Abstract
Introduction:
While several mechanisms may explain metal-related health effects, the exact cellular processes are not fully understood. We evaluated the association between leukocyte telomere length (LTL) and urine arsenic (ΣAs), cadmium (Cd) and tungsten (W) exposure in the Strong Heart Study (SHS, N=1,702) and in the Strong Heart Family Study (SHFS, N=1,793).
Methods:
Urine metal concentrations were measured using ICP-MS. Arsenic exposure was assessed as the sum of inorganic arsenic, monomethylarsonate and dimethylarsinate levels (ΣAs). LTL was measured by quantitative polymerase chain reaction.
Results:
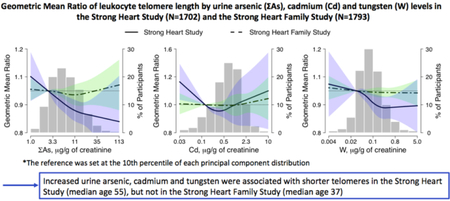
In the SHS, median levels were 1.09 for LTL, and 8.8, 1.01 and 0.11 µg/g creatinine for ΣAs, Cd, and W, respectively. In the SHFS, median levels were 1.01 for LTL, and 4.3, 0.44, and 0.10 µg/g creatinine. Among SHS participants, increased urine ΣAs, Cd, and W was associated with shorter LTL. The adjusted geometric mean ratio (95% confidence interval) of LTL per an increase equal to the difference between the percentiles 90th and 10th in metal distributions was 0.85 (0.79, 0.92) for ΣAs, 0.91 (0.84, 1.00) for Cd and 0.93 (0.88, 0.98) for W. We observed no significant associations among SHFS participants. The findings also suggest that the association between arsenic and LTL might depend on the exposure levels or age.
Conclusions:
Additional research is needed to confirm the association between metal exposures and telomere length.
Keywords: American Indians, Arsenic, Cadmium, Tungsten, Telomeres
Graphical Abstract
INTRODUCTION
Environmental exposure to toxic heavy metals such us arsenic, cadmium and tungsten, have been associated with several health effects, including cardiovascular disease (1, 2), oxidative stress (3, 4), stroke (5), diabetes (6) and some types of cancer (7, 8). However, the mechanisms of action for these associations, especially at the cellular levels, are still not fully understood. Some studies suggest that these associations could be in part related with telomere shortening (9). Telomeres are distinctive structures that are found at each end of the chromosomes, protecting DNA from damage and instability. Telomere shortening occurs naturally every time a cell divides, and when telomeres reach a critical length, the cell can no longer be divided and becomes senescence. Telomere length (TL) has been established as a biomarker of aging and age-related diseases (10), including cardiovascular diseases (11, 12), diabetes (13), cancer and mortality (14). While a decrease of TL with age is inevitable, telomere shortening rate could be influenced by modifiable factors such as diet (15), physical activity (16) or metal exposures (9).
Several epidemiologic studies have investigated the association between exposure to arsenic, cadmium and other metals with TL with mixed results. Most of the studies on the relationship between TL and arsenic have been conducted among populations affected by high arsenic exposure. In particular, small studies from Nepal (17), Argentina (18, 19), Bangladesh (20), and India (21) suggested a positive association between arsenic and TL, while a study from Italy found a borderline inverse association (22). Cadmium exposure, however, has been consistently associated with shorter telomeres in Nepal (17) and NHANES 1999–2002 (23). No study to our knowledge has investigated the association between tungsten exposure and TL.
American Indian communities in the United States are affected by a high burden of diabetes and other age-related diseases compared to other US populations, as well as higher exposure levels to arsenic (24), cadmium (25) and probably other toxic metals. Our objective was therefore to assess the cross-sectional association between arsenic, cadmium and tungsten exposure, assessed as concentrations in urine, with leucocyte telomere length among American Indian men and women from Arizona, Oklahoma, North and South Dakota participating in the Strong Heart Study (SHS), and also among participants of the Strong Heart Family Study (SHFS), which recruited multigenerational families from the same SHS communities.
MATERIALS AND METHODS
Study Population
The SHS is an ongoing cohort study of cardiovascular disease and its risk factors among American Indian men and women. A total of 4,549 participants from Arizona, Oklahoma, North and South Dakota (median age: 55 years) were enrolled between 1989 and 1991 (phase I). Participants returned for follow-up visits in 1993–1995 (phase II) and 1998– 1999 (phase III). For the present study, we will use data from phase I only. Among them, 2,242 participants had urine metals and leucocyte telomere length data available at baseline visit. For the present study, 451 participants from one of the communities were excluded due to withdraw of consent to conduct research. We further excluded participants with missing urine creatinine (n=2), educational level (n=2), body mass index (n=3), and glomerular filtration rate (n=46), and 36 participants with outlier values of TL data, resulting in 1,702 SHS participants for the analyses.
The SHFS is an auxiliary family-based study that recruited multigenerational families from the same communities as the original SHS participants. In the SHFS, 3,838 men and women (median age: 37 years) have baseline data collected in 1998–1999 (phase III) and 2001–2004 (phase IV), and follow-up data in 2001–2004 (for some participants recruited in phase III) and 2006–2009 (phase V). 2,277 participants have baseline urine metal concentrations and telomere length determinations measured at phase IV, all of them free of diabetes at baseline visit. After excluding participants from the community that withdrew consent (n=469), and participants with missing data on educational level (n=7), smoking status (n=1), body mass index (n=6), and glomerular filtration rate (n=1), a total of 1,793 SHFS individuals remained for the present study.
The study design and data collection methods for both cohorts have been described previously in detail (26, 27) and are also available at the website (http://www.strongheartstudy.org). The Indian Health Service, institutional review boards, and the participating tribes approved the study protocol. All participants provided informed consent.
Urine metal levels
In both cohorts, spot urine samples were collected the morning of the baseline visit and frozen for measurements. Levels of total arsenic (As), cadmium (Cd), and tungsten (W) in urine were measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) at the Trace Element Laboratory of University of Graz, Austria following a standardized protocol (28). Urine arsenic speciation was conducted to evaluate inorganic arsenic exposure avoiding the contribution of non-toxic seafood arsenicals. The concentrations of inorganic arsenic (iAs, i.e. arsenite plus arsenate), monomethylarsonate (MMA), dimethylarsinate (DMA), and arsenobetaine were determined by High Performance Liquid Chromatography (HPLC) ICP-MS. We used the sum of urine iAs, MMA and DMA (ΣAs) as our biomarker of inorganic arsenic. The limits of detection were 0.1 µg/L for total arsenic and arsenic species, 0.015 µg/L for cadmium, and 0.005 µg/L for tungsten. We imputed urine metal concentrations below the limit of detection in the SHS (96 for iAs, 13 for MMA, 0 for DMA, 1 for Cd, and 10 for W), and in the SHFS (199 for iAs, 58 for MMA, 0 for DMA, 94 for Cd, and 60 for W), as the limit of detection divided the square root of 2. In the SHS, the inter-assay coefficients of variation were 6.0% for IAS, 3.5% for MMA, 4.4% for DMA, 6.0% for Cd and 14.5% for W. In the SHFS, the inter-assay coefficients of variation were 5.6% for iAs, 6.3% for MMA, 3.5% for DMA, 8.7% for Cd and 18.1% for W. Urine cadmium concentrations were corrected for molybdenum (Mo) oxide interference using the formula Cdcorr=Cd-0.0016*Mo (28). A more detailed description of the standards, procedures and certified reference materials for the urine metal determinations has been published (28). Metal urine concentrations were divided by urine creatinine to account for urine dilution and expressed as µg/g of creatinine.
Leucocyte telomere length
For both SHS and SHFS, blood samples were collected into EDTA tubes following standard protocols. Genomic DNA from peripheral blood was isolated according to standard methods and stored at −80ºC. Leukocyte telomere length (LTL) was measured by quantitative polymerase chain reaction (qPCR) at Dr. Elizabeth Blackburn’s laboratory of the University of California, San Francisco, using a high-throughput telomere length assay system as previously described (13, 29, 30). For both SHS and SHFS, all participants with available DNA samples were assayed. In particular, LTL was measured as the ratio of the telomeric product vs. the single-copy gene (T/S ratio), which is unitless. Specifically, the longer the telomeres are in each sample, the more PCR product will be generated in PCR reactions using primers specific for the telomeric DNA. The average PCR efficiencies of S and T runs were 95% and 84%, respectively. T/S ratio was calculated by taking the difference between the mean of two T values and two S values attained for each of the three replicates. Each DNA sample was assayed three times and the mean of the three T/S ratios was used for the analysis. More detailed methods for LTL measurement and quality control procedures have been described elsewhere (13). The same reference sample/primers were used in the telomere length measurement of both study samples.
Other variables
At baseline visit, each participant underwent an in-person interview and a physical examination. Information on age, sex, educational level and smoking status was collected using standard questionnaires (26). Anthropometric measures were performed according to a standardized protocol by certified staff. Body mass index was calculated as weight in kilograms divided by the square of height in meters. All participants provided blood samples after at least 8 hours of fasting. Diabetes status was defined as having fasting glucose levels ≥126 μg/dL, 2-hour post-load plasma glucose levels ≥200 μg/dL, HbA1c levels ≥6.5%, or using of diabetes medication (31). Urine and serum creatinine levels were measured by an automated alkaline picrate methodology. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology equation (32).
Statistical methods
All statistical analyses described here were performed in both SHS and SHFS samples. The distribution of ΣAs, Cd and W were log-transformed for the analyses. As in other studies, LTL was also log-transformed to improve normality (23, 33–35). We described the median and interquartile range of LTL and metal levels across participant’s characteristics. Differences between subgroups were analyzed using Kruskal-Wallis tests.
We estimated the geometric mean ratios (GMR) and 95% confidence intervals (CI) of LTL based on ΣAs, Cd and W levels in separate models. Each metal was included in the models as continuous and the associations were reported per an increase equal to the difference between the percentile 90th versus percentile 10th of the corresponding metal distribution. These percentiles were obtained from the joint distribution of the two study samples. Models were conducted with gradual degrees of adjustment in order to identify the effect of selected groups of covariates. Model 1 was adjusted for age, gender and educational level. Model 2 was further adjusted for smoking status, body mass index, eGFR and diabetes status (only for SHS analyses). Generalized linear models (GLM) were used for the analyses in the SHS, while generalized estimating equations (GEE) were used in the SHFS to control for dependency among family members.
To explore potential non-linear relationships, metals were also modeled as tertiles and the estimates were reported by comparing the 2 highest tertiles to the lowest one. We further graphically showed the dose-response relationship between LTL and metals using restricted quadratic splines functions with knots at the 10th, 50th and 90th percentiles of each metal distribution.
Because the associations between metals and TL might vary depending on age or geographic differences, we repeated the analyses stratifying by age (<50 and ≥50 years old) and study region. As exploratory analyses, we also analyzed the differential associations by gender, smoking status, study region, low eGFR (no/yes), diabetes status, and metal concentrations (categorized below and above the median) by including the interaction term of the metal and the subgroup variable in fully-adjusted models (see Supplemental material). The statistical significance of the interaction terms was examined using Wald tests. We additionally ran several sensitivity analyses to evaluate the robustness of the findings. First, we repeated the main association in models further adjusted for the distribution of the other two metals (see Supplemental material). Second, we conducted the associations between LTL and metals in analyses restricted to individuals without reduced kidney function (eGFR ≥ 60 ml/min/1.73m2) and never smokers in separate analyses (see Supplemental material). Similarly, we also repeated the analyses excluding participants with prevalent cardiovascular disease (112 and 51 cases in the SHS and the SHFS, respectively) with essentially identical results (not shown). We also re-evaluated the associations accounting for urine dilution by treating urine creatinine and specific gravity (separately) as covariates in regression models with consistent results (not shown). Due to potential collinearity between eGFR and diabetes status, we repeated the associations in the SHS not adjusting for diabetes status, with identical results (not shown).
RESULTS
Metals and telomere length levels and pairwise Spearman’s correlations in the SHS and the SHFS
Among the 1,702 SHS participants, the median (interquartile range) was 1.09 (0.86, 1.47) for LTL, and 8.8 (5.4, 14.5), 1.01 (0.63, 1.59) and 0.11 (0.06, 0.22) for ΣAs, Cd and W (in µg/g), respectively (Table 1). Among the 1,793 SHFS participants, the median (interquartile range) was 1.01 (0.88, 1.16) for LTL, and 4.3 (2.9, 7.2), 0.44 (0.20, 0.86) and 0.10 (0.06, 0.19) for ΣAs, Cd and W (in µg/g), respectively. In both cohorts, younger participants tended to have longer telomeres compared to the older ones, although the differences were not statistically significant in the SHS, probably due to the smaller age range. Participants with shorter telomeres were more likely men, less educated, from Arizona and heavier. Male participants generally had lower urine metal concentrations than females. ΣAs and W concentrations were higher in Arizona, while cadmium was higher in North and South Dakota. Non-current smokers, and higher-educated participants had lower metal levels. In the SHS, participants with diabetes had higher ΣAs and W but lower Cd levels.
Table 1.
Median (IQR) of leucocyte telomere length and urine arsenic, cadmium and tungsten concentrations (µg/g creatinine) by participant’s characteristics among Strong Heart Study (n=1,702) and Strong Heart Family Study (n=1,793) participants
| Strong Heart Study | Strong Heart Family Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | LTL | ΣAs | Cd | W | n | LTL | ΣAs | Cd | W | |
| Overall | 1702 | 1.09 (0.86, 1.47) | 8.8 (5.4, 14.5) | 1.01 (0.63, 1.59) | 0.11 (0.06, 0.22) | 1793 | 1.01 (0.88, 1.16) | 4.3 (2.9, 7.2) | 0.44 (0.20, 0.86) | 0.10 (0.06, 0.19) |
| Age, years | ||||||||||
| < 55 | 874 | 1.11 (0.88, 1.49) | 8.8 (5.5, 14.3) | 0.94 (0.60, 1.44) | 0.11 (0.06, 0.22) | 678 | 1.09 (0.97, 1.24) | 4.1 (2.7, 6.8) | 0.21 (0.10, 0.43) | 0.11 (0.06, 0.21) |
| 55 – 64 | 541 | 1.09 (0.83, 1.50) | 9.1 (5.4, 14.8) | 1.05 (0.69, 1.66) | 0.11 (0.06, 0.21) | 746 | 0.98 (0.85, 1.11) | 4.5 (2.9, 7.2) | 0.55 (0.31, 0.98) | 0.10 (0.06, 0.18) |
| ≥ 65 | 287 | 1.06 (0.86, 1.42) | 8.0 (5.1, 14.3) | 1.09 (0.66, 1.67) | 0.11 (0.06, 0.23) | 369 | 0.93 (0.80, 1.04) | 4.5 (3.0, 7.6) | 0.74 (0.43, 1.22) | 0.10 (0.06, 0.19) |
| P | 0.16 | 0.58 | <0.001 | 0.50 | <0.001 | 0.01 | <0.001 | 0.05 | ||
| Sex | ||||||||||
| Men | 714 | 1.08 (0.84, 1.42) | 8.1 (4.8, 13.5) | 0.75 (0.49, 1.14) | 0.10 (0.05, 0.19) | 704 | 1.01 (0.88, 1.14) | 4.0 (2.8, 6.4) | 0.31 (0.15, 0.59) | 0.10 (0.06, 0.20) |
| Women | 988 | 1.11 (0.87, 1.50) | 9.5 (5.8, 15.2) | 1.19 (0.78, 1.83) | 0.12 (0.07, 0.24) | 1089 | 1.02 (0.88, 1.16) | 4.6 (3.0, 7.5) | 0.55 (0.27, 1.02) | 0.11 (0.06, 0.19) |
| P | 0.02 | <0.001 | <0.001 | <0.001 | 0.20 | <0.001 | <0.001 | 0.17 | ||
| Smoking status | ||||||||||
| Never | 459 | 1.11 (0.88, 1.51) | 8.7 (5.2, 14.7) | 0.88 (0.54, 1.44) | 0.12 (0.07, 0.23) | 742 | 1.02 (0.89, 1.16) | 4.1 (2.7, 6.4) | 0.34 (0.17, 0.66) | 0.10 (0.06, 0.20) |
| Former | 542 | 1.07 (0.86, 1.43) | 8.1 (5.1, 13.2) | 0.80 (0.55, 1.26) | 0.11 (0.06, 0.22) | 366 | 0.99 (0.87, 1.14) | 4.2 (2.8, 7.1) | 0.53 (0.25, 0.92) | 0.11 (0.06, 0.18) |
| Current | 701 | 1.10 (0.84, 1.49) | 9.6 (5.7, 15.2) | 1.21 (0.84, 1.88) | 0.11 (0.06, 0.21) | 685 | 1.01 (0.88, 1.15) | 4.8 (3.1, 7.8) | 0.53 (0.24, 1.07) | 0.10 (0.06, 0.19) |
| P | 0.27 | <0.001 | <0.001 | 0.15 | 0.09 | <0.001 | <0.001 | 0.76 | ||
| Education | ||||||||||
| No HS | 332 | 1.03 (0.82, 1.42) | 12.7 (7.9, 18.3) | 1.19 (0.78, 1.86) | 0.12 (0.07, 0.22) | 198 | 1.04 (0.90, 1.17) | 5.8 (3.6, 9.6) | 0.39 (0.13, 0.88) | 0.13 (0.07, 0.27) |
| Some HS | 410 | 1.11 (0.85, 1.50) | 8.3 (5.1, 14.3) | 1.08 (0.68, 1.70) | 0.11 (0.07, 0.24) | 341 | 1.07 (0.90, 1.22) | 4.5 (2.9, 7.6) | 0.34 (0.15, 0.86) | 0.12 (0.07, 0.25) |
| Completed HS | 960 | 1.11 (0.88, 1.49) | 7.9 (4.9, 13.1) | 0.91 (0.58, 1.39) | 0.11 (0.06, 0.21) | 1254 | 1.00 (0.87, 1.13) | 4.1 (2.8, 6.6) | 0.47 (0.24, 0.85) | 0.10 (0.06, 0.18) |
| P | 0.008 | <0.001 | <0.001 | 0.02 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| BMI, kg/m2 | ||||||||||
| < 25 | 308 | 1.14 (0.88, 1.51) | 10.5 (5.8, 16.5) | 1.27 (0.84, 1.95) | 0.11 (0.07, 0.21) | 423 | 1.04 (0.92, 1.21) | 4.2 (2.9, 6.8) | 0.38 (0.15, 0.85) | 0.12 (0.06, 0.21) |
| 25–29 | 600 | 1.08 (0.85, 1.45) | 8.8 (5.3, 14.7) | 1.02 (0.64, 1.63) | 0.11 (0.06, 0.22) | 522 | 1.01 (0.88, 1.17) | 4.3 (2.9, 7.1) | 0.50 (0.23, 0.96) | 0.10 (0.06, 0.20) |
| ≥ 30 | 794 | 1.08 (0.86, 1.49) | 8.4 (5.0, 13.6) | 0.90 (0.58, 1.38) | 0.11 (0.06, 0.22) | 848 | 1.00 (0.86, 1.13) | 4.4 (2.9, 7.2) | 0.43 (0.22, 0.81) | 0.10 (0.06, 0.19) |
| P | 0.20 | <0.001 | <0.001 | 0.99 | <0.001 | 0.69 | 0.001 | 0.02 | ||
| Study region | ||||||||||
| Arizona | 168 | 1.03 (0.88, 1.44) | 14.7 (9.8, 20.2) | 0.72 (0.51, 1.16) | 0.21 (0.12, 0.34) | 181 | 0.98 (0.86, 1.12) | 7.7 (5.3, 12.8) | 0.40 (0.18, 0.83) | 0.20 (0.11, 0.39) |
| Oklahoma | 582 | 1.22 (0.97, 1.64) | 5.3 (3.6, 7.7) | 0.80 (0.54, 1.23) | 0.09 (0.05, 0.16) | 758 | 1.00 (0.87, 1.12) | 3.8 (2.6, 5.5) | 0.46 (0.23, 0.83) | 0.09 (0.05, 0.17) |
| N/S Dakota | 952 | 1.05 (0.77, 1.40) | 10.9 (7.1, 16.1) | 1.16 (0.77, 1.80) | 0.12 (0.07, 0.21) | 854 | 1.04 (0.90, 1.19) | 4.5 (2.9, 7.5) | 0.42 (0.18, 0.88) | 0.10 (0.06, 0.18) |
| P eGFR, mL/minute/1.73m2 |
<0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.14 | <0.001 | ||
| ≥ 60 | 1553 | 1.09 (0.85, 1.47) | 9.0 (5.5, 14.6) | 1.00 (0.64, 1.57) | 0.11 (0.06, 0.22) | 1716 | 1.02 (0.89, 1.16) | 4.3 (2.9, 7.1) | 0.43 (0.19, 0.85) | 0.10 (0.06, 0.19) |
| < 60 | 149 | 1.14 (0.94, 1.49) | 7.4 (4.6, 13.7) | 1.07 (0.61, 1.79) | 0.10 (0.06, 0.21) | 77 | 0.91 (0.79, 1.02) | 4.6 (3.0, 7.2) | 0.62 (0.34, 0.89) | 0.10 (0.06, 0.17) |
| P | 0.12 | 0.005 | 0.45 | 0.57 | <0.001 | 0.70 | 0.003 | 0.28 | ||
| Diabetes | ||||||||||
| No DM | 1027 | 1.09 (0.88, 1.46) | 8.4 (5.1, 13.8) | 1.03 (0.66, 1.63) | 0.11 (0.06, 0.21) | |||||
| DM | 675 | 1.10 (0.83, 1.49) | 9.5 (5.9, 15.5) | 0.96 (0.60, 1.52) | 0.12 (0.07, 0.23) | |||||
| P | 0.33 | <0.001 | 0.009 | 0.005 | ||||||
Abbreviations: LTL, leucocyte telomere length; IQR, interquartile range; HS, high school; BMI, body mass index; eGFR, estimated glomerular filtration rate; DM, diabetes mellitus;
P, p-value.
P-values obtained by Kruskal-Wallis tests.
In the SHS, the pairwise Spearman correlations of LTL with the three metals were negative and small, but statistically significant (P-values < 0.04) (Figure 1). In the SHFS, telomere length was also negatively correlated with ΣAs and Cd, but not correlated with W.
Figure 1. Pairwise Spearman’s correlations (p-value) between leukocyte telomere length and creatinine-corrected urine arsenic, cadmium and tungsten concentrations, among Strong Heart Study (n=1,702) and Strong Heart Family Study (n=1,793) participants.
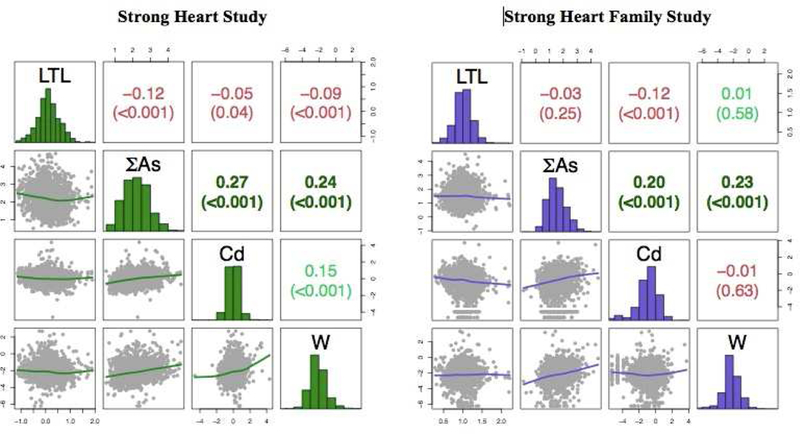
Metals were corrected for urine dilution by dividing for urine creatinine. The diagonal panel shows the histograms of the log-transformed distribution of leukocyte telomere length and each metal. The upper panel represents the Spearman pairwise correlation coefficients. The lower panel shows the pairwise scatterplots. The axes indicate values in the log-scale. Negative correlations coefficients were colored in red, positive correlations were colored in green. Abbreviations: LTL, leukocyte telomere length.
Association of urine metal concentrations with telomere length in the SHS
Higher concentrations of urine ΣAs were associated with shorter telomeres (Table 2). In particular, the fully-adjusted GMR (95% CI) of LTL per an increase equal to the difference between the percentiles 90th and 10th in ΣAs was 0.85 (0.79, 0.92) (Table 2, model 2). We found inverse associations between Cd and W levels with telomere length in the fully-adjusted model (GMR (95% CI): 0.91 (0.84, 1.00) for Cd and 0.93 (0.88, 0.98) for W), although these associations were no longer significant after adjustment for the concentrations of the other metals (GMR (95% CI): 0.95 (0.87, 1.04) for Cd and 0.95 (0.90, 1.00) for W, Supplemental Table 1). The same conclusions were derived from flexible models with metals introduced as tertiles (Table 2) and restricted quadratic splines (Figure 2).
Table 2.
Geometric mean ratios (95% confidence intervals) of leukocyte telomere length associated with urine arsenic, cadmium and tungsten concentrations among Strong Heart Study participants (n=1,702) and among Strong Heart Family Study participants (n=1,793).
| Strong Heart Study | Strong Heart Family Study | |||||
|---|---|---|---|---|---|---|
| n | Model 1 | Model 2 | n | Model | Model 2 | |
| ΣAs | ||||||
| Tertil1 | 567 | 1 (Reference) | 1 (Reference) | 601 | 1 (Reference) | 1 (Reference) |
| Tertil2 | 568 | 0.96 (0.91, 1.01) | 0.97 (0.92, 1.02) | 596 | 0.99 (0.96, 1.03) | 0.99 (0.96, 1.02) |
| P value | 0.12 | 0.20 | 0.61 | 0.55 | ||
| Tertil3 | 567 | 0.90 (0.85, 0.95) | 0.91 (0.86, 0.96) | 596 | 1.00 (0.97, 1.03) | 1.00 (0.96, 1.03) |
| P value | <0.001 | <0.001 | 0.97 | 0.81 | ||
| p90 vs. p10* | 1702 | 0.84 (0.78, 0.91) | 0.85 (0.79, 0.92) | 1793 | 1.00 (0.94, 1.05) | 0.99 (0.94, 1.05) |
| P value | <0.001 | <0.001 | 0.92 | 0.80 | ||
| Cd | ||||||
| Tertil1 | 573 | 1 (Reference) | 1 (Reference) | 599 | 1 (Reference) | 1 (Reference) |
| Tertil2 | 563 | 0.95 (0.90, 1.01) | 0.96 (0.91, 1.01) | 598 | 0.99 (0.97, 1.02) | 1.00 (0.97, 1.02) |
| P value | 0.08 | 0.14 | 0.72 | 0.78 | ||
| Tertil3 | 566 | 0.93 (0.88, 0.99) | 0.94 (0.89, 1.00) | 596 | 1.01 (0.99, 1.04) | 1.01 (0.98, 1.04) |
| P value | 0.02 | 0.05 | 0.36 | 0.50 | ||
| p90 vs. p10* | 1702 | 0.90 (0.83, 0.98) | 0.91 (0.84, 1.00) | 1793 | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.03) |
| P value | 0.01 | 0.05 | 0.41 | 0.47 | ||
| W | ||||||
| Tertil1 | 573 | 1 (Reference) | 1 (Reference) | 606 | 1 (Reference) | 1 (Reference) |
| Tertil2 | 564 | 0.92 (0.88, 0.97) | 0.93 (0.88, 0.98) | 593 | 1.02 (0.99, 1.05) | 1.02 (0.99, 1.04) |
| P value | 0.003 | 0.004 | 0.18 | 0.25 | ||
| Tertil3 | 565 | 0.92 (0.88, 0.97) | 0.93 (0.88, 0.98) | 594 | 0.99 (0.97, 1.02) | 0.99 (0.97, 1.02) |
| P value | 0.005 | 0.009 | 0.69 | 0.53 | ||
| p90 vs. p10* | 1702 | 0.92 (0.87, 0.97) | 0.93 (0.88, 0.98) | 1793 | 0.99 (0.97, 1.02) | 0.99 (0.97, 1.01) |
| P value | 0.003 | 0.007 | 0.59 | 0.40 | ||
Model 1 adjusted for age (years), sex and educational level (< high school, some high school, completed high school).
Model 2 further adjusted for smoking status (never, former, current), body mass index, estimated glomerular filtration rate (mL/minute per 1.73m2) and diabetes mellitus (yes, no, only in SHS models).
In the SHS, the tertiles cutoffs are 6.4 and 12.4 μg/g for ΣAs, 0.74 and 1.30 μg/g for Cd, and 0.08 and 0.17 μg/g for W.
In the SHFS, the tertiles cutoffs are 3.3 and 5.8 μg/g for ΣAs, 0.27 and 0.67 μg/g for Cd, and 0.07 and 0.15 μg/g for W.
90th and the 10th percentiles obtained from the distribution resulted from combining the corresponding SHS and SHFS metal distributions.
The 90th and the 10th percentiles are 17.4 and 2.5 μg/g for ΣAs; 2.00 and 0.16 μg/g for Cd; and 0.41 and 0.04 μg/g for W.
Figure 2. Dose response relationship of leukocyte telomere length by urine arsenic, cadmium and tungsten concentrations among Strong Heart Study participants (n=1,702) and among Strong Heart Family Study participants (n=1,793).
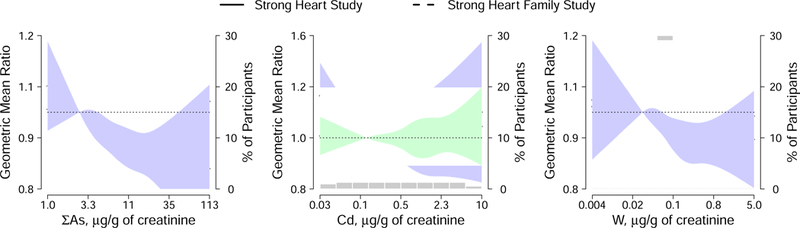
Lines and shaded areas represent adjusted geometric mean ratios and 95% confidence intervals for leukocyte telomere length based on restricted quadratic splines for log-transformed urine metals concentrations with knots at 10th, 50th and 90th percentiles. The reference value was set at 10th percentile of each urine metal distribution. Percentiles for each metal were obtained from the joint distribution of both cohorts.
Models are adjusted for age (years), sex, educational level (< high school, some high school, completed high school), smoking status (never, former, current), body mass index (kg/m2), estimated glomerular filtration rate (mL/minute per 1.73m2) and diabetes mellitus (yes, no, only for SHS models) (model 2 adjustment).
In stratified analyses by study region, however, the association between ΣAs and LTL was positive, although not statistically significant, in Arizona (GMR (95% CI): 1.11 (0.91, 1.36)), and inverse in Oklahoma (GMR (95% CI): 0.91 (0.84, 0.99)) (Supplemental Table 2, Supplemental Figure 1). Similarly, the association between W and LTL was inverse in Oklahoma (GMR (95% CI): 0.88 (0.82, 0.94)), but null in Arizona and North and South Dakota. The association between Cd and LTL did not differ by study region. In stratified analyses by age, the associations were consistent in older participants but not significant in younger participants (Supplemental Table 2). We did not find any significant effect modification of the associations in exploratory interactions analyses (Supplemental Table 3). Sensitivity analyses conducted within participants with normal eGFR and non-current smokers showed essentially identical results than with the overall study sample (Supplemental Figure 1).
Association of urine metal concentrations with telomere length in the SHFS
No significant associations were found between ΣAs, Cd or W and LTL (Table 2). In particular, the GMR (95% CI) of LTL per an increase of the difference between the percentiles 90th and 10th in metal distributions was 0.99 (0.94, 1.05) for ΣAs, 1.01 (0.99, 1.03) for Cd and 0.99 (0.97, 1.01) for W. We found differential associations in stratified analyses by study region or age (Supplemental Table 2), nor in interaction analyses (Supplemental Table 4).
DISCUSSION
In this study conducted among American Indian men and women, we found that increasing environmental exposures to arsenic, cadmium and tungsten, assessed by urine concentrations, were associated with shorter telomeres among participants of the SHS sample. The associations of cadmium and tungsten with LTL, however, were attenuated and no longer statistically significant after adjustment for the other metals. The inverse associations of arsenic and tungsten with TL were consistent among older participants (≥50 years old) but weaker and no longer significant in younger participants (<50 years old). On the other hand, arsenic, cadmium and tungsten exposure was not associated with LTL among participants of the SHFS, which were younger and generally less exposed than participants of the SHS.
Arsenic, cadmium and tungsten are heavy metals naturally occurring in the soil, rocks and/or groundwater. Arsenic exposure occurs via drinking-water (37), although intake of cereals, rice, and other crops may be the main source in populations less exposed to arsenic in water (38). In our study population, Arizona is a region naturally affected by higher arsenic exposure levels, whereas in Oklahoma participants live in more urban areas and are less exposed to arsenic. Interestingly, the direction of the association between arsenic and LTL in the SHS was positive in Arizona (n=168) and inverse in Oklahoma (n=582). Nevertheless, these stratified results must be taken with caution due to the small sample sizes, especially for Arizona. While arsenic has a relatively short half-life in the body (7–10 days), urinary arsenic has been considered a reliable biomarker for long-term exposure assessment under chronic and constant conditions of exposure (37). Tobacco is a major source of cadmium for humans. In non-smokers, cadmium exposure generally comes from vegetables (39), shellfish (40), and organ and processed meats (25) intake. Cadmium is generally accumulated in the liver and kidneys, it has an estimated half-life in the body of 10–30 years and its excretion is primarily through urine (39). While the main exposure source of tungsten is occupational in the heavy metal industry, drinking-water, food and air may be a source of exposure for the general population (41, 42). Most of tungsten uptake in the body is rapidly excreted through urine or feces. There is, however, a slow component in tungsten half-life (43). The exposure levels of arsenic, cadmium and tungsten in the SHS and the SHFS are considered moderate-low, especially lower for the SHFS. In particular, the creatinine-corrected urine arsenic, cadmium and tungsten median concentrations in the SHS (1989–1991) were 8.9, 1.0 and 0.11 μg/g, respectively, and in the SHFS (2001–2004) were 4.3, 0.44 and 0.10 μg/g, respectively, while in NHANES data from 2004–2005 the median levels were 5.6, 0.17, 0.07 μg/g for ΣAs, Cd and W, respectively.
Some epidemiologic studies have explored the association between arsenic exposure and telomere length, generally reporting positive associations (17–22), i.e, increased arsenic exposure associated with longer telomeres. However, most of those studies are small and conducted in populations exposed to high levels of arsenic in drinking-water. A study on 351 Nepali adolescents (12–16 years old) found a positive, but not statistically significant, association between urine arsenic (geometric mean ΣAs 114.5 μg/L) and salivary TL (17). Arsenic exposure, as measured in urine, was positively associated with TL in two different regions from the Andes Mountains in northern Argentina with elevated arsenic concentrations in drinking-water (264 and 169 adult participants with median urine ΣAs 196 and 80 μg/L, respectively) (18), and among 202 women from the same area (median urine ΣAs 230 μg/L) (19). Among 167 Bangladeshi adults participating in the HEALS cohort, telomeres were significantly longer among participants classified in the high exposure group (mean urine ΣAs 856 μg/g) as compared to those in the lower exposure group (mean urine ΣAs 61.9 μg/g) (20). Similarly, a study in India selecting 120 participants (60 with skin lesions and 60 without, studied separately) from areas with high levels of arsenic in drinking-water (levels 30–620 μg/L), and 60 participants from a district where arsenic in water was within the WHO safe limit, found longer telomeres in the high exposure groups (mean urine ΣAs 318 and 290 μg/L in participants without and with skin lesions, respectively), compared to unexposed controls (mean urine ΣAs 30.5 μg/L) (21). On the other hand, a study of 241 young Italian adults (20–46 years old), is the only study to our knowledge reporting an inverse association (although borderline significant) between urine arsenic and TL (22).
Few observational studies have investigated the association between cadmium exposure and telomere shortening. In particular, a NHANES 1999–2002 study showed an inverse association of urine cadmium (n=2,090, geometric mean 0.28 μg/L) and blood cadmium (n=6,796, geometric mean 0.44 μg/L) with LTL (23). An inverse association between urine cadmium (geometric mean 0.19 μg/L) and salivary TL was also found among 351 adolescents from Nepal (17). A study on 227 Chinese women reported a statistically significant inverse correlation between placenta cadmium levels (median 0.076 μg/g) and placental TL. However, null association was found between blood cadmium (geometric mean 0.18 μg/L) and TL among 99 children from Poland (8 years old) (44). In vitro studies with mouse embryos and mouse embryonic stem cells also concluded that exposure to cadmium and to cigarette smoke condensate results in shorter telomeres and DNA damage (45, 46). Although no epidemiological or experimental studies to our knowledge have investigated the association of tungsten and telomere length, some studies support that tungsten exposure may be related with DNA damage (47, 48).
The suggested inverse associations of arsenic and cadmium with telomere length in the SHS are biologically plausible, since exposures to these metals have been associated with mechanisms that promote telomere shortening, such us oxidative stress, inflammation and inhibition of DNA repair (49–52). Our finding of arsenic being positively related with LTL at higher exposure levels while inversely related with LTL at lower exposure levels in the SHS is consistent with previous epidemiologic studies, but contrary to the evidence from experimental data. In particular, in vitro studies on human cells reported that low levels of arsenite (iAsIII) exposure (<1 μM) produced telomere maintenance and increased telomerase activity, while higher levels of arsenite exposure (≥1 μM) decreased telomere length and telomerase activity (53, 54). Another study showed that arsenic trioxide at 0.75–1.0 μM inhibited telomerase and resulted in chromosomal instability (55). The mixed results on experimental settings could be in part related with telomerase activity. Telomerase is an enzyme that helps maintenance of TL and plays a paradoxical role in tumorigenesis, since telomerase activity is frequently found in advanced tumors (56), while for noncancerous cells, telomere loss could lead to cancer formation (55).
Limitations of this study include, first of all, its observational nature, by which residual or non-measured confounding could have occurred. Other limitations are the use of one single measure for metal exposure assessment, the lack of information about past metal exposures, the unavailability of blood cell count information, a potential confounder of the evaluated associations, and the cross-sectional design. Our study also has several strengths: the high quality of the protocol and laboratory methods; assessment of arsenic exposure in urine, a biomarker that accounts for arsenic from different sources of exposure; the availability and comparison of two study samples with large sample sizes; and the assessment of the association between tungsten exposure and TL for the first time.
CONCLUSIONS
In conclusion, exposure to arsenic, cadmium and tungsten was associated with telomere shortening among participants of the Strong Heart Study, but not among participants of the Strong Heart Family Study, who included younger participants and were exposed to generally lower arsenic and cadmium concentrations. The findings add to the body of evidence evaluating the relationship between telomere length and arsenic by using data from two large samples from populations exposed to moderate (SHS) and low (SHFS) arsenic concentrations in drinking-water. Further research, however, is needed to confirm the associations of metal exposures on telomere length and its related health consequences at different exposure levels and ages.
Supplementary Material
Highlights.
The cellular mechanisms for metal-related health effects are not fully understood
The Strong Heart and Strong Heart Family studies have telomere and urine metal data
Arsenic, cadmium and tungsten were related with shorter telomeres in SHS (median age 55)
In the SHFS (median age 37), the corresponding associations were null
Additional research is needed to confirm the association of metal exposures with telomere length
Acknowledgments
Funding
This study was supported by the National Institute of Health Sciences (R01ES021367, R01ES025216, P42ES010349, P30ES009089), and by the National Heart, Lung, and Blood Institute (cooperative agreements grants U01-HL41642, U01-HL41652, U01-HL41654, U01-HL65520, U01-HL65521 and research grants R01-HL109315, R01-HL109301, R01-HL109284, R01-HL109282 and R01-HL109319 and R01-HL090863).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors have declared that they do not have any conflict of interest.
REFERENCES
- 1.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Current atherosclerosis reports 2013; 15(10):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M. Environmental Metals and Cardiovascular Disease in Adults: A Systematic Review Beyond Lead and Cadmium. Current environmental health reports 2016; 3(4):416–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free radical biology & medicine 2011; 51(2):257–81. [DOI] [PubMed] [Google Scholar]
- 4.Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Veterinary medicine international 2011; 2011:457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell J, Galloway TS, Abo-Zaid G, Melzer D, Depledge MH, Osborne NJ. High urinary tungsten concentration is associated with stroke in the National Health and Nutrition Examination Survey 1999–2010. PloS one 2013; 8(11):e77546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Current diabetes reports 2013; 13(6):831–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS, Kim YJ, Seo YR. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. Journal of cancer prevention 2015; 20(4):232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulin JJ, Wild P, Romazini S, Lasfargues G, Peltier A, Bozec C, et al. Lung cancer risk in hard-metal workers. American journal of epidemiology 1998; 148(3):241–8. [DOI] [PubMed] [Google Scholar]
- 9.Langie SA, Koppen G, Desaulniers D, Al-Mulla F, Al-Temaimi R, Amedei A, et al. Causes of genome instability: the effect of low dose chemical exposures in modern society. Carcinogenesis 2015; 36 Suppl 1:S61–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi H, Li C, Ren F, Zhang H, Zhang L. Telomere, aging and age-related diseases. Aging clinical and experimental research 2013; 25(2):139–46. [DOI] [PubMed] [Google Scholar]
- 11.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ (Clinical research ed) 2014; 349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circulation research 2004; 94(5):575–84. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Zhu Y, Lin J, Matsuguchi T, Blackburn E, Zhang Y, et al. Short leukocyte telomere length predicts risk of diabetes in american indians: the strong heart family study. Diabetes 2014; 63(1):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Seminars in cancer biology 2011; 21(6):349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafie N, Golpour Hamedani S, Barak F, Safavi SM, Miraghajani M. Dietary patterns, food groups and telomere length: a systematic review of current studies. European journal of clinical nutrition 2017; 71(2):151–8. [DOI] [PubMed] [Google Scholar]
- 16.Mundstock E, Zatti H, Louzada FM, Oliveira SG, Guma FT, Paris MM, et al. Effects of physical activity in telomere length: Systematic review and meta-analysis. Ageing research reviews 2015; 22:72–80. [DOI] [PubMed] [Google Scholar]
- 17.Fillman T, Shimizu-Furusawa H, Ng CF, Parajuli RP, Watanabe C. Association of cadmium and arsenic exposure with salivary telomere length in adolescents in Terai, Nepal. Environmental research 2016; 149:8–14. [DOI] [PubMed] [Google Scholar]
- 18.Ameer SS, Xu Y, Engstrom K, Li H, Tallving P, Nermell B, et al. Exposure to Inorganic Arsenic Is Associated with Increased Mitochondrial DNA Copy Number and Longer Telomere Length in Peripheral Blood. Frontiers in cell and developmental biology 2016; 4:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Engstrom K, Vahter M, Broberg K. Arsenic exposure through drinking water is associated with longer telomeres in peripheral blood. Chemical research in toxicology 2012; 25(11):2333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Roy S, Tong L, Argos M, Jasmine F, Rahaman R, et al. Arsenic exposure, telomere length, and expression of telomere-related genes among Bangladeshi individuals. Environmental research 2015; 136:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee D, Bhattacharjee P, Sau TJ, Das JK, Sarma N, Bandyopadhyay AK, et al. Arsenic exposure through drinking water leads to senescence and alteration of telomere length in humans: A case-control study in West Bengal, India. Molecular carcinogenesis 2015; 54(9):800–9. [DOI] [PubMed] [Google Scholar]
- 22.Borghini A, Faita F, Mercuri A, Minichilli F, Bustaffa E, Bianchi F, et al. Arsenic exposure, genetic susceptibility and leukocyte telomere length in an Italian young adult population. Mutagenesis 2016; 31(5):539–46. [DOI] [PubMed] [Google Scholar]
- 23.Zota AR, Needham BL, Blackburn EH, Lin J, Park SK, Rehkopf DH, et al. Associations of cadmium and lead exposure with leukocyte telomere length: findings from National Health and Nutrition Examination Survey, 1999–2002. American journal of epidemiology 2015; 181(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grau-Perez M, Kuo CC, Gribble MO, Balakrishnan P, Jones Spratlen M, Vaidya D, et al. Association of Low-Moderate Arsenic Exposure and Arsenic Metabolism with Incident Diabetes and Insulin Resistance in the Strong Heart Family Study. Environmental health perspectives 2017; 125(12):127004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olmedo P, Grau-Perez M, Fretts A, Tellez-Plaza M, Gil F, Yeh F, et al. Dietary determinants of cadmium exposure in the Strong Heart Family Study. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2017; 100:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. American journal of epidemiology 1990; 132(6):1141–55. [DOI] [PubMed] [Google Scholar]
- 27.North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, et al. Genetic and Environmental Contributions to Cardiovascular Disease Risk in American Indians: The Strong Heart Family Study. American journal of epidemiology 2003; 157(4):303–14. [DOI] [PubMed] [Google Scholar]
- 28.Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Analytical methods : advancing methods and applications 2012; 4(2):406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic acids research 2002; 30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods 2010; 352(1– 2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Dibetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care 2011; 34 Suppl 1:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 1999; 130(6):461–70. [DOI] [PubMed] [Google Scholar]
- 33.Scinicariello F, Buser MC. Urinary antimony and leukocyte telomere length: An analysis of NHANES 1999–2002. Environmental research 2016; 150:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menke A, Casagrande S, Cowie CC. Leukocyte telomere length and diabetes status, duration, and control: the 1999–2002 National Health and Nutrition Examination Survey. BMC endocrine disorders 2015; 15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber KA, Heaphy CM, Rohrmann S, Gonzalez B, Bienstock JL, Agurs-Collins T, et al. Influence of In Utero Maternal and Neonate Factors on Cord Blood Leukocyte Telomere Length: Clues to the Racial Disparity in Prostate Cancer? Prostate cancer 2016; 2016:3691650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 2002; 21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 37.ATSDR. Public Health Statement of Arsenic. Agency for toxic substances and disease registry 2007; (https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3).
- 38.European Food Safety Authority. EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on Arsenic in Food. EFSA Journal 2009; 7(10):1351 [ 198 pp.]. doi:10.2903/j.efsa.2009.1351 . 10.2903/j.efsa.2009.1351http://www.efsa.europa.eu/en/scdocs/scdoc/1351.htm. http://www.efsa.europa.eu/en/scdocs/scdoc/1351.htm [accessed 12 December 2011] 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ATSDR. Public Health Statement of Cadmium. Agency for toxic substances and disease registry 2012. (https://www.atsdr.cdc.gov/csem/csem.asp?csem=6&po=12).
- 40.Olmedo P, Pla A, Hernandez AF, Barbier F, Ayouni L, Gil F. Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environment international 2013; 59:63–72. [DOI] [PubMed] [Google Scholar]
- 41.Bolt AM, Mann KK. Tungsten: an Emerging Toxicant, Alone or in Combination. Current environmental health reports 2016; 3(4):405–15. [DOI] [PubMed] [Google Scholar]
- 42.Lemus R, Venezia CF. An update to the toxicological profile for water-soluble and sparingly soluble tungsten substances. Critical reviews in toxicology 2015; 45(5):388–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ATSDR. Public Health Statement of Tungsten. Agency for toxic substances and disease registry 2005; (https://www.atsdr.cdc.gov/phs/phs.asp?id=804&tid=157).
- 44.Pawlas N, Plachetka A, Kozlowska A, Broberg K, Kasperczyk S. Telomere length in children environmentally exposed to low-to-moderate levels of lead. Toxicology and applied pharmacology 2015; 287(2):111–8. [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Okuka M, McLean M, Keefe DL, Liu L. Telomere susceptibility to cigarette smoke-induced oxidative damage and chromosomal instability of mouse embryos in vitro. Free radical biology & medicine 2010; 48(12):1663–76. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Okuka M, Lu W, Tsibris JC, McLean MP, Keefe DL, et al. Telomere shortening and DNA damage of embryonic stem cells induced by cigarette smoke. Reproductive toxicology (Elmsford, NY) 2013; 35:89–95. [DOI] [PubMed] [Google Scholar]
- 47.Guilbert C, Kelly AD, Petruccelli LA, Lemaire M, Mann KK. Exposure to tungsten induces DNA damage and apoptosis in developing B lymphocytes. Leukemia 2011; 25(12):1900–4. [DOI] [PubMed] [Google Scholar]
- 48.Kelly AD, Lemaire M, Young YK, Eustache JH, Guilbert C, Molina MF, et al. In vivo tungsten exposure alters B-cell development and increases DNA damage in murine bone marrow. Toxicological sciences : an official journal of the Society of Toxicology 2013; 131(2):434–46. [DOI] [PubMed] [Google Scholar]
- 49.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free radical biology & medicine 2004; 37(12):1921–42. [DOI] [PubMed] [Google Scholar]
- 50.Galaris D, Evangelou A. The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Critical reviews in oncology/hematology 2002; 42(1):93–103. [DOI] [PubMed] [Google Scholar]
- 51.Liu S, Sun Q, Wang F, Zhang L, Song Y, Xi S, et al. Arsenic induced overexpression of inflammatory cytokines based on the human urothelial cell model in vitro and urinary secretion of individuals chronically exposed to arsenic. Chemical research in toxicology 2014; 27(11):1934–42. [DOI] [PubMed] [Google Scholar]
- 52.Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Pro-inflammatory properties of cadmium. Acta biochimica Polonica 2012; 59(4):475–82. [PubMed] [Google Scholar]
- 53.Zhang TC, Schmitt MT, Mumford JL. Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis 2003; 24(11):1811–7. [DOI] [PubMed] [Google Scholar]
- 54.Ferrario D, Collotta A, Carfi M, Bowe G, Vahter M, Hartung T, et al. Arsenic induces telomerase expression and maintains telomere length in human cord blood cells. Toxicology 2009; 260(1–3):132–41. [DOI] [PubMed] [Google Scholar]
- 55.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 1999; 96(5):701–12. [DOI] [PubMed] [Google Scholar]
- 56.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science (New York, NY) 1994; 266(5193):2011–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


