Abstract
Enk neurons in CeA modulate the activity of the amygdala projection neurons and it is very likely that changes of Enk signaling cause the heightened anxiety that accompanies chronic pain. We use chemogenetics and transgenic mice to investigate the effects of acute and continuous activation of the amygdala Enk neurons on persistent pain and anxiodepressive-like behavior in mice.
Enk-cre mice were injected bilaterally into the CeA with cre-activated AAV-DREADD/Gq/mCherry, while neuropathic pain was induced by sciatic nerve constriction. A single injection of DREADD’s ligand CNO decreased the anxiety-like behavior in both, uninjured mice and in mice with neuropathic pain and produced robust analgesia that lasted for 24 hours. Furthermore, the activation of Enk neurons by the DREADD ligand led to increased c-Fos expression in PKC-δ interneurons of the CeA and in non-serotonergic neurons in the ventrolateral periaqueductal gray (vlPAG), a brain structure that is an essential part of the descending pain inhibitory system. Next, we added CNO to the drinking water of the experimental mice for 14 days in order to assess the effects of continuous activation of CeA Enk interneurons on anxiodepressive-like behavior, which is affected by chronic pain. The prolonged activation of the CeA Enk interneurons reduced neohypophagia in the novelty suppressed feeding test and increased ΔFosB (a marker for sustained neuronal activation) in the vlPAG of mice with chronic pain.
All together, the results of our experiments point to an important role of the CeA Enk neurons in the control of both nociception and emotion. Activation of Enk neurons resulted in sustained analgesia accompanied by anxiolysis and antidepressant effects. Very likely, these effects of CeA Enk neurons are result of the activation of vlPAG, a brain region that is essential not only for descending inhibition of pain but it is also a core element in the resilience to stress.
Keywords: central amygdala, enkephalin interneurons, descending pain modulation, anxiety-like behavior, persistent pain
Introduction
One of the main amygdala functions is integration of sensory signals to coordinate a range of emotional responses and trigger adaptive behavior (Yu et al., 2016). These amygdala functions depend upon outputs that modify a variety of endocrine, autonomic, and sensory systems. Painful stimuli recruit these amygdala functions, including the rapid processing of nociception, producing a response, and modulating subsequent sensitivity to painful stimuli. It plays a further role in production of persistent anxiety and altered pain sensitivity caused by chronic pain conditions. The CeA receives processed nociceptive input from the basolateral amygdala (BLA) and direct nociceptive input via the spinoparabrachial-amygdaloid pathway (Missig et al., 2017, Bernard et al., 1989, Braz et al., 2005, McDonald et al., 1999), and consists of mainly inhibitory GABAergic neurons categorized as projection neurons and interneurons. The CeA interneurons are the interface between the neuronal input and output of the nucleus (Sun et al., 1994). Enkephalin (Enk) and dynorphin are endogenous opioid neuropeptides with wide distribution through the CNS including CeA (Poulin et al., 2008, Marchant et al., 2007). κ-, δ- and μ-opioid receptors are found in the CeA (Mansour et al., 1994), supporting functional importance of opioid neuropeptides in the CeA (Poulin et al., 2008, Marchant et al., 2007). Dynorphin is expressed mainly in the medial subdivision of the CeA by a group of projection neurons, which also express CRF (Marchant et al., 2007) while Enk is synthesized and limited to a subset of interneurons in the centrolateral and centrocentral part of the CeA (Poulin et al., 2008). A high number of Enk neurons are found to overlap with CeA neurons that express PKC-δ (Haubensak et al., 2010). The PKC-δ cells, also called “OFF cells”, are considered to be the master switch of amygdala output (Haubensak et al., 2010, Amano et al., 2012). Thus, many Enk interneurons likely engage in feed-forward inhibition of CeA interneurons and projection neurons (Bajo et al., 2011) and are a key element for regulation of CeA output and pain sensitivity.
Chronic pain causes changes in the CeA that include up and down regulation of neurotransmitters synthesis and changes in receptors expression (Narita et al., 2006, Thompson and Neugebauer, 2017, Shinohara et al., 2017, Jiang et al., 2014). Chronic pain also decreases inhibitory GABAergic tone in the CeA and increases excitability of CeA outputs (Narita et al., 2006, Thompson and Neugebauer, 2017, Shinohara et al., 2017, Jiang et al., 2014). This occurs in parallel with down regulation of the opioid receptors (Narita et al., 2006, Jiang et al., 2014). These changes may contribute to anxiogenic and nociceptive effects of chronic pain via outputs to vlPAG, which is a part of the descending pain inhibitory system (Ossipov et al., 2014). With its role in regulation of CeA outputs, impaired Enk interneuron function is a good candidate to account for changes in CeA inhibitory tone and CeA output to these regions. We hypothesize that acute and long-lasting activation of the inhibitory CeA Enk interneurons will have analgesic effects via indirect activation of the vlPAG, and can oppose the effects of chronic pain on nociception and anxiety. We set forward experiments to investigate the effects of acute and chronic activation of the Enk neurons on mice behavior and pain perception. The targeted activation of CeA Enk neurons was achieved by bilateral injections of cre-activated DREADDs into the CeA of transgenic knockin mice with selective expression of Cre recombinase by a subset of enkephalinergic interneurons.
Methods
Animals.
Wild type C57BL/6J and transgenic B6;129S-Penktm(cre)Hze/J male mice (30–35g) were purchased from Jackson Laboratories and were group housed at 10/14 hours light/dark daily cycle with lights on at 7:00 am. The surgeries and behavior experiments were carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington DC, 2011) and the guidelines of the Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science. All behavior experiments were conducted between 9.00 am and 1.00 pm and were preceded by at least 1 hour of acclimatization to the testing room. The mice that participated in more than one test were given a few days of rest between tests but each mouse was tested only once by various behavior tests. The test area was isolated from the rest of the experimental room with a curtain and the illumination of the test arena was adjusted between 120 Lux to 250 Lux for different tests.
Stereotaxic Surgery.
All surgery was performed under isoflurane anesthesia and aseptic technique. After induction with gas anesthesia, the mice were positioned in Stoelting stereotaxic frame and the top of the skull was exposed by a longitudinal incision. A 32 G needle was lowered with coordinates - 1.3 mm, ± 2.5 mm and - 4.5 mm in respect to bregma through 1 mm holes drilled into the parietal bones. 200 nl of the Cre activated adeno-associated virus (AAVhSyn-DIO-hM3D(Gq)-mCherry, titer ≥ 5.4 X1012, UNC Vector core, Chapel Hill, NC) was injected bilaterally into the CeA by an infusion pump (Microsyringe pump, World Precision Instruments, Sarasota, FL) at rate of 1.5 nl /second. The needle was withdrawn slowly 5 minutes after the injection. The wounds were closed with clips and NSAID analgesia and fluids provided for the next three days. The same surgical technique was used to inject control wild type C57BL/6J mice with control virus from the same vendor (AAV-hSyn-EGFP, titer ≥ 4.3 X1012, UNC Vector core, Chapel Hill, NC).
Sciatic Nerve Constriction by Cuffing.
We followed the method of sciatic nerve constriction by application of a small plastic tube as first described by Benbouzid et al. (Benbouzid et al., 2008). This nerve cuffing surgery occurred 3 weeks after stereotaxic surgery. The surgery was performed again under isoflurane anesthesia after cleaning the shaved skin of the upper left thigh with antiseptic. The main branch of the sciatic nerve was exposed with forceps and a 4 mm long piece of P90 sterile polyethylene tubing (cuff) that was split lengthwise (inner diameter 0.86 mm and outer diameter 1.27 mm; Becton Dickinson Intramedic, Franklin Lakes, NJ) was placed onto the nerve. The nerve with the cuff was returned to its position and the incision was closed with wound clips. Again, as after the viral injections, analgesia with NSAID was given for the following three days.
Nociceptive and Behavior Testing.
Mice were given at least one hour to acclimatize to the testing room before any nociceptive or behavior testing. Thermal sensitivity was assed by Hargreaves test using a Heat-Flux Radiometer (Ugo Basile, Italy). Mice were placed in individual compartments on a platform with a glass floor. A beam of infrared light was aimed at the lateral side of the hind paw of mice when they had a paw firmly pressed against the glass, while not locomoting. The exposure of the paw to the infrared beam caused an abrupt withdraw, shake and sometimes licking of the affected paw by the animal, reactions that were automatically detected by the device. The average of three readings taken over an hour was used to determine the reaction latency to the heat. Tactile sensitivity was measured using von Frey filaments with different stiffness applied to the plantar surface of the hind paw through a mesh floor (Chaplan et al., 1994). Each filament was applied with enough force to bend it to near a right angle for 5 seconds. Three quick withdraws out of 5 filament applications was considered a positive reaction. The starting filament was 1 gram and the 50% mechanical thresholds were calculated by Dixon’s up and down method (Dixon, 1991). Mechanical thresholds were obtained before and following sciatic nerve constriction from both paws up to 45 days after the surgery.
Elevated O-maze.
The test equipment included a standard O-maze platform for mice with two open and two closed quadrants (Stoelting Co., Chicago, IL), which was set in an area separated from the rest of the testing room with curtains. A video camera attached to the ceiling above the O-maze was used to record mice behavior. The mice were placed in any one of the open quadrants and their behavior was recorded for 5 minutes. The video record started at the moment when the mouse first entered a closed quadrant. The anxiety like behavior was assessed from the video records. The time spent in the open quadrants (the entire body of the mouse is in the open quadrant) was calculated as a percent of the total time on the maze.
Light/Dark box.
The Light/Dark box (depth 40 cm, width 40 cm, and height 35 cm) consisted of two equal acrylic compartments, one opaque and one translucent that were separated by a divider with a 10 × 3.2 cm opening at the floor level. Each mouse was placed in the center of the dark compartment and allowed to explore the entire box for 10 minutes. The time taken to exit from the dark to the light compartment and the percent of total time spent in the light compartment was calculated from video records and used to evaluate the anxiety-like behavior of the mice. The mice tend to spend more time in the dark, protected compartment and increased exploration of the light compartment was interpreted as reduced anxiety-like behavior.
Novelty suppressed feeding.
The testing arena consisted of an open field box positioned under bright light (250 Lux). A piece of white paper was placed in the middle of the arena with a single pellet of regular rodent chow. Mice were food deprived overnight. After at least one hour of acclimatization to the testing room, the mice were taken to the arena and their behavior was recorded with a ceiling video camera for 10 minutes. The time until the first bite with an audible crunch was accepted as “Time to feed”, while sniffing, pushing the piece around or holding it without biting were not considered as positive signs. The mice were moved after the test to individual boxes, which contained predetermined amounts of rodent chow. The weight of food consumed was recorded after one hour in the box after which the mice were returned to their home cages.
Drugs.
Clozapine N-Oxide (CNO) (Tocris, Ellisville, MO), an inert compound and ligand for the DREADD receptors, was injected (1 mg/kg, i.p) or administered via drinking water in lightproof containers (1 mg/kg in 5 ml of drinking water (Andreoli et al., 2017).
Immunohistochemistry.
Mice were euthanized 24 hours after testing with an i.p injection of pentobarbital sodium (Vedco Inc., Saint Joseph, MO) and perfused transcardially with 0.01M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. The collected tissue was sectioned into 40 μm thick coronal sections using Leica VT1000S vibrotome.
The brain sections were washed with 0.01M PBS, incubated in 3% H2O2 for 15 minutes, again washed with 0.01M PBS, and then incubated in a blocking solution (0.01M PBS, 0.05% Triton X-100, 3% normal donkey serum) for 2 hours at room temperature. All sections were incubated with primary antibodies diluted in the same blocking solution at 4°C for 48 hours on rotating platform. The dilutions of primary antibody were as follows: 1:1K rabbit antibody to Methionine enkephalin (anti-Enk; catalog 20065, Immunostar, Hudson, WI), 1:1K mouse antibody to PKC-δ (anti-PKC-δ; catalog 610398, BD Biosciences, San Jose, CA), 1:1K goat antibody to serotonin (anti-5HT; catalog GT20079, Neuromics, Edina, MN), 1:2K rabbit antibody to ΔFosB (anti-FosB; catalog 14695; Cell Signaling Technology, Danvers, MA) and 1:10K rabbit against c-Fos (anti-c-Fos; catalog 2250; Cell Signaling Technology, Danvers, MA) and 1:5K chicken antibody to mCherry, (anti-mCherry, catalog CPCA-mCherry, EnCor Biotechnology Inc., Alachua, FL).
Following incubation with the primary antibody, sections for c-Fos, ΔFosB, and Met-Enk and were incubated with biotinylated secondary antibodies (Jackson ImmunoResearch Inc. West Grove, PA), 1:2K dilution for 2 hours, followed by incubation in avidin-biotin complex (ABC kit, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. The fluorescent signal was developed by incubation for 12 minutes in 20 nmol of tyramide conjugated Alexa Fluor 488 or Alexa Fluor 405 fluorescent dyes. Direct secondary antibodies conjugated to Alexa 595 in dilution 1:400 were used to visualize mCherry and 5HT immunoreactivity.
Microscopy.
A Leica DM 5500B epifluorescence microscope was used to acquire 16-bit images of amygdala and vlPAG. The immunoreactivity for the various markers was evaluated on four to six sections per animal. The atlas-matched brain sections covered the CeA (1.1 mm - 1.8 mm to bregma) and vlPAG (4.2 mm - 4.8 mm to bregma). Analysis of the immunohistochemistry results was done using the ImageJ software (ImageJ, NIH image, Research Service Branch, Bethesda, MD). The multichannel images were split into 8 bit single channel images and the individual cells were labeled with a multi-point tool. The colocalization was established by merging the single channels with already labeled cells. The number of immunofluorescent cells above background was averaged per bregma level and subsequently pooled together for each animal that had a complete set of sections.
Statistical analysis.
Each animal was randomly assigned to a vehicle or CNO treated group. A between-subjects experimental design was used for all behavior analyses and a within-subject experimental design was chosen for the analysis of pain. A separate cohort of wild type mice and non-DREADD viral constructs were used as additional controls. The selected experimental design is acceptable alternative to a standard 2 by 2 design, which includes DREADD and non-DREADD viruses with CNO and vehicle treatments (Smith et al., 2016). Data are presented as mean ± SEM and analyzed with Graph Prism 7 software. Student’s T-test was used for two group comparisons, while Two-way repeated measures ANOVA was used to evaluate the development of thermal hyperesthesia and mechanical allodynia. The accepted level of significance was P < 0.05 in all tests.
Results
Chemogenetic activation of Enk neurons in the CeA causes anxiolysis.
If chronic pain and other conditions produce anxiety by dysregulation of CeA outputs, acute activation of Enk neurons should be able to regulate anxiety behaviors. In the first set of experiments we determined the effects of acute chemogenetic activation of Enk neurons in the CeA on mice anxiety as measured by two classic anxiety tests, namely elevated O-maze and Light/Dark box. Activation of Enk neurons (DREADD-Gq receptors, CNO, 1 mg/kg, i.p.) increased the percent of time in the open quadrant of the O-maze in comparison to the vehicle-injected control group, (Vehicle group: 14.8 ± 2.8 % versus CNO group: 26.2 ± 3.6 %, T-test, t8 = 2.5, P < 0.05, n = 5 per group). Similar results were obtained by the Light/Dark Box test for anxiety. Activation of Enk neurons in the CeA increased the time in the light compartment (Vehicle group: 16.9 ± 3.2 % versus CNO group: 32 ± 3.5 %, T-test, t8 = 3.1, P < 0.05, n = 5 per group) and decreased the time to exit from the dark compartment (Vehicle group: 154 ± 47 sec versus CNO group: 25 ± 10 sec, T-test, t8 = 2.9, P < 0.05, n = 5 per group).
Chemogenetic activation of Enk neurons in the CeA causes lasting analgesia in mice with persistent pain.
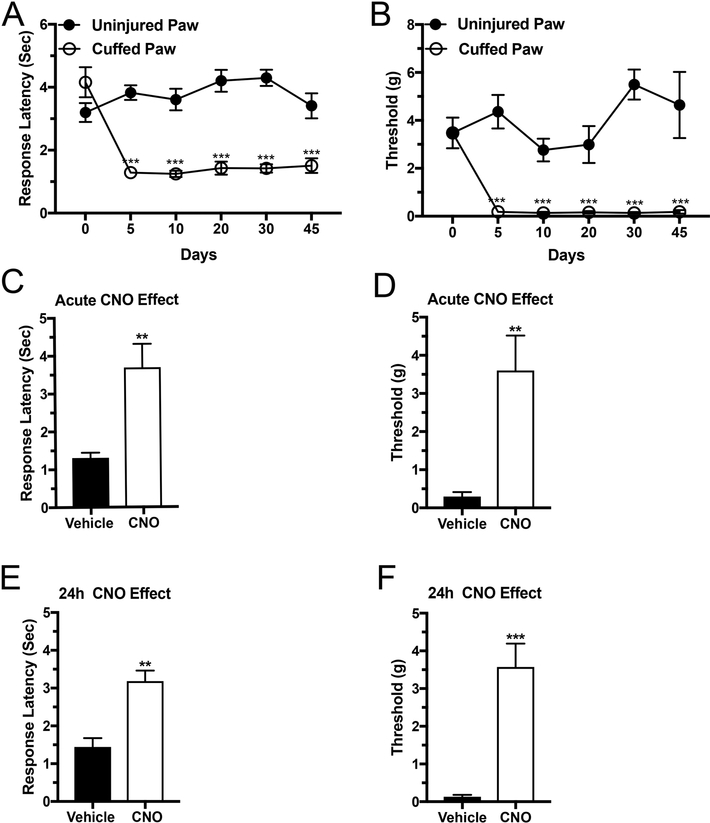
If hyperalgesia caused by chronic pain is partly due to increased CeA output that suppresses vlPAG, increasing the activity of CeA Enk inhibitory interneurons should result in increased vlPAG activity and diminish hyperalgesia. Enk-cre mice were subjected to cuffing surgery to induce persistent neuropathic pain. To verify effectiveness of this procedure in these animals, thermal hyperesthesia and mechanical allodynia were measured from the fifth postsurgical day until the end of the experiment or 45 days. The thermal threshold as measured by the response latency to heat decreased from 4.1 ± 1.5 sec before the surgery down to 1.3 ± 0.3 sec at the 5th day, 1.3 ± 0.3 sec on the 10th day, 1.4 ± 0.4 sec on the 20th day, 1.4 ± 0.4 sec on the 30th day and 1.6 ± 0.6 sec on the 45th (Two-way Repeated ANOVA, Cuffing X Time, F(5,45) = 12.5, P < 0.001 for interaction, n = 10 per group, Figure 1 A). A similar decrease was observed in the mechanical thresholds where the tactile sensitivity decreased from 3.5 ± 2 grams before the surgery down to 0.18 ± 0.09 grams at the 5th day, 0.13 ± 0.14 grams on the 10th day, 0.16 ± 0.15 grams on the 20th day, 0.14 ± 0.11 grams on the 30th day and 0.25 ± 0.27 grams on the 45th day (Two-way Repeated ANOVA, Cuffing X Time, F(5,45) = 8.3, P < 0.001 for interaction, n = 10 per group, Figure 1 B)
Figure 1: Chemogenetic activation of Enk neurons in the CeA causes lasting analgesia in mice with persistent pain.
Insertion of a plastic cuff onto the sciatic nerve led to development of thermal hypersensitivity (A) and mechanical allodynia (B), which were detected at the 5th postsurgical day and lasted for 45 days or to the end of the experiment. Panels C and D show decreased thermal sensitivity (C) and mechanical allodynia (D), when the mice were tested 30 to 40 minutes after a single CNO injection. Panels E and F show decreased thermal sensitivity (E) and mechanical allodynia (F) when the same mice were tested again 24 hours later. Time course for pain development was evaluated by Two-way repeated measures ANOVA test, *** P < 0.001 cuffing versus time. CNO treatment was evaluated using T-test, ** P < 0.01 and *** P < 0.001, n = 10 per group.
Acute activation of CeA Enk interneurons (CNO, 1 mg/kg, i.p.) increased the thresholds for both thermal sensitivity as measured by the response latency (control group: 1.3 ± 0.1 sec versus CNO treated group: 3.7 ± 0.6 sec, T-tests, t8 = 3.7, P < 0.01, n = 5 per group, Figure 1 C) and mechanical allodynia as measured by the mechanical thresholds (control group: 0.3 ± 0.1 grams versus CNO treated group: 3.6 ± 0.9 grams, T-tests, t8 = 3.6, P < 0.01, n = 5 per group, Figure 1D). We were surprised to find that the analgesia induced by the acute activation of Enk neurons in the CAmy by a single intraperitoneal injection of CNO lasted for 24 hours when measured by both Hargreaves (control group: 1.4 ± 0.2 sec versus CNO treated group: 3.2 ± 0.2 sec, T-tests, t8 = 4.8, P < 0.01, n = 5 per group, Figure 1 E) and von Frey (control group: 0.1 ± 0.05 grams versus CNO treated group: 3.5 ± 0.6 grams, T-tests, t8 = 5.5, P < 0.001, n = 5 per group, Figure 1F).
The effects of Enk activation were also examined in anxiety behaviors during chronic pain in the same animals. Activation of Enk interneurons (CNO, 1 mg/kg, i.p.) decreased the latency to feed in a novelty-suppressed feeding task when mice were tested 45 days after the onset of pain (control group: 375 ± 61 sec versus CNO treated group: 178 ± 38 sec, T-tests, t8 = 2.3, P < 0.05, n = 5 per group). Total food consumption was not affected by the CNO injection (the amount of food consumed for one hour after the experiment was an average of 0.31 ± 0.02 grams in the control group and 0.29 ± 0.03 grams in the CNO injected group, P > 0.05, n = 5 per group). These results support the hypothesis that Enk interneurons of the CeA can reduce hyperalgesia and anxiety caused by chronic pain.
Chemogenetic activation of Enk neurons in the CeA increased the expression of c-Fos by PKC-δ cells in CeA and non 5-HT-ir cells in vlPAG.
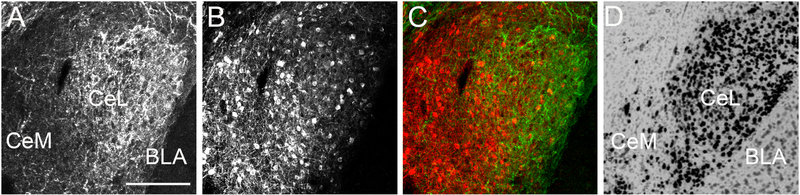
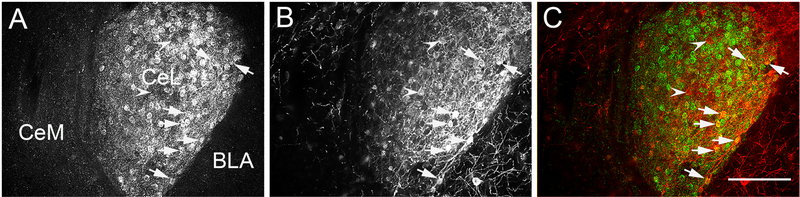
The PKC-δ neurons regulate the activity of the CeA projection neurons, which originate from the medial portion of the CeA and directly target vlPAG (Amano et al., 2012, Haubensak et al., 2010). Approximately 40% of the PKC-δ neurons express Enk mRNA (Haubensak et al., 2010). In our experiments, the expression of AAV-hSyn-DIO-hM3D(Gq)-mCherry in Enk-Cre mice was restricted to the CeA expression of met-enkephalin as detected by immunofluorescence (Figure 2A to C) and the pattern of expression was similar to the pattern of preproenkephalin mRNA distribution in the amygdala as shown by Allen Brain Atlas (reference number Penk - RP_060315_01_A07 - coronal, Figure 2D). An equal number of PKC-δ positive neurons were found in both groups (control group with an average of 45 ± 7 PKC-δ-ir neurons and the CNO injected group with an average of 49 ± 6 PKC-δ-ir neurons, T-test, t9 = 0.4, P > 0.05, n = 5 and 6 per group). Additionally, a similar number of PKC-δ-ir positive cells in the CeA were transduced with DREADDs (control group with 30.6 % ± 5.4 PKC-δir/DREADD-mCherry-ir colocalization and CNO treated group with 28.7 % ± 5.8 PKC-δ-ir/DREADD-mCherry-ir colocalization, T-test, t9 = 0.8, P > 0.05, n = 5 and 6 per group, Figure 3).
Figure 2: The expression of the cre activated AAV-hSyn-DIO-hM3D(Gq)-mCherry virus injected into the CeA of Enk-cre mice was restricted to the anatomical borders of the nucleus and overlapped with the expression of Enk immunoreactivity and Enk mRNA.
Panel A shows the Enk-ir, panel B shows mCherry-ir and panel C shows Enk/mCherry-ir (green/red) in the CeA. The panel D shows the expression of preproenkephalin mRNA in the CeA of mouse brain (Image credit: Allen Institute). Abbreviations: BLA - basolateral nucleus of the amygdala, CeL - centrolateral subdivision of the CeA and CeM - centromedial subdivision of the CeA. Scale bar = 100 μm.
Figure 3: Numerous PKC-δ-ir neurons colocalized with AAV-hSyn-DIO-hM3D(Gq)-mCherry in the CeA of Enk-cre mice.
Panel A shows the expression of PKC-δ-ir, panel B shows the expression of mCherry-ir and panel C is a merged image where the arrows point to PKC-δ-ir neurons (green) that colocalized with mCherry-ir (red) neurons. Arrowheads point to single labeled mCherry-ir neurons. Abbreviations: BLA - basolateral nucleus of the amygdala, CeL - centrolateral subdivision of the CeA and CeM - centromedial subdivision of the CeA. Scale bar = 100 μm.
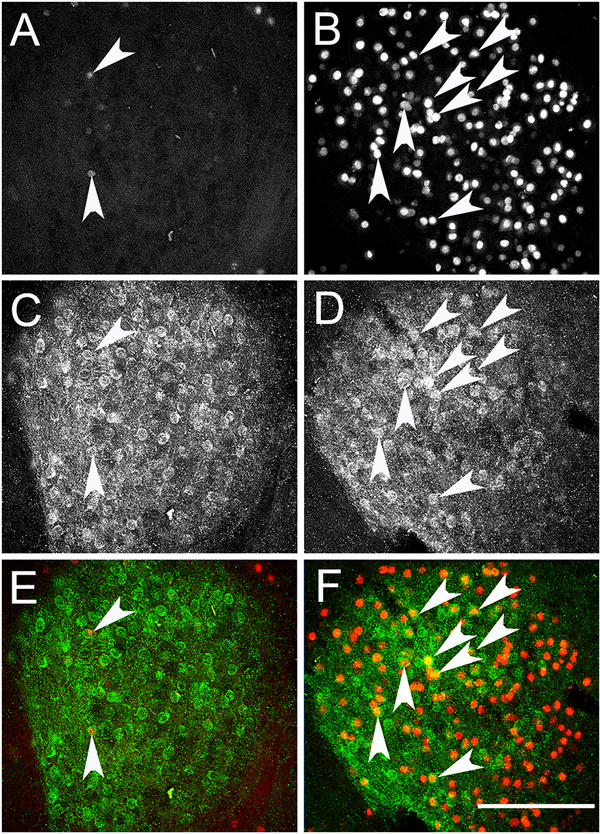
To determine the circuitry of how CeA Enk neurons might influence pain sensitivity and anxiety, we examined the effect of CeA Enk neuron activation on c-Fos expression as an index of neuron activation. Activation of DREADD-Gq in Enk neurons (1 mg/kg, i.p.) increased c-Fos-ir expression in the CeA (vehicle group: 34 ± 4 cells versus CNO group: 128 ± 16 cells, T-tests, t9 = 7.2, P < 0.001, n = 5 and 6 per group, Figure 4 A and B). Furthermore, CNO treatment increased the number of activated PKC-δ-ir cells as detected by the increased double-labeled c-Fos/PKC-δ-ir neurons (vehicle group: 3 ± 1 neurons versus CNO group: 14 ± 3 neurons, T-test, t9 = 3.7, P < 0.01, n = 5 and 6 per group, Figure 4 E and F). This verifies the effectiveness of DREADD-Gq as an approach for activation of CeA neuronal population and even PKC-δ-ir positive neurons.
Figure 4: Chemogenetic activation of Enk neurons in the CeA increases the expression of c-Fos by PKC-δ-ir cells.
Panels A and B show c-Fos-ir in the CeA of the vehicle (A) and CNO (B) injected mice. Panels C and D show the expression of PKC-δ-ir in the CeA of vehicle (C) and CNO (D) injected mice. Panels E and F show the colocalization of c-Fos (red) and PKC-δ-ir (green) in the CeA of vehicle (E) and CNO (F) injected mice. Arrowheads point to double labeled (c-Fos/ PKC-δ-ir) neurons. Scale bar = 100 μm.
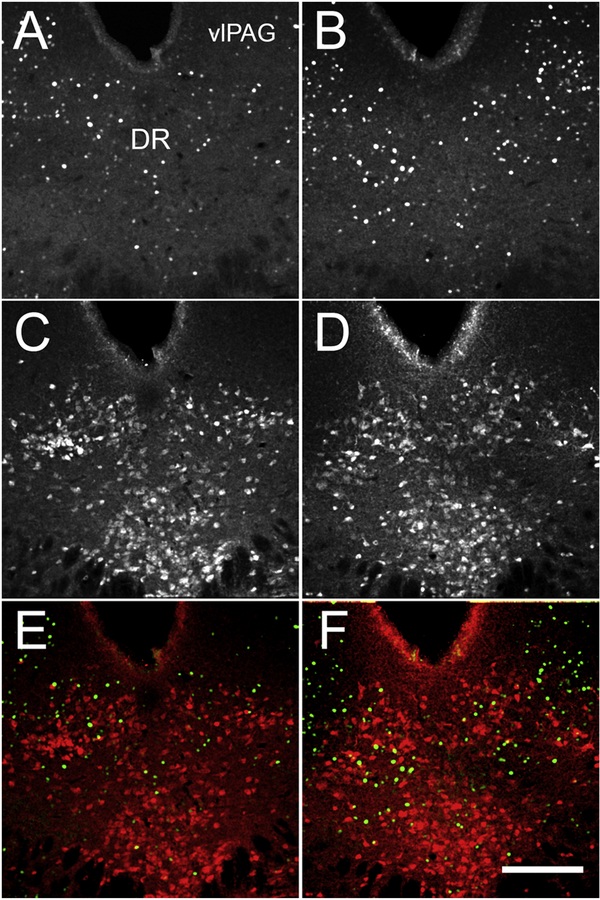
If Enk neurons in the CeA engage the descending pain modulation their activation would be expected to indirectly activate vlPAG. To answer this question, we assessed the expression of c-Fos-ir in vlPAG after activation of the CeA Enk neurons (CNO, 1 mg/kg, i.p.). The serotonin immunoreactivity (5-HT-ir) was used to outline the dorsal region of the dorsal raphe nucleus (DR) and the adjacent vlPAG (Figure 5). Activation of CeA Enk neurons caused increased number of c-Fos-ir expressing cells in vlPAG (control group: 56 ± 10 cells versus CNO group: 83 ± 8 cells, T-test, t9 = 3.2, P < 0.01, n = 5 and 6 per group but not in the neighboring DR, where a very few 5-HT-ir neurons were colocalized with c-Fos-ir (T-test, t9 = 1.1, P > 0.05, n = 5 and 6 per group (Figure 5). This supports a model where activation of Enk interneurons of the CeA suppresses CeA inhibitory projections, and permits increased activity of vlPAG to promote the removal of hyperalgesia.
Figure 5: Chemogenetic activation of Enk neurons in the CeA increases the expression of c-Fos in vlPAG.
Panels A and B show c-Fos-ir in the DR and vlPAG of the vehicle (A) and CNO (B) injected mice. Panels C and D show the expression of 5-HT-ir in the DR and vlPAG of vehicle (C) and CNO (D) injected mice. Panels E and F show that despite the increased number of c-Fos (green) positive cells in CNO treated mice, a very few of these c-Fos cells were colocalized with 5-HT-ir (red) in the vehicle (E) and in CNO (F) injected mice. Abbreviations: DR - dorsal raphe nucleus, vlPAG - ventrolateral periaqueductal gray. Scale bar = 200 μm.
Sustained chemogenetic activation of Enk neurons in CeA prevents the development of anxiodepressive-like behavior in mice with persistent pain.
Persistent pain lasting 4 to 6 weeks reliably induces anxiety-like and depression-like behavior in rodents, which can be objectively quantified (Benbouzid et al., 2008, Narita et al., 2006, Dimitrov et al., 2014). Next, we investigated if sustained activation of Enk neurons in the CeA would affect not only the pain thresholds but also the anxiodepressive-like behavior in mice with long-lasting pain. Enk-cre transgenic mice were injected bilaterally into the CeA with AAV-hSyn-DIO-hM3D(Gq)-mCherry and underwent cuffing surgeries, as in the experiments described above. Thirty days after the cuffing surgery, water soluble CNO was dissolved in the drinking water of the experimental group (1 mg per 5 ml of water) to cause sustained activation of CeA Enk neurons, while the control animals received only tap water via the same water bottles as the experimental group. Prolonged treatment with CNO reduced both the thermal hypersensitivity and the mechanical thresholds in mice with pain. The average response latency to thermal stimulus was 1.2 ± 0.2 sec in the controls and 2.8 ± 0.2 sec in the experimental group (T-test, t8 = 5.5, P < 0.001, n = 5 per group). The threshold for mechanical allodynia was similarly elevated from an average of 0.1 ± 0.03 grams in the control group to an average of 4.9 ± 1.1 grams in the CNO group (T-test, t8 = 4.2, P < 0.01, n = 5 per group).
The mice that were treated with CNO for 14 days also showed significantly shorter latency to feed than their respective control group in the novelty-suppressed feeding task (control group: 486 ± 70 sec versus CNO group: 181 ± 3 sec, T-tests, t8 = 4, P < 0.01, n = 5 per group). Similar to the previous experiment with a single CNO injection, the amount of consumed food for one hour after the experiment completion was not affected by the prolonged CNO treatment (control group: 0.70 ± 0.2 grams and CNO group: 0.84 ± 0.1 grams, T-tests, t8 = 0.6, P > 0.05, n = 5 per group).
Sustained chemogenetic activation of Enk neurons in CeA increases the expression of ΔFosB-ir in vlPAG of mice with persistent pain.
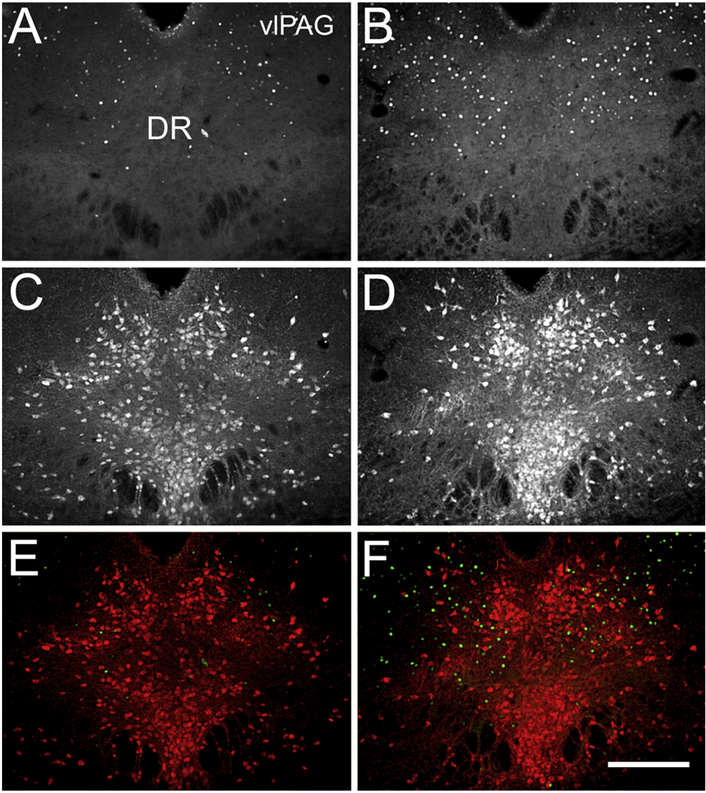
We hypothesized, based on the well-known contribution of vlPAG and the serotonin signaling to central regulation of pain that the DR and vlPAG will show signs of activation. To our surprise, the number of 5-HT-ir neurons did not change with the CNO treatment (control group: 70 ± 3 cells versus CNO group: 73 ± 6 cells, t8 = 0.5, P > 0.05, n = 5 per group) and almost none of the serotonin cells expressed ΔFosB, a marker for prolonged neuronal activation (Figure 6). Nonetheless, the number of activated neurons as indicated by the ΔFosB-ir expression in vlPAG and the adjacent DR was much higher after the CNO treatment (control group: 28 ± 5 ΔFosB-ir cells versus CNO group: 50 ± 6 ΔFosBir cells, t8 = 3.2, P < 0.05, n = 5 per group, Figure 6).
Figure 6: Sustained chemogenetic activation of Enk neurons in CeA increases the expression of ΔFosB-ir in vlPAG of mice with persistent pain.
Panels A and B show ΔFosB-ir in the DR and vlPAG of the vehicle (A) and CNO (B) treated mice. Panels C and D show the expression of 5-HT-ir in the DRD and vlPAG of vehicle (C) and CNO (D) treated mice. Panels E and F show both, ΔFosB-ir (green) and 5-HT-ir (red) in the control (E) and in CNO (F) treated mice. Increased numbers of ΔFosB-ir positive cells are clearly visible in the area of vlPAG presented on the right (B and F) panels. It is also evident that the ΔFosB-ir expressing cells are not 5-HT-ir cells (F). Abbreviations: DR - dorsal raphe nucleus, vlPAG - ventrolateral periaqueductal gray. Scale bar = 200 μm.
Control experiments.
Despite the fact that the chemogenetic technique has become widely popular in the neuroscience community since the first description of the DREADD technology (Armbruster et al., 2007), many questions regarding the use of CNO and more specifically clozapine and CNO metabolism, half-life, penetration of the blood-brain barrier and interaction with other drugs still remain in discussion (Manvich et al., 2018). In the current study, the best approach was a within subjects design, requiring comparison of CNO with vehicle. We therefore verified major results using alternative approaches.
First, we tested if CNO itself can be the cause of some of the observed effects. We injected wild type mice with a control non-DREADD vector (AAV-hSyn-EGFP) into the CeA and three weeks later tested the mice for anxiety-like behavior after a single injection of CNO (1 mg/kg, i.p.). The CNO treatment did not affect the mice behavior (Elevated O-maze, control group with 11.2 ± 1.8 % versus CNO group with 10.5 ± 2.7 % time spent in the open quadrants, T-test, t13 = 0.2, P > 0.05, n = 7 and 8 per group; Light/Dark Box, control group with 26.7 ± 2.5 % versus CNO group with 26.1 ± 3.9 % time spent in the light field, T-test, t13 = 0.9, P > 0.05, n = 7 and 8 per group). CNO injection also failed to change of the mechanical thresholds of these wild type mice after induction of neuropathic pain (control group: 0.2 ± 0.02 g versus CNO group: 0.2 ± 0.03 g, T-test, t28 = 0.6, P > 0.05, n = 15 per group).
We also tested if some of the effects of CNO treatment can be reproduced with compound 21, a different clozapine derivate with proven bioavailability in CNS (Chen et al., 2015). Enk-cre mice with DREADD-Gq in the CeA were generated, as above. Mice were injected with vehicle or Compound 21 and tested for anxiety-like behavior on elevated O-maze. The intraperitoneal injection of 3mg/kg dose of Compound 21 resulted in significant anxiolysis as measured by the time spent in the open quadrants of the elevated O-maze (control group: 13.3 ± 4.3 % versus CNO group: 33.4 ± 5.6 %, T-test, t10 = 2.6, P < 0.01, n = 6 per group).
Discussion
The CeA is a complex brain region that regulates a wide variety of autonomic and non-autonomic functions, including modulation of ongoing pain. The CeA mediates both pro- and anti-analgesic effects through the activation of different cell groups, receptors and neurotransmitters (Andreoli et al., 2017, Missig et al., 2017, Cai et al., 2018, Werka, 1994). We designed experiments to investigate a possible brain circuitry by which the CeA enkephalinergic neurons, the largest population of interneurons in the nucleus, modify the anxiety-like behavior and pain perception in mice.
The CeA is a source of robust amygdala outputs to regions that modulate both anxiety and pain sensitivity. Two primary outputs include CeA projections to the vlPAG, which regulate pain via the descending pain inhibitory system (Oliveira and Prado, 2001), and projections to the adjacent DR, which control defensive and anxiety responses to a variety of aversive stimuli (Biagioni et al., 2013, Teissier et al., 2015). This would suggest that activation of CeA inhibitory projections would suppress vlPAG and support hyperalgesia, and that any CeA element that regulates CeA inhibitory projections would remove this suppression and permit regulation of pain. However, this is complicated by the finding that major targets of CeA inhibitory projections to the vlPAG and DR are local GABAergic populations (Weissbourd et al., 2014) whose activation produces hyperalgesia (Samineni et al., 2017). In addition, CeA projections are not only GABAergic, but contain various neuropeptides such as CRF, dynorphin and neurokinin B as co-transmitters (Marchant et al., 2007). It is not surprising, then, that the role of CeA in pain perception depends on the circumstances, producing either analgesia or algesia (Carrasquillo and Gereau, 2008, Andreoli et al., 2017, Veinante et al., 2013). However, activation of CeA opioid receptors causes analgesia (Wilson and Junor, 2008, Primeaux et al., 2006, Ragnauth et al., 2001, Konig et al., 1996, Zhang et al., 2013), partly by inhibiting the CeA GABAergic input to vlPAG (Finnegan et al., 2005), indicating that the CeA has opioid elements, which produce analgesia by suppressing of CeA outputs.
Our data are consistent with the above-described circuitry. Chemogenetic activation of CeA Enk neurons affected both behavior and nociception in mice. First, the activation of CeA Enk interneurons resulted in anxiolysis and raised the thermal and mechanical thresholds in mice with sciatic nerve constriction. The analgesia induced by acute activation of Enk neurons lasted for at least 24 hours. Second, two weeks of continuous chemogenetic activation of CeA Enk neurons also resulted in anxiolysis and analgesia without any evidence for diminished efficiency by the end of the test period. Using various neuronal markers, we were able to show a potential brain circuitry in which the CeA Enk neurons exert their anxiolytic and analgesic properties. The circuitry begins with activation of enkephalinergic and Enk/PKC-δ interneurons in the CeA that control the output of the nucleus (Haubensak et al., 2010). vlPAG, which receives direct projections from the CeA and orchestrates and maintains the endogenous opioid analgesia (Oliveira and Prado, 2001, Weissbourd et al., 2014), was the second brain region activated by acute as well as sustained activation of Enk neurons in the CeA.
While the CeA projection and interneuronal populations are GABAergic, the cells also express a variety on neuropeptidergic co-transmitters. Our experiments did not eliminate the possibility that the activation of Enk interneurons by DREADDs led to simultaneous release of additional transmitters. However, the fact that a single injection of CNO produced analgesia that lasted for 24 hours support the notion that the endogenous opioids are engaged by the DREADD activation. A published report describes an injury-induced μ-opioid receptor constitutive activity in the spinal cord that depends on endogenous enkephalin release and it is responsible for pain suppression via central mechanisms (Corder et al., 2013). We speculate that the long-lasting analgesia observed after a single injection of CNO likely demonstrates engagement of the descending pain inhibitory system in addition to the spinal opioid receptors that are already tonically overactive after the nerve injury.
The CeA is the interface between nociception and response to pain and undergoes substantial changes as part of the central sensitization triggered by chronic pain. The excitability of the CeA projection neurons increases in chronic pain models and reflects the plastic changes associated with pain. The increased signaling by nociceptive efferents of the pituitary adenylate cyclase-activating polypeptide (PACAP) and calcitonin gene-related peptide (CGRP), accompanied by increased afferent CRF signaling, is believed to modulate the excitability of the CeA projections neurons, and is responsible for the development of hypersensitivity and pain chronification (Missig et al., 2017, Andreoli et al., 2017, Shinohara et al., 2017, Han et al., 2005). On the other hand, long lasting pain reduces the activity of the μ- and δ-opioid receptors in the CeA, to which the enkephalin is a natural ligand, therefore reducing the inhibitory Enk transmission in the CeA (Narita et al., 2006). Our experiment with sustained activation of CeA Enk very likely compensated for the opioid receptors downregulation by pain by increasing the secretion of enkephalin in the nucleus.
Our anatomical and functional data are consistent with already established micro-circuitry of the CeA, and with the functional anatomy of the descending pain inhibitory system. A paper by Haubensak et al. showed that a significant percentage of the Enk in the CeA belonged to a group of PKC-δ interneurons (Haubensak et al., 2010). According to the study, activation of the PKC-δ neurons suppresses the CeA output to the vlPAG area. Another recent paper employed modern tracing techniques to determine that CeA projections to DR and vlPAG are GABAergic, and that the projections target local GABA neurons rather than the serotoninergic DR neurons (Weissbourd et al., 2014). These publications provide a good explanation for our results, which show that chemogenetic activation of Enk causes activation of a high number PKC-δ neurons with associated activation of non-serotonergic neurons in DR and adjacent vlPAG, and support the assignment of a CeA Enk population to the descending pain inhibitory system.
As stated above, we believe that the robust and sustained analgesia, which is a result of the activation of the CeA Enk, is the primary cause for the observed decrease of anxiodepressive behavior. However, the anxiolysis caused by acute activation of the CeA in uninjured mice cannot be attributed to any concurrent analgesic activity. The DR is a suitable candidate to be a part of the brain circuitry by which CeA Enk neurons exert their anxiolytic activity because of the established involvement of DR in anxiety control (Teissier et al., 2015) and the direct efferents that the nucleus receives from the CeA (Weissbourd et al., 2014). Our experiments did not provide any compelling evidence for activation of serotoninergic neurons in the DR; nonetheless, the acute anxiolysis could be explained by the activation of the same brain region of the vlPAG, which according to a recent publication by Berton et al., is responsible for decreased anxiety in a chronic stress model (Berton et al., 2007). A strong correlation between the expression of ΔFosB in the vlPAG and mouse resistance to chronic stress indicates that the overactivity of vlPAG may contribute to stress resilience. The expression of ΔFosB was induced primarily in substance P (SP) containing neurons rather than serotonergic cell, and that led to decreased release of SP in nucleus accumbens, which Berton et al. proposed to be the putative mechanism for the observed antidepressant effect (Berton et al., 2007). In our experiments, both the acute and sustained activation of the CeA Enk were found to cause activation of non-serotonergic neurons in the vlPAG. It is possible that the vlPAG controls both the expression of pain and anxiety as two independent processes and therefore the decreased anxiety in mice with chronic pain after activation of the CeA Enk is not associated with a direct pain relieve.
In conclusion, our work shows that Enk in the CeA not only modulates ongoing pain but that continuous activation of CeA Enk population leads to powerful analgesia which buffers the effects of chronic pain on anxiodepressive behavior.
Highlights.
Activation of the CeA Enk interneurons decreases anxiety-like behavior.
Activation of the CeA Enk interneurons decreases the thermal hypersensitivity and mechanical allodynia in mice with neuropathic pain.
The CeA Enk interneurons are essential for the descending modulation of pain.
Acknowledgement
We thank Dr. Michel Barrot, Universite de Strasbourg for his comments on our preliminary data, and Dr. Amiel Rosenkranz and Dr. Carl White, RFUMS for their insights into the manuscript contents and editing help. This work is supported by National Institute of Mental Health, award number R01MH105528 to E.D.
Footnotes
Conflicts of interest
No potential conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Amir A, Goswami S and Pare D (2012) ‘Morphology, PKCdelta expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons’, J Neurophysiol, 108(12), pp. 3196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli M, Marketkar T and Dimitrov E (2017) ‘Contribution of amygdala CRF neurons to chronic pain’, Exp Neurol, 298(Pt A), pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S and Roth BL (2007) ‘Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand’, Proc Natl Acad Sci U S A, 104(12), pp. 5163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Madamba SG and Siggins GR (2011) ‘Neuroadaptation of GABAergic transmission in the central amygdala during chronic morphine treatment’, Addict Biol, 16(4), pp. 551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ and Barrot M (2008) ‘Sciatic nerve cuffing in mice: a model of sustained neuropathic pain’, Eur J Pain, 12(5), pp. 591–9. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Peschanski M and Besson JM (1989) ‘A possible spino (trigemino)-ponto-amygdaloid pathway for pain’, Neurosci Lett, 100(1–3), pp. 83–8. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL and Nestler EJ (2007) ‘Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses’, Neuron, 55(2), pp. 289–300. [DOI] [PubMed] [Google Scholar]
- Biagioni AF, de Freitas RL, da Silva JA, de Oliveira RC, de Oliveira R, Alves VM and Coimbra NC (2013) ‘Serotonergic neural links from the dorsal raphe nucleus modulate defensive behaviours organised by the dorsomedial hypothalamus and the elaboration of fear-induced antinociception via locus coeruleus pathways’, Neuropharmacology, 67, pp. 379–94. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN and Basbaum AI (2005) ‘Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor’, Neuron, 47(6), pp. 787–93. [DOI] [PubMed] [Google Scholar]
- Cai YQ, Wang W, Paulucci-Holthauzen A and Pan ZZ (2018) ‘Brain Circuits Mediating Opposing Effects on Emotion and Pain’, J Neurosci, 38(28), pp. 6340–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo Y and Gereau R. W. t. (2008) ‘Hemispheric lateralization of a molecular signal for pain modulation in the amygdala’, Mol Pain, 4, pp. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM and Yaksh TL (1994) ‘Quantitative assessment of tactile allodynia in the rat paw’, J Neurosci Methods, 53(1), pp. 55–63. [DOI] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL and Jin J (2015) ‘The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs’, ACS Chem Neurosci, 6(3), pp. 476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE and Taylor BK (2013) ‘Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence’, Science, 341(6152), pp. 1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov EL, Tsuda MC, Cameron HA and Usdin TB (2014) ‘Anxiety- and depression-like behavior and impaired neurogenesis evoked by peripheral neuropathy persist following resolution of prolonged tactile hypersensitivity’, J Neurosci, 34(37), pp. 12304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ (1991) ‘Staircase bioassay: the up-and-down method’, Neurosci Biobehav Rev, 15(1), pp. 47–50. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR and Pan HL (2005) ‘Effect of the {mu} opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala’, J Pharmacol Exp Ther, 312(2), pp. 441–8. [DOI] [PubMed] [Google Scholar]
- Han JS, Li W and Neugebauer V (2005) ‘Critical role of calcitonin generelated peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior’, J Neurosci, 25(46), pp. 10717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A and Anderson DJ (2010) ‘Genetic dissection of an amygdala microcircuit that gates conditioned fear’, Nature, 468(7321), pp. 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Fang D, Kong LY, Jin ZR, Cai J, Kang XJ, Wan Y and Xing GG (2014) ‘Sensitization of neurons in the central nucleus of the amygdala via the decreased GABAergic inhibition contributes to the development of neuropathic pain-related anxiety-like behaviors in rats’, Mol Brain, 7, pp. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ and Zimmer A (1996) ‘Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin’, Nature, 383(6600), pp. 535–8. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H and Watson SJ (1994) ‘Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study’, J Comp Neurol, 350(3), pp. 412–38. [DOI] [PubMed] [Google Scholar]
- Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH and Weinshenker D (2018) ‘The DREADD agonist clozapine Noxide (CNO) is reverse-metabolized to clozapine and produces clozapinelike interoceptive stimulus effects in rats and mice’, Sci Rep, 8(1), pp. 3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS and Osborne PB (2007) ‘Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division’, J Comp Neurol, 504(6), pp. 702–15. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C and Davis M (1999) ‘Cortical afferents to the extended amygdala’, Ann N Y Acad Sci, 877, pp. 309–38. [DOI] [PubMed] [Google Scholar]
- Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, Hammack SE and May V (2017) ‘Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain’, Biol Psychiatry, 81(8), pp. 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M and Suzuki T (2006) ‘Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala’, Neuropsychopharmacology, 31(4), pp. 739–50. [DOI] [PubMed] [Google Scholar]
- Oliveira MA and Prado WA (2001) ‘Role of PAG in the antinociception evoked from the medial or central amygdala in rats’, Brain Res Bull, 54(1), pp. 55–63. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K and Porreca F (2014) ‘Descending pain modulation and chronification of pain’, Curr Opin Support Palliat Care, 8(2), pp. 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Castonguay-Lebel Z, Laforest S and Drolet G (2008) ‘Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat’, J Comp Neurol, 506(6), pp. 943–59. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, McDonald AJ, Mascagni F and Wilson MA (2006) ‘The role of delta opioid receptors in the anxiolytic actions of benzodiazepines’, Pharmacol Biochem Behav, 85(3), pp. 545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ and Pfaff DW (2001) ‘Female preproenkephalin-knockout mice display altered emotional responses’, Proc Natl Acad Sci U S A, 98(4), pp. 1958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samineni VK, Grajales-Reyes JG, Copits BA, O’Brien DE, Trigg SL, Gomez AM, Bruchas MR and Gereau R. W. t. (2017) ‘Divergent Modulation of Nociception by Glutamatergic and GABAergic Neuronal Subpopulations in the Periaqueductal Gray’, eNeuro, 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Watabe AM, Nagase M, Okutsu Y, Takahashi Y, Kurihara H and Kato F (2017) ‘Essential role of endogenous calcitonin gene-related peptide in pain-associated plasticity in the central amygdala’, Eur J Neurosci, 46(6), pp. 2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW and Mahler SV (2016) ‘DREADDS: Use and application in behavioral neuroscience’, Behav Neurosci, 130(2), pp. 137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yi H and Cassell MD (1994) ‘Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala’, J Comp Neurol, 340(1), pp. 43–64. [DOI] [PubMed] [Google Scholar]
- Teissier A, Chemiakine A, Inbar B, Bagchi S, Ray RS, Palmiter RD, Dymecki SM, Moore H and Ansorge MS (2015) ‘Activity of Raphe Serotonergic Neurons Controls Emotional Behaviors’, Cell Rep, 13(9), pp. 1965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JM and Neugebauer V (2017) ‘Amygdala Plasticity and Pain’, Pain Res Manag, 2017, pp. 8296501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Yalcin I and Barrot M (2013) ‘The amygdala between sensation and affect: a role in pain’, J Mol Psychiatry, 1(1), pp. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K and Luo L (2014) ‘Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons’, Neuron, 83(3), pp. 645–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werka T (1994) ‘Post-stress analgesia in rats with partial amygdala lesions’, Acta Neurobiol Exp (Wars), 54(2), pp. 127–32. [PubMed] [Google Scholar]
- Wilson MA and Junor L (2008) ‘The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models’, Neuropsychopharmacology, 33(12), pp. 2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Garcia da Silva P, Albeanu DF and Li B (2016) ‘Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors’, J Neurosci, 36(24), pp. 6488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Zhang M, Li A, Pan L, Berman BM, Ren K and Lao L (2013) ‘DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia’, Neuroscience, 252, pp. 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]