Abstract
Background:
There is preliminary evidence linking physical activity to better prostate cancer (PCa) outcomes, though the molecular mechanisms underlying this association are not clear.
Methods:
In a Seattle-based cohort of patients diagnosed with clinically localized PCa and prospective follow-up for outcomes (n=1354), we studied the association between self-reported vigorous physical activity and PCa progression to a metastatic-lethal phenotype. A subset of patients have prostate cancer tissue samples available for investigating DNA methylation (Infinium® HumanMethylation450 BeadChip array) and exercise (n=524).
Results:
Patients who had vigorous physical activity at least once per week during the year before diagnosis (~79% of the cohort) were significantly less likely to progress to metastatic-lethal PCa compared to those who had vigorous physical activity less frequently (adjusted hazard ratio =0.63, p-value=0.029). Among the subset of men who had radical prostatectomy as primary treatment and tumor tissue available, a differentially methylated region (DMR) was identified (family-wise error rate=0.03, hypo-methylated in the weekly exercise group), with 9 methylation probes located in the promoter region of CRACR2A. This gene encodes a calcium binding protein involved in innate immune response. The methylation level of the nine CpGs was inversely correlated with CRACR2A gene expression (average correlation coefficient= – 0.35).
Conclusions:
Vigorous physical activity before diagnosis is associated with epigenetic alterations of CRACR2A and PCa metastatic lethal progression.
Impact:
This analysis provides strong evidence for the association between vigorous physical activity and a less likelihood to develop metastatic lethal progression, and a suggestive link between exercise and DNA methylation in CRACRA2A gene.
Keywords: Physical activity, prostate cancer, DNA methylation, epigenetics
INTRODUCTION
Prostate cancer (PCa) is the most frequently diagnosed cancer among men in the United States (1–2). There are also close to 3 million prostate cancer survivors in the US, yet over 27,000 men die each year from the disease (1–2). More than 80% of PCa patients are diagnosed with localized disease with excellent long-term survival. However, a subset of men with localized PCa will progress to develop metastasis and cancer-specific mortality. Among men diagnosed with clinically localized PCa, there is growing evidence linking physical activity with better outcomes (3–5), including a reduction in cancer progression (3), PCa-specific mortality (4–5), and all-cause mortality (4–5); however, the molecular pathways underlying these associations remain unclear. The hypothesized mechanisms include physical activity inducing alterations in circulating factors such as insulin-like grow factor I (IGF1), inflammatory cytokines and tumor vascularization that may inhibit proliferation and promote apoptosis of prostate cancer cells (6–9).
Numerous studies have been conducted to investigate exercise-induced molecular alterations, including DNA methylation in blood and some cancer tissues (10). While observational studies have reported weak correlations between physical activity and DNA methylation, interventional studies have consistently identified a significant impact of exercise on DNA methylation of a number of genes involved in metabolism, muscle growth and inflammation across different tissues (muscle, adipose, blood). This impact may depend on the genetic pathways involved, tissue-specificity, and intensity of exercise. The DNA methylation changes associated with exercise have not always correlated with changes in gene expression (10). For PCa, it has been observed that regular exercise improves patient outcomes. It is therefore of interest to investigate alterations of DNA methylation induced by exercise and explore potential beneficial effects of exercise that may be mediated by DNA methylation. One study reported that transcripts of cell cycle and DNA repair genes may be modulated in normal prostate tissue by vigorous activity (11). However, we are not aware of any studies that have focused on exercise and DNA methylation in primary PCa tissues.
We hypothesize that physical activity alters DNA methylation profiles in prostate tumor tissue, and these changes may be molecular intermediaries that lead to better PCa outcomes. To test this hypothesis we conducted a methylome-wide analysis in a cohort of men with localized PCa for whom self-reported vigorous physical activity data before diagnosis and long-term follow-up data for metastatic lethal progression were both available. The study aimed to investigate the relationship between vigorous physical activity and PCa metastatic lethal progression, to identify differentially methylated positions (DMPs) and regions (DMRs) in prostate tumor tissue that are associated with vigorous physical activity, and to assess whether the changes in methylation, if any, were associated with corresponding changes in mRNA expression and metastatic lethal progression.
Materials and Methods
Study population
Participants of two previously described population-based studies of men with histologically confirmed, clinically localized PCa (n=1354) were included in the analysis (12–13). Men from the first study were diagnosed at ages 40 to 64 years during 1993–1996 and men from the second study were diagnosed at ages 35 to 74 years during 2002–2005. Information on demographics, medical history and environmental/lifestyle exposures up to and including the date of diagnosis was collected by in-person interviews occurring within 1 year after diagnosis (median elapse time from diagnosis to interview is 254 days), and men were asked for consent to access medical records and tumor tissue collected at biopsy and surgery. Vigorous physical activities were defined to be any type of leisure time activities that last more than 20 minutes or work up for a sweat in the questionnaire. Data collected on number of days in a week having vigorous physical activity in the year prior to PCa diagnosis were analyzed. Light and moderate activity data were not collected in one of the two studies and therefore not analyzed. Men were grouped into three categories of vigorous physical activity frequency in this analysis— greater than 3 times a week, 1–3 time a week, or <1 time per week vigorous physical activity. Gleason score, PSA at diagnosis, tumor stage, and primary treatment were collected from the SEER cancer registry. Prostate cancer recurrence status was determined from prospectively collected information from follow-up surveys, review of medical records, and/or physician follow-up as needed. Metastatic progression was confirmed by positive bone scan, MRI, CT, or biopsy. In this analysis, patients who developed metastases or died from prostate cancer were combined to a metastatic-lethal phenotype category. This study was approved by Fred Hutchinson Cancer Research Center’s Institutional Review Board, and all subjects signed informed consent.
Tumor sample preparation, methylation and gene expression profiling
Tumor sample collection was restricted to the subset of men who had radical prostatectomy as their primary treatment and for whom formalin-fixed paraffin-embedded (FFPE) prostatectomy specimens were available (n=566). FFPE blocks from radical prostatectomy specimens were used to make H&E slides, which were reviewed by a prostate pathologist to confirm the presence and location of PCa. Areas containing ≥75% tumor tissue were marked and two 1-mm cores were taken from the dominant cancer focus for DNA (using RecoverAll® Total Nucleic Acid Isolation Kit—Applied Biosciences) and RNA (RNeasy® FFPE Kit—Qiagen Inc.) purification.
Tumor DNA samples were bisulfite converted using the EZ DNA methylation kit (Zymo Research). The Infinium® Human Methylation450 BeadChip (Illumina) measured genome-wide CpG methylation using beads with target-specific probes designed to interrogate >485,000 CpG sites (14). The correlations between blind duplicates ranged from 0.96 to 0.99, and were >0.99 for replicates across plates. Subset-quantile within array normalization (SWAN) (15) and batch adjustment through ComBat (16) were completed. After processing, methylation profiles on 478,998 CpG sites were available for 523 men. The DNA methylation β-value ranges from 0 (unmethylated) to 1 (fully methylated) as an estimate of the percentage of DNA in the tissue sample that is methylated at each CpG probe.
Matched tumor gene expression data for >29,000 transcripts were available for 469 (90%) men with methylation data. The Whole-Genome cDNA-mediated Annealing, Selection, extension and Ligation (DASL®) HT Assay (Illumina) developed specifically for use with archival FFPE specimens was used (17). Data were quantile normalized, log2 transformed and batch effects were removed using ComBat (16). The procedures for quality control and data processing have been described elsewhere (18).
Statistical methods
Descriptive statistics were used to summarize the demographic and clinical characteristics of patients stratified by weekly exercise frequency. Survival analysis and the Cox proportional hazards model were employed to assess the association between physical activity and the risk of metastatic-lethal progression, adjusting for relevant covariates. Covariates that may be associated with exercise were described in Table 1. A subset of these covariates who had p-value less than 0.1 in the Cox model were retained for adjustment, including age, smoking, education, study, Gleason sum, PSA level, and primary treatment. We identified individual differentially methylated positions (DMPs) by vigorous physical activity using linear regression coupled with empirical Bayes shrinkage as implemented in the Bioconductor limma package (19). We adjusted for body mass index, age at diagnosis, smoking history, race, and study. The differentially methylated region (DMR) analysis was conducted by the bumphunting method implemented in the Bioconductor Minfi package (20). We also evaluated whether the significant DMPs and DMRs were correlated with changes in mRNA expression of corresponding genes using Spearman correlations. Analyses were performed using R (https://www.r-project.org/).
Table 1.
Characteristics of patients with clinically localized prostate cancer by weekly vigorous physical activity frequency in the year prior to diagnosis.
| Characteristic | Vigorous physical frequency | |||
|---|---|---|---|---|
| <1 time/week n=283 | 1–3 times/week n=713 | >3 times/week N=357 | ||
| Study | HIM | 142 (40%) | 337 (47%) | 106 (37%) |
| PROS | 215 (60%) | 376 (53%) | 177(63%) | |
| Age | Median (25%,75%) | 60 (56,65) | 59 (54,63) | 60 (56,64) |
| Race | Caucasian | 255 (90%) | 662 (93%) | 325 (91%) |
| African American | 28 (10%) | 51 (7%) | 32 (9%) | |
| Education | High school or less | 75 (27%) | 109 (15%) | 53 (15%) |
| College/vocational school | 85(30%) | 166 (23%) | 70 (20%) | |
| Bachelor degree | 55(19%) | 219(31%) | 110 (31%) | |
| Graduate school | 68(24%) | 219(32%) | 124 (35%) | |
| BMI | < 25 | 75 (27%) | 135 (30%) | 73 (37%) |
| 25.0–29.9 | 212(48%) | 367 (51%) | 134 (51%) | |
| >30 | 131(26%) | 181 (19%) | 45 (12%) | |
| Alcohol | < 1 drink/week | 64 (23%) | 137 (19%) | 65 (18%) |
| ≥1 drinks/week | 219(77%) | 576 (81%) | 292(82%) | |
| Smoking | Never | 105 (37%) | 315 (44%) | 165 (46%) |
| Former | 127 (45%) | 331 (46%) | 172 (48%) | |
| Current | 51 (18%) | 67 (10%) | 20 (6%) | |
| PSA level at diagnosis | 0–3.9 | 25(10%) | 102(15%) | 60 (18%) |
| 4–9.9 | 162 (63%) | 426 (64%) | 207(62%) | |
| 10–19.9 | 49 (19%) | 98 (15%) | 43 (13%) | |
| >20 | 22 (8%) | 41(6%) | 22 (6%) | |
| Gleason sum | 2–6 | 161(57%) | 408 (57%) | 203 (57%) |
| 3+4 | 78 (28%) | 212 (30%) | 102 (29%) | |
| 4+3 | 19 (7%) | 42(6%) | 22 (6%) | |
| 8–10 | 24 (9%) | 50(7%) | 29 (8%) | |
| Risk category (D’Amico) | Low | 106(37%) | 295(41%) | 143 (40%) |
| Intermediate | 71(25%) | 165(23%) | 90 (25%) | |
| High | 106(37%) | 253(35%) | 124(35%) | |
| Primary treatment | Radical prostatectomy | 158 (56%) | 460(65%) | 228(64%) |
| Radiation or Radiation + ADT | 87 (31%) | 181(25%) | 93 (26%) | |
| ADT | 9 (3%) | 11(2%) | 4 (1%) | |
| Other treatment | 29(10%) | 61(9%) | 32(9%) | |
| Metastatic-lethal status as of the last follow-up (Dec. 2016) | No metastatic-lethal progression | 245(87%) | 653(92%) | 327(92%) |
| Metastatic-lethal progression | 38(13%) | 60(8%) | 30(8%) | |
RESULTS
The demographic and clinical characteristics of the 1354 patients with clinically localized PCa are shown in Table 1, stratified by the frequency of vigorous physical activity in the year prior to diagnosis (< 1 time per week or 1–3 times per week or > 3 times per week). Linear regression or Chi-square test of independence, when appropriate, were employed to assess univariate associations between these characteristics and three categories of vigorous physical activity (p-values not shown in Table1). Compared to the patients who had vigorous physical activity less than 1 time per week, the patient groups who had vigorous physical activity more than once weekly (including 1–3 times per week and >3 times per week) were more likely to have higher education level (bachelor degree or graduate school), less likely to be over-weighted (BMI>30), less likely to be current smoker. The median age of the group who exercised vigorously 1–3 times per week was 1 year younger than the other two exercise groups. The clinical characteristics of PCa, e.g. baseline PSA levels, Gleason sum, D’Amico risk score, did not differ significantly between the three exercise groups. Notably, the proportion of patients who progressed to metastatic lethal phenotype was significant lower among patients who exercised vigorously more than once weekly (p-value=0.037), and no difference was shown between 1–3 times per week and >3 times per week (8% for both groups, Table 1).
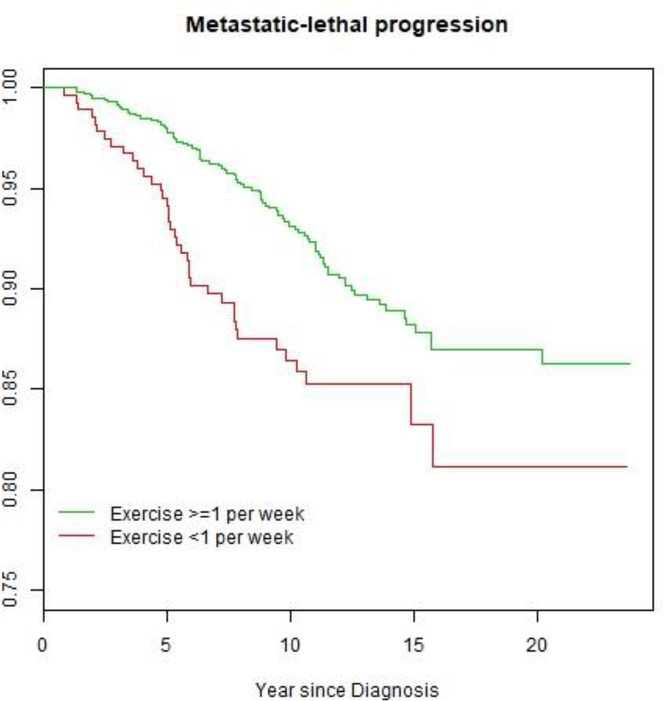
The association of vigorous physical activity and metastatic lethal progression was assessed among the cohort of 1354 patients using a time-to-event analysis. A total of 128 metastatic lethal events were developed in follow-up. The median follow-up time since diagnosis is 11.3 years. In a Cox proportional hazards model and when compared to the group with vigorous physical activity less than once per week, the hazard ratio of developing metastatic lethal progression is 0.56 for the group with 1–3 times per week (p=0.008), 0.64 for the group with >3 times per week (p=0.09), adjusting for age, smoking, education, study, Gleason sum, PSA level, and primary treatment. Since there is no material difference between the hazard ratios in the two exercise groups who exercised more than once per week, consistent with the results in Table 1, the two groups were combined in the coming analysis. Patients who exercised vigorously at least once weekly were consistently less likely to develop metastatic-lethal progression during follow-up (hazard ratio in the Cox model adjusting for the aforementioned covariates 0.63; 95% C.I. [0.42,0.95], p-value=0.029). When only adjusted for age at diagnosis, the hazard ratio for the association of weekly vigorous physical activity and metastatic-lethal progression is 0.57 (95% C.I. [0.39,0.84]). Figure 1 shows the Kaplan-Meier plot for time to metastatic-lethal event, stratified for the two exercise groups.
Figure 1.
Survival curve for developing metastatic-lethal progression in men diagnosed with clinically localized prostate cancer, stratify by the frequency of vigorous physical activity.
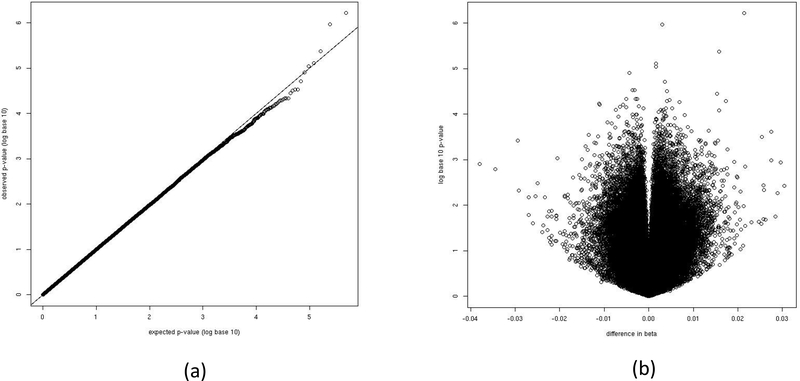
A subset of patients with localized prostate cancer who had radical prostatectomy as primary treatment and tumor samples available were analyzed by tumor DNA methylation profiling (n=524). Differential methylation probes were investigated using the Limma software in R/Bioconductor. Figure 2(a) shows the quantile-quantile plot of p-values for ~450,000 probes that passed quality control. The majority of p-values reside on the diagonal line, suggesting that there is no systematic bias in the DMP analysis, though there are a few off-diagonal data points there is little evidence of significant DMPs. The minimal p-value among all probes being evaluated did not meet either the family-wise error rate or false discovery rate 0.05 level. Figure 2(b) shows the volcano plot of p-values and the corresponding differences in methylation beta values between the two exercise groups, adjusting for age, smoking, race, study and a number of other potential confounders. The differences of methylation beta values between the two exercise groups range from −0.05 to 0.05.
Figure 2.
Results of differentially methylated probe (DMP) analysis. The beta-values of methylation probes were regressed on weekly vigorous exercise frequency, adjusting for age, smoking, race, Gleason sum and other potential confounders. (a) The q-q plot of the p-values; (b) The volcano plot of p-values and differences of beta- values by vigorous physical activity.
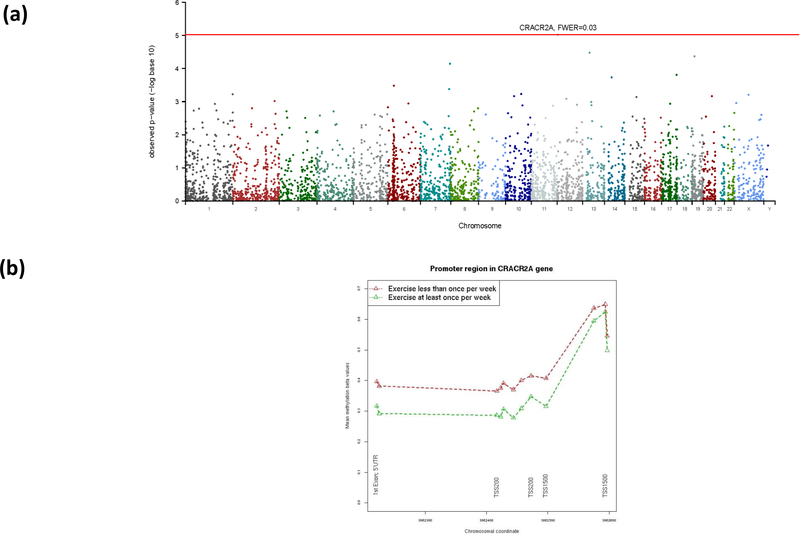
The results of the differential methylated region (DMR) analysis are shown in Figure 3. The bumphunting method yielded over 4000 bumps (regions), though the majority of the bumps are not statistically significant after accounting for multiple testing. The top ranked DMR, which contains nine CpG probes in the promoter region of the CRACR2A gene on chromosome 12, reaches the family-wise error rate level <0.05 (Figure 3a). Patients who exercised vigorously at least weekly had approximately 10% lower methylation beta values at all nine CpG probes, compared to patients who exercised vigorously less than weekly (Figure 3b). These nine CpG probes are located from TSS 200 to 5’UTR and the first exon region (Table 2), with methylation levels inversely correlated with CRACR2A gene expression levels (correlation coefficient ~ −0.3). After adjusting for age, smoking and other potential confounding covariates, the difference of beta values was smaller at the 5% level. The differences of beta values were less pronounced in the three CpG probes upstream in the TSS1500 region.
Figure 3:
DMR results using the Bumphunting method. (a) Manhattan plot for the DMR p-values. The bump with the smallest p-value reaches a family wise error rate of 0.026 and contains nine probes for the CRACR2A gene. (b) The probe-level methylation beta-values for the identified DMR in the promoter region of the CRACR2A gene. The first nine probes are in the DMR.
Table 2.
Characteristics and statistics for the 12 CpGs in the CRACR2A gene associated with weekly vigorous physical activity frequency.
| Probe ID | Genome Coordinate | In DMR | Gene annotation | Mean beta(exercise <=1, exercise>1) | Adjusted difference in beta† | DMP p-value | Correlation with mRNA expression |
|---|---|---|---|---|---|---|---|
| cg09172548 | 3732482 | Yes | 1st Exon; 5’ UTR | (0.40, 0.32) | −0.047 | 0.06 | −0.34 |
| cg04804877 | 3732486 | Yes | 1st Exon; 5’ UTR | (0.38, 0.29) | −0.057 | 0.03 | −0.35 |
| cg0819230 | 3732678 | Yes | TSS200 | (0.37,0.29) | −0.050 | 0.01 | −0.35 |
| cg03397307 | 3732684 | Yes | TSS200 | (0.38,0.28) | −0.061 | 0.01 | −0.34 |
| cg16133088 | 3732689 | Yes | TSS200 | (0.39,0.31) | −0.054 | 0.01 | −0.35 |
| cg05972871 | 3732705 | Yes | TSS200 | (0.37,0.28) | −0.060 | 0.02 | −0.34 |
| cg26955987 | 3732718 | Yes | TSS200 | (0.40, 0.31) | −0.060 | 0.03 | −0.33 |
| cg03485694 | 3732734 | Yes | TSS200 | (0.42, 0.35) | −0.044 | 0.01 | −0.34 |
| cg12710510 | 3732758 | Yes | TSS200 | (0.41,0.32) | −0.060 | 0.004 | −0.33 |
the difference in beta estimated in linear regression adjusted for potential confounders.
Table 3 shows the associations of the nine DMR probes in the CRACR2A gene with the time to metastatic-lethal progression, adjusting for the weekly vigorous physical activity. The analysis was conducted among the subset of patients with localized PCa who had methylation data available (n=463 men, 29 metastatic- lethal progression events). All nine probes show moderately significant hazard ratios with 10% decrease in methylation (0.80~0.88), some of which reach nominal statistical significance at p-value ≤0.05 level. The three most significant CpG probes associated with metastatic-lethal progression in Table 3 are also significantly differentially methylated comparing the two exercise groups (Table 2). Because this analysis has been adjusted for vigorous physical activity, the results in Table 2 and 3 rule out the possibility that physical activity may independently associated with the CpG probes and metastatic-lethal progression, suggesting the mediation role of these CpG methylation sites in the influence of physical activity on metastatic-lethal progression. The direction of the associations supports the hypothesis that more vigorous exercise promotes lower methylation levels in the promoter region of the CRACR2A gene, which in turn is associated with a lower risk of developing metastatic- lethal diseases.
Table 3.
Association of the nine DMR CpGs in the CRACR2A gene with metastatic-lethal progression, adjusting for weekly vigorous physical activity and other covariates.
| Hazard ratio for 10% decrease of beta value | 95% CI | P-value | |
|---|---|---|---|
| cg09172548 | 0.87 | (0.74,1.02) | 0.085 |
| cg04804877 | 0.86 | (0.74,1.00) | 0.049 |
| cg0819230 | 0.84 | (0.71,1.01) | 0.065 |
| cg03397307 | 0.86 | (0.73,1.00) | 0.050 |
| cg16133088 | 0.83 | (0.70,0.98) | 0.032 |
| cg05972871 | 0.85 | (0.73,1.00) | 0.051 |
| cg26955987 | 0.83 | (0.71,0.97) | 0.018 |
| cg03485694 | 0.78 | (0.63,0.95) | 0.016 |
| cg12710510 | 0.81 | (0.69,0.95) | 0.012 |
DISCUSSION
In a well-characterized cohort of patients with localized PCa, men who exercised vigorously at least once per week during the year before diagnosis (~79% of the cohort) were significantly less likely to develop metastatic-lethal progression when compared to those who exercised vigorously but less frequently (HR=0.63, p-value=0.029). Vigorous physical activity was not associated with disease characteristics at diagnosis such as PSA level and Gleason sum. The time-to-analysis analysis was adjusting for potential confounding variables including age, smoking status, education, study, primary treatment method, Gleason sum. This result adds rigorous evidence to the existing literature on the association of vigorous physical activity and a lower likelihood to develop metastatic-lethal progression.
This analysis is the first to investigate the association between vigorous physical activity and DNA methylation in primary prostate tissues. While there is evidence for DMPs with exercise frequency in this methylome-wide search, no CpG probes reached genome-wide significance in single-probe analysis, likely due to high dimensionality of the CpG probes being investigated and limited sample size. The Bumphunting method, however, was able to combine regional probe-level associations and detected a differentially methylated region (DMR) (family-wise error rate=0.03) with nine methylation probes located in the promoter region of CRACR2A. All nine CpGs associated with higher exercise frequency had lower methylation levels (hypomethylation), which corresponded higher expression levels of the mRNA transcript. The differences in beta values are around 0.10, without adjusting for confounding variables. Furthermore, lower methylation levels of these CpGs were associated with reduced risk of developing metastatic- lethal progression during follow-up after adjusting for vigorous physical activity, supporting the hypothesis that differential methylation in the promoter region of CRACR2A may be part of the molecular intermediaries reflected in the beneficial impact of vigorous physical activity on PCa metastatic-lethal progression. All these molecular and epidemiologic data corroborate with each other, supporting the mediation role of CRACR2A in exercise and PCa metastatic-lethal progression. An independent validation study is warranted to confirm this molecular finding on mechanisms of vigorous physical activity influencing DNA methylation.
Calcium is an intracellular messenger essential for various biological processes including cell proliferation, apoptosis and metastasis. In non-excitable cells, store-operated calcium entry (SOCE) is the principle influx of Ca2+. CRACR2A is a gene encoding a calcium binding protein that is a key regulator of Ca2+ (CRAC) channels that mediate SOCE (21). This protein is involved in innate immune response and neutrophil degranulation, which may be associated with intense exercise. Similar observations include associations of a higher methylation level of CACNA2D3 gene, another calcium channel gene, with less exercise and worse prognostics of gastric cancers (22–23). As a ca2+ sensor, CRACR2A is highly expressed in T cells and play key roles in regulating SOCE. Evidence showed that CRACR2A directly interact with ORAI1 and STIM1, which are critical components of CRAC channels that regulate SOCE in immune cells, thereby forming a ternary complex. STIM1 locates in the endoplasmic reticulum (ER) as a Ca2+ sensor, it’s oligomerized with the cytoplasmic transmembrane protein ORAI1 to form pores for Ca2+ influx when the ER Ca2+ store is depleted. Inhibition of STIM1 or ORAI have been suggested to suppress cell proliferation, migration and invasion in various cancer models (24–28), including PCa (28). With the elevation of calcium ion concentration, CRACR2A would dissociate from ORAI1 and STIM1, affecting their translocation and clustering. Based on our discovery, we speculate that CRACR2A could act as an upstream regulator of PCa progression through acting on STIM1 and ORAI1. Further experimental validation is needed to explore the molecular basis of this regulation.
The strengths of this study include a well-characterized PCa patient cohort with over 10 years follow-up for cancer outcomes and the large number of patients having both genome-wide methylation and gene expression data. The newly identified CpGs in the CRACR2A gene promoter show concerted associations with vigorous physical activity (hypo-methylation) and RNA gene expression levels and the metastatic- lethal progression (reduced risk), consistent with the existing literature reporting hypo-methylation in Calcium channel genes associated with vigorous exercise and better prognostic outcomes. There are several limitations of this analysis. First, while the sample size for the patient cohort is over 1000, on a subset of them have methylation profiling data available, proving a limited power to detect differential methylation in a genome-wide interrogation. Though interesting and plausible, the findings in the CRACR2A gene is exploratory and need to be validated separately. Second, the physical activity data were reported, harmonized between the two study cohorts. The recall bias and measurement error may cause a lack of finer categorization and dose association between the group who exercised vigorously less than weekly and the group who exercised vigorously more than once a week. We also did not have adequate data for analyzing light and moderate physical activities. The group which had vigorous physical activity less than once per week may contain some men with light and moderate physical activity, therefore may attenuate the estimates of the association parameters.
Acknowledgment
Funding:
This work was supported by grants from the National Cancer Institute (R01 CA 222833, R01 CA056678, R01 CA092579, K05 CA175147 (JLS), P30 CA015704, and P50 CA097186), with additional support provided by the Fred Hutchinson Cancer Research Center.
Footnotes
Conflict of interest disclosures: None
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Cancer Among Men. http://www.cdc.gov/cancer/dcpc/data/men.htm. Accessed September 27, 2017.
- 2.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 65:5–29, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Richman EL, Kenfield SA, Stampfer MJ et al. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Research. 2011;71:3889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenfield SA, Stampfer MJ, Giovannucci E et al. Physical activity and survival after prostate cancer diagnosis in the Health Professionals Follow-up Study. Journal of Clinical Oncology. 2011;29:726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonn SE, Sjolander A, Lagerros YT, et al. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiology, Biomarkers & Prevention. 2015;24: 57–64. [DOI] [PubMed] [Google Scholar]
- 6.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 2008;114:23–37. [DOI] [PubMed] [Google Scholar]
- 7.Barnard RJ, Ngo TH, Leung PS, Aronson WJ, Golding LA. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate 2003;56:201–6. [DOI] [PubMed] [Google Scholar]
- 8.Haverkamp J, Charbonneau B, Ratliff TL. Prostate inflammation and its potential impact on prostate cancer: a current review. J Cell Biochem 2008;103:1344–53. [DOI] [PubMed] [Google Scholar]
- 9.Jones LW, Antonelli J, Masko EM et al. (2012) Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol 113:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiologica. 2015;213: 39–59. [DOI] [PubMed] [Google Scholar]
- 11.Magbanua MJ, Richman EL, Sosa EV, et al. Physical activity and prostate gene expression in men with low-risk prostate cancer. Cancer Causes and Control. 2014;25: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. American Journal of Epidemiology. 2008;168: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanford JL, Wicklund KG, McKnight B, Daling JR, Brawer MK. Vasectomy and risk of prostate cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1999;8: 881–886. [PubMed] [Google Scholar]
- 14.Bibikova M, Barnes B, Tsan C, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98: 288–295. [DOI] [PubMed] [Google Scholar]
- 15.Maksimovic J, Gordon L, Oshlack A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome Biology. 2012;13: R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8: 118–127. [DOI] [PubMed] [Google Scholar]
- 17.Fan JB, Yeakley JM, Bibikova M, et al. A versatile assay for high-throughput gene expression profiling on universal array matrices. Genome Research. 2004;14: 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S, Geybels MS, Leonardson A, et al. Epigenome-wide tumor DNA methylation profiling identifies novel prognostic biomarkers of metastatic-lethal progression in men diagnosed with clinically localized prostate cancer. Clinical Cancer Research. 2016;23:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology. 2004;3: Article3. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe AE, Murakami P, Lee H, Leek JT, Fallin DM, Feinberg AP and Irizarry RA (2012). “Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies.” International journal of epidemiology, 41(1), pp. 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikanth S, Jung HJ, Kim K, et al. (2012) A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nature Cell Biology, 12:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanajo A, Sasaki A, Nagasaki H, et al. 2008. Methylation of the calcium channel-related gene, CACNA2D3, is frequent and a poor prognostic factor in gastric cancer. Gastroenterology 135, 580–590. [DOI] [PubMed] [Google Scholar]
- 23.Yuasa Y, Nagasaki H, Akiyama Y, et al. 2009. DNA methylation status is inversely correlated with green tea intake and physical activity in gastric cancer patients. Int J Cancer 124, 2677–2682. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Zhang JJ, Huang XY. Oria1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009;15:124–134. [DOI] [PubMed] [Google Scholar]
- 25.Chen YF, Chiu WT, Chen YT et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A 2011;108(37):15225–15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang N, Tang Y, Wang F et al. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett 2013;330(2):163–169. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Zhang Z, Wang R et al. Suppression of STIM1 inhibits human glioblastoma cell proliferation and induces G0/G1 phase arrest. 2013;32:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Yibin, Gu Peng, Li Jian et al. Suppression of STIM1 inhibits the migration and invasion of human prostate cancer cells is associated with PI3K/Akt signaling inactivation. 2017;38:2629–26. [DOI] [PMC free article] [PubMed] [Google Scholar]