Abstract
Occupational agricultural dust exposure can cause severe lung injury, including COPD and asthma exacerbations. Cell-derived extracellular vesicles can mediate inflammatory responses and immune activation, but the contribution of diet-derived extracellular vesicles to these processes is poorly understood. We investigated whether bovine milk-derived extracellular vesicles modulate inflammatory responses to agricultural dust exposures in a murine model. C57BL/6 mice were fed either a extracellular vesicle-enriched modification of the AIN-93G diet with lyophilized bovine milk (EV), or a control diet wherein the milk was pre-sonicated, disrupting the milk extracellular vesicles and thereby leading to RNA degradation (DEV). Mice were maintained on the diets for 5 – 7 weeks and challenged with a single (acute) intranasal instillation of a 12.5% organic dust extract (DE), or with 15 instillations over 3 weeks (repetitive exposure model). Through these investigations, we identified significant interactions between diet and DE when considering numerous inflammatory outcomes, including lavage inflammatory cytokine levels and cellular infiltration into the lung airways. DE-treated peritoneal macrophages also demonstrated altered polarization, with EV-fed mouse macrophages exhibiting an M1 shift compared to an M2 phenotype in DEV-fed mice (IL-6, TNF, IL-12/23 all significantly elevated, and IL-10 and arginase decreased in EV macrophages, ex vivo). In complementary in vitro studies, mouse macrophages treated with purified milk-derived EV were found to express similar polarization phenotypes upon DE stimulation. These results suggest a role for dietary extracellular vesicles in the modulation of lung inflammation in response to organic dust which may involve macrophage phenotype polarization.
Keywords: Organic dust, lung inflammation, macrophages, milk extracellular vesicles
1. Introduction
Aerosolized organic dusts elicit potent airway inflammatory responses. Individuals working in agricultural industries experience acute and chronic lung inflammatory symptoms and disease including rhinosinusitis, bronchitis, and obstructive pulmonary disease[1–5]. While agriculture workers are at increased risk for these lung inflammatory diseases, the mechanisms underlying the disparities in lung disease susceptibility in this population are unclear.
A major environmental factor that may modulate immune function is diet. In addition to essential macro- and micronutrient acquisition by diet, recent studies have identified that host physiological processes, including immune cell activities, may be modified by diet-derived extracellular vesicles containing RNA, lipids, and proteins that retain bioactivity in the host[6–8]. For example, extracellular vesicles in bovine milk contain numerous microRNA species, and milk consumption alters human gene expression that is regulated by these microRNAs and modifies circulating inflammatory cytokine levels[6]. However, it is unclear how bovine milk-derived extracellular vesicles affect immune responses to inflammatory challenges such as those elicited by aerosolized environmental particles.
In this context, we sought to determine how the lung inflammatory and immune responses to organic dusts are altered by bovine milk-derived dietary extracellular vesicles. Using diets containing bovine milk, with (DEV) or without (EV) prior sonication to disrupt extracellular vesicles, we assessed the inflammatory responses in the lungs and peritoneal macrophages of mice exposed to extracts of dust derived from swine confinement facilities (DE) in both acute and repetitive exposure models. Furthermore, we assessed the impacts of isolated milk extracellular vesicles on mouse alveolar macrophage polarization during DE treatment in vitro. Through these experiments, we identified marked differences in innate immune responses to DE, whereby mice fed diets containing bovine milk-derived extracellular vesicles consistently exhibited enhanced inflammatory signaling and M1 macrophage polarization in response to dust exposure, while mice on diets in which bovine milk-derived extracellular vesicles were disrupted via sonication exhibited M2 macrophage phenotype responses. Together, these results indicate that diet-derived milk extracellular vesicles have the capacity to modulate innate immune responses to aerosolized challenges, and suggest diet may be a contributing factor in determining susceptibility to inflammatory disease in agriculture workers.
2. Materials & Methods
2.1. Reagents
Cell culture medium RPMI and physiological buffers were obtained from Gibco/Life technologies, (Grand Island, NY), ELISA reagents and ancillary materials were from R & D Systems (Minneapolis, MN), microtiter plates from Corning Life Sciences (Tewksbury, MA). Falcon tissue culture plastic was purchased from Fisher Scientific (Hampton, NH). All other materials not specifically identified in the text were from Millipore/Sigma (St. Louis, MO).
2.2. Swine confinement dust extract.
Aqueous extracts of agricultural dust samples were prepared as previously reported[9]. Briefly, settled dust was collected from horizontal surfaces >1 m above the floor in concentrated animal feeding operation (CAFO) facilities housing 500–800 swine located in northeastern Nebraska. CAFO dust was screened through a coarse (0.25mm) mesh, and was then suspended in Hanks balanced salt solution (HBSS) at a concentration of 100 mg/mL, and incubated with stirring for 1 h at room temp. The resulting saturated slurry was then centrifuged (4,000 rpm, 20 min.), the supernate collected and re-centrifuged. The cleared extract was filter sterilized and frozen in aliquots at −80°C for future use (100% DE). Prepared in this manner, DE has been characterized and is free of particulates larger than 0.2 nm dia., contains both gram– and gram+ bacterial components (>90% g+) and fungal proteins (ergosterol)[10].
2.3. Animals.
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were 6–8 weeks of age at the beginning of experiments. Mice were group-housed in a pathogen-free AAALAC-approved facility located on the campus of the Univ. Nebraska Medical Center, and all experiments were approved by the university’s Animal Care and Use Committee. Mice were acclimatized in micro-isolator cages for at least one week during which they were allowed ad libitum access to standard rodent chow and water.
2.4. Extracellular vesicle-defined diets.
Diets containing bovine milk, with or without prior sonication to disrupt extracellular vesicles (EV: no sonication/no disruption of extracellular vesicles; DEV: sonication to disrupt extracellular vesicles) were prepared as previously described [6]. Briefly, the AIN-93G diet formula was modified to substitute evaporated fat-free bovine milk for the casein and cornstarch in the diet to achieve 10% total calories from the milk. Remaining dietary casein was replaced with soy protein (Bob’s Red Mill) to eliminate additional milk-derived extracellular vesicles. For the DEV diet, the bovine milk was ultrasonicated for 1 hour to transiently disrupt all extracellular vesicles and constituent cargo prior to lyophilization and use in diet preparation. No other diet ingredients are sonicated. We have previously identified that milk sonication results in transient disruption of milk extracellular vesicles, leading to a 20% reduction in total extracellular vesicle content of the diet and greater than 98% of RNA cargos in the reformed milk extracellular vesicles [11].
2.5. DE exposure model.
Mice were randomly assigned to treatment groups: mice were placed 5 per cage, and half of the cages were randomly chosen to receive EV diet, while the other cages were given DEV diet. Within each diet group, half of the cages were given DE challenge, while the remaining cages were given a saline challenge. For challenges, mice were lightly sedated (isofluorane) and given either a 12.5% solution of DE or vehicle (saline) by intranasal instillation (I.N., 50 μL) once (acute exposure model) or on 15 consecutive weekdays (repeated exposure model) as previously reported [12]. A schematic of the treatment protocols is provided in Figure 1. Mice were euthanized 5 h following the final DE exposure, and tracheas cannulated for bronchoalveolar lavage (BAL; 3×1 mL fractions of normal saline). Inflammatory cells were recovered from the pooled BAL fluid (BALF) and total cellularity was quantified. In addition, cells were cytocentrifuged and the resulting slides fixed and stained for differential cell counts (Cytopro, Wescor, Logan, UT, and DiffQuick, Dade-Behring, Newark, DE). Cell-free BAL supernates were collected for cytokine analysis by ELISA. Following lavage, lung vasculature was perfused, whole lungs removed en bloc, fixed by inflation in a solution of 10% formalin in PBS (24 h), and embedded in paraffin for sectioning and stained with hematoxylin/eosin (Univ. Nebraska Med. Center Tissue Sciences Facility). For some experiments, peritoneal macrophages were recovered from the abdominal cavity for ex vivo culture.
Figure 1. Schematic Overview of Treatment Strategies.
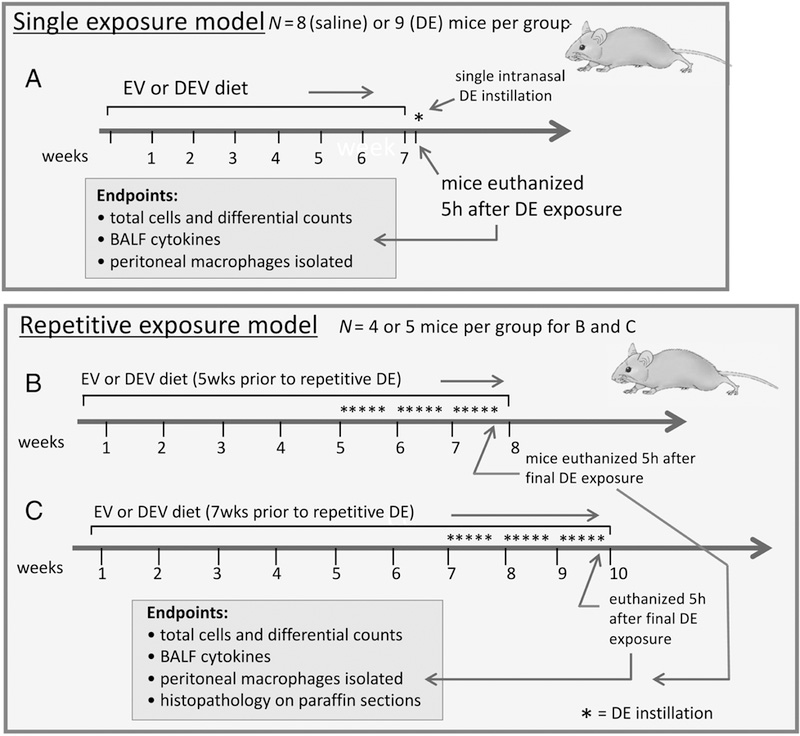
Mice were maintained on an extracellular vesicle enriched (EV) or sonicated/disrupted extracellular vesicle (DEV) diet for 7 wks, followed by a single exposure to DE (A). Alternatively, mice were fed EV or DEV diets for 5 wks (B), or 7 wks (C) after which DE was administered daily for 3 wks while remaining on the assigned diets.
2.6. Histopathology scoring.
For the repetitive-exposure experiments, hematoxylin and eosin-stained lung sections (4–5 μm thickness) were analyzed by a pathologist blinded to treatment groups as described previously [12]. Indicators of inflammatory pathology including peri-bronchiolar and peri-vascular lymphoid aggregates, and inflammatory features in alveolar and bronchiolar compartments were assessed across the entire lung section. Lymphoid aggregates were counted, and inflammatory scores were assigned based on a numerical index (0 – 3) where no inflammation = 0, and severe inflammation = 3.
2.7. Cytokine measurements.
Undiluted cell-free BALF and peritoneal macrophage culture supernates were assayed for cytokines by commercially-available ELISA kits according to the manufacturer’s directions (Duoset ELISA development kit, R&D Systems, Minneapolis, MN). Arginase activity was quantified in peritoneal macrophage lysates using a microplate-based assay (QuantiChrom arginase assay kit, BioAssay Systems, Hayward, CA). The limits of detection for each assay were: 30 pg/mL, 25 pg/mL, 15 pg/mL, 18 pg/mL, 20 pg/mL, 12 pg/mL, and 0.3 U/L for IL-6, TNF-α, CXCL1, AREG, IL-12/23, IL-10, and arginase, respectively. Because IL-12(p40) and IL-23 normally exist as a heterodimer, the assay chosen detects this dimer (12/23). For mouse BALF, samples were measured in duplicate on the assays for a total of 10 technical replicates per condition.
2.8. Ex vivo peritoneal macrophage cultures.
Mice were maintained on EV or DEV diets and challenged with or without DE (as above). Cells were recovered from the abdominal cavity of euthanized mice by lavage with sterile buffered saline. Red blood cells were lysed using an ammonium chloride lysis buffer, and peritoneal immune cells were counted by hemacytometer (1.1 × 106 –3.1 × 106 cells per mouse). Cells from each mouse were suspended in extracellular vesicle-free medium (RPMI 1640 containing 10% serum replacement (KnockOut SR, Life Technologies, Grand Island, NY) and antibiotics (Pen-Strep, HyClone, South Logan UT)) and plated in duplicate wells of a 24-well cluster plate. Cells were allowed to adhere for 18 hours, and wells were rinsed and re-fed with extracellular vesicle-free medium to remove nonadherent cells. This process results in adherent cultures which exhibit a predominant macrophage-like phenotype. Cells in one of the duplicate wells were treated with 1% DE, and the other with medium alone (con), and were incubated at 37ºC for 36 h. Cell-free culture supernates were assayed in duplicate for murine chemokines IL-6, TNF-α, IL-12/23, and IL-10. Adherent cells were removed from the plates (trypsin/EDTA) lysed in detergent-free lysis buffer (MgCl2 and EGTA in Tris buffer) containing protease-inhibitors (broad-spectrum protease inhibitor cocktail, Millipore-Sigma) sonicated and centrifuged. Cleared lysates were assayed in duplicate for arginase activity. Results are normalized to cell number and expressed as pg/mL/106 cells (or U/L/106 cells for arginase).
2.9. Isolation and characterization of milk-derived EVs.
Pasteurized and homogenized fat-free bovine milk was purchased at a local grocery. Milk nanovesicles were isolated using a modification of previously published methods [13,14]. Briefly, milk was centrifuged at 3,000 x g, 15’ to remove residual fat and cellular debris, and the supernate was centrifuged at 12,000 x g, and again at 35,000 x g. Clarified milk whey was mixed with equal volumes of 0.25 M EDTA (30’ on ice) to precipitate casein and casein-associated nanovesicles, and re-centrifuged at 48,000 x g for 1.5 h. Supernates were collected and EVs precipitated using ExoQuick reagent (System Biosciences, Palo Alto, CA), (850 µL reagent in 1.6 mL whey, 4ºC, 18h). Samples were filtered through a 0.22 µm syringe filter, centrifuged at 1,500 x g, 30’ and the isolated EV pellet was re-suspended in sterile PBS. Extracellular vesicles recovered using this method were characterized using a Nanosight LM10 nanoparticle tracking analysis system, using NTA 2.3 software (Malvern Panalytical, Westborough, MA). Concentrations of EVs recovered from several preps ranged from 2.6 to 3.6 × 109 particles/mL and particle sizes ranged from 47 to 144 nm, consistent with the reported size range for exosomes. The EV concentration in these preps was confirmed using a CD63 microplate assay (ExoELISA-ULTRA, System Biosciences) which returned comparable concentration values (1.9 to 6.3 × 109 particles / mL).
2.10. In vitro cell culture model.
Immortalized murine macrophages (MH-S, ATCC, Manassas, VA) were grown to 80% confluency on 12-well plates in growth medium (ATCC, 30–2001 supplemented with 10% FCS and pen/strep). Immediately before initiating experiments, complete medium was replaced with extracellular vesicle-free medium (30–2001 containing 10% serum replacement (KnockOut SR, Life Technologies, Grand Island, NY)) for 2 h. MH-S cells were treated with medium alone (con) 1% DE alone, con + milk EVs, or 1% DE + milk EVs, and incubated 24 h. Culture supernates were harvested for cytokine measurements, cells were counted and lysates were prepared for arginase assays. The quantity of EVs used was adjusted to approximate the nanovesicle content in a 10% fat-free milk solution, thus, 24.8 × 106 exosomes were added to each of the exosome-treated wells. Three independent experiments were performed with triplicate wells for each condition, and inflammatory markers were measured in duplicate on immunoassays (N=18 technical replicates).
2.11. Statistical analysis.
Box plots depict the medians and the 25th and 75th quartiles for the entire data set for each condition. Differences in cytokines (each mouse was measured twice) were determined using repeated measures two-way ANOVA. Differences in total and differential cell counts were determined using two-way ANOVA. The diet by dust interaction was examined in each analysis. If an interaction was statistically non-detectable (p ≥ 0.05), a main effects two-way ANOVA was used. In nearly all cases, analyses were conducted on the log base 2 of the measured value due to the data scale exhibiting a mean-variance relationship. ANOVA on the transformed variable tests the equality of geometric means. Instances where the analyses were conducted on the data scale are annotated in the associated table and/or figure. A significance level of 0.05 was used to determine significance for each comparison; Scheffe’s adjustment for multiple comparisons was utilized when determining the significance of differences in levels of the dust by diet interaction. Boxplots of the data are presented on the data scale for each condition and were created using Graphpad Prism software. The data analysis for this paper was generated using SAS software, version 9.4 for Windows.
Bar graphs are depicted using mean +/− standard error of the mean for each group.
3. Results
3.1. Effects of dietary bovine milk-derived extracellular vesicles on airway inflammation associated with acute DE exposure
We sought to determine whether milk-derived extracellular vesicles alter the lung inflammatory response associated with an acute, single exposure to DE. In these investigations, mice were placed on the EV or DEV diet for 7 weeks then subjected to a single intranasal challenge with 12.5% DE. Mice were sacrificed 5h after the dust exposure. (See Figure 1 for treatment scheme overview). We did not identify any interaction between the diet and DE exposure variables when considering the total number of cells in the BALF, although dust exposure significantly increased total cell influx as well as neutrophil influx (Figure 2A). Interestingly, an interaction between diet and DE exposure was identified (p = 0.0001) when considering lavage macrophage levels, where we identified significant increases in macrophage influx in mice fed the DEV diet compared to those fed the EV diet (Figure 2B). Furthermore, when considering the impact of diet and DE exposure on cytokine/mediator production, we identified a significant interaction of diet on dust-induced IL-6 release (p < 0.0001; Figure 3A), CXCL1 release (p < 0.0001; Figure 3C), and AREG release (p = 0.0402; Figure 3D), wherein DEV-fed mice exhibited significant reductions in each of these mediators as compared to EV-fed mice. While TNF-α was significantly increased by DE treatment, diet was not found to modify this interaction (Figure 3B). These findings indicate significant changes in the acute lung inflammatory response elicited by DE may be mediated by the alteration of the extracellular vesicles cargo in the diet.
Figure 2. Neutrophil recruitment is stimulated in mice fed the EV diet followed by acute DE exposure compared to DEV diet mice.
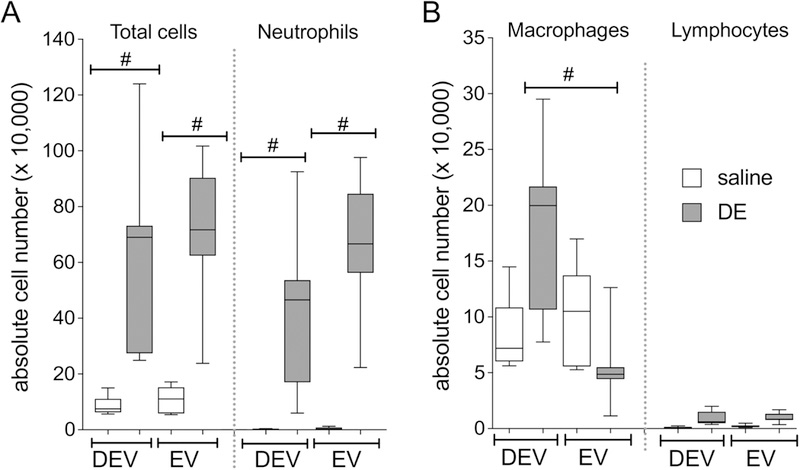
Total cell counts and neutrophils (A) and macrophage and lymphocyte counts (B) in mice fed EV or DEV diet for 7 wks followed by a single DE challenge. Results are from 2 independent parallel experiments with N=8 (saline) or N=9 (DE) mice per group. #p<0.001, by ANOVA.
Figure 3. Inflammatory mediators are elevated in BALF from mice fed EV diet vs. DEV diet following acute DE exposure.
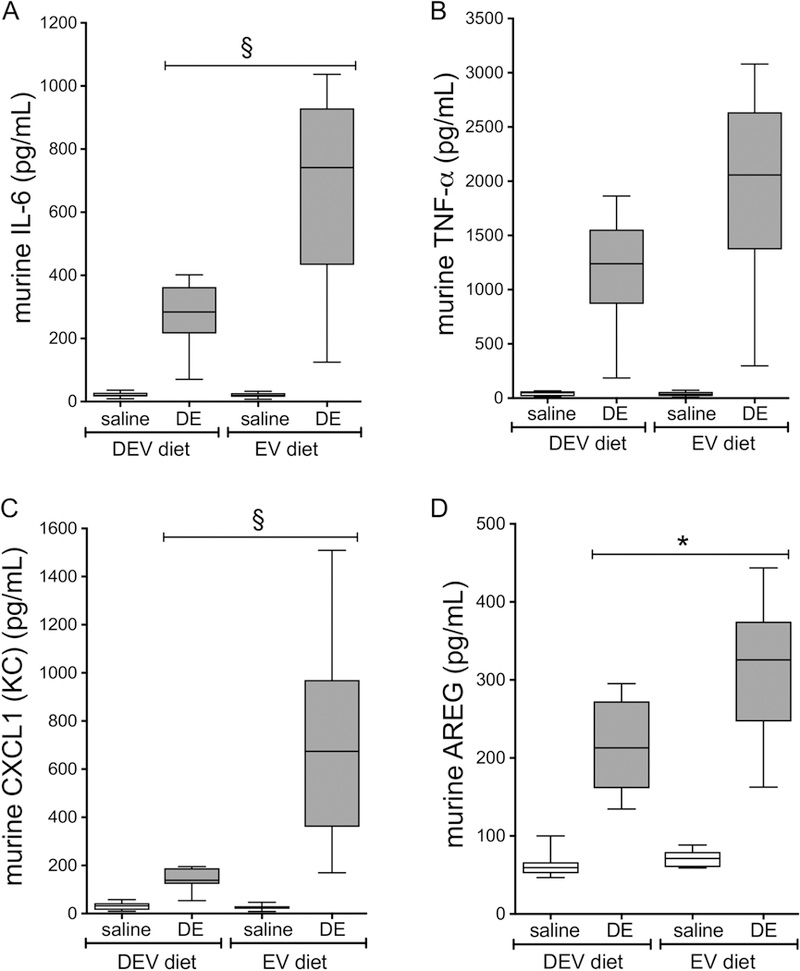
Mice were fed EV or DEV diet for 7 wks followed by a single DE challenge. IL-6 (A), TNF-α (B), CXCL1 (C), and AREG (D) were measured 5 h following DE intranasal challenge. Medians and quartiles are shown for N=8 (saline) or N=9 (DE) mice per group. *p<0.05; §p<0.0001, by ANOVA.
3.2. Effects of dietary bovine milk-derived extracellular vesicles on airway inflammation associated with repetitive DE exposure
To analyze the impact of diet-derived milk extracellular vesicles on the inflammatory response associated with repetitive DE exposure, mice were fed the DEV or EV diets for 5 weeks or 7 weeks prior to initiation of 15 consecutive weekday challenges of DE (See Figure 1 for treatment scheme). In these investigations, we did not identify any interactions between diet (DEV vs. EV), diet duration (5 vs. 7 weeks) and DE exposure when considering total and differential cellularity in the lavage fluid; although each factor independently was found to exert an effect on outcomes, whereby DE exposure was associated with significantly increased total cell influx as compared to saline-treated mice (p < 0.0001; Figure 4), which corresponded with increased neutrophil levels (p < 0.0001; Figure 5). Animals fed the DEV diet exhibited reduced total BALF cellularity (p = 0.0047; Figure 4) and neutrophilia (p = 0.0003; Figure 5), while mice on the diets for 7 weeks also exhibited increased overall BALF cellularity (p = 0.0005; Figure 4) and neutrophil influx (p = 0.0049; Figure 5) compared to the 5-week diet. When considering macrophages, similar results were identified, where DE exposure (p < 0.0001) and increased diet duration (p = 0.0108) were associated with increased BALF macrophages (Figure 5), although EV vs. DEV diet did not produce any significant differences in macrophage recruitment in this exposure model. Furthermore, we identified significant increases in BALF inflammatory cytokines IL-6, TNF-α, and CXCL1 in mice repetitively exposed to DE compared to saline-challenged mice (p < 0.0001; Figure 6A-C). In the case of TNF-α and CXCL1 levels, two-way interactions were identified between diet duration and DE (p = 0.0081 and p = 0.0212, respectively) and diet and DE (p = 0.0012 and p = 0.0101) whereby mice fed the EV diet and mice fed the diet for 7 weeks as opposed to 5 weeks exhibited increased TNF-α and CXCL1 BALF levels (Figure 6A-C). Interestingly, in the repetitive exposure studies, while DE exposure was associated with increased AREG BALF levels (p < 0.0001; Figure 6D), there was a significant interaction between DE and diet (p < 0.0001), and mice fed the EV diet exhibited a trend towards reduced AREG BALF levels (p = 0.0682). In addition to BALF cellularity, neutrophilia, and cytokine profile changes, we identified significant alterations in inflammatory lung pathology associated with these exposures. As shown in Figure 7, mice fed either the EV or DEV diet for 5 weeks demonstrated a vigorous inflammatory cell influx into alveolar, peri-vascular and peri-bronchiolar compartments in response to DE insult (B, D) compared to intranasal saline treatment (A, C). Mice on the EV diet however, exhibited a strikingly dense accumulation of effector cells, and numerous large lymphoid aggregates in response to DE (D), which were less prominent in the DEV group (B). To more quantitatively assess these pathological features, inflammatory scores were assigned to lung tissue sections in a blinded fashion (Figure 7E, F). Mice fed the EV diet for 5 wks and were repeatedly challenged with DE showed significantly increased lymphoid aggregate numbers (Figure 7E) and enhanced alveolar inflammation (Figure 7F) compared to mice fed the DEV diet. Bronchiolar inflammation was not significantly different between the two groups (Figure 7F). While lung inflammatory scores exhibited similar trends in mice fed the diets for 7 wks prior to repetitive DE exposure, these differences did not reach statistical significance in post-hoc analyses (data not shown). Together, these findings support the proposition that dietary bovine milk-derived extracellular vesicles influence the lung inflammatory response to repetitive dust exposure, with intake duration impacting the strength of the extracellular vesicles’ influence on immune outcomes.
Figure 4. Repetitive DE-mediated immune cell influx is enhanced in mice fed EV diet for 5 or 7 wks vs. mice on DEV diet.
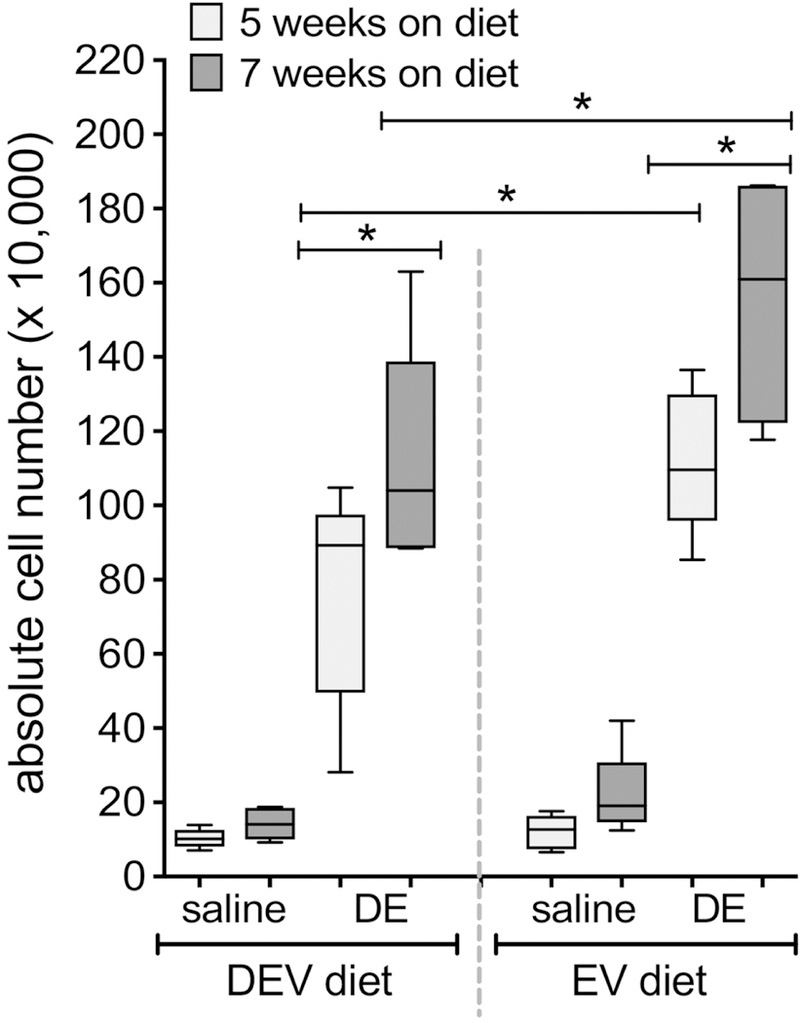
Mice were fed EV or DEV diet for 5 or 7 weeks prior to initiation of 15 consecutive weekday intranasal challenges with DE. Total cell numbers were enumerated in lavage fluid collected 5 h following the final DE instillation. N=5 mice per condition (except EV diet, 7 wks; N=4). *p<0.05, by ANOVA.
Figure 5. Repetitive DE challenge elicits lung neutrophil infiltration; the effect is amplified by both extracellular vesicle composition and diet duration.
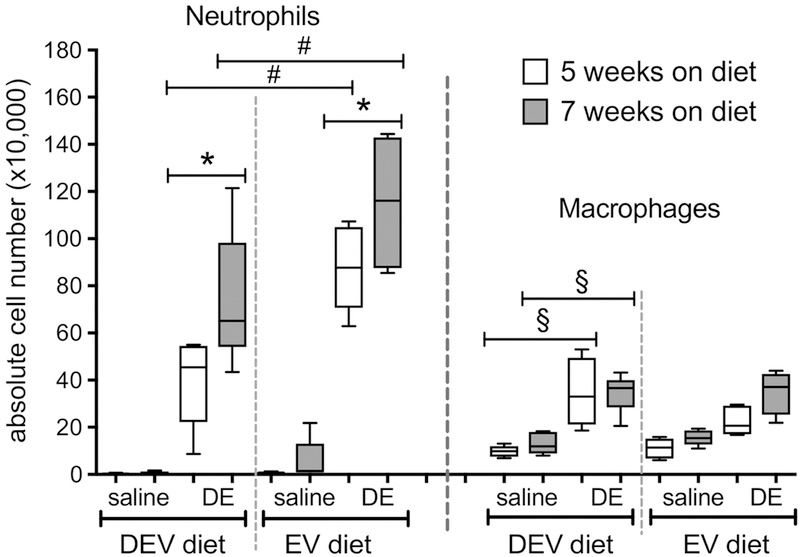
Mice were fed EV or DEV diet for 5 or 7 wks prior to initiation of 15 consecutive weekday intranasal challenges with DE. Neutrophils and macrophages were counted in lavage fluid collected 5 h following the final DE instillation. N= 4 or 5 mice per condition (as in Fig. 4). *p<0.05; #p<0.001, §p<0.0001by ANOVA.
Figure 6. Duration of diet regimen exacerbates repetitive DE-induced cytokine effects in EV-fed mice.

Mice were fed EV or DEV diet for 5 or 7 wks prior to initiation of 15 consecutive weekday intranasal challenges with DE. At 5 h following the final DE instillation, lavage was collected and assayed for IL-6 (A), TNF-α (B), CXCL1 (C), and AREG (D). N= 4 or 5 mice per condition (as in Fig. 4). #p<0.001; §p<0.0001, by ANOVA.
Figure 7. Mice fed the EV diet for 5 wks followed by 3 wks of daily DE exposure display exaggerated inflammatory lung pathology vs. DEV diet mice.
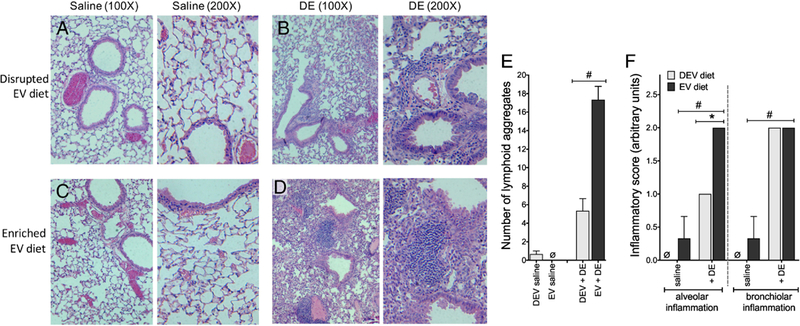
Mice were fed EV or DEV diet for 5 wks prior to 15 consecutive weekday intranasal challenges with DE. Inflated, fixed and paraffin-embedded lung tissues were stained with hematoxylin and eosin and assessed for histopathological markers of inflammation. Micrographs were made at 100X and 200X magnification: (A) DEV, saline instillation; (B) DEV, DE instillation; (C) EV, saline instillation; (D) EV, DE instillation; (E) enumeration of lymphoid aggregates per lung lobe; (F) scoring of alveolar and bronchiolar inflammation. The mean inflammatory scores for 3 mice per group are reported. *p<0.05; #p<0.001, by ANOVA.
3.3. Effects of dietary milk-derived extracellular vesicles on peritoneal macrophage phenotype
In addition to impacting the local lung inflammatory response to DE exposure, we assessed how milk-derived extracellular vesicle intake may alter peripheral immune cell activities by investigating the polarization of peritoneal macrophages obtained from mice fed the EV and DEV diets. Characterization of phagocytic macrophage polarization by utilizing chemokine profiles is well established [15,16]. The classically-activated M1 (pro-inflammatory) macrophage phenotype is typically characterized by release of high levels of IL-6, TNF, IL-12 and IL-23, and low levels of IL-10 and arginase. In contrast, the alternatively-activated M2 (protective/repair) phenotype exhibits a profile low in inflammatory mediators (IL-1, IL-6 and TNF), iNOS and IL-12/23, and increased levels of IL-10 and arginase. Thus, we chose to characterize the potential functional polarization of macrophages from mice fed EV and DEV diets by examining the differential production of these chemokines in the context of DE from in vivo exposure, or post-collection stimulation ex vivo. In peritoneal macrophages isolated from mice fed the EV or DEV diets for 7 weeks prior to receiving a single DE challenge, we identified significant interactions between DE and diet for all mediators assessed (Figure 8). Macrophages isolated from DE-challenged EV-fed mice and cultured for 36 hours ex vivo with 1% DE exhibited dramatically heightened production of the M1 polarization-associated cytokines TNF-α (p = 0.0018) and IL-12/23 (p = 0.0063; Figure 8B-C), and reduced M2-associated mediators IL-10 (p < 0.0001) and arginase (p < 0.0001; Figure 8D-E). Even when considering peritoneal macrophages from mice challenged with DE that received no additional ex vivo stimulus, macrophages from DEV-fed mice released significantly lower levels of TNF-α (p < 0.0001) and IL-6 (p < 0.0001) and significantly higher levels of IL-10 (p = 0.0019) and arginase (p < 0.0001) compared to cells from EV-fed mice.
Figure 8. Mouse peritoneal macrophages exhibit M1 cytokine profile bias in the presence of EV diet following acute DE insult.
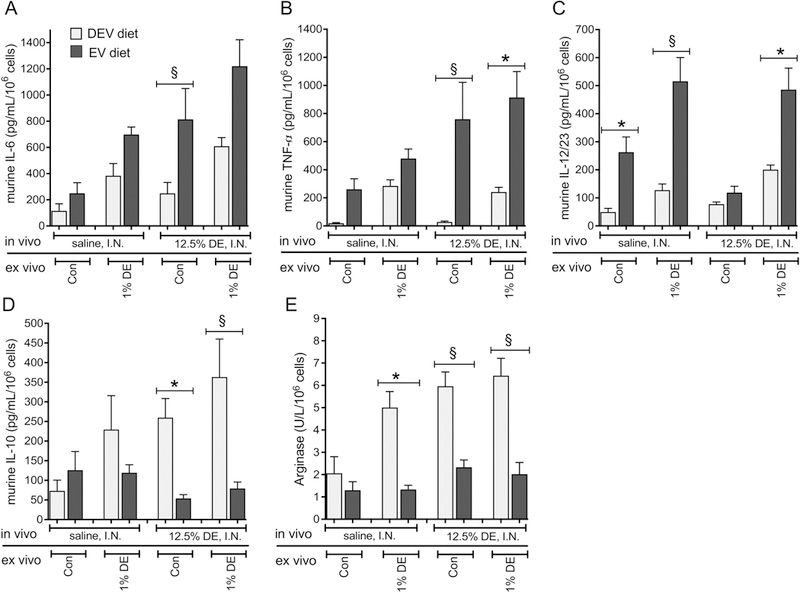
Peritoneal macrophages were recovered from mice fed EV or DEV diets for 7 wks and exposed to a single I.N. DE or saline treatment. Recovered macrophages were cultured in extracellular vesicle-free medium in the presence or absence of 1% DE ex vivo for 36 h. IL-6 (A), TNF-α (B), IL12/23 (C), and IL-10 (D) were measured in culture supernates. Arginase (E) was measured in cell lysates. N= 8 (saline) or 9 (DE) mice per condition. *p<0.05; §p<0.001, by ANOVA.
When peritoneal macrophages were collected and assayed in the setting of the repetitive DE exposure model, an interaction was identified between diet and DE when considering all mediator outcomes except for arginase (Figure 9). Here, in mice fed an EV diet for 5 weeks followed by repetitive DE exposure, ex vivo stimulation of peritoneal macrophages with 1% DE led to increased release of IL-6 (p<0.0001), TNF-α (p = 0.0002) and IL-12/23 (p = 0.0006), along with reduced release of IL-10 (p = 0.0011) compared to mice fed the DEV diet. While there was no interaction identified between diet and DE exposure identified when considering arginase levels, diet did reduce arginase levels independent of DE exposure (p = 0.0003). Similar effects were observed in mice fed the EV diet for 7 weeks followed by repetitive DE exposure; significant interactions between DE exposure and diet were identified in all assayed mediators except arginase (Figure 10). In the case of arginase, EV diet was found to be associated with reduced arginase levels independent of DE exposure (p = 0.0227). With respect to the other mediators assayed, macrophages isolated from EV-fed mice exposed to DE that were stimulated ex vivo with 1% DE, demonstrated significant increases in IL-6 (p = 0.0162) and IL-12/23 (p < 0.0001), a trend towards increased TNF-α (p = 0.0876), and reduced IL-10 (p = 0.0003) compared to DEV-fed mice under the same conditions. Together, these results indicate a shift in peritoneal macrophage polarization in mice fed the EV versus DEV diet and exposed to DE, suggesting bovine milk-derived extracellular vesicles may prime the host for different immune responses during both acute and repetitive inflammatory challenges.
Figure 9. Peritoneal macrophages from mice fed EV diet for 5 wks express M1 cytokines following repetitive DE exposure.
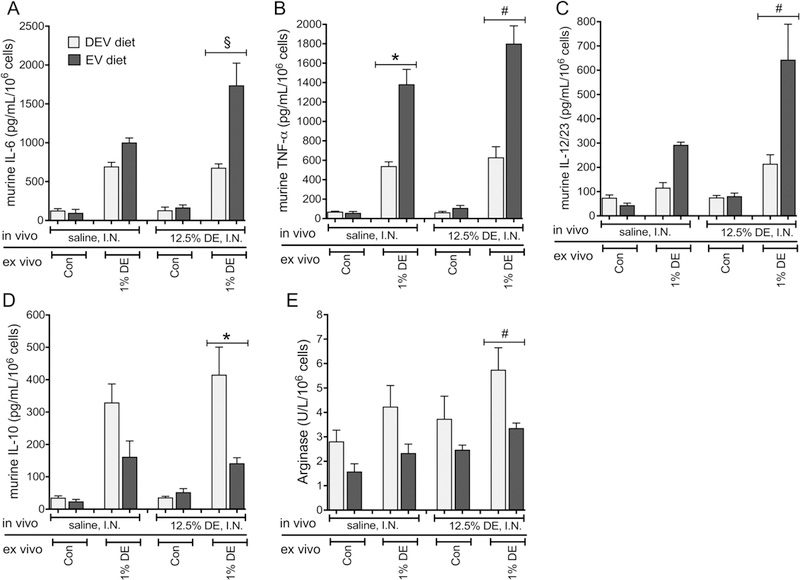
Peritoneal macrophages were recovered from mice fed EV or DEV diets for 5 wks prior to 15 consecutive weekday challenges with DE. Isolated macrophages were cultured in exosome-free medium in the presence or absence of 1% DE ex vivo for 36 h. IL-6 (A), TNF-α (B), IL12/23 (C), and IL-10 (D) were measured in culture supernates. Arginase (E) was measured in cell lysates. N= 5 mice per condition *p<0.05; #p<0.001; §p<0.0001, by ANOVA.
Figure 10. Repetitive DE-induced M1 polarization of peritoneal macrophages from mice fed the EV diet for 7 wks persists.
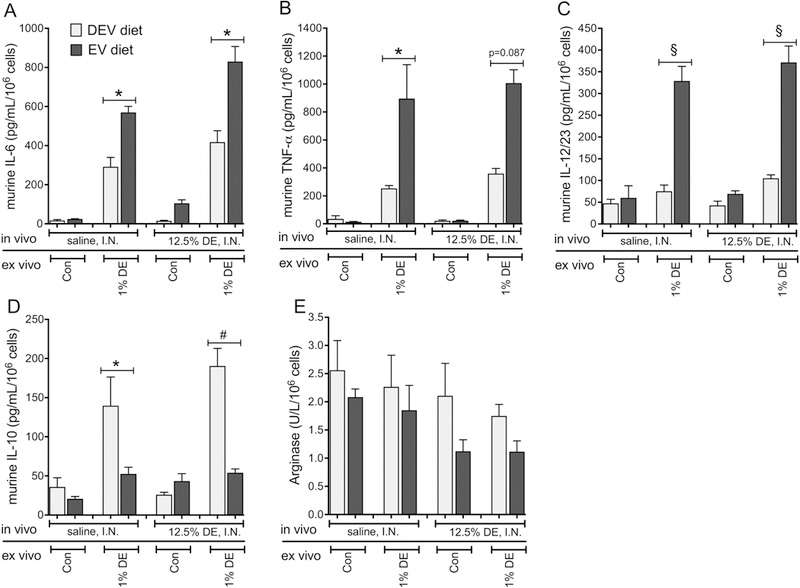
Peritoneal macrophages were recovered from mice fed EV or DEV diets for 7 wks prior to initiation of 15 consecutive weekday challenges with DE. Macrophages were cultured in exosome-free medium in the presence or absence of 1% DE for 36 h. IL-6 (A), TNF-α (B), IL12/23 (C), and IL-10 (D) were measured in culture supernates. EV diet was associated with reduced arginase across all comparisons, independent of DE exposure (p=0.0227, E). N= 5 mice per condition (except EV diet + DE, N=4). *p<0.05; #p<0.001; §p<0.0001, by ANOVA.
3.4. Effects of milk-derived extracellular vesicles on murine macrophage phenotype in vitro
To assess whether or not the macrophage polarization phenotype identified in the peritoneal macrophages isolated from EV- and DEV-fed mice was driven by the extracellular vesicles found in milk, we performed complementary studies in vitro using the MH-S murine alveolar macrophage cell line and purified milk-derived extracellular vesicles. Here, MH-S macrophages were treated with 1% DE in the presence or absence of exogenous milk-derived extracellular vesicles for 24 hours. For all mediators assayed in these in vitro studies except arginase, we identified a significant interaction between DE and diet (Figure 11A-E). Treatment with milk-derived extracellular vesicles and DE elicited significant increases in IL-6 (p < 0.0001), TNF-α (p < 0.0001), and IL-12/23 (p < 0.0001) that indicate an M1-like polarization, while significantly decreasing IL-10 (p < 0.0001) levels predictive of M2-polarized macrophages. Arginase levels were impacted by milk extracellular vesicles, where the vesicle treatment reduced arginase production (p < 0.0001) independently from DE exposure. Further analyses of the extracellular vesicles isolated from the milk confirm that the preparation yields fairly pure, uniform extracellular vesicle distribution where particles primarily range from 70 to 100 nm (Figure 11G), and are largely free of milk protein contaminants (Figure 11F). These findings corroborate our findings from both our in vivo animal model and ex vivo peritoneal macrophage studies and support our hypothesis that the milk-derived extracellular vesicles are modulating inflammatory responses at least in part through modifying macrophage activities during DE exposure.
Figure 11. Purified milk-derived extracellular vesicles modulate macrophage responses to DE in vitro.

Murine macrophage (MH-S) cultures were exposed to 1% DE in the presence or absence of exogenous milk-derived extracellular vesicles for 24 h, and supernates were assayed for inflammatory mediators. The presence of milk extracellular vesicles amplified the DE-induced release of proinflammatory cytokines IL-6, TNF-α, and IL-12/23 (A, B, C), while attenuating the pro-repair mediators IL-10 (D), and arginase in cell lysates (E). EV diet reduced arginase levels independent of DE treatment (p<0.0001). Data from 3 parallel experiments (N=18 technical replicates per condition). Analysis of the isolated milk extracellular vesicles (Nanosight LM10) indicates a suspension containing few large milk protein (e.g. casein) contaminants (F), and a large fraction of particles measuring 70 to 100 nm (G). §p<0.0001, by ANOVA.
4. Discussion
Occupational exposures in agriculture settings increase workers’ risk for various lung diseases, including chronic bronchitis, asthma-like syndrome, and chronic obstructive pulmonary disease [1–5]. Yet, it is unclear why only certain individuals experience these negative consequences following organic dust exposure. Numerous factors likely play a role in an individual’s susceptibility to lung disease following exposure to inflammatory environmental aerosols, including genetic and other environmental factors. In the study presented here, we have assessed the role of consumed bovine milk as a potential source of bioactive extracellular vesicles in modulating the inflammatory response to dust exposures. Our findings identify that consumption of bovine milk containing unsonicated extracellular vesicles alters immune responses to both acute and repetitive inflammatory dust exposures, including regulation of macrophage polarization during DE treatment, suggesting diet is an important component in determining lung inflammatory outcomes to agricultural dust exposures.
Membrane-bound extracellular vesicles contain DNA, RNA, lipids and proteins, and act as extracellular messengers for cell-to-cell communication. Endogenously produced cell-derived extracellular vesicles have been shown to promote inflammatory responses and immune activation [17–19], with recognized roles in lung disease pathogenesis [20,21]. Diet-derived extracellular vesicle signaling is however, less well understood. Extracellular vesicle fractions in human breastmilk have clear immune-modulatory activities, including inhibition of IL-2 and IFN-γ following stimulation of PBMC, and increased T regulatory cell populations, following incubation with milk extracellular vesicles [22]. A recent study found that bovine milk extracellular vesicle microRNA target genes are altered in human peripheral blood mononuclear cells following consumption of bovine milk [6]. Together, these findings suggest that human and bovine milk-derived extracellular vesicles are bioactive and have the capacity to alter gene expression in immune cells. However, it is unclear how these changes in immune cell gene expression may lead to altered immune responses.
Indeed, milk consumption is of critical importance in early mammalian physiology and is a known epigenetic regulator of early life development, including immunity [23–25]. Consumption of cross-species milk beyond the typical early postnatal period may contribute to the upregulation of developmental genes that are key regulators of health and disease, including IGF1, NFKBI, and FOXP3, among others [23–26]. Interestingly, consumption of farm-produced milk has been linked to a reduced incidence of asthma and allergy [27,28], and a recent report identified that farm exposures and consumption of unpasteurized farm milk were associated with decreased numbers of circulating dendritic cells in children [29]. Our results described herein using a murine model of inflammatory dust exposures indicate that bovine milk extracellular vesicles derived from pasteurized milk have a dramatic impact on immune outcomes. Consumption of milk extracellular vesicles was consistently found to be associated with heightened pro-inflammatory responses and M1 macrophage polarization in response to DE stimulation across several experimental designs reflecting both acute and repetitive dust exposures. Additional in vitro studies using the MH-S mouse alveolar macrophage cell line support this hypothesis, where we identified that pretreating macrophages with isolated extracellular vesicles from bovine milk recapitulated our findings of M1 polarization following DE challenge. In the setting of the repetitive DE exposure model, these responses were associated with detrimental pathologic outcomes, whereby heightened peri-bronchiolar and peri-vascular inflammation and lymphoid aggregate formation were identified in mouse lungs following repetitive DE exposure. These deleterious pathologic findings also corresponded with significantly reduced pro-repair mediator AREG production in EV-fed mice. In contrast, DEV-fed mice have reduced overall inflammatory outcomes and lung pathology, coincident with significantly increased AREG production following repetitive DE exposure, along with an M2-polarization bias in DE-treated peritoneal macrophages. In this scenario, the M2-like polarization and immunomodulatory phenotype conveyed by the absence of the dietary extracellular vesicles appears to have provided a protective advantage in the context of these organic dust exposures. It is important to note, though, that numerous studies attribute a protective effect of farm milk consumption in early life to the prevention of allergic airway disease (typically driven by an M2-polarized immune response) [27,28,30,31]. It is thus feasible that extracellular vesicle-mediated M1 polarization may provide a protective advantage in the setting of environmentally-induced allergic and/or asthmatic disease. Although the present study does not address the multitude of specific extracellular vesicle constituents which may be responsible for the immune modulatory effects observed, experiments could be devised which might target various classes of effector molecules (e.g. RNA, protein) to narrow the field of suspects. Future studies investigating the role of bovine milk extracellular vesicle-containing diets in the context of an allergic model of airway inflammation are thus warranted and will provide further understanding of the impact of milk consumption on immune performance.
Taken together, these observations identify an important role for diet in immune performance, and indicate bioactive extracellular vesicles in bovine milk regulate inflammatory physiology. Using a murine model of acute and repetitive organic dust exposures, we have identified that a diet containing complete bovine milk confers an M1-polarization status and enhanced inflammatory response to DE compared to a reduced inflammatory, immunomodulatory phenotype conferred by a diet deplete of milk-derived extracellular vesicles. These findings suggest dietary intake of bovine milk may be a factor altering lung inflammatory outcomes to organic dust exposure, potentially regulating lung disease susceptibility in exposed individuals.
Highlights.
A diet containing complete bovine milk confers an M1-polarization status and enhanced inflammatory response to dust extract compared to a reduced inflammatory, immunomodulatory phenotype conferred by a diet devoid of bovine milk-derived exosomes.
Dietary intake of bovine milk may be a factor altering lung inflammatory outcomes to environmental dust exposure.
There may be a role for dietary exosomes in the modulation of lung inflammation in response to organic dust which may involve macrophage phenotype polarization.
Acknowledgments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kirkhorn SR, Garry VF. Agricultural lung diseases. Environ Health Perspect 2000;108:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Langley RL. Consequences of respiratory exposures in the farm environment. N C Med J 2011;72:477–480. [PubMed] [Google Scholar]
- [3].American Thoracic Society. Respiratory health hazards in agriculture. Am J Respir Crit Care Med 1998;158:S1–S76. [DOI] [PubMed] [Google Scholar]
- [4].Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 2003;9:185–196. [DOI] [PubMed] [Google Scholar]
- [5].Nordgren TM, Bailey KL. Pulmonary health effects of agriculture. Curr Opin Pulm Med 2016;22:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 2014;144:1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zempleni J Milk exosomes: beyond dietary microRNAs. Genes Nutr 2017;12:12-017-0562-6. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cui J, Zhou B, Ross SA, Zempleni J. Nutrition, microRNAs, and Human Health. Adv Nutr 2017;8:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol 2002;93:289–296. [DOI] [PubMed] [Google Scholar]
- [10].Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A 2010;73:684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leiferman A, Shu J, Grove R, Cui J, Adamec J, Zempleni J. A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J Nutr Biochem 2018;59:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 2009;296:L1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pieters BC, Arntz OJ, Bennink MB, Broeren MG, van Caam AP, Koenders MI, van Lent PL, van den Berg, Wim B, de Vries M, van der Kraan, Peter M Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS one 2015;10:e0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. American Journal of Physiology-Cell Physiology 2016;310:C800–C807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- [16].Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A 2004;101:4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu AT, Lu JT, Ran ZH, Zheng Q. Exosome in intestinal mucosal immunity. J Gastroenterol Hepatol 2016;31:1694–1699. [DOI] [PubMed] [Google Scholar]
- [18].Carriere J, Barnich N, Nguyen HT. Exosomes: From Functions in Host-Pathogen Interactions and Immunity to Diagnostic and Therapeutic Opportunities. Rev Physiol Biochem Pharmacol 2016;172:39–75. [DOI] [PubMed] [Google Scholar]
- [19].Nazimek K, Bryniarski K, Askenase PW. Functions of Exosomes and Microbial Extracellular Vesicles in Allergy and Contact and Delayed-Type Hypersensitivity. Int Arch Allergy Immunol 2016;171:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nana-Sinkam SP, Acunzo M, Croce CM, Wang K. Extracellular Vesicle Biology in the Pathogenesis of Lung Disease. Am J Respir Crit Care Med 2017;196:1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kubo H Extracellular Vesicles in Lung Disease. Chest 2018;153:210–216. [DOI] [PubMed] [Google Scholar]
- [22].Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007;179:1969–1978. [DOI] [PubMed] [Google Scholar]
- [23].Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J 2013;12:103-2891-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Melnik BC, Schmitz G. Milk’s Role as an Epigenetic Regulator in Health and Disease. Diseases 2017;5:10.3390/diseases5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Melnik BC, John SM, Carrera-Bastos P, Schmitz G. Milk: a postnatal imprinting system stabilizing FoxP3 expression and regulatory T cell differentiation. Clin Transl Allergy 2016;6:18–016-0108–9. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qin LQ, He K, Xu JY. Milk consumption and circulating insulin-like growth factor-I level: a systematic literature review. Int J Food Sci Nutr 2009;60 Suppl 7:330–340. [DOI] [PubMed] [Google Scholar]
- [27].Waser M, Michels KB, Bieli C, Floistrup H, Pershagen G, von Mutius E, Ege M, Riedler J, Schram-Bijkerk D, Brunekreef B, van Hage M, Lauener R, Braun-Fahrlander C, PARSIFAL Study team. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy 2007;37:661–670. [DOI] [PubMed] [Google Scholar]
- [28].Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E, ALEX Study Team. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 2001;358:1129–1133. [DOI] [PubMed] [Google Scholar]
- [29].Martikainen MV, Kaario H, Karvonen A, Schroder PC, Renz H, Kaulek V, Dalphin JC, von Mutius E, Schaub B, Pekkanen J, Hirvonen MR, Roponen M. Farm exposures are associated with lower percentage of circulating myeloid dendritic cell subtype 2 at age 6. Allergy 2015;70:1278–1287. [DOI] [PubMed] [Google Scholar]
- [30].Jiang Z, Zhu L. Update on the role of alternatively activated macrophages in asthma. J Asthma Allergy 2016;9:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saradna A, Do DC, Kumar S, Fu QL, Gao P. Macrophage polarization and allergic asthma. Transl Res 2018;191:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


