8) | 29 (15.4) | 19 (25.0) | 10 (8.9) |
| Married | 98 (38.9) | 20 (17.4) | 78 (56.9) | 80 (42.6) | 16 (21.1) | 64 (57.1) |
| Widowed | 28 (11.1) | 18 (15.7) | 10 (7.3) | 21 (11.2) | 13 (17.1) | 8 (7.1) |
| Divorced/separated | 81 (32.1) | 44 (38.3) | 37 (27.0) | 58 (30.9) | 28 (36.8) | 30 (26.8) |
| Smoking status§ | ||||||
| Current | 50 (19.8) | 41 (35.7) | 9 (6.6) | 34 (18.1) | 26 (34.2) | 8 (7.1) |
| Former | 85 (33.7) | 29 (25.2) | 56 (40.9) | 65 (34.6) | 20 (26.3) | 45 (40.2) |
| Never | 117 (46.4) | 45 (39.1) | 72 (52.6) | 89 (47.3) | 30 (39.5) | 59 (52.7) |
| BMI category§ | ||||||
| Normal (<25) | 60 (23.8) | 12 (10.4) | 48 (35.0) | 48 (25.5) | 8 (10.5) | 40 (35.7) |
| Overweight (25–30) | 71 (28.2) | 23 (20.0) | 48 (35.0) | 51 (27.1) | 16 (21.0) | 35 (31.3) |
| Obese (≥30) | 121 (48.0) | 80 (69.6) | 41 (30.0) | 89 (47.3) | 52 (68.4) | 37 (33.0) |
Body mass index
Differences by race and study completion assessed with t-tests for continuous variables and Chi-square for categorical variables
p<0.0001 for differences by race; no significant differences were observed by study completion status
Includes 13 subjects (10 complete study) in AA who did not finish high school; no EA subjects had education <high school
Mean ENL excretion levels across the four study visits are shown for treatment group 1 and group 2 in Figures 2a and 2b, respectively. Compared to AA women, EA women had higher mean ENL excretion levels at each visit. After the flaxseed intervention, ENL excretion levels were significantly elevated, and the magnitude of the increase was similar between the two treatment groups and the two races. ENL levels remained stable among the three habitual time-points, with no significant carry-over effect observed.
Figure 2.
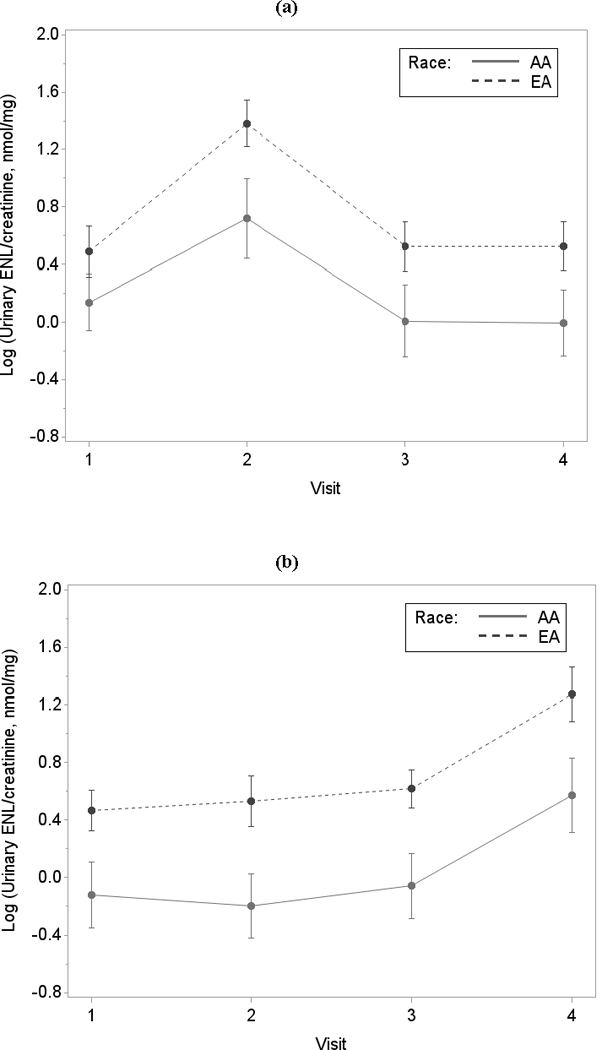
Figure 2 details the mean (95% confidence intervals) log-transformed creatinine-adjusted urinary enterolactone levels across visits in AA and EA for (a) 93 participants (41 AA and 52 EA) in treatment group 1; and for (b) 95 participants (35 AA and 60 EA) in treatment group 2
Supplemental Table 1 provides the information of the selected SNPs in the analysis and Supplemental Tables 2 and 3 detail the associations of the selected SNPs with ENL excretion levels at baseline and with change in excretion after flaxseed intervention, stratified by race and adjusted for age and BMI. The p-values for these associations are summarized in Figures 3a and 3b (baseline ENL excretion) and Figures 4a and 4b (change in excretion after flaxseed intervention). Among EA women, baseline ENL excretion was associated with rs6738028 in BCL2L11, rs4869739 in ESR1, and rs6259 in SHBG. Collectively, these 3 SNPs explained 12% of the variation in ENL excretion. Different SNPs were associated with baseline ENL excretion in AA women: rs3020418 in ESR1, rs740160 in ARPC1A, rs11761528 in ZSCAN5, rs11974702 and rs7809615 in CYP3A5, and rs4633 in COMT which collectively explained 16% of the variation in ENL excretion. There was no overlap in the SNPs associated with ENL excretion between EA and AA women. It should be noted that none of the above associations remained statistically significant after correction for multiple testing, and none were significant in both AA and EA women.
Figure 3.
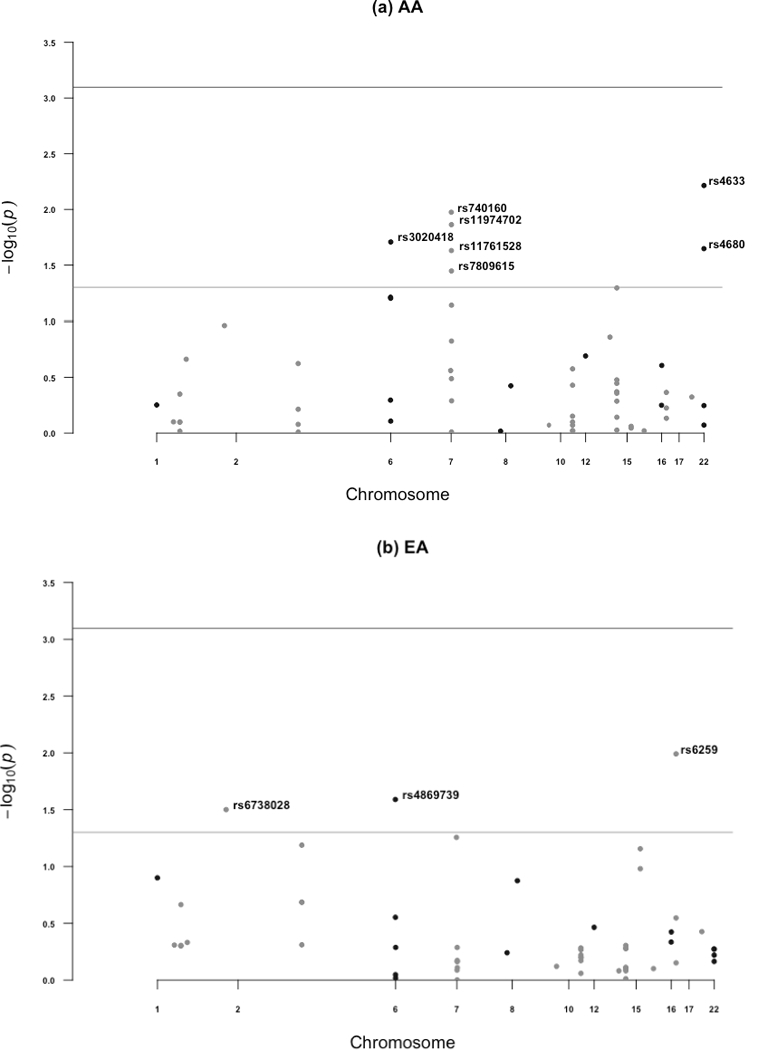
Manhattan plots summarizing p-values from associations between SNPs and baseline urinary enterolactone excretion, adjusted for baseline age and BMI in (a) AA and (b) EA. In both figures, the light gray horizontal line represents p=0.05; the darker gray horizontal line represents Bonferroni corrected statistical significance.
Figure 4.
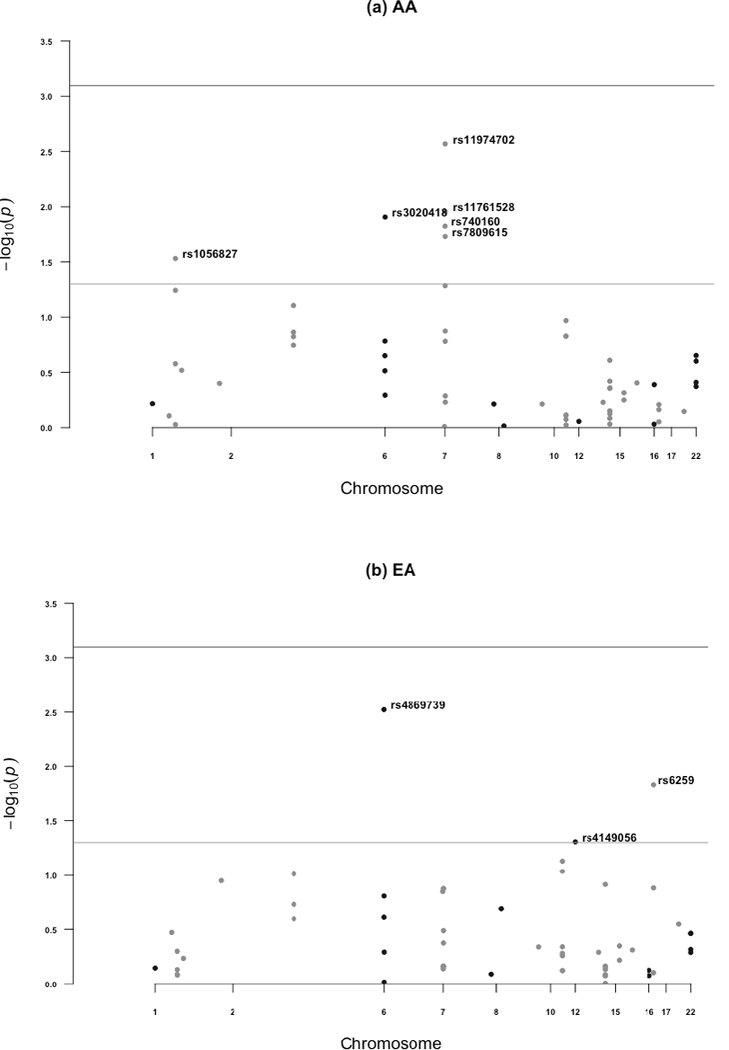
Manhattan plots summarizing p-values from associations between SNPs and post-intervention urinary enterolactone excretion, adjusted for baseline age and BMI in (a) AA and (b) EA. In both figures, the light gray horizontal line represents p=0.05; the darker gray horizontal line represents Bonferroni corrected statistical significance
Several SNPs were also nominally associated with ENL excretion in response to the flaxseed intervention. Among EA women, ENL excretion after flaxseed intervention was associated with rs4869739 in ESR1, rs6259 in SHBG, and rs4149056 in SLCO1B1. These 3 SNPs explain 17% of the variation in ENL excretion in response to the flaxseed intervention. Among AA women, several different SNPs were associated with ENL excretion after the intervention: rs1056827 in CYP1B1, rs3020418 in ESR1, rs740160 in ARPC1A, rs11761528 in ZSCAN5, and rs11974702 and rs7809615 in CYP3A5. These 6 SNPs explained 13% of the variation in ENL excretion after flaxseed consumption. However, none of the SNPs were shared across the two groups.
Among AA women, with the exception of rs4633 in COMT and rs1056827 in CYP1B1, variation in SNPs associated with ENL excretion at baseline were also associated with excretion after the intervention. Similarly, among EA women, variation in rs4869739 in ESR1 and rs6259 in SHBG was associated with both baseline and post-intervention ENL excretion.
Discussion
Few previous studies have investigated the impact of genetic variation on urinary ENL excretion and individual differential response to lignan exposure (13, 28, 73, 74), and, to our knowledge, none have included substantial numbers of AA participants. In the present study, we were able to examine a large number of SNPs in genes related to steroid hormone metabolism, thus expanding on previous investigations. We observed several SNPs associated with ENL excretion, and different SNPs were identified in the two populations. Further, the impact of genotype on excretion was comparable regardless of whether we examined excretion at baseline or in response to the intervention. Given this consistency and that we also observed lower ENL excretion at all time-points for AA compared to EA women, our finding suggest that genetic variation may, in part, be involved in the racial differences in ENL excretion.
We observed considerable disparities by race for the effect of genetic variations on urinary ENL excretion. Consistent with some previous studies (33, 75), polymorphisms in ESR1 were associated with ENL excretion (p < 0.05) among both AAs and EAs, although different SNPs were identified in each population. Notably, the allele frequencies for both ESR1 rs4869739 and rs3020418 differ drastically by race, with the minor allele in one population being the major allele in the other population. Relatively little is known about the functional significance of these two ESR1 SNPs. In a GWAS meta-analysis in the GEFOS/GENOMOS Consortium, lower bone density was observed in participants with greater prevalence of the variant alleles in rs4869739. The other SNP rs3020418 was identified in another GWAS of human height, with the A allele associated with greater height, which is also much more common in the AA population. ESR1 codes for the estrogen receptor type 1 which is a DNA-binding transcription factor and also has ligand-binding properties (53). Lignans are weak phytoestrogens that can function as competitive inhibitors of endogenous estrogen binding. If the variant alleles are resulting in reduced availability of receptors for binding of estrogens and/or lignans, increased availability of circulating lignans could be channeled into enterohepatic recycling for eventual excretion.
In addition to polymorphisms in ESR1, among AA women, we also found urinary ENL excretion associated with SNPs in several hormone metabolizing genes including CYP3A5, COMT, and CYP1B1. There is limited research on steroid hormone metabolism related genes and lignan metabolism, especially for AAs. A previous flaxseed intervention study reported that the changes in the 2OHE1:16OHE1 ratio increased with increasing numbers of variant alleles in CYP1B1 and COMT genes in predominantly white postmenopausal women, which supported the putatively protective functions of these SNPs (13). CYP1B1 contributes to hydroxylation of E1 at the 4- and 2-positions, and variations in this gene have been shown to have higher activities (76). COMT, in particular, contributes to the excretion of hydroxylated estrogen metabolites through methylation and subsequent excretion (77). Given the structural similarity of ENL to estradiol, similar mechanisms may affect lignan excretion. In the present study, associations between ENL excretion and variation in CYP1B1 and COMT were observed only among AA women. This may help explain the lower urinary ENL levels in AA compared to EA women in the study, and may also attribute to the higher circulating steroid hormone levels in AA women than EAs found in previous literature (78). Thus, further research on the SNPs in these two genes is of great importance to investigate the race disparity in hormone-related cancers.
Regarding postmenopausal EA women, three polymorphisms in SHBG, BCL2L11 and SLCO1B1 were associated with lignan metabolism in the study (p < 0.05). We found that the variant allele A of rs6259 in SHBG was inversely associated with urinary ENL excretion both at baseline and in response to FS intervention. Similar inverse association was also shown between rs4149056 polymorphism in SLCO1B1 with ENL excretion after exposure to a lignan-rich diet. SLCO1B1 gene encodes the protein solute carrier organic anion transporter family member 1B1 (SLCO1B1), which assists the transport of compounds from the blood into the liver. A GWAS found significant relationships between rs4149056 variant and estrone conjugate (E1C) plasma concentrations (79). The study suggested that this variant resulted in reduced transporter activity and higher E1C plasma concentrations. Given that lignans have properties similar to estrogen, the same mechanism may exist for ENL. On the other hand, previous studies investigating lignan exposure and breast cancer risk have varied in assessment methods (dietary intake vs. enterolignan excretion) which may also contribute to inconsistencies in associations. Given our experimental design, this is unlikely to be a concern in the present study. However, future investigations of lignan exposure and breast cancer risk will need to give appropriate consideration to exposure assessment.
A potential limitation of this study is the small sample size and the lack of a replication population. Also, because of the relatively small sample size, a GWAS approach was not feasible, and we relied on a candidate SNP approach to focus on major contributors to steroid hormone metabolism and xenobiotic metabolism. Further, SNPs were chosen to have minor allele frequency (>10%) sufficient for statistical analysis, and thus the influence of less common SNPs cannot be studied.
Our study has several notable strengths. We utilized a cross-over design where each participant serves as her own control, and thus the influence of confounding covariates is reduced. Notably, this study is reasonably comprehensive research to investigate genetic effects on lignan metabolism. We included a relatively large number of SNPs in related genes, and involved both AA and EA populations. In addition, we performed ancestral analysis to detect hidden population substructure, which makes our findings of race disparities more reliable. Although none of the selected SNPs were significantly associated with ENL excretion after correction for multiple comparisons, the results of this study nevertheless may shed light on the potential impact of genetic variation on lignan metabolism of postmenopausal women and stimulate further research.
In summary, we observed that variation in a number of genes related to steroid hormone metabolism were associated with lignan excretion at baseline and after flaxseed intervention among postmenopausal women, which may help explain the difference in urinary ENL levels among AAs and EAs. To our knowledge, this is the first report suggesting potential explanations related to genetic variation for racial disparities in lignan metabolism among AA and EA postmenopausal women. Future chemoprevention and diet intervention strategies to reduce breast cancer incidence and mortality may benefit by incorporation of genetic variation and examination by race.
Supplementary Material
Acknowledgements.
This work was supported by National Cancer Institute (NCI) grant U01CA161809(S. McCann, J. Lampe, M. Hullar, S. Yao, D. Tritchler) and grant P30CA016056 (S. McCann,S. Yao) involving the use of Roswell Park Comprehensive Cancer Center’s Databank and Biorepository and the Genomics Shared Resource.
Contributor Information
Huiru Chang, Department of Biostatistics, University at Buffalo, Buffalo, NY.
Song Yao, Department of Cancer Prevention and Control, Roswell Park Comprehensive Cancer Center, Buffalo, NY.
David Tritchler, Department of Biostatistics, University at Buffalo, Buffalo, NY.
Meredith A. Hullar, Fred Hutchinson Cancer Research Center, Seattle, WA
Johanna W. Lampe, Fred Hutchinson Cancer Research Center, Seattle, WA
Lilian U. Thompson, Department of Nutritional Sciences, University of Toronto, Toronto, Canada
Susan E. McCann, Department of Cancer Prevention and Control, Roswell Park Comprehensive Cancer Center, Buffalo, NY .
Reference List
- (1).Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 1998;90(17):1292–9. [DOI] [PubMed] [Google Scholar]
- (2).Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer 2005;12(4):1071–82. [DOI] [PubMed] [Google Scholar]
- (3).Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94(8):606–16. [DOI] [PubMed] [Google Scholar]
- (4).Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst 2004;96(24):1856–65. [DOI] [PubMed] [Google Scholar]
- (5).Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed influences urinary lignan excretion in a dose-dependent manner in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2000;9(10):1113–8. [PubMed] [Google Scholar]
- (6).Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr 2005;93(3):393–402. [DOI] [PubMed] [Google Scholar]
- (7).Landete JM. Plant and mammalian lignans: a review of sourve, intake, metabolism, intestinal bacteria and health. Food Res International 2012;46:410–24. [Google Scholar]
- (8).Quartieri A, Garcia-Villalba R, Amaretti A, et al. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol Nutr Food Res 2016;60(7):1590–601. [DOI] [PubMed] [Google Scholar]
- (9).Landete JM, Arques J, Medina M, et al. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit Rev Food Sci Nutr 2016;56(11):1826–43. [DOI] [PubMed] [Google Scholar]
- (10).Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood ) 2005;230(8):558–68. [DOI] [PubMed] [Google Scholar]
- (11).Mense SM, Hei TK, Ganju RK, Bhat HK. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Perspect 2008;116(4):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Brooks JD, Ward WE, Lewis JE, et al. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr 2004;79(2):318–25. [DOI] [PubMed] [Google Scholar]
- (13).McCann SE, Wactawski-Wende J, Kufel K, et al. Changes in 2-hydroxyestrone and 16alpha-hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiol Biomarkers Prev 2007;16(2):256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer 2001;39(1):58–65. [DOI] [PubMed] [Google Scholar]
- (15).Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer 2002;43(2):187–92. [DOI] [PubMed] [Google Scholar]
- (16).Chen J, Wang L, Thompson LU. Flaxseed and its components reduce metastasis after surgical excision of solid human breast tumor in nude mice. Cancer Lett 2006;234(2):168–75. [DOI] [PubMed] [Google Scholar]
- (17).Chen MN, Lin CC, Liu CF. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric 2015;18(2):260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Edel AL, Patenaude AF, Richard MN, et al. The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young, healthy adults. Eur J Nutr 2016;55(2):651–63. [DOI] [PubMed] [Google Scholar]
- (19).McCann SE, Thompson LU, Nie J, et al. Dietary lignan intakes in relation to survival among women with breast cancer: the Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Res Treat 2010;122(1):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Seibold P, Vrieling A, Johnson TS, et al. Enterolactone concentrations and prognosis after postmenopausal breast cancer: assessment of effect modification and meta-analysis. Int J Cancer 2014;135(4):923–33. [DOI] [PubMed] [Google Scholar]
- (21).Velentzis LS, Cantwell MM, Cardwell C, et al. Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies. Br J Cancer 2009;100(9):1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Atkinson C, Lampe JW, Scholes D, et al. Lignan and isoflavone excretion in relation to uterine fibroids: a case-control study of young to middle-aged women in the United States. Am J Clin Nutr 2006;84(3):587–93. [DOI] [PubMed] [Google Scholar]
- (23).Franco OH, Burger H, Lebrun CE, et al. Higher dietary intake of lignans is associated with better cognitive performance in postmenopausal women. J Nutr 2005;135(5):1190–5. [DOI] [PubMed] [Google Scholar]
- (24).Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer. Lancet 1997;350(9083):990–4. [DOI] [PubMed] [Google Scholar]
- (25).Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr 2013;32(5):671–8. [DOI] [PubMed] [Google Scholar]
- (26).Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 1998;83(7):2223–35. [DOI] [PubMed] [Google Scholar]
- (27).Touillaud MS, Thiebaut AC, Fournier A, et al. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst 2007;99(6):475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Low YL, Tai ES. Understanding diet-gene interactions: Lessons from studying nutrigenomics and cardiovascular disease. Mutat Res 2007. [DOI] [PubMed] [Google Scholar]
- (29).Keinan-Boker L, van der Schouw YT, Grobbee DE, Peeters PH. Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr 2004;79(2):282–8. [DOI] [PubMed] [Google Scholar]
- (30).Linseisen J, Piller R, Hermann S, Chang-Claude J. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int J Cancer 2004;110(2):284–90. [DOI] [PubMed] [Google Scholar]
- (31).McCann SE, Muti P, Vito D, et al. Dietary lignan intakes and risk of pre- and postmenopausal breast cancer. Int J Cancer 2004;111(3):440–3. [DOI] [PubMed] [Google Scholar]
- (32).McCann SE, Kulkarni S, Trevisan M, et al. Dietary lignan intakes and risk of breast cancer by tumor estrogen receptor status. Breast Cancer Res Treat 2006. [DOI] [PubMed] [Google Scholar]
- (33).Low YL, Dunning AM, Dowsett M, et al. Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiol Biomarkers Prev 2007;16(5):1009–16. [DOI] [PubMed] [Google Scholar]
- (34).McCann SE, Moysich KB, Freudenheim JL, Ambrosone CB, Shields PG. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J Nutr 2002;132(10):3036–41. [DOI] [PubMed] [Google Scholar]
- (35).Piller R, Verla-Tebit E, Wang-Gohrke S, Linseisen J, Chang-Claude J. CYP17 Genotype Modifies the Association between Lignan Supply and Premenopausal Breast Cancer Risk in Humans. J Nutr 2006;136(6):1596–603. [DOI] [PubMed] [Google Scholar]
- (36).Sonestedt E, Ivarsson MI, Harlid S, et al. The Protective Association of High Plasma Enterolactone with Breast Cancer Is Reasonably Robust in Women with Polymorphisms in the Estrogen Receptor {alpha} and {beta} Genes. J Nutr 2009. [DOI] [PubMed] [Google Scholar]
- (37).Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science 2002;296(5576):2225–9. [DOI] [PubMed] [Google Scholar]
- (38).Marsh EE, Shaw ND, Klingman KM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab 2011;96(10):3199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ademuyiwa FO, Olopade OI. Racial differences in genetic factors associated with breast cancer. Cancer Metastasis Rev 2003;22(1):47–53. [DOI] [PubMed] [Google Scholar]
- (40).Law MH, Bishop DT, Lee JE, et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet 2015;47(9):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Goes FS, McGrath J, Avramopoulos D, et al. Genome-wide association study of schizophrenia in Ashkenazi Jews. Am J Med Genet B Neuropsychiatr Genet 2015;168(8):649–59. [DOI] [PubMed] [Google Scholar]
- (42).Ruth KS, Campbell PJ, Chew S, et al. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur J Hum Genet 2016;24(2):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Varenhorst C, Eriksson N, Johansson A, et al. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur Heart J 2015;36(29):1901–12. [DOI] [PubMed] [Google Scholar]
- (44).Korostishevsky M, Steves CJ, Malkin I, et al. Genomics and metabolomics of muscular mass in a community-based sample of UK females. Eur J Hum Genet 2016;24(2):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).He M, Xu M, Zhang B, et al. Meta-analysis of genome-wide association studies of adult height in East Asians identifies 17 novel loci. Hum Mol Genet 2015;24(6):1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Fejerman L, Ahmadiyeh N, Hu D, et al. Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun 2014;%20;5:5260. doi: 10.1038/ncomms6260.:5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Cornelis MC, Byrne EM, Esko T, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry 2015;20(5):647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Wood AR, Esko T, Yang J, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 2014;46(11):1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lu X, Wang L, Lin X, et al. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet 2015;24(3):865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Rietveld CA, Esko T, Davies G, et al. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proc Natl Acad Sci U S A 2014;111(38):13790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511(7510):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Shin SY, Fauman EB, Petersen AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet 2014;46(6):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Moayyeri A, Hsu YH, Karasik D, et al. Genetic determinants of heel bone properties: genome-wide association meta-analysis and replication in the GEFOS/GENOMOS consortium. Hum Mol Genet 2014;23(11):3054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Chen Z, Tao S, Gao Y, et al. Genome-wide association study of sex hormones, gonadotropins and sex hormone-binding protein in Chinese men. J Med Genet 2013;50(12):794–801. [DOI] [PubMed] [Google Scholar]
- (55).Michailidou K, Hall P, Gonzalez-Neira A, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet 2013;45(4):353–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Liu M, Ingle JN, Fridley BL, et al. TSPYL5 SNPs: association with plasma estradiol concentrations and aromatase expression. Mol Endocrinol 2013;27(4):657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Coviello AD, Haring R, Wellons M, et al. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple Loci implicated in sex steroid hormone regulation. PLoS Genet 2012;8(7):e1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Prescott J, Thompson DJ, Kraft P, et al. Genome-wide association study of circulating estradiol, testosterone, and sex hormone-binding globulin in postmenopausal women. PLoS One 2012;7(6):e37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478(7367):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Suhre K, Shin SY, Petersen AK, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011;477(7362):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Kato N, Takeuchi F, Tabara Y, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet 2011;43(6):531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Zhai G, Teumer A, Stolk L, et al. Eight common genetic variants associated with serum DHEAS levels suggest a key role in ageing mechanisms. PLoS Genet 2011;7(4):e1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Lango AH, Estrada K, Lettre G, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010;467(7317):832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Kang TW, Kim HJ, Ju H, et al. Genome-wide association of serum bilirubin levels in Korean population. Hum Mol Genet 2010;19(18):3672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Imielinski M, Baldassano RN, Griffiths A, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet 2009;41(12):1335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009;41(6):666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Johnson AD, Kavousi M, Smith AV, et al. Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet 2009;18(14):2700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Adlercreutz H, Fotsis T, Bannwart C, et al. Isotope dilution gas chromatographic-mass spectrometric method for the determination of lignans and isoflavonoids in human urine, including identification of genistein. Clin Chim Acta 1991;199(3):263–78. [DOI] [PubMed] [Google Scholar]
- (70).Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 2003;164(4):1567–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 2007;7(4):574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 2009;9(5):1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Low YL, Taylor JI, Grace PB, et al. Phytoestrogen exposure, polymorphisms in COMT, CYP19, ESR1, and SHBG genes, and their associations with prostate cancer risk. Nutr Cancer 2006;56(1):31–9. [DOI] [PubMed] [Google Scholar]
- (74).Sonestedt E, Borgquist S, Ericson U, et al. Enterolactone is differently associated with estrogen receptor beta-negative and -positive breast cancer in a Swedish nested case-control study. Cancer Epidemiol Biomarkers Prev 2008;17(11):3241–51. [DOI] [PubMed] [Google Scholar]
- (75).Beckmann L, Husing A, Setiawan VW, et al. Comprehensive analysis of hormone and genetic variation in 36 genes related to steroid hormone metabolism in pre- and postmenopausal women from the breast and prostate cancer cohort consortium (BPC3). J Clin Endocrinol Metab 2011;96(2):E360–E367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Paracchini V, Pedotti P, Raimondi S, et al. A common CYP1B1 polymorphism is associated with 2-OHE1/16-OHE1 urinary estrone ratio. 43 ed. 2005. p. 702–6. [DOI] [PubMed] [Google Scholar]
- (77).Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996;6(3):243–50. [DOI] [PubMed] [Google Scholar]
- (78).Dunn BK, Agurs-Collins T, Browne D, Lubet R, Johnson KA. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat 2010;121(2):281–92. [DOI] [PubMed] [Google Scholar]
- (79).Dudenkov TM, Ingle JN, Buzdar AU, et al. SLCO1B1 polymorphisms and plasma estrone conjugates in postmenopausal women with ER+ breast cancer: genome-wide association studies of the estrone pathway. Breast Cancer Res Treat 2017;164(1):189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


