Abstract
Intracranial atherosclerosis (ICAS) is a progressive pathological process that causes progressive stenosis and cerebral hypoperfusion and is a major cause of stroke occurrence and recurrence around the world. Multiple factors contribute to the development of ICAS. Angiography imaging techniques can improve the diagnosis of and the selection of appropriate treatment regimens for ICAS. Neither aggressive medication nor endovascular interventions can eradicate stroke recurrence in patients with ICAS. Non-pharmacological therapies such as remote ischemic conditioning and hypothermia are emerging. Comprehensive therapy with medication in combination with endovascular intervention and/or non-pharmacological treatment may be a potential strategy for ICAS treatment in the future. We summarized the epidemiology, pathophysiological mechanisms, risk factors, biomarkers, imaging and management of ICAS.
Keywords: Intracranial atherosclerosis, Angiography, Biomarker, Diagnosis, Treatment, Ischemic conditioning
1. Introduction
Atherosclerosis, a lifelong and systemic disease, is a major cause of stroke, myocardial infarction, ischemic gangrene and death. Despite the control of risk factors, atherosclerosis shows a gradual progression. Intracranial atherosclerosis (ICAS) can result in thromboembolism and subsequent transient or permanent cerebral ischemic events and is a major cause of ischemic stroke worldwide. Compared with other stroke subtypes, patients with ICAS are at a higher risk of recurrent ischemic events and death. Thus, investigation of the underlying mechanism of ICAS progression, prevention, and treatment may greatly decrease the incidence and mortality of ischemic stroke.
2. Epidemiology
ICAS ranges from 3.5% to 13% in asymptomatic individuals (Suri and Johnston, 2009). In a large population-based study among individuals of white descent, Bos and his colleagues found that the overall prevalence of intracranial internal carotid artery calcification, an indicator of ICAS, was 82.2% in the general population (Bos et al., 2012). Based on population, race, and method of detection, the estimated prevalence of symptomatic intracranial stenosis ranges from 20% to 53% (Mazighi et al., 2008) with higher risk in Asian, African, and Hispanic ancestry (Caplan et al., 1986; Mazighi et al., 2008; Mazighi et al., 2006; Wang et al., 2014b). In a case-control autopsy study, Mazighi et al. found that the prevalence of intracranial plaques and stenosis in ischemic stroke patients were 62.2% and 43.2%, respectively. Stenosis graded 30% to 75% might be associated with fatal stroke (Mazighi et al., 2008). In a retrospective study, Hallevi et al. demonstrated that the majority of subcortical ischemic stroke patients presented ICAS during neuroimaging and that the progression of neurological deficit was related to ICAS (Hallevi et al., 2012). As a result, stroke patients manifesting symptomatic ICAS may have a severe prognosis including worsened functional outcome and even increased morbidity and mortality. The annual recurrence rate of ischemic stroke in patients with ICAS varies from 10% to 50%, and the 2-year risk of recurrent stroke in the territory of the stenotic artery is 38.2% (Mazighi et al., 2006). In addition, annual mortality rates were reported to be 12.4% per year for intracranial internal carotid artery stenosis, 6.8% for middle cerebral artery (MCA) stenosis, and 11.6% for vertebrobasilar stenosis (Komotar et al., 2006).
3. Pathophysiological mechanism
Recent studies have indicated that atherosclerosis is a chronic inflammatory process. High-risk factors, such as hypertension, diabetes, and smoking, cause vascular endothelial dysfunction and increased permeability. This leads to accumulation of cholesterol-containing low-density lipoproteins (LDL) in the intima, which initiates a complex series of inflammatory and biochemical reactions involving accumulation of extracellular matrix, activation of the endothelium, infiltration of monocytes and T cells, intimal thickening, fibrous cap formation, and angiogenesis (Hansson, 2009). As a result of escalated inflammatory activation, the plaque grows gradually, leading to progressive stenosis. The rupture of an unstable atherosclerotic lesion triggers platelet activation and thrombus formation, occluding brain vessels and leading to ischemia and infarction (Yeh and Khan, 2006).
3.1. Differences between intra- and extra-cranial arteries
Compared to extracranial arteries that are rich in elastin filaments in the tunica media, intracranial arteries are muscular arteries with few elastic fibers. Without an external elastic lamina, the internal elastic lamina of the intracranial arteries is denser and fenestrated differently. Their adventitia is less abundant and their media is thinner than that of similarly sized extracranial vessels (Chen et al., 2008). The vessel wall metabolism in intracranial arteries is distinct. The composition of uronic acid, sulfur, and hexosamine, the proportion of chondroitin sulfates and hyaluronic acid in total glycosaminoglycans as well as the ratio of ester to total cholesterol are lower, and the percentage of heparin sulfate is higher in intima-media preparations of healthy intracranial arteries than those in unaffected coronary and aortic arteries (Ritz et al., 2014). Due to the different anatomic and hemodynamic peculiarities of arteries, the atherosclerotic process of different arteries may have different characteristics. Compared with extracranial vessels, proliferative fibrosis with fewer complicated lesions is more prevalent than lipid infiltration of the intima or adventitia in ICAS.
3.2. Progression of intracranial atherosclerosis with age
Due to the higher activities of antioxidant enzymes in intracranial arteries, the occurrence of atherosclerosis is less in intracranial arteries than in extracranial arteries. Reduplication and fragmentation of the internal elastic lamina are frequently observed in the aorta and coronary arteries of infants and young juveniles. Lipids are fewer in intracranial arteries of individuals below 15 years old. However, the antioxidant protection of intracranial arteries decreases markedly with the increase of age, contributing to a swift buildup of plaque in intracranial arteries in older people (D’Armiento et al., 2001). Atherosclerotic progression is linear over all ages with respect to extracranial arteries while atherogenesis is less extensive with respect to intracranial arteries and occurs later. As age progresses, intracranial arteries respond with a gradual loss of elastic fibers and medial muscular elements, and with an increase in collagen tissue replacing muscle fibers in the media. Combined with intimal thickening, reduplication and splitting of the thick internal elastic lamina frequently occur from the second to third decade of life, and fibrosis and hyalinization of media and adventitia prevail in this period (Qureshi and Caplan, 2014). From the fifth to sixth decade, intimal necrosis and thickenings are the most prominent manifestation in ICAS. Vasa vasorum extending into the vascular media, a marker of vascular damage, is mainly found in proximal intracranial segments, and is usually associated with erythrocyte leak, plaque neovascularization, and intraplaque hemorrhage (Labadzhyan et al., 2011). Dense mineral deposits, especially calcium, indicate the degeneration of media of intracranial arteries. The absence of an external elastic lamina (Masuoka et al., 2010), prominent expression of proinflammatory proteasomes (Sun et al., 2012), and reduced expression of inflammatory inhibitors (Tulamo et al., 2010) might result in susceptibility of intracranial arteries to the cascade of inflammatory reactions and plaque instability. Unstable atherosclerotic plaques rich in high-lipid content, neovasculature, and inflammatory components appear after the fifth decade.
3.3. Mechanisms of ischemic stroke related to intracranial arterial stenosis
The pathophysiology of ischemic stroke related to ICAS involves multiple mechanisms. ICAS may incite downstream ischemia in a specific arterial territory due to hypoperfusion, artery-to-artery embolism, plaque extension over small perforator artery ostia, or combined mechanisms. The inflammatory process not only affects the onset, development and dynamic variations of the arterial atherosclerotic plaque, but also contributes to the appearance and evolution of atherothrombosis. Both luminal stenosis and plaque composition are significant parameters that reflect subsequent ischemic events. Kasner et al. emphasized that severe stenosis (≥70%) was the strongest predictor of ischemic stroke associated with ICAS (Kasner et al., 2006). Microembolic signals were detected in 36.1% of patients with symptomatic MCA atherosclerotic stenosis during the acute phase of a cerebral ischemic event (Shi et al., 2012), suggesting that embolism is very common in patients and that the existence of unstable atherosclerotic plaques underlies intracranial stenosis. Neuroimaging showed characteristic distal wedge-shaped territorial infarct or the presence of multiple cortico-subcortical lesions, and provided indirect evidence of the participation of an artery-to-artery embolism in ischemic stroke (Arenillas et al., 2005). When hypoperfusion occurs distally to high-grade stenosis, it prevents the clearing of emboli from the cerebral circulation and results in their accumulation at low perfusion pressure regions, suggesting that stenosis and embolism can act synergistically to induce ischemia (Gonzalez et al., 2013).
4. Traditional cardiovascular risk factors
4.1. Age
Age, a non-modifiable risk factor, is an important independent risk factor for both intra- and extra-cranial atherosclerosis and is associated with increasing prevalence and severity of ICAS with a distinct disease progression. With increased age, intracranial arteries are more susceptible to oxidative stress and inflammatory reactions than extracranial arteries due to differential hemodynamics, histology and metabolism. A cross-sectional study of 1208 subjects aged 40 years and above, with no history of ischemic cerebrovascular events, suggested that age, hypertension and diabetes mellitus are independent predictors for the presence and severity of asymptomatic intracranial atherosclerosis (AsIA) (Bae et al., 2007). Transcranial Doppler (TCD) showed that numbers of stenoses were higher in subjects aged ≥65 years than in those aged < 65 years. In an anatomic study, severe ICAS was identified in 43% of individuals in the sixth decade, 65% in the seventh decade, and 80% in the eighth and ninth decades (Baker et al., 1967).
4.2. Race
The locations of cerebral atherosclerosis vary among different racial and ethnic groups. Compared with extracranial arteries, atherosclerosis is more prone to occur in intracranial arteries in Asian, Hispanic, and African populations. The Barcelona-AsIA study showed that the prevalence of AsIA and moderate-severe AsIA were 8.6% and 3.3%, respectively, among stroke-free Caucasians with moderate-to-high vascular risk (Lopez-Cancio et al., 2012a). Chinese patients with at least one vascular risk factor of hypertension, diabetes, or hyperlipidemia had asymptomatic MCA stenosis (12.6%) (Wong et al., 2007b), while the prevalence of AsIA was 15% in Japanese patients (Uehara et al., 1998). The most common vascular lesion in Caucasians is extracranial carotid stenosis, and ICAS accounts for only about 10% of stroke (Tsivgoulis et al., 2014); however, ICAS causes about 33% to 50% of stroke and > 50% of transient ischemic attack (TIA) in Chinese patients, 20% to 25% of stroke in Korean patients, 47% in Thailand and 47.9% in Singapore (Wong, 2006). Overall, the racial and ethnic differences in the prevalence of ICAS may be attributed to differences in genetic susceptibility, and/or risk factors and lifestyles between races. As Asians, Africans and Hispanics make up the majority of the world’s population, ICAS can likely be one of the most common vascular lesions in stroke patients throughout the world.
4.3. Hypertension
Hypertension induces endothelial dysfunction and is the direct cause of damage to the arterial wall, thereby resulting in disturbances to the laminar flow. Furthermore, the flow separation and oscillatory shear stress lead to damage in the vessels in a cycle. Some studies have reported that the higher incidence of hypertension in Asian and African ancestries may contribute to their higher prevalence of ICAS (Alkan et al., 2009). In the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial, the most relevant risk factors for recurrent stroke and other vascular events in patients with symptomatic ICAS were mean systolic blood pressure (SBP) of 140 mmHg or higher, total cholesterol concentration higher than 200 mg/dL, and alcohol consumption (Chaturvedi et al., 2007).
4.4. Dyslipidemia
Dyslipidemia, an established risk factor for coronary and extracranial atherosclerosis, refers to abnormal levels of lipid profiles that include hypercholesterolemia, a high ratio of LDL to high-density lipoprotein (HDL) cholesterol, and a high ratio of apolipoprotein B (apoB) to apolipoprotein AI (apoAI). Accumulation of LDL in the intima initiates the development and progression of atherosclerosis, and eventually leads to the formation of plaque. HDL plays an anti-atherosclerotic role by promoting cholesterol effluxion from macrophages, inhibiting oxidation of LDL and migration of smooth muscle cell (SMC) and aggregation of platelets. ApoAI, the major apolipoprotein in HDL, can initiate reverse cholesterol transport from blood vessels to the liver, and also has anti-inflammatory and antioxidant effects. ApoB, which can represent the total number of potentially atherogenic properties, generates multiple proinflammatory products and aggravates atherogenesis within the vascular wall through oxidation reaction. Lopez-Cancio et al. found that the triglyceride/HDL ratio was significantly associated with moderate to severe AsIA, but was not an independent predictor for AsIA (Lopez-Cancio et al., 2012b). In a prospective study including 464 patients with acute ischemic stroke, the ICAS group showed a greater apoB/apoAI ratio than extracranial atherosclerosis (ECAS) and no cerebral atherosclerotic stenosis groups (Park et al., 2011). There was a positive correlation between the apoB/apoAI ratio and the presence and severity of ICAS, suggesting that the apoB/apoAI ratio may be a promising biomarker for predicting the risk and severity of ICAS. Furthermore, in patients with ICAS, a high apoB/apoAI ratio is probably an indicator for deep subcortical ischemic burden, a potential risk factor for cognitive decline and recurrent stroke. In addition, patients with pre-existing brain infarct had a lower level of apoAI than those without pre-existing brain infarct, whereas the apoB level did not differ significantly (Park et al., 2013). In a substudy of the Trial of Cilostazol in Symptomatic Intracranial Stenosis 2 (TOSS-2), the progression of ICAS of patients with acute symptomatic stenosis in the M1 segment of the MCA or basilar artery was evaluated with magnetic resonance angiography (MRA) (Kim et al., 2012b). The results showed that compared with baseline, HDL increased while remnant lipoproteins (products of partially catabolized chylomicrons and very low-density lipoprotein) decreased in the non-progression group at 7 months. At 7 months, the apoB/apoAI ratio was lower and HDL was higher in non-progressors than in progressors. Post-stroke HDL elevation, along with remnant lipoprotein reduction and a low apoB/AI ratio might play a protective function in the prevention of symptomatic ICAS progression. These results suggest that selective loss of antioxidant and anti-atherosclerotic protection may be a potential mechanism of ICAS.
4.5. Metabolic syndrome
A patient is diagnosed with metabolic syndrome when 3 or more of the following parameters are present: abdominal obesity for the European population (≥94 cm in men and≥80 cm in women); arterial blood pressure≥130/ ≥85 mmHg in baseline visit; level of triglycerides ≥150 mg/dL; low HDL cholesterol (in men ≥40 mg/dL and in women ≥50 mg/dL); and fasting plasma glucose ≥100 mg/dL or history of diabetes mellitus or taking antidiabetic medications (Alberti et al., 2009). Accumulation of LDL in the intima triggers the evolution of atherosclerosis. In this process, metabolic syndrome favors a hypercoagulable and proinflammatory state mainly mediated by insulin resistance, which can promote the reduction of antioxidant protection and endogenous fibrinolytic activity of intracranial circulation. In line with this, Lopez-Cancio et al. found that age, metabolic syndrome and insulin resistance are significant risk factors for moderate to severe ICAS in the Barcelona-AsIA study (Lopez-Cancio et al., 2012b). Moreover, metabolic syndrome was detected in approximately half of the individuals with ICAS and predicted a substantially high risk of ischemic event recurrence (Ovbiagele et al., 2006).
4.6. Other risk factors
Cigarette smoking increases the oxidative modification of LDL and alters the biosynthesis of nitric oxide (NO) to influence the inception and progression of atherosclerosis. Smoking was more common in patients with ICAS than in those with ECAS, and smoking may play a part in the development of ICAS in patients with acute ischemic stroke (Kim et al., 2012c).
5. Biological markers
5.1. Adiponectin
Adiponectin, a protein secreted by adipose cells, has a protective effect against atherosclerosis and plaque formation. Previous studies have suggested that adiponectin has beneficial effects on cerebrovascular function and flow via stimulating the production of NO in endothelial cells, improving insulin sensitivity, reducing insulin resistance, and increasing glucose uptake. It plays anti-inflammatory and anti-atherogenic roles by suppressing the expressions of inflammatory cytokines and adhesion molecules that are related to leukocyte trafficking and neointimal formation, and through attenuating the expression of scavenger receptors to reduce uptake of cholesterol and to inhibit transformation of macrophages to foam cells. Furthermore, adiponectin can inhibit secondary inflammation and promote angiogenesis following brain ischemia (Song et al., 2014). Hypoadiponectinemia is associated with obesity, type 2 diabetes, insulin resistance, metabolic syndrome, dyslipidemia, hypertension, and oxidative stress. In addition, hypoadiponectinemia is related to impaired arterial endothelium-dependent vasodilation (Tan et al., 2004). As a result, hypoadiponectinemia increases the risk of systemic atherosclerosis, ischemic stroke and coronary heart disease (Savopoulos et al., 2011). Kim et al. showed that the level of serum adiponectin was lower in patients with strokes due to large artery atherosclerosis (LAA) than in those with non-LAA strokes, supporting the anti-atherogenic properties of adiponectin (Kim et al., 2012a). Bang et al. demonstrated that compared with other ischemic stroke subtypes, lower adiponectin levels were present in patients with symptomatic ICAS, and there was a relationship between the extent of hypoadiponectinemia and the severity of ICAS: the lower the level of adiponectin, the more advanced the ICAS (Bang et al., 2007). Thus, adiponectin may be a useful marker for identifying ICAS, especially advanced ICAS.
5.2. Interleukin-6
Interleukin-6 (IL-6), an upstream inflammatory cytokine, is highly upregulated upon acute infections, chronic inflammatory conditions, obesity, cancer development, physiologic stress and ischemic stroke (Hartman and Frishman, 2014). Triggered by vasoactive peptides, reactive oxygen species (ROS) and other cytokines, a variety of cells including monocytes and macrophages as well as resident cells of the affected vasculature can express and release IL-6 (Schuett et al., 2009). IL-6 plays a crucial role in driving the downstream inflammatory reaction accountable for atherosclerosis through the activation of endothelial cells, promotion of endothelial dysfunction, acceleration of SMC proliferation and migration, induction of lymphocyte differentiation, stimulation of hepatic synthesis of acute-phase reactants as well as increased coagulability. IL-6 is an important regulator that impacts the development, progression, and complications of atherosclerosis at different stages, as reflected by a prospective study demonstrating that both the baseline and the progression of carotid mean-maximal intimamedia thickness (mmIMT) are positively correlated with long-term average IL-6 levels (Okazaki et al., 2014). Thus, chronic elevation of the serum IL-6 level was an independent predictor of accelerated atherosclerosis in patients with conventional vascular risk factors, and IL-6 may be used as a potential therapeutic target for the progression of atherosclerosis. Echolucent plaque characterized by rich lipid, increased density of macrophages and a thin fibrous cap represents rupture-vulnerability and is a risk factor for ischemic stroke. Yamagami et al. demonstrated that there was an inverse association between serum IL-6 levels and plaque echogenicity of carotid artery plaques, suggesting that IL-6 contributed to the formation of unstable plaques and their thrombotic complications (Yamagami et al., 2004). A study including 226 Japanese patients without a history of cerebrovascular disease found that mean IL-6 levels in ICAS patients were higher than in those without ICAS, indicating that elevated IL-6 concentration was related to subclinical ICAS (Hoshi et al., 2008). In addition, high circulating levels of IL-6 within 48 h after stroke onset were positively correlated with future ICAS progression, supporting the premise that IL-6 may play an important role in the development of ICAS (Shimizu et al., 2013).
5.3. C-reactive protein
C-reactive protein (CRP), a sensitive biomarker for systemic inflammation, is secreted by vascular resident and infiltrated cells upon increased IL-6 stimulation and contributes actively to all stages of atherosclerotic progression. It is a significant indictor of the inflammatory activity and destabilization of atherosclerotic lesions. CRP is an independent and strong predictor of first and recurrent ischemic heart disease or stroke and of all-cause mortality (Kaptoge et al., 2010). In addition, CRP is also a modulator that maintains and enhances inflammatory response in cerebral arteries and brain injury. Through a positive feedback mechanism, CRP activates a complement cascade. CRP not only initiates the chemotaxis of leukocyte and expression of adhesion molecules, but also induces apoptosis through a caspase-dependent mechanism. Chan et al. found that high-sensitivity C-reactive protein (hs-CRP) can be a useful biomarker and predictor of both short- and long-term mortality after stroke (Chan et al., 2012). A large community-based study reported that the level of CRP was positively associated with the prevalence of AsIA (Zhang et al., 2013), suggesting that inflammation plays an important role in the pathogenesis of ICAS, and hs-CRP can be used as an independent predictor of AsIA and high atherosclerotic burden of intracranial arteries (Wang et al., 2014a). In a prospective study with first-ever intracranial large artery (ILA) atherosclerosis, blood levels of CRP and plasminogen activator inhibitor-1 (PAI-1) were detected 3 months after the qualifying stroke or TIA, and all patients underwent long-term follow-up to assess progression of ILA by TCD (Arenillas et al., 2008). Researchers found that CRP and PAI-1 were independent predictors of ILA progression and could contribute to a higher recurrence of ischemic stroke, suggesting that ICAS progression is associated with a proinflammatory state and defective fibrinolysis, two main features of metabolic syndrome. Arenillas and Alvarez-Sabin found that high levels of hs-CRP were detected for at least 3 months after stroke onset in patients with first-ever symptomatic ILA occlusive disease and were associated with an increased risk of recurrent ischemic events (Arenillas and Alvarez-Sabin, 2005). CRP may therefore be a useful biomarker to estimate the progression and destabilization of ICAS.
5.4. Matrix metalloproteinases
Matrix metalloproteinases (MMPs) can be upregulated by tissue plasminogen activator (tPA) and are produced in a latent form requiring activation. Once activated, these act as shedders at the cell surface to control activation of death receptors, growth factors, and other signaling molecules, thus participating in many physiological and pathological processes. MMPs trigger dysfunction and apoptosis of neurons and oligodendrocytes by disrupting cell-cell and cell-matrix signaling. MMPs can also cause plaque instability and rupture via proteolysis of the matrix, promotion of inflammatory cell infiltration and apoptosis of SMC.
MMP levels may vary due to the different natures of atherosclerotic plaques. MMP-2 activity is higher in SMC-rich lesions, whereas MMP-9 activity is higher in lesions rich in macrophages (Sluijter et al., 2006). Furthermore, by degrading neurovascular substrates such as extracellular matrix, basal lamina, and tight junctions in endothelial cells, MMPs increase the permeability of the blood-brain barrier that underlies neuronal inflammation, hemorrhagic transformation, and vasogenic edema. They play a deleterious and important role in the pathogenesis of atherosclerosis, cerebral ischemia and hemorrhage. It has been suggested that plasma levels of MMP-2 and MMP-9 were positively correlated with stroke severity and the extent of neuronal injury after stroke, and a high level of MMP-9 increased the risk of hemorrhagic transformation after infarction (Heo et al., 1999). In a rat model of acute focal cerebral ischemia, Park et al. found that plasma levels of pro-MMP-9 increased progressively over the course of 24 h, and were positively associated with final infarct volumes (Park et al., 2009). A study including 177 consecutive subjects with chronic cerebral infarction showed that reduced plasma levels of MMP-2 were more closely correlated with ICAS than with ECAS, while ECAS was related to ulceration and hemorrhage compared to ICAS (Jeon et al., 2012). Overconsumption of MMP-2 in ICAS patients may contribute to the lower plasma level. In addition, MMPs also actively take part in modulating brain matrix, brain plasticity and vascular remodeling during the recovery status after acute stroke, and this also contributes to the lower plasma level of MMPs. Therefore, MMPs may serve as causative biomarkers of ICAS pathophysiology.
5.5. The ankle-brachial index (ABI)
The ankle-brachial index (ABI), an indicator of atherosclerosis or arterial stiffness, is calculated as systolic blood pressure as measured at the ankle divided by systolic blood pressure as measured at the arm (brachial artery) while lying down. A ratio of < 0.9 is considered abnormal (Curry et al., 2018; Jin, 2018). Low ABI can be used as a simple and non-invasive tool to identify AsIA (Jimenez et al., 2014). In a prospective study including 73 ICAS patients with only a single episode of stroke, lower ABI was correlated with an increased risk of stroke recurrence in patients with symptomatic ICAS, suggesting that ABI is a useful indicator to assess high-risk patients (Massot et al., 2014).
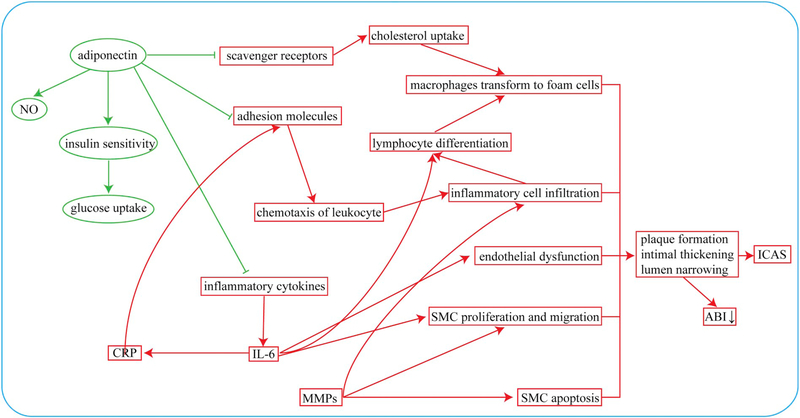
The interrelations between different biological makers for ICAS are schematically summarized in Fig. 1.
Fig. 1.
The interrelations between different biological markers for ICAS. Factors that can enhance or inhibit the formation of ICAS are denoted by red and green lines, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
6. The effect of ICAS on brain function
ICAS affects sensorimotor function, cognitive function, and psychiatric symptoms, and these clinical manifestations are correlated with the severity and location of stenosis. ICAS is a high-risk factor for cerebral ischemia and may trigger TIA or cause ischemic stroke.
ICAS causes chronic, progressive stenosis and cerebral hypoperfusion. The brain adapted to progressive hypoperfusion either by establishing collateral circulation or tolerating lower energy supply; therefore, patients with ICAS usually do not show neurological dysfunction at earlier stages. Hippocampus, which is closely related to memory, is vulnerable to ischemia, and therefore patients with ICAS usually start with memory dysfunction. ICAS patients present with various degrees of cognitive impairment, which could be attributed to subtle cortical and subcortical ischemic lesions or damage in anatomic integrity and perfusion deficiency caused by ICAS (Hilal et al., 2017). In the Atherosclerosis Risk in Communities (ARIC) Study, Dearborn et al. (Dearborn et al., 2017) found that the prevalence of ICAS in participants with normal cognition, mild cognitive impairment, and dementia was 35.0%, 37.9%, and 56.6%, respectively. Specifically, presence of anterior cerebral artery plaque, stenosis > 50% or > 2 vascular territories with plaque was associated with dementia. A memory clinic case-control study showed that patients with ICAS are more likely to have vascular cognitive impairment no dementia (VCIND) and dementia independent of cardiovascular risk factors and MRI markers (Hilal et al., 2017). Post-mortem studies indicated that Circle of Willis atherosclerosis was more severe in subjects with Alzheimer’s disease (AD, 34%) and vascular dementia (VaD, 53%) than in control subjects (18%), and atherosclerotic grade increased the odds ratios of both AD and VaD (Beach et al., 2007). Importantly, cognitive dysfunction caused by ICAS could not be improved with aggressive medical management to enhance reperfusion, based on two recent clinical trials: the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial, and revascularization with percutaneous angioplasty and stenting, calling into doubt the hypothesis that reperfusion of stenotic cerebral arteries would improve cognition (Turan et al., 2017). Furthermore, ICAS itself is associated with post-stroke cognitive impairment (Guo et al., 2018; Hilal et al., 2015). Thus, ICAS may be an indicator to identify those at risk of future cognitive decline. However, the mechanisms underlying how ICAS leads to cognitive decline and dementia remains under-explored.
Depression is a common manifestation in patients with ICAS, and 17% of patients with cerebral, coronary, or peripheral atherosclerosis had symptoms of depression (Grool et al., 2011). There was a significant association between post-stroke depression (PSD) and ICAS at both the acute and chronic phases of stroke (Chen et al., 2016), and the presence of ICAS approximately doubled the probability of PSD occurrence and thus may be an independent predictor of PSD. Patients with ICAS may also have other psychiatric dysfunction, such as mood disturbance, anxiety, stimulation (arousal), psychotic symptoms, sleep disturbance, apathy, and a series of others. The normal function of neural circuits related to neuropsychiatric modulation might be interfered with by a significant decrease in cerebral perfusion. ICAS may contribute to mood by hypoperfusion of cerebral blood flow globally or in specific brain regions, damage to the blood-brain barrier caused by a dysfunction of the endothelium of the brain’s vessels, or destruction of neurons initiated by activation of immunological processes (Bidzan et al., 2014).
ICAS is a high-risk factor for TIA and ischemic stroke. The recurrence rate of cerebrovascular events in patients with ICAS is about 12.32/100 patients yearly (Gouveia et al., 2014). Intracranial plaques and stenoses were identified in 45–62% of patients with ischemic stroke on autopsy (Mazighi et al., 2008). Lacunar and subcortical infarctions are two most common infarctions in patients with intracranial stenosis (Man et al., 2009; Mull et al., 1997). Stroke patients with ICAS had a higher risk to develop VCI and PSD.
In addition, vascular alterations in the brain can manifest as parkinsonism, and the predominant clinical presentation of vascular parkinsonism is gait impairment, manifested as lower body parkinsonism. Vascular parkinsonism is most commonly a result of brain white matter lesions and basal ganglia lacunes (Korczyn, 2015). In an analytical prospective cross-sectional study, ICAS was found in one out of three stroke-free Pakistanis on MRI. Kamal et al. (Kamal et al., 2014) demonstrated that the distribution of ICAS in young Pakistanis is common and equally distributed across gender and is related to modifiable risks.
7. Diagnostic imaging
As the traditional criterion for assessment of cerebral vasculatures, digital subtract angiography (DSA) can provide essential information regarding hemodynamic status, degree of stenosis in intracranial vessels, and collateral circulation in patients. However, DSA is invasive and may cause a small but real risk of permanent neurological deficit. In addition, DSA can cause radiation exposure, potential allergy to iodine contrast material and contrast media-induced kidney damage, thereby limiting the application of DSA. Several noninvasive screening tests that provide safer and less expensive ways to evaluate intracranial vessels are widely used for the diagnosis of ICAS such as TCD, computed tomography angiography (CTA), magnetic resonance angiography (MRA), and high-resolution magnetic resonance imaging (HR-MRI).
7.1. TCD
TCD is an inexpensive, noninvasive and safe method that can provide information about real-time flow and direction of flow, microembolic signals and steal phenomenon, and vasodilatory capacity of arteries and arterioles due to stimuli. In the Neuroimaging of Intracranial Atherosclerosis (SONIA) study, an international multicenter study for diagnosis of ICAS with TCD and DSA, ICAS was identified with ≥50% stenosis (Zhao et al., 2011). Compared with DSA, TCD SONIA in MCA had 78% sensitivity, 93% specificity, 73% positive predictive value (PPV), 94% negative predictive value (NPV), and overall accuracy of 90%, similar to stenosis in vertebral artery (69%, 98%, 88%, 93%, and 92%, respectively). Addition of a stenotic/prestenotic ratio or low-velocity criteria improved predictive values of TCD in testing severe disease and showed good agreement with DSA. However, the PPV and NPV of TCD for diagnosis of ICAS in another multicenter study was lower, 50% and 85%, respectively (Qureshi and Caplan, 2014). Due to insufficient temporal bone window, unfavorable insonation angle, or low-flow velocity or volume, it may be difficult to detect ICAS. Ultrasound contrast agents may be helpful to improve the TCD signal quality and allow more arterial signals to be insonated. Injection of Levovist increased the diagnosis of intracranial artery segments from 12% to 63% (Hansberg et al., 2002). Transcranial colour-coded duplex may be useful to increase the diagnostic accuracy of ICAS (Nedelmann et al., 2009). Moreover, microembolic signals detected by TCD may be linked with subsequent progression of neurological deficits during the acute phase of ischemic stroke, which may be an indicator of stroke recurrence in patients with symptomatic ICAS. Vasomotor reactivity quantification measured by TCD, a potential risk for stroke, may reflect the capacity of cerebral autoregulation (Alexandrov et al., 2012). Thus, TCD is a well-established technique for screening ICAS, but its accuracy and predictive value may vary depending on the sonographer’s skills and patient’s status.
7.2. CTA
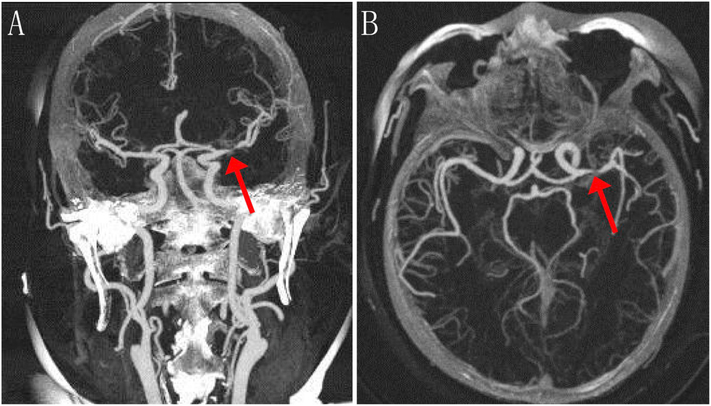
CTA is an accurate and efficient noninvasive approach in the evaluation of the etiology of acute stroke and provides better delineation of the anatomy of intracranial arteries. CTA can not only detect and calculate intraluminal narrowing, but also provide information about calcification of the arterial wall as well as characteristics of plaque morphology and collateralization in ICAS. Furthermore, CTA may detect ischemic lesions and regional hypoperfusion when combined with concurrent CT/CT perfusion. Using DSA as the gold standard, the sensitivity and specificity of CTA in detecting complete large arterial occlusions were 100%. For detection of ≥50% stenosis, CTA had a sensitivity of 97.1%, specificity of 99.5% and NPV of 99.8% (Nguyen-Huynh et al., 2008). In the evaluation of post-stenotic or post-occlusive arterial segments of low flow or turbulence and vessel patency, CTA might be superior to DSA (Skutta et al., 1999). CTA also revealed higher sensitivity for intracranial stenosis (98% vs. 70%) and occlusion (100% vs. 87%), and a higher PPV for stenosis (93% vs. 65%) and occlusion (100% vs. 59%) than that of MRA (Bash et al., 2005). Combined with CTA, the sensitivity of detecting ≥50% intracranial stenosis with MRA increased from 92% to 100% and the specificity from 91% to 99% (Hirai et al., 2002). For discrimination of calcified, intermediate, and soft plaques, CTA has a good consistency with both histopathology and intravascular ultrasound. It detected thrombi with a sensitivity of 92%, a specificity of 89% and an accuracy of 90%, and can be used to localize and identify thrombosis and inflamed plaques (Aziz et al., 2008). Compared with histopathology, CTA had 93.9% sensitivity and 98.7% specificity in detecting plaque ulceration (Saba et al., 2007). For acute ischemic stroke, both time-to-reperfusion and residual cerebral blood flow in the ischemic territory contribute to the final infarct volume after thrombolysis, which is influenced by leptomeningeal collateralization and recanalization. The CTA collateralization score was inversely correlated with final infarct volume, which can be an independent predictor of final infarct volume in acute intracranial arterial stroke (Angermaier et al., 2011) and recurrent stroke events (McVerry et al., 2012). CTA has some limitations including radiation exposure, loss of laminar flow hemodynamic information, and artefactual narrowing or non-visualization of intracranial internal carotid artery due to the susceptibility gradient near the sphenoid sinus. Thus, CTA can replace DSA in evaluating ICAS to some extent (Fig. 2).
Fig. 2.
Representative CTA images of ischemic stroke with stenosis. Coronal (a) and axial (b) CTA images were taken 4 days after left MCA infarction in a 55-year-old woman with right hemiparesis and aphasia. A high-grade stenosis at the proximal left MCA (M1 segment) is denoted with red arrows. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
7.3. MRA
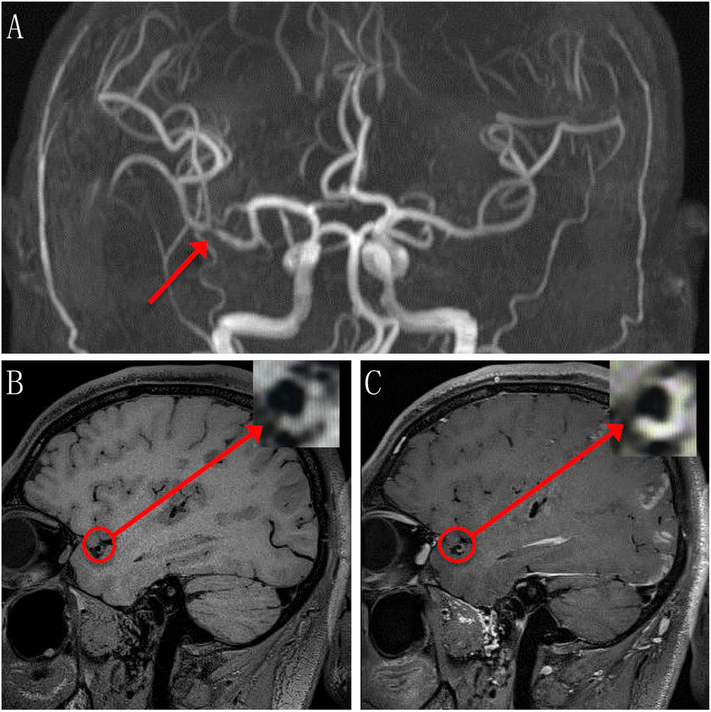
MRA provides simultaneous flow and anatomical information and has been used widely to evaluate intracranial arteries. Using DSA as the criterion standard, time-of-flight magnetic resonance angiography (TOF-MRA) had 100% sensitivity, 99% specificity, 87% PPV and 100% NPV in detecting complete occlusion of intracranial arteries, and 78% to 85% sensitivity, 95% specificity, 75% to 79% PPV and 95% to 97% NPV in detecting high-grade stenosis (50% to 99%) (Choi et al., 2007). TOF-MRA is not applicable in the assessment of intracranial in-stent stenosis due to artifacts from the stent or coils. Quantitative MRA, which uses traditional time-of-flight and phase-contrast MRI to measure blood flow and visualize vascular anatomy, had a sensitivity of 100%, specificity of 92%, PPV of 67%, and NPV of 100% in the detection of radiographic restenosis that may be an indicator of clinical events (Prabhakaran et al., 2009). Percentage of maximal luminal stenosis and hemodynamic effects of lesion factors determine the outcomes of symptomatic ICAS and the risk of recurrent ischemic stroke. Fractional flow that may reflect hemodynamic impact can be assessed by TOF-MRA signal intensity ratio (SIR). Liebeskind et al. found that compared to the distal/proximal SIR of the symptomatic intracranial stenosis ≥0.9, the hazard ratio (HR) for stroke in the territory of the symptomatic artery with SIR < 0.9 was 5.2 (1.8, 15.3; P < .001), and in those with < 70% stenosis, a SIR < 0.9 was significantly associated with recurrent stroke in the territory (P = .006), with a 2-year event rate of 17.3% (Liebeskind et al., 2015). In addition, in patients with symptomatic ICAS in the anterior circulation, there was a remarkable negative correlation (P = .011) between SIR values and infarct volumes on diffusion-weighted MRI (Leng et al., 2013b). SIR across an ICAS on TOF-MRA was demonstrated to be of both high intra-observer and interobserver reproducibility (Leng et al., 2013a). Therefore, diminished TOF-MRA signal intensity distal to the symptomatic artery that reflects hemodynamic impairment may be a predictor of high-risk intracranial lesions. MRA has no radiation exposure; however, MRA requires longer imaging times than DSA or CTA, frequently resulting in image quality degradation and artifacts due to patient motion. MRA also has a lower spatial resolution that may reduce the ability to represent severe stenosis in small vessels or may overestimate the degree of stenosis due to the differential flow velocities or turbulent flow within the stenosis (Bash et al., 2005). Hence, MRA may be an effective screening tool for the detection of intracranial atherosclerotic steno-occlusive disease that requires the need for high-resolution MR imaging (Degnan et al., 2012) (Fig. 3).
Fig. 3.
Representative TOF-MRA and HR-MRI images of ischemic stroke with stenosis. a, 3-dimesional TOF-MRA image shows severe stenosis (arrow) at the proximal portion of the right MCA (M2 segment) in a 38-year-old male. Non-contrast (b) and contrast (c) image of T1-weighted HR-MRI shows the inward remodeling of plaque (cycle). Magnified image of plaque in inlet shows slightly hyperintense (b) and enhanced (c) signals of plaque.
7.4. HR-MRI
HR-MRI is an advanced MRI modality that can render intracranial arterial wall, and can disclose intracranial atherosclerotic plaque burden and vulnerability with a sensitivity of 85% and specificity of 92% (Larose et al., 2005). HR-MRI may produce more precise information about future risk related to ICAS and provide a more in-depth understanding of pathophysiology and diagnosis of ICAS for designing individualized treatment options for ICAS (Leng et al., 2014). It not only indicates the degree of stenosis, but also shows intraplaque factors including plaque morphology, plaque components, and inflammation, which are predictors of vulnerable atherosclerotic lesions. Compared with stable plaque, vulnerable plaque has a thinner fibrous cap, larger lipid core and a myriad of inflammatory cells, which is related to positive remodeling, greater plaque thickness or a higher ratio of plaque thickness to patent lumen, intra-plaque hemorrhage and contrast enhancement on HR-MRI (Ryu et al., 2014). Positive remodeling refers to a compensatory vessel enlargement due to an increased plaque burden and is significantly correlated with plaque rupture and acute ischemic symptoms. On the other hand, negative remodeling with fibrotic change induces local shrinkage of the vessel and is more resistant to rupture. Using HR-MRI as an in vivo modality for identifying the morphological characteristics of intracranial arteries, Chung et al. found that in addition to the degree of MCA stenosis, outward remodeling of the stenotic area (85.7% vs. 37.5%, P = .011) was remarkably higher in the symptomatic MCA stenosis group than in the asymptomatic group (Chung et al., 2012). In a retrospective study, Xu et al. found that the occurrence rate of intra-plaque hemorrhage, a risk factor for ischemic stroke, was significantly higher in symptomatic MCA stenosis vs. asymptomatic patients (19.6% vs. 3.2%, P = .01) (Xu et al., 2012). Strong contrast enhancement of the plaque, an indicator of inflammation, is associated with increased endothelial permeability and vascular supply to the plaque. Skarpathiotakis et al. found that strong pathologic enhancement of intracranial atherosclerotic plaque was observed on the high-resolution vessel wall MR imaging in all patients performed within 1 month of the onset of ischemic stroke, and the strength and presence of enhancement of the atherosclerotic plaque decreased with time after the initial stroke presentation (Skarpathiotakis et al., 2013), suggesting that intracranial plaque enhancement could be an indicator of plaque instability and related to ischemic events. In addition, HR-MRI is able to determine the pathophysiologic mechanisms underlying intracranial atherosclerotic ischemic stroke. By showing the image of a luminal wall, cross-sectional HR-MRI can provide evidence of the stroke patterns of intracranial atherosclerotic arteries such as penetrating artery disease or large-artery atherosclerosis that includes parent artery plaques occluding a penetrating artery and artery-to-artery embolisms (Gao et al., 2014). Furthermore, HR-MRI has moderate inter-observer reproducibility (k = 0.75) and excellent intra-observer reproducibility (k = 0.81) in determining abnormal and normal walls (Li et al., 2009). HR-MRI can provide direct visualization of atherosclerotic lesions of intracranial arteries and may be a useful technology in risk stratification and treatment selection (Fig. 3).
The major manifestations, advantages and disadvantages of different imaging methods for the diagnosis of ICAS are summarized in the table below.
8. Management and treatment
A number of pharmacological neuroprotectants targeting different pathophysiological mechanisms of ischemic brain injury such as free radical scavengers, mitochondria potassium channel openers, calcium channel blockers, anti-oxidative agents, excitatory amino acid antagonists, anti-inflammatory drugs, and stem cells have been shown to be effective in animal stroke models, but have failed in human clinical trials (Chen et al., 2014; Selim and Wang, 2015; Wang et al., 2015). So far, rapid administration of intravenous recombinant tissue-type plasminogen activator (tPA) to appropriate patients remains the only pharmacotherapy approved by the Food and Drug Administration for early treatment of acute ischemic stroke (Jauch et al., 2013). However, the application of tPA thrombolysis in clinical settings is restricted by the narrow therapeutic time window, and fewer than 5% of patients receive tPA treatment. Endovascular intervention can restore blood flow in a timely manner, and thrombectomy with stent retrievers is now recommended as the standard care for acute ischemic strokes with a proximal large vessel occlusion, but again treatment is limited to a small number of stroke patients. Furthermore, effective and aggressive medical management does not completely prevent stroke recurrence in symptomatic ICAS patients. A systemic review on outcome after stenting for ICAS showed that the cumulative probability of a follow-up event (stroke or death) was nearly 14% at 30 months, and the total occurrence of in-stent restenosis (defined as > 50% according to WASID criteria) was 14.4% in long-term follow-up (Groschel et al., 2009). Here we review the status of the prevention and treatment of stroke with ICAS.
8.1. Risk-factor control
Higher incidence of recurring stroke and other vascular events in patients with symptomatic ICAS was associated with alcohol consumption, mean SBP of 140 mmHg or higher and total cholesterol concentration exceeding 200 mg/dL (Chaturvedi et al., 2007). Thus, patients with ICAS may benefit from risk factor modification. Although some researchers worry about lowering blood pressure because it could lead to hypoperfusion and ischemic events, the WASID study indicated that the recurrence rates of ischemic events in the territory of the symptomatic stenotic artery were higher in those with SBP of 160 mmHg (Turan et al., 2007). The 30-day rate of stroke or death was 10.7% and the 1-year rate of the primary end point was 25% in patients treated with aspirin or warfarin plus standard management of risk factors in the WASID trial (Zaidat et al., 2008). However, the corresponding rates in SAMMPRIS were 5.8% and 12.2%, respectively, supporting the importance of risk-factor control in the recurrence and outcomes for patients with symptomatic ICAS. In line with this, the investigators set more aggressive targets for risk factor control in the SAMMPRIS study: < 140 mmHg for SBP (< 130 mmHg in the case of patients with diabetes), < 70 mg/dL for LDL-C, and encouraging and supporting each patient to achieve lifestyle modifications including smoking cessation, dietary adjustment, exercise and stress management (Prabhakaran and Romano, 2012). The results indicated significant reductions in the use of tobacco (7–10%), levels of LDL (20–25 mg/dL) and SBP (11 mmHg), and a modest increase in moderate or vigorous exercise (22–27%) in the fourth month. Compared with WASID, in which SBP lower than 140 mmHg and LDL-C < 70 mg/dL were attained in 49% and 6% of patients at enrollment and in 50% and 12% of patients at 1 year (Chaturvedi et al., 2007), the same risk factor levels were achieved in 34% (SBP) and 24% (LDL-C) of patients at enrollment and in 70% and 62% of patients at 1 year in SAMMPRIS (Derdeyn et al., 2014). Furthermore, escalating from “usual” to “aggressive” risk factor management was estimated to contribute to an additional 15% risk reduction in outcome events (Sila, 2012). As a result, for patients with symptomatic ICAS, statins to lower LDL to < 70 mg/dL and even subscribing to tight regulation of hypertension beyond the acute stroke period can be recommended (Chimowitz et al., 2011). Furthermore, Bang et al. found that premorbid statin usage, which can modulate plaque enhancement in symptomatic intracranial atherosclerotic plaque, was independently associated with a decrease in large cortical lesions in patients with intracranial atherosclerotic stroke, suggesting the importance of appropriate prestroke statin treatment for patients with ICAS (Chung et al., 2016).
8.2. Antithrombotic therapy
Several antithrombotic drugs have been routinely used for the prevention of primary or recurrent stroke, including drugs targeting coagulation such as warfarin, low-molecular-weight heparin (LMWH), dabigatran and rivaroxaban, and antiplatelet drugs such as aspirin, clopidogrel, and cilostazol. Although several new drugs have been developed, aspirin is still the most commonly used antiplatelet agent and has been the gold standard for evaluating the efficiency of other anticoagulants.
Warfarin, the most widely prescribed oral anticoagulant in the USA, plays its anticoagulation role via blocking vitamin K1. Its efficacy for stroke prevention was compared with aspirin in the WASID trial, which enrolled 569 patients with stroke or TIA caused by intracranial stenosis within the previous 90 days (Chimowitz et al., 2005). The results showed that 1) the two-year rate of ischemic stroke in the aspirin group (19.7%) was higher than that of the warfarin group (17.2%); 2) the mortality in the warfarin group was 9.7%, which was almost two times of that in the aspirin group; 3) the incidence of major hemorrhages was 8.3% in the warfarin group and 3.2% in the aspirin group. Since warfarin had significantly higher rates of adverse events and had no benefit over aspirin, aspirin was recommended for symptomatic intracranial arterial stenosis patients in place of warfarin. Due to the high recurrence rate of ischemic stroke despite taking aspirin or warfarin, other therapies such as aggressive management of risk factors, alternative antiplatelet regimens, combinations of different anticoagulants, and endovascular treatment should be considered.
LMWH is a new class of anticoagulants derived from unfractioned heparin with several advantages over unfractioned heparin due to reduced non-specific binding to proteins or cells. Its efficacy on prevention of stroke has been investigated by three trials: the larger Fraxiparine in Ischemic Stroke (FISS)-bis study, the Trial of ORG 10172 in Acute Stroke Treatment (TOAST), and the Tinzaparin in Acute Ischemic Stroke Trial (TAIST). The results indicated that there was no short- or long-term benefit of immediate full-dose anticoagulant therapy in acute stoke (Bath et al., 2000; Gubitz et al., 2004). Systematic reviews and meta-analyses showed that the routine use of any type of anticoagulant in acute ischemic stroke was not recommended. The efficacy of combined nadroparin calcium with anti-factor Xa on prevention of recurrent stroke was investigated in the FISS-2 study, in which 353 acute ischemic stroke patients with large artery occlusive disease were enrolled (Wong et al., 2007a). The result showed that the proportion of patients with good outcomes at 6 months was 73% in the LMWH group and 69% in the aspirin group, and the occurrences of hemorrhagic transformation of infarct and adverse events were similar in both groups. In other words, LMWH had no definitive benefit over aspirin in acute stroke patients (predominantly due to intracranial atherosclerosis).
Clopidogrel, a derivative of thienopyridine, irreversibly blocks the platelet surface P2Y12 receptor and thus limits adenosine diphosphate (ADP)-induced platelet aggregation. Clopidogrel exerts anti-thrombotic effects that are different from and additive to those of aspirin (Hurst et al., 2013). The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, a clinical study of clopidogrel versus aspirin monotherapy for secondary prevention in patients with atherosclerotic disease, showed that clopidogrel was superior to aspirin in lowering a cluster of ischemic events, including stroke, myocardial infarction, and vascular death, with a favorable safety profile (CAPRIE Steering Committee, 1996) (1). However, clopidogrel alone was not significant in reducing the risk of stroke (Yi et al., 2014). Short-term combination of aspirin and clopidogrel has been demonstrated to be effective in reducing microembolic signals, lowering the risk of recurrent stroke in ICAS patients in the Clopidogrel plus Aspirin for Infarction Reduction (CLAIR) study (Wang et al., 2013). The SAMMPRIS trial indicated that aggressive medical management with clopidogrel (75 mg/day) and a high dose of aspirin (325 mg/day) were superior to percutaneous transluminal angioplasty and stenting (Derdeyn et al., 2014). Dual antiplatelet therapy played a crucial part in reducing the early risk of stroke in patients with recent symptomatic intracranial stenosis or who are at high risk of harboring intracranial stenosis (Derdeyn et al., 2014).
Cilostazol, a potent PDE3 inhibitor, inhibits the platelet activation and aggregation pathway via increasing the intracellular level of cyclic adenosine monophosphate (Bhogal et al., 2016). In addition to its antiplatelet function, cilostazol can not only improve lipid metabolism and suppress the proliferation of arterial SMCs, but can also alleviate inflammatory reactions, improve vascular endothelial functions and dilate blood vessels (Zhang et al., 2015). A combined therapy of cilostazol and aspirin was more effective to prevent the progression of symptomatic ICAS than aspirin alone (Kwon et al., 2005). Meta-analysis studies showed that cilostazol might be a more effective and safer alternative to aspirin for reducing the progression of ICAS and decreasing the recurrence of ischemic stroke (Zhang et al., 2015). However, aspirin combined with either cilostazol or clopidogrel showed no significant difference in preventing the progression of ICAS in the TOSS-2 study (Kwon et al., 2011). Nevertheless, some benefits were observed for aspirin and cilostazol combinatorial therapy (e.g. favorable ICAS progression as well as fewer hemorrhagic complications), suggesting its usefulness and efficacy as a possible long-term therapeutic strategy for symptomatic ICAS.
8.3. Endovascular management
With the advance of neuroimaging techniques, it is now possible to determine irreversible infarcted brain tissue and perfusion of collateral circulation, two critical factors for the selection of eligible patients for endovascular therapy, which causes faster and more complete recanalization. This has greatly facilitated the development and application of endovascular therapy in ischemic stroke. In the 2015 update of evidence-based guidelines for endovascular treatment of acute ischemic stroke by the American Heart and Stroke Association (Powers et al., 2015), thrombectomy is now recommended as the standard care for acute ischemic strokes with a proximal large vessel occlusion in the anterior circulation.
8.3.1. Endovascular management for acute stroke related to ICAS
Endovascular intervention is a minimally invasive method to recanalize an occluded vessel, and it can reduce the incidence of watershed infarcts secondary to chronic cerebral ischemia caused by hypoperfusion (Osbun and Kim, 2014). Several published trials have now proven the benefit of endovascular treatment of acute ischemic stroke (Berkhemer et al., 2015; Campbell et al., 2015; Goyal et al., 2015; Jovin et al., 2015; Saver et al., 2015). Berkhemer et al. demonstrated that intra-arterial treatment for patients with proximal intracranial occlusion of the anterior circulation was safe and effective when administered < 6 h after onset of symptoms (Berkhemer et al., 2015). The ECTEND-IA trial indicated that patients could greatly benefit from endovascular management through the use of CT perfusion imaging to exclude patients with large ischemic cores or unsalvageable ischemic brains, and to allow for shorter intervals (30 mins) to the start of treatment (interval between alteplase administration and randomization). As a result of CT perfusion, angiographic revascularization was observed in 86% of patients, with blood flow restored to > 50% of the affected region (Campbell et al., 2015). The results of the ESCAPE trial indicated that rapid endovascular management could benefit patients with acute ischemic stroke due to intracranial occlusion in the anterior circulation artery (Goyal et al., 2015), and good outcomes can be achieved with small infarct cores, shorter intervals from onset of symptom to treatment initiation, low rates of general anesthesia, moderate to good collateral circulation and high rates of successful reperfusion. As a result, emergent intracranial angioplasty with or without stenting in patients with hyperacute stroke secondary to ICAS may be a feasible and safe approach with a high rate of successful revascularization and favorable outcomes, and patients with acute ischemic stroke secondary to ICAS should be considered as candidates for intracranial intervention (Al Kasab et al., 2017; Yoon et al., 2015). However, three clinical trials including the Interventional Management of Stroke III trial, Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy trial, and Endovascular Treatment for Acute Ischemic Stroke (SYNTHESIS) trial showed that endovascular intervention has no significant advantage over intravenous tPA (Broderick et al., 2013; Ciccone et al., 2013; Kidwell et al., 2013), likely due to longer time to reperfusion in patients after moderate to severe stroke. These controversial trials indicated that rapid reperfusion and imagebased enrollment of eligible stroke patients are crucial for the success of future acute endovascular trials.
8.3.2. Endovascular intervention for a high-risk subgroup
Concurring with SAMMPRIS, the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trial showed a worse primary end point in patients treated with stent than that in patients treated with the medical therapy (24% vs. 9% at 30 days, 36% vs. 15% at 12 months follow-up), suggesting that the high risk of endovascular therapy was not unique to a specific device (Zaidat et al., 2015). As a result, aggressive medical management remains the standard strategy for patients with ICAS (Kernan et al., 2014), although controversy still exists as to whether the high-risk subgroup of patients who are refractory to medical treatment could benefit from revascularization (Banerjee and Chimowitz, 2017; van den Wijngaard et al., 2016).
In a retrospective study, Yu et al. found that angioplasty and stenting led to immediate luminal gain from baseline in both the short term (12 months) and the long term (80 months) in patients with ICAS, compared with medical therapy, and the arterial lumen after angioplasty and stenting can probably be well maintained (Yu et al., 2017). So far, in clinical practice or trial recruitment, patient selection for endovascular intervention has been mainly based on the severity of the symptomatic stenosis lesions (70–99% stenosis), an independent predictor for stroke recurrence (Leng et al., 2016). Pu et al. demonstrated that cerebral hemodynamic criteria could optimize the diagnosis and treatment strategies for symptomatic ICAS patients, and should be used to screen patients for endovascular treatment (Pu et al., 2017). Miao et al. found that the successful recanalization was 97.3%, and that the 30-day rate of stroke, TIA and death was 4.3% in patients with severe symptomatic ICAS combined with poor collaterals who received endovascular stenting therapy (Miao et al., 2015). The short-term safety of endovascular stenting for patients with severe symptomatic ICAS in China was acceptable, which was contrary to the primary outcome in the SAMMPRIS and VISSIT trials. Liu et al. also found that the rate of 30-day stroke, TIA and death was 7.1%, with 100% successful stent deployment rate in patients with severe symptomatic intracranial vertebrobasilar artery stenosis who were treated with stenting (Liu et al., 2016). The researchers considered that the lower event rate and high successful revascularization could be attributed to a number of factors, including: the selection of patients for which the etiology was hypoperfusion but not perforator stroke; a longer interval between the qualifying event to stent, which allowed for better medical preparation for the procedure; a proper choice of devices to maximize success rate and minimize procedural complications; and experienced clinicians (Liu et al., 2016; Miao et al., 2015). Clinical outcomes were more favorable in those patients with non-progressive symptoms in the subacute period and those receiving statin therapy and regular antiplatelet therapy (Alexander et al., 2016). Age, history of diabetes mellitus, preprocedural mRS score and lesion site in BA were risk factors for serious adverse events (Cheng et al., 2016). A long (> 5 mm), eccentric, or tortuous lesion under Mori classification may increase the periprocedural event rate of intravascular intervention. Stenting not only cannot reestablish the occluded perforators, but also may result in a “snow-plowing” effect, exacerbating perforator occlusion in patients with perforator occlusion from atheromatous branch disease (Sui et al., 2015).
In a post hoc analysis, Lutsep et al. found that the SAMMPRIS trial provided no evidence to support the use of percutaneous transluminal ballon angioplasty (PTA) compared with medical treatment in any examined subgroup of patients with symptomatic ICAS, including those with hypoperfusion symptoms (Lutsep et al., 2015). To increase the efficacy of intravascular intervention, identification of high-risk patients is very important. First, intracranial arterial stenosis with other pathogenesis such as vasculitis or moyamoya disease should be excluded. Second, patients with hypoperfusion but not artery-to-artery embolism might benefit from intravascular therapy. Third, morphological features may influence the outcomes of endovascular procedure, and lesions with negative remodeling may be prone to vessel injury, resulting in vessel dissection or hemorrhage (Zhu et al., 2016). The criteria eligible for angioplasty and stenting may include severe symptomatic intracranial stenosis (> 70%), at least two strokes caused by intracranial stenosis despite maximal medical management, and an interval from the most recent stroke of > 7 days (Osbun and Kim, 2014). The China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial, an ongoing, prospective, multicenter and randomized trial with experienced operators and qualified patient selection, aims to determine whether intracranial stenting demonstrates a benefit over medical management for patients with severe symptomatic ICAS (Gao et al., 2015). In the 14th World Federation of Interventional Radiology and Therapy, Jiao et al. reported early results from phase I of the CISSISS study, in which the incidence of periprocedural complications was about 2%, much lower than that in the SAMMPRIS trial.
8.3.3. Complications of endovascular intervention for ICAS
Endovascular management of the intracranial vasculature is technically more challenging than that of the extracranial carotid artery (Broderick, 2011) due to the following characteristics of intracranial vasculature: tortuous structure, floating freely in the cerebrospinal fluid, smaller arterial diameters, thinner arterial walls, and small penetrating brain arteries arising at right angles which are often near or at the site of the placement of the stent. Endovascular approaches for intracranial atherosclerosis include PTA, balloon-mounted stents or self-expanding intracranial stents. Success rates varied from 71% to 100% with a median of 96.9%; the combined periprocedural minor or major stroke and death rates during intracranial stenting ranged from 0% to 50% with a median of 7.7% (Groschel et al., 2009). There was no difference in periprocedural complications between the two methods although SES self-expanding intracranial stents resulted in more frequent restenosis ≥50%.
Due to a high risk of periprocedural complications in the stenting arm, the SAMMPRIS trial was halted prematurely. Derdeyn et al. analyzed the types and mechanisms of periprocedural stroke after intracranial angioplasty and stenting in the SAMMPRIS trial, and found that the most frequent types of stroke were local perforator ischemic stroke, primary intraparenchymal hemorrhage, and subarachnoid hemorrhage, while distal symptomatic embolic stroke was uncommon (Derdeyn et al., 2013). Perforator occlusion by displaced or disrupted atheromatous debris may be the cause of regional perforator stroke after stenting. Hyperperfusion or autoregulatory dysfunction may be a mechanism of intraparenchymal hemorrhage, which was associated with a higher baseline degree of stenosis in addition to the combination of a high procedural activated clotting time (> 300 s) and a loading dose of clopidogrel. Subarachnoid hemorrhages were associated with wire perforation and vessel rupture. As a result, potential approaches to reduce the risk of these complications may include improving patient selection using different imaging, optimizing management of patients with high risk for reperfusion injury or perforator stroke, advancing device selection adapted to individual vessel morphology, and enhancing physician experience (Miao, 2014). Ecker et al. advised that AsIA patients should be treated medically unless they have a severe perfusion abnormality in the region due to large arteries (Ecker et al., 2006). On the other hand, endovascular interventions could be considered along with aggressive medical management in symptomatic ICAS patients with recurrent cerebral ischemic events.
8.4. Non-pharmacological therapies
Accumulating studies suggest that non-pharmacological therapies, such as therapeutic hypothermia, ischemic/hypoxic conditioning, acupuncture, certain medical gases and other strategies might further salvage affected brain tissue and provide new therapeutic opportunities for stroke patients (Chen et al., 2014).
8.4.1. Hypothermia
Induced hypothermia has proved to be beneficial and safe in cases of ischemia caused by cardiac arrest or hypoxic-ischemic encephalopathy in newborns (Nagel et al., 2008). Laboratory studies have indicated that therapeutic hypothermia is a promising treatment for ischemic stroke; however, clinical trials have not shown clear and definitive evidence for the therapeutic effects, safety and feasibility of the therapy (Chen et al., 2014; Han et al., 2015). Hypothermia decreases the severity of ischemic brain damage (Chen et al., 2014; Han et al., 2015) via multiple mechanisms including: decreasing metabolism, inhibiting inflammatory and immune response, regulating gene expression, balancing the pathways between cell survival and death, and preventing blood-brain barrier disruption.
8.4.2. Ischemic conditioning
Ischemic tolerance of the brain, also called “conditioning”, is a transient, sublethal ischemia which results in tolerance to later lethal cerebral ischemia. The protective mechanisms underlying brain ischemic conditioning are complex, including the improvement of cerebral blood flow (CBF), the upregulation of protective membrane proteins, a reduction of inflammation, increased autophagy function, and the regulation of survival/death signaling, among others (Chen et al., 2014). Ischemic conditioning could be performed before ischemia (ischemic preconditioning–IPC), during ischemia (perconditioning), or after ischemia (postconditioning). Ischemic conditioning could be performed by interrupting cerebral blood supply or by remote ischemic conditioning (RIC) via mechanical interruptions of blood flow in distal organs.
Preconditioning by a transient interruption of cerebral blood supply has been well investigated in animal models of focal and global ischemia. The highest protection occurs when preconditioning is applied prior to the onset of cerebral ischemia, but it is less effective when applied at 12 or 48 h before ischemia (Hess et al., 2013). TIAs may represent a clinical model of ischemic tolerance and protect the brain against a subsequent stroke (Moncayo et al., 2000; Weih et al., 1999). However, acute stroke is unpredictable, and it is unrealistic to interrupt cerebral blood supply in clinic.
A more clinically relevant RIC has become popular and has been studied in animals and patients for its neuroprotection alone or in combination with alteplase. Remote limb preconditioning and perconditioning reduced infarct size, edema, and blood-brain barrier permeability in a rodent model of cerebral ischemia (Ren et al., 2011; Ren et al., 2018; Ren et al., 2009). RIC elevates CBF and collateral circulation and promotes vascular Notch signaling activity and arteriogenesis in the ischemic brain, which is correlated with the functional prognosis after stroke. Several animal studies have indicated that RIC can also alleviate inflammation and white matter damage, decrease amyloid accumulation and improve cognition in mouse models for VCI and vascular dementia (Hess et al., 2015). In a prospective and randomized study (Meng et al., 2012), patients with symptomatic ICAS were enrolled to evaluate the protective effects of repetitive bilateral arm ischemic preconditioning (BAIPC). Patients received BAIPC treatment along with standard medical treatment (n = 38) while the control group underwent standard medical treatment only (n = 30). The results indicated that the BAIPC group had significantly lower recurrent stroke incidence at 90 and 300 days, shorter average time to recovery (mRS score < 1) as well as remarkable improvement in cerebral perfusion status vs. the control group. Further study indicated that BAIPC alleviates plasma biomarkers of inflammation and coagulation (Meng et al., 2015). Thus, the ischemic remote preconditioning method may be a feasible and potential approach to inhibit stroke recurrence in patients with ICAS. Ischemic preconditioning is suitable for primary and secondary stroke prevention in multiple settings, including symptomatic and asymptomatic intra- and perhaps extra-cranial arterial occlusive lesions, as an adjunctive method with or without reperfusion therapy for acute ischemic stroke, and as a prophylactic therapy against delayed ischemic injury in subarachnoid hemorrhage (Selim and Wang, 2015). Large multicenter randomized clinical studies are still needed to determine the optimal protocol for the ischemic conditioning procedure and to further evaluate the potential benefits of ischemic conditioning in human ischemic injury (Vasdekis et al., 2013).
These non-pharmacological therapies have proven to be effective and have potential in protecting the brain from ischemia and injury.
The translation into a possible clinical benefit through large clinical trials to confirm their safety and efficacy is still needed. In addition, the optimal protocol, including timing, frequency, and duration, eligible population and combining with other therapies also need to be addressed.
9. Conclusion
In summary, intracranial atherosclerosis, a lifetime, systemic and progressive disease, is a major cause of ischemic stroke worldwide. Multiple mechanisms such as endothelial dysfunction, inflammation, oxidative stress, and cell apoptosis are involved in the development and progression of atherosclerosis. Unhealthy lifestyles, metabolic disturbance, and traditional cardiovascular risk factors are risk factors for ICAS, whereas inflammation may play an important role in the pathogenesis and destabilization of ICAS. Noninvasive screening imaging techniques including TCD, CTA, MRA, and HR-MRI are widely used for the diagnosis of ICAS and for the selection of patients for endovascular treatment. Aggressive management of risk factors and short-term dual antiplatelet therapy followed by aspirin monotherapy are recommended. tPA and endovascular treatment in selected patients are two effective approaches for the treatment of stroke patients with ICAS. Non-pharmacological therapies may have synergistic effects with either tPA and endovascular treatment but need to be further verified in larges-cale clinical trials.
| Images | Manifestations | Advantages | Disadvantages |
|---|---|---|---|
| TCD | Detects real-time flow velocity and direction of flow, microembolic signals and steal phenomenon, and vasoreactivity. | Inexpensive, noninvasive and safe. | The sonographer’s skills and patient’s status may influence the accuracy and predictive value. |
| CTA | Detects intraluminal narrowing by visualization of the arterial lumen. Provides information about calcification, plaque morphology and collateralization. | Accurate, efficient and minimally invasive. Higher acquisition speed and less susceptible to motion artifacts than MRA. | Radiation exposure, the necessity of iodinated contrast material use, and lack of laminar flow hemodynamic information. |
| MRA | Depicts and visualizes the arterial lumen based on the so-called inflow effect of unsaturated spins. | No radiation. | Overestimation of the stenosis degree due to the flow-related artifacts. |
| DSA | Measures hemodynamic status, degree of stenosis in intracranial vessels, and collateral circulation. | The gold standard for the diagnosis of ICAS. | Invasive, radiation exposure, costs and several risks such as contrast media-induced kidney damage, allergy, and ischemic stroke. |
| HR-MRI | Discloses intracranial arterial wall and the stenosis grade. Identifies plaque components, burden, vulnerability, and remodeling pattern in vivo. | Determines causes of stenosis, identifies stroke mechanism, and directs the treatment strategy. | Inability to cover a large volume of intracranial artery due to time constraints and anisotropic spa- tial resolution with low spatial resolution in slice-select direction. |
Acknowledgements
This study was sponsored by the 12th Five-Year Plan for Energy, Science and Technology support program 2013BAI07B01 (to X.J.) and the Chinese National Natural Science Foundation 81371289 (to X.J.), and by National Institutes of Health/NINDS grants NS079345 (to G.C.) and the Department of Veterans Affairs Merit Review grants BX002346 and BX003923 (to G.C.).
Abbreviations:
- ABI
Ankle-brachial index
- AD
Alzheimer’s disease
- apoAI
Apolipoprotein AI
- apoB
Apolipoprotein B
- ARIC
Atherosclerosis Risk in Communities
- AsIA
Asymptomatic intracranial atherosclerosis
- ASPECTS
Alberta Stroke Program Early CT Score
- BAIPC
Bilateral arm ischemic preconditioning
- CBF
Cerebral blood flow
- CRP
C-reactive protein
- CTA
Computed tomography angiography
- CVD
Cerebrovascular disease
- DSA
Digital subtraction angiography
- ECAS
Extracranial atherosclerosis
- ESCAPE
Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times
- EXTEND-IA
Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial
- FISS
Fraxiparine in Ischemic Stroke
- HDL
High-density lipoprotein
- HR-MRI
High-resolution magnetic resonance imaging
- hs-CRP
High-sensitivity C-reactive protein
- ICAS
Intracranial atherosclerosis
- IL-6
Interleukin-6
- ILA
Intracranial large artery
- LAA
Large artery atherosclerosis
- LDL
Low-density lipoproteins
- LMWH
Low-molecular-weight heparin
- MCA
Middle cerebral artery
- mmIMT
Mean-maximal intima-media thickness
- MMPs
Matrix metalloproteinases
- MRA
Magnetic resonance angiography
- MR CLEAN
Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands
- mRS
Modified Rankin score
- NIHSS
National Institutes of Health Stroke Scale
- NO
Nitric oxide
- NPV
Negative predictive value
- PAI-1
Plasminogen activator inhibitor-1
- PPV
Positive predictive value
- PSD
Post-stroke depression
- PTA
Percutaneous transluminal balloon angioplasty
- ROS
Reactive oxygen species
- RIC
Remote ischemic conditioning
- SAMMPRIS
Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis
- SBP
Systolic blood pressure
- SIR
Signal intensity ratio
- SMC
Smooth muscle cell
- SONIA
Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis
- SPECT
Single-photon emission computed tomography
- SYNTHESIS
Endovascular Treatment for Acute Ischemic Stroke
- TAIST
Tinzaparin in Acute Ischemic Stroke Trial
- TCD
Transcranial doppler
- TIA
Transient ischemic attack
- TICI
Thrombolysis in cerebral ischemia
- TOAST
Trial of ORG 10172 in Acute Stroke Treatment
- TOF-MRA
Time-of-flight magnetic resonance angiography
- TOSS-2
Trial of Cilostazol in Symptomatic Intracranial Stenosis 2
- tPA
Tissue plasminogen activator
- VA
Vertebral artery
- VaD
Vascular dementia
- VCI
Vascular cognitive impairment
- WASID
Warfarin-Aspirin Symptomatic Intracranial Disease
Footnotes
Conflict of interest
All authors have no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning of the submitted work.
References
- Al Kasab S, et al. , 2017. Endovascular treatment for AIS with underlying ICAD. J. Neurointerventional Surg. 9, 948–951. 10.1136/neurintsurg-2016012529. [DOI] [PubMed] [Google Scholar]
- Alberti KG, et al. , 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645. 10.1161/circulationaha.109.192644. [DOI] [PubMed] [Google Scholar]
- Alexander MD, et al. , 2016. Lesion location, stability, and pretreatment management: factors affecting outcomes of endovascular treatment for vertebrobasilar atherosclerosis. J Neurointerv Surg. 8, 466–470. 10.1136/neurintsurg-2014-011633. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, et al. , 2012. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J. Neuroimaging 22, 215–224. 10.1111/j.1552-6569.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- Alkan O, et al. , 2009. Intracranial cerebral artery stenosis with associated coronary artery and extracranial carotid artery stenosis in Turkish patients. Eur. J. Radiol. 71, 450–455. 10.1016/j.ejrad.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Angermaier A, et al. , 2011. CT-angiographic collateralization predicts final infarct volume after intra-arterial thrombolysis for acute anterior circulation ischemic stroke. Cerebrovasc. Dis. 31, 177–184. 10.1159/000321868. [DOI] [PubMed] [Google Scholar]
- Arenillas JF, Alvarez-Sabin J, 2005. Basic mechanisms in intracranial large-artery atherosclerosis: advances and challenges. Cerebrovasc. Dis. 20 (Suppl. 2), 75–83. 10.1159/000089359. [DOI] [PubMed] [Google Scholar]
- Arenillas JF, et al. , 2005. Angiogenesis in symptomatic intracranial atherosclerosis: predominance of the inhibitor endostatin is related to a greater extent and risk of recurrence. Stroke 36, 92–97. 10.1161/01.STR.0000149617.65372.5d. [DOI] [PubMed] [Google Scholar]
- Arenillas JF, et al. , 2008. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke 39, 1456–1463. 10.1161/strokeaha.107.498600. [DOI] [PubMed] [Google Scholar]
- Aziz K, et al. , 2008. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation 117, 2061–2070. 10.1161/circulationaha.106.652313. [DOI] [PubMed] [Google Scholar]
- Bae HJ, et al. , 2007. Risk factors of intracranial cerebral atherosclerosis among asymptomatics. Cerebrovasc. Dis. 24, 355–360. 10.1159/000106982. [DOI] [PubMed] [Google Scholar]
- Baker A, et al. , 1967. The geographic pathology of atherosclerosis: a review of the literature with some personal observations on cerebral atherosclerosis. J. Chronic Dis. 20, 685–706. [Google Scholar]
- Banerjee C, Chimowitz MI, 2017. Stroke Caused by Atherosclerosis of the Major Intracranial arteries. Circ. Res. 120, 502–513. 10.1161/circresaha.116.308441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, et al. , 2007. Adiponectin levels in patients with intracranial atherosclerosis. Neurology 68, 1931–1937. 10.1212/01.wnl.0000263186.20988.9f. [DOI] [PubMed] [Google Scholar]
- Bash S, et al. , 2005. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am. J. Neuroradiol. 26, 1012–1021. [PMC free article] [PubMed] [Google Scholar]
- Bath PM, et al. , 2000. Low-molecular-weight heparins and heparinoids in acute ischemic stroke: a meta-analysis of randomized controlled trials. Stroke 31, 1770–1778. [DOI] [PubMed] [Google Scholar]
- Beach TG, et al. , 2007. Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 113, 13–21. 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA, et al. , 2015. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 372, 11–20. 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- Bhogal P, et al. , 2016. Cilostazol: an antiplatelet agent for the neurointerventionist? J Neurointerv Surg. 8, 208–209. 10.1136/neurintsurg-2014-011570. [DOI] [PubMed] [Google Scholar]
- Bidzan M, et al. , 2014. Neuropsychiatric symptoms in patients with Alzheimer’s disease with a vascular component. Ann. Agric. Environ. Med. 21, 412–415. 10.5604/1232-1966.1108615. [DOI] [PubMed] [Google Scholar]
- Bos D, et al. , 2012. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke 43, 1878–1884. 10.1161/strokeaha.111.648667. [DOI] [PubMed] [Google Scholar]
- Broderick JP, 2011. The challenges of intracranial revascularization for stroke prevention. N. Engl. J. Med. 365, 1054–1055. 10.1056/NEJMe1108394. [DOI] [PubMed] [Google Scholar]
- Broderick JP, et al. , 2013. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N. Engl. J. Med. 368, 893–903. 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, et al. , 2015. Endovascular therapy for ischemic stroke with perfusionimaging selection. N. Engl. J. Med. 372, 1009–1018. 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- Caplan LR, et al. , 1986. Race, sex and occlusive cerebrovascular disease: a review. Stroke 17, 648–655. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee, 1996. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Chan CP, et al. , 2012. Multiple atherosclerosis-related biomarkers associated with short- and long-term mortality after stroke. Clin. Biochem. 45, 1308–1315. 10.1016/j.clinbiochem.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, et al. , 2007. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology 69, 2063–2068. 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- Chen XY, et al. , 2008. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc. Dis. 25, 74–80. 10.1159/000111525. [DOI] [PubMed] [Google Scholar]
- Chen F, et al. , 2014. Non-pharmaceutical therapies for stroke: mechanisms and clinical implications. Prog. Neurobiol. 115, 246–269. 10.1016/j.pneurobio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YK, et al. , 2016. Intracranial atherosclerosis and poststroke depression in Chinese patients with ischemic stroke. J. Stroke Cerebrovasc. Dis. 25, 998–1004. 10.1016/j.jstrokecerebrovasdis.2015.12.038. [DOI] [PubMed] [Google Scholar]
- Cheng L, et al. , 2016. Risk factors associated with in-hospital serious adverse events after stenting of severe symptomatic intracranial stenosis. Clin. Neurol. Neurosurg. 147, 59–63. 10.1016/j.clineuro.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, et al. , 2005. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N. Engl. J. Med. 352, 1305–1316. 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, et al. , 2011. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N. Engl. J. Med. 365, 993–1003. 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CG, et al. , 2007. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T. AJNR Am. J. Neuroradiol. 28, 439–446. [PMC free article] [PubMed] [Google Scholar]
- Chung GH, et al. , 2012. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur. J. Radiol. 81, 4069–4074. 10.1016/j.ejrad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Chung JW, et al. , 2016. Previous statin use and high-resolution magnetic resonance imaging characteristics of intracranial atherosclerotic plaque: the intensive statin treatment in acute ischemic stroke patients with intracranial atherosclerosis study. Stroke 47, 1789–1796. 10.1161/strokeaha.116.013495. [DOI] [PubMed] [Google Scholar]
- Ciccone A, et al. , 2013. Endovascular treatment for acute ischemic stroke. N. Engl. J. Med. 368, 904–913. 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SJ, et al. , 2018. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: US Preventive Services Task Force Recommendation Statement. JAMA 320, 177–183. 10.1001/jama.2018.8357. [DOI] [PubMed] [Google Scholar]
- D’Armiento FP, et al. , 2001. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 32, 2472–2479. [DOI] [PubMed] [Google Scholar]
- Dearborn JL, et al. , 2017. Intracranial atherosclerosis and dementia: the Atherosclerosis Risk in Communities (ARIC) study. Neurology 88, 1556–1563. 10.1212/wnl.0000000000003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan AJ, et al. , 2012. MR angiography and imaging for the evaluation of middle cerebral artery atherosclerotic disease. AJNR Am. J. Neuroradiol. 33, 1427–1435. 10.3174/ajnr.A2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard IR, et al. , 2016. Treatment and imaging of intracranial atherosclerotic stenosis: current perspectives and future directions. Brain Behav. 6, e00536 10.1002/brb3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CP, et al. , 2013. Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery 72, 777–795. discussion 795. 10.1227/NEU.0b013e318286fdc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CP, et al. , 2014. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 383, 333–341. 10.1016/s0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker RD, et al. , 2006. Current concepts in the management of intracranial atherosclerotic disease. Neurosurgery 59, S210–S218. discussion S213–213. 10.1227/01.neu.0000237326.06732.aa. [DOI] [PubMed] [Google Scholar]
- Gao T, et al. , 2014. Mechanisms of ischemic stroke in patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study. Exp. Ther. Med. 7, 1415–1419. 10.3892/etm.2014.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, et al. , 2015. China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS): a new, prospective, multicenter, randomized controlled trial in China. Interv. Neuroradiol. 21, 196–204. 10.1177/1591019915581778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez NR, et al. , 2013. Intracranial arterial stenoses: current viewpoints, novel approaches, and surgical perspectives. Neurosurg. Rev. 36, 175–184. discussion 184–175. 10.1007/s10143-012-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia A, et al. , 2014. Recurrence in intracranial atherosclerotic disease: a stenosisbased analysis. J. Stroke Cerebrovasc. Dis. 23, 2080–2084. 10.1016/j.jstrokecerebrovasdis.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Goyal M, et al. , 2015. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 372, 1019–1030. 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- Grool AM, et al. , 2011. Location of cerebrovascular and degenerative changes, depressive symptoms and cognitive functioning in later life: the SMART-Medea study. J. Neurol. Neurosurg. Psychiatry 82, 1093–1100. 10.1136/jnnp.2010.232413. [DOI] [PubMed] [Google Scholar]
- Groschel K, et al. , 2009. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke 40, e340–e347. 10.1161/strokeaha.108.532713. [DOI] [PubMed] [Google Scholar]
- Gubitz G, et al. , 2004. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst. Rev, CD000024 10.1002/14651858.CD000024.pub2. [DOI] [PubMed] [Google Scholar]
- Guo J, et al. , 2018. Elevated CRP at admission predicts post-stroke cognitive impairment in Han Chinese patients with intracranial arterial stenosis. Neurol. Res. 40, 292–296. 10.1080/01616412.2018.1438224. [DOI] [PubMed] [Google Scholar]
- Hallevi H, et al. , 2012. Intracranial atherosclerosis is associated with progression of neurological deficit in subcortical stroke. Cerebrovasc. Dis. 33, 64–68. 10.1159/000333388. [DOI] [PubMed] [Google Scholar]
- Han Z, et al. , 2015. Therapeutic hypothermia for stroke: where to go? Exp. Neurol. 272, 67–77. 10.1016/j.expneurol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Hansberg T, et al. , 2002. Effects of the ultrasound contrast-enhancing agent Levovist on the detection of intracranial arteries and stenoses in chinese by transcranial Doppler ultrasound. Cerebrovasc. Dis. 14, 105–108. [DOI] [PubMed] [Google Scholar]
- Hansson GK, 2009. Atherosclerosis–an immune disease: the Anitschkov Lecture 2007.Atherosclerosis 202, 2–10. 10.1016/j.atherosclerosis.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Hartman J, Frishman WH, 2014. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev 22, 147–151. 10.1097/crd.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Heo JH, et al. , 1999. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 19, 624–633. 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Hess DC, et al. , 2013. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke 44, 1191–1197. 10.1161/strokeaha.112.678482. [DOI] [PubMed] [Google Scholar]
- Hess DC, et al. , 2015. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 11, 698–710. 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- Hilal S, et al. , 2015. Intracranial stenosis, cerebrovascular diseases, and cognitive impairment in chinese. Alzheimer Dis. Assoc. Disord. 29, 12–17. 10.1097/wad.0000000000000045. [DOI] [PubMed] [Google Scholar]
- Hilal S, et al. , 2017. Intracranial stenosis in cognitive impairment and dementia. J. Cereb. Blood Flow Metab. 37, 2262–2269. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, et al. , 2002. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. AJNR Am. J. Neuroradiol. 23, 93–101. [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, et al. , 2008. Relation between interleukin-6 level and subclinical intracranial large-artery atherosclerosis. Atherosclerosis 197, 326–332. 10.1016/j.atherosclerosis.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Hurst NL, et al. , 2013. Clopidogrel “resistance”: pre- vs post-receptor determinants. Vasc. Pharmacol. 59, 152–161. 10.1016/j.vph.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Jauch EC, et al. , 2013. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44, 870–947. 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Jeon SB, et al. , 2012. Biomarkers and location of atherosclerosis: matrix metalloproteinase-2 may be related to intracranial atherosclerosis. Atherosclerosis 223, 442–447. 10.1016/j.atherosclerosis.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Jimenez M, et al. , 2014. Ankle-brachial index in screening for asymptomatic carotid and intracranial atherosclerosis. Atherosclerosis 233, 72–75. 10.1016/j.atherosclerosis.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Jin J, 2018. Screening for Peripheral Artery Disease with Ankle-Brachial Index. JAMA 320, 212 10.1001/jama.2018.9112. [DOI] [PubMed] [Google Scholar]
- Jovin TG, et al. , 2015. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 372, 2296–2306. 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- Kamal AK, et al. , 2014. Clinical, lifestyle, socioeconomic determinants and rate of asymptomatic intracranial atherosclerosis in stroke free Pakistanis. BMC Neurol. 14, 155 10.1186/s12883-014-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge S, et al. , 2010. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375, 132–140. 10.1016/s0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasner SE, et al. , 2006. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 113, 555–563. 10.1161/circulationaha.105.578229. [DOI] [PubMed] [Google Scholar]
- Kernan WN, et al. , 2014. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45, 2160–2236. 10.1161/str.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, et al. , 2013. A trial of imaging selection and endovascular treatment for ischemic stroke. N. Engl. J. Med. 368, 914–923. 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, et al. , 2012a. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J. Neurol. Sci. 312, 117–122. 10.1016/j.jns.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Kim DE, et al. , 2012b. Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study.Stroke 43, 1824–1830. 10.1161/strokeaha.112.653659. [DOI] [PubMed] [Google Scholar]
- Kim DE, et al. , 2012c. Associations of cigarette smoking with intracranial atherosclerosis in the patients with acute ischemic stroke. Clin. Neurol. Neurosurg. 114, 1243–1247. 10.1016/j.clineuro.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Komotar RJ, et al. , 2006. Natural history of intracranial atherosclerosis: a critical review. Neurosurgery 58, 595–601. discussion 595–601. 10.1227/01.neu.0000204102.88016.33. [DOI] [PubMed] [Google Scholar]
- Korczyn AD, 2015. Vascular parkinsonism–characteristics, pathogenesis and treatment. Nat. Rev. Neurol. 11, 319–326. 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- Kwon SU, et al. , 2005. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 36, 782–786. 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- Kwon SU, et al. , 2011. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke 42, 2883–2890. 10.1161/strokeaha.110.609370. [DOI] [PubMed] [Google Scholar]
- Labadzhyan A, et al. , 2011. Histopathologic evaluation of basilar artery atherosclerosis. J. Neurol. Sci. 307, 97–99. 10.1016/j.jns.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larose E, et al. , 2005. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation 112, 2324–2331. 10.1161/circulationaha.105.538942. [DOI] [PubMed] [Google Scholar]
- Leng X, et al. , 2013a. Interobserver reproducibility of signal intensity ratio on magnetic resonance angiography for hemodynamic impact of intracranial atherosclerosis. J. Stroke Cerebrovasc. Dis. 22, e615–e619. 10.1016/j.jstrokecerebrovasdis.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, et al. , 2013b. Magnetic resonance angiography signal intensity as a marker of hemodynamic impairment in intracranial arterial stenosis. PLoS One 8, e80124 10.1371/journal.pone.0080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, et al. , 2014. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke 45, 645–651. 10.1161/strokeaha.113.002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, et al. , 2016. The contemporary management of intracranial atherosclerotic disease. Expert. Rev. Neurother. 16, 701–709. 10.1080/14737175.2016.1179111. [DOI] [PubMed] [Google Scholar]
- Li ML, et al. , 2009. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis 204, 447–452. 10.1016/j.atherosclerosis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS, et al. , 2015. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis. J. Neuroimaging 25, 87–91. 10.1111/jon.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. , 2016. Stenting for symptomatic intracranial vertebrobasilar artery stenosis: 30-day results in a high-volume stroke center. Clin. Neurol. Neurosurg. 143, 132–138. 10.1016/j.clineuro.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Lopez-Cancio E, et al. , 2012a. The Barcelona-Asymptomatic Intracranial Atherosclerosis (AsIA) study: prevalence and risk factors. Atherosclerosis 221, 221–225. 10.1016/j.atherosclerosis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Lopez-Cancio E, et al. , 2012b. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke 43, 2712–2719. 10.1161/strokeaha.112.661702. [DOI] [PubMed] [Google Scholar]
- Lutsep HL, et al. , 2015. Does the stenting versus aggressive medical therapy trial support stenting for subgroups with intracranial stenosis? Stroke 46, 3282–3284. 10.1161/strokeaha.115.009846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man BL, et al. , 2009. Lesion patterns and stroke mechanisms in concurrent atherosclerosis of intracranial and extracranial vessels. Stroke 40, 3211–3215. 10.1161/STROKEAHA.109.557041. [DOI] [PubMed] [Google Scholar]
- Massot A, et al. , 2014. Predictive value of ankle-brachial index and PAI-1 in symptomatic intracranial atherosclerotic disease recurrence. Atherosclerosis 233, 186–189. 10.1016/j.atherosclerosis.2013.12.034. [DOI] [PubMed] [Google Scholar]
- Masuoka T, et al. , 2010. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol. Med. Chir. (Tokyo) 50, 179–182. [DOI] [PubMed] [Google Scholar]
- Mazighi M, et al. , 2006. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology 66, 1187–1191. 10.1212/01.wnl.0000208404.94585.b2. [DOI] [PubMed] [Google Scholar]
- Mazighi M, et al. , 2008. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 39, 1142–1147. 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- McVerry F, et al. , 2012. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am. J. Neuroradiol. 33, 576–582. 10.3174/ajnr.A2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R, et al. , 2012. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 79, 1853–1861. 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- Meng R, et al. , 2015. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics 12, 667–677. 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, 2014. Intracranial angioplasty and stenting before and after SAMMPRIS: “from simple to complex strategy - the Chinese experience”. Front. Neurol. 5, 129 10.3389/fneur.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, et al. , 2015. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke 46, 2822–2829. 10.1161/strokeaha.115.010549. [DOI] [PubMed] [Google Scholar]
- Moncayo J, et al. , 2000. Do transient ischemic attacks have a neuroprotective effect? Neurology 54, 2089–2094. [DOI] [PubMed] [Google Scholar]
- Mull M, et al. , 1997. Cerebral hemispheric low-flow infarcts in arterial occlusive disease.Lesion patterns and angiomorphological conditions. Stroke 28, 118–123. [DOI] [PubMed] [Google Scholar]
- Nagel S, et al. , 2008. Therapeutic hypothermia in experimental models of focal and global cerebral ischemia and intracerebral hemorrhage. Expert. Rev. Neurother. 8, 1255–1268. 10.1586/14737175.8.8.1255. [DOI] [PubMed] [Google Scholar]
- Nedelmann M, et al. , 2009. Consensus recommendations for transcranial color-coded duplex sonography for the assessment of intracranial arteries in clinical trials on acute stroke. Stroke 40, 3238–3244. 10.1161/strokeaha.109.555169. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huynh MN, et al. , 2008. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke 39, 1184–1188. 10.1161/strokeaha.107.502906. [DOI] [PubMed] [Google Scholar]
- Okazaki S, et al. , 2014. Association of interleukin-6 with the progression of carotid atherosclerosis: a 9-year follow-up study. Stroke 45, 2924–2929. 10.1161/strokeaha.114.005991. [DOI] [PubMed] [Google Scholar]
- Osbun JW, Kim LJ, 2014. Internal carotid artery stenting for intracranial atherosclerosis. Methodist DeBakey Cardiovasc. J. 10, 245–250. 10.14797/mdcj-10-4-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, et al. , 2006. Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology 66, 1344–1349. 10.1212/01.wnl.0000210530.46058.5c. [DOI] [PubMed] [Google Scholar]
- Park KP, et al. , 2009. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke 40, 2836–2842. 10.1161/strokeaha.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, et al. , 2011. High levels of apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis. Stroke 42, 3040–3046. 10.1161/strokeaha.111.620104. [DOI] [PubMed] [Google Scholar]
- Park JH, et al. , 2013. Deep subcortical infarct burden in relation to apolipoprotein B/AI ratio in patients with intracranial atherosclerotic stenosis. Eur. J. Neurol. 20, 671–680. 10.1111/ene.12021. [DOI] [PubMed] [Google Scholar]
- Powers WJ, et al. , 2015. 2015 American Heart Association/American Stroke Association Focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46, 3020–3035. 10.1161/str0000000000000074. [DOI] [PubMed] [Google Scholar]
- Prabhakaran S, Romano JG, 2012. Current diagnosis and management of symptomatic intracranial atherosclerotic disease. Curr. Opin. Neurol. 25, 18–26. 10.1097/WCO.0b013e32834ec16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, et al. , 2009. The utility of quantitative magnetic resonance angiography in the assessment of intracranial in-stent stenosis. Stroke 40, 991–993. 10.1161/strokeaha.108.522391. [DOI] [PubMed] [Google Scholar]
- Pu Y, et al. , 2017. Intracranial atherosclerosis: from anatomy to pathophysiology. Int. J.Stroke 12, 236–245. 10.1177/1747493016685716. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Caplan LR, 2014. Intracranial atherosclerosis. Lancet 383, 984–998. 10.1016/s0140-6736(13)61088-0. [DOI] [PubMed] [Google Scholar]
- Ren C, et al. , 2009. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 1288, 88–94. 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, et al. , 2011. Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol. Res. 33, 514–519. 10.1179/016164111X13007856084241. [DOI] [PubMed] [Google Scholar]
- Ren C, et al. , 2018. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav. Brain Res. 340, 87–93. 10.1016/j.bbr.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Ritz K, et al. , 2014. Cause and mechanisms of intracranial atherosclerosis. Circulation 130, 1407–1414. 10.1161/circulationaha.114.011147. [DOI] [PubMed] [Google Scholar]
- Ryu CW, et al. , 2014. High-resolution MRI of intracranial atherosclerotic disease.Neurointervention. 9, 9–20. 10.5469/neuroint.2014.9.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba L, et al. , 2007. Efficacy and sensitivity of axial scans and different reconstruction methods in the study of the ulcerated carotid plaque using multidetector-row CT angiography: comparison with surgical results. AJNR Am. J. Neuroradiol. 28, 716–723. [PMC free article] [PubMed] [Google Scholar]
- Saver JL, et al. , 2015. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 372, 2285–2295. 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- Savopoulos C, et al. , 2011. Adipokines and stroke: a review of the literature. Maturitas 70, 322–327. 10.1016/j.maturitas.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Schuett H, et al. , 2009. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost. 102, 215–222. 10.1160/th09-05-0297. [DOI] [PubMed] [Google Scholar]
- Selim M, Wang M, 2015. Ischemic Preconditioning: the Long-Awaited Savior of Neuroprotection. Has it Arrived? Neurotherapeutics 12, 655–656. 10.1007/s13311-015-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi MC, et al. , 2012. Compensatory remodeling in symptomatic middle cerebral artery atherosclerotic stenosis: a high-resolution MRI and microemboli monitoring study.Neurol. Res. 34, 153–158. 10.1179/1743132811y.0000000065. [DOI] [PubMed] [Google Scholar]
- Shimizu K, et al. , 2013. Association between inflammatory biomarkers and progression of intracranial large artery stenosis after ischemic stroke. J. Stroke Cerebrovasc. Dis.22, 211–217. 10.1016/j.jstrokecerebrovasdis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Sila C, 2012. Medical treatment of intracranial atherosclerosis has been shown to be superior. J Neurointerv Surg. 4, 83–84. 10.1136/neurintsurg-2012-010270. [DOI] [PubMed] [Google Scholar]
- Skarpathiotakis M, et al. , 2013. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am. J. Neuroradiol. 34, 299–304. 10.3174/ajnr.A3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutta B, et al. , 1999. Intracranial stenoocclusive disease: double-detector helical CT angiography versus digital subtraction angiography. AJNR Am. J. Neuroradiol. 20, 791–799. [PMC free article] [PubMed] [Google Scholar]
- Sluijter JP, et al. , 2006. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke 37, 235–239. 10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- Song J, et al. , 2014. Association between risk factors for vascular dementia and adiponectin. Biomed. Res. Int. 2014, 261672 10.1155/2014/261672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui B, et al. , 2015. Distribution and features of middle cerebral artery atherosclerotic plaques in symptomatic patients: a 3.0 T high-resolution MRI study. Neurol. Res. 37, 391–396. 10.1179/1743132815y.0000000023. [DOI] [PubMed] [Google Scholar]
- Sun R, et al. , 2012. High expression of ubiquitin conjugates and NF-kappaB in unstable human intracranial atherosclerotic plaques. J. Cell. Physiol. 227, 784–788. 10.1002/jcp.22790. [DOI] [PubMed] [Google Scholar]
- Suri MF, Johnston SC, 2009. Epidemiology of intracranial stenosis. J. Neuroimaging 19 (Suppl. 1), 11S–16S. 10.1111/j.1552-6569.2009.00415.x. [DOI] [PubMed] [Google Scholar]
- Tan KC, et al. , 2004. Hypoadiponectinemia is associated with impaired endotheliumdependent vasodilation. J. Clin. Endocrinol. Metab. 89, 765–769. 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, et al. , 2014. Prevalence of symptomatic intracranial atherosclerosis in Caucasians: a prospective, multicenter, transcranial Doppler study. J. Neuroimaging 24, 11–17. 10.1111/j.1552-6569.2012.00707.x. [DOI] [PubMed] [Google Scholar]
- Tulamo R, et al. , 2010. Lack of complement inhibitors in the outer intracranial artery aneurysm wall associates with complement terminal pathway activation. Am. J.Pathol. 177, 3224–3232. 10.2353/ajpath.2010.091172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan TN, et al. , 2007. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 115, 2969–2975. 10.1161/circulationaha.106.622464. [DOI] [PubMed] [Google Scholar]
- Turan TN, et al. , 2017. Is there Benefit from Stenting on Cognitive Function in Intracranial Atherosclerosis? Cerebrovasc. Dis. 43, 31–35. 10.1159/000450964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, et al. , 1998. Frequency and clinical correlates of occlusive lesions of cerebral arteries in Japanese patients without stroke. Evaluation by MR angiography.Cerebrovasc. Dis. 8, 267–272. [DOI] [PubMed] [Google Scholar]
- Vasdekis SN, et al. , 2013. The role of remote ischemic preconditioning in the treatment of atherosclerotic diseases. Brain Behav. 3, 606–616. 10.1002/brb3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. , 2013. The effectiveness of dual antiplatelet treatment in acute ischemic stroke patients with intracranial arterial stenosis: a subgroup analysis of CLAIR study. Int. J. Stroke 8, 663–668. 10.1111/j.1747-4949.2012.00828.x. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. , 2014a. Associations of high sensitivity C-reactive protein levels with the prevalence of asymptomatic intracranial arterial stenosis. Eur. J. Neurol. 21, 512–518. 10.1111/ene.12342. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. , 2014b. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) study. Stroke 45, 663–669. 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. , 2015. Ischemic conditioning-induced endogenous brain protection: applications pre-, per- or post-stroke. Exp. Neurol. 272, 26–40. 10.1016/jexpneurol.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih M, et al. , 1999. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke 30, 1851–1854. [DOI] [PubMed] [Google Scholar]
- Wong LK, 2006. Global burden of intracranial atherosclerosis. Int. J. Stroke 1, 158–159. 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Wong KS, et al. , 2007a. Low-molecular-weight heparin compared with aspirin for the treatment of acute ischaemic stroke in Asian patients with large artery occlusive disease: a randomised study. Lancet Neurol. 6, 407–413. 10.1016/s1474-4422(07)70079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KS, et al. , 2007b. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology 68, 2035–2038. 10.1212/01.wnl,0000264427.09191.89. [DOI] [PubMed] [Google Scholar]
- Xu WH, et al. , 2012. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann. Neurol. 71, 195–198. 10.1002/ana.22626. [DOI] [PubMed] [Google Scholar]
- Yamagami H, et al. , 2004. Higher levels of interleukin-6 are associated with lower echogenicity of carotid artery plaques. Stroke 35, 677–681. 10.1161/01.str.0000116876.96334.82. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Khan BV, 2006. The potential role of antiplatelet agents in modulating inflammatory markers in atherothrombosis. J. Thromb. Haemost. 4, 2308–2316. 10.1111/j.1538-7836.2006.02202.x. [DOI] [PubMed] [Google Scholar]
- Yi X, et al. , 2014. A comparative study of dual versus monoantiplatelet therapy in patients with acute large-artery atherosclerosis stroke. J. Stroke Cerebrovasc. Dis. 23, 1975–1981. 10.1016/j.jstrokecerebrovasdis.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Yoon W, et al. , 2015. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery 76, 680–686. discussion 686. 10.1227/neu.0000000000000694. [DOI] [PubMed] [Google Scholar]
- Yu SCH, et al. , 2017. Long-term evolutionary change in the lumen of intracranial atherosclerotic stenosis following angioplasty and stenting. Oper. Neurosurg. 10.1093/ons/opx097. [DOI] [PubMed] [Google Scholar]
- Zaidat OO, et al. , 2008. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology 70, 1518–1524. mailto:https://doi.org/101212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidat OO, et al. , 2015. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 313, 1240–1248. 10.1001/jama.2015.1693. [DOI] [PubMed] [Google Scholar]
- Zhang S, et al. , 2013. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur. J. Neurol. 20, 1479–1485. 10.1111/ene.12210. [DOI] [PubMed] [Google Scholar]
- Zhang WH, et al. , 2015. Effects of cilostazol on the progression and regression of symptomatic intracranial artery stenosis: it reduces the risk of ischemic stroke. Neural Regen. Res. 10, 667–672. 10.4103/1673-5374.155443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, et al. , 2011. Velocity criteria for intracranial stenosis revisited: an international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke 42, 3429–3434. 10.1161/strokeaha.111.621235. [DOI] [PubMed] [Google Scholar]
- Zhu XJ, et al. , 2016. High-resolution magnetic resonance vessel wall imaging for intracranial arterial stenosis. Chin. Med. J. 129, 1363–1370. mailto: 10.4103/0366-6999.182826. [DOI] [PMC free article] [PubMed] [Google Scholar]