Abstract
OBJECTIVES:
Congenital human cytomegalovirus (cCMV) is a leading cause of pediatric hearing loss. Recent literature has suggested that valganciclovir (VGCV) therapy can improve hearing outcomes. The objective of this study was to evaluate the long-term hearing outcomes among symptomatic CMV patients treated with VGCV.
METHODS:
A retrospective chart review of symptomatic CMV patients treated with VGCV was completed. The primary endpoint was the change in best ear hearing scores prior to treatment and after follow-up audiograms. A paired-sample t-test was used to evaluate the data.
RESULTS:
A total of 16 children were included in the study and participants were followed for an average of 3.2 years. There was a measurable worsening, but not a statistically significant change in the best ear hearing scores, where the mean change was 11.9dB (p-value=0.070). However, 14/16 patients (87.5%, p-value<0.001) were found to have clinically significant worsening of hearing. The mean change in hearing scores for the left and right ear was 14.2 dB (p-value=0.023) and 15.5 dB (p-value=0.032), respectively. Mean elapsed time for progressive loss was 2.6±0.2 years. When comparing the better or worse ear, there was no pattern for which ear deteriorated earlier or more frequently.
CONCLUSIONS:
Our data did show a measurable, but not a statistically significant worsening outcome in best ear hearing. There was a significant change in both left and right ear hearing. Our results suggest that VGCV may provide only a short-term improvement in hearing outcomes; however, these preliminary post-hoc findings suggest the need for a more rigorous evaluation.
Keywords: CMV, Sensorineural Hearing Loss, Infection, Pediatric Otolaryngology
1. INTRODUCTION:
Congenital human cytomegalovirus (cCMV) is a Herpes virus associated with intellectual impairment, sensorineural hearing loss (SNHL) and multisystem organ failure in children. However, a majority of children with cCMV infection have no significant clinical findings at birth or long-term disabilities related to the infection. It is a global health problem affecting approximately 0.3-1.7 % of all newborns [1,2]. The infection can be asymptomatic or cause severe symptomatic disease, which in part is attributed to when the infection occurred during pregnancy [3]. Among children with severe disease associated with cCMV infection, approximately 30-50% of those children will have sensorineural hearing loss, which has been found to be progressive and fluctuating in nature [4,5]. In fact, cCMV is a leading non-genetic cause of SNHL worldwide [1].
Unlike many forms of SNHL, cCMV may be treatable. The antiviral drug valganciclovir (VGCV) has been proposed to improve hearing, speech, and language outcomes associated with this infection [6]. A recent publication from the CASG (Collaborative Antiviral Study Group) reported that a six-month course of VGCV therapy provides a modest improvement in hearing and neurocognitive outcomes when compared to a shorter six-week course of therapy [7]. While this data is promising, there is little information regarding the long-term hearing outcomes following VGCV therapy. Within our own institution, we have noticed that some children with symptomatic cCMV previously treated with VGCV will go on to develop worsening hearing loss years later. These findings have prompted a more in-depth evaluation of the hearing outcomes among these patients to determine what the long-term hearing outcomes are for children with symptomatic cCMV-related disease.
2. MATERIALS AND METHODS:
A retrospective chart review of symptomatic cCMV patients treated with VGCV was completed. Patients were deemed symptomatic if they had one or more of the following conditions related to their cCMV infection: thrombocytopenia, petechiae, hepatomegaly, splenomegaly, intrauterine growth restriction, hepatitis, microcephaly, intracranial calcifications, abnormal cerebrospinal fluid indexes, or chorioretinitis. All patients were confirmed to have cCMV infection through urine or saliva specimens within the first three weeks of life, or dried-blood spot testing as part of the metabolic screening during the first days of life. All infants diagnosed with symptomatic congenital cytomegalovirus infection were offered VGCV therapy. This recommendation is based on two randomized clinical trials that demonstrated a neurocognitive and hearing benefit from this treatment [7,11], Children were initially offered a six-week course at 16 mg/kg bid. After the 2015 Kimberlin et al. paper demonstrated greater efficacy with a longer treatment course of six months, this approach was offered [7]. The outcome index of treatment was primarily speech and language, specifically receptive and language composite scores. While the therapeutic purpose of VGCV therapy was the ability to have some recovery or improvement in hearing.
Multiple outcomes were collected from patients with symptomatic cCMV seen in our pediatric otolaryngology clinics at Primary Children’s Hospital from 2003-2017. A baseline brainstem auditory evoked response (ABR) was performed prior to the initiation of any therapeutic intervention. A summary of the auditory testing included this baseline ABR, otoacoustic emission (OAE) if feasible, distortion product otoacoustic emissions (DPOE) testing and tympanometry for patient younger than six months of age. For older children, Visual Reinforcement Audiometry (VRA) or Conditioned Play Audiometry (CPA) was used. A Vivosonic was used for ABR testing. For behavior evaluations the following devices were utilized: GSI TympStar Pro for tympanometry, Biologic Aud-X for OAE, and a GSI Audio Star Pro for audiometry.
Patients were then followed with regular audiologic assessments, with an average of 5.8 assessments being performed on each study participant. The primary endpoint was the change between baseline and follow-up in the hearing scores corresponding to the best ear at each audiologic follow-up time point. This measure was used as a functional assessment in daily living. For example, a child with mild hearing loss in one ear and profound loss in the contralateral ear will function like a child with a mild hearing impairment [8]. Secondary endpoints included change in mean thresholds for both the right and left ear, time to hearing deterioration after treatment, and the proportion of best versus worse ear that deteriorates first. The hearing score in each ear is defined by averaging the minimum response levels at two frequencies (2.0 kHz and 4.0 kHz). Clinically significant worsening of hearing was defined as the occurrence of either: a) 10 dB or greater increase in minimum response level (MRL) at both 2 and 4 kHz, b) 15 dB or greater increase at either frequency, or c) cochlear implantation. A paired-sample t-test was used to evaluate differences in the mean change of hearing scores. The institutional review board (IRB) at our institution approved our study protocol.
3. RESULTS:
Sixteen symptomatic cCMV infected children who underwent treatment with VGCV were included in this study, of which 10 (62.5%) patients were female and 6 (37.5%) were male. A total of nine patients were started on therapy before one month of age. While seven patients were started on therapy after one month of age, with an average start of therapy at 13.3 months of age among those patients. Approximately one-third of children had two or more of the major symptoms associated with cCMV, with neurologic findings including gliosis, intracranial calcifications, microcephaly, cortical dysplasia, cystic changes and demyelination. None of the patients included in the study had normal hearing at baseline. All patients were treated with VGCV therapy and the average duration of treatment was 92.7 days. The breakdown on the length of therapy is as follows: six patients less than six weeks therapy, seven patients between six weeks and six months, and three patients for more than six months. Average audiological follow-up was 3.2 years (range 0.3-10.0 years). Approximately 37.5% of patients were followed for at least five years. A summary of the patient characteristics can be seen in Table 1.
TABLE 1:
Characteristics of Study Participants
| Characteristic | |
|---|---|
| Age VGCV Started | No (%) |
| < 1 month | 8 (50.0%) |
| > 1 month | 8 (50.0%) |
| Length of VGCV Treatment | No (%) |
| <6 weeks | 6 (37.5%) |
| >6 weeks, <6 months | 7 (43.8%) |
| 6 months | 3 (18.7%) |
| Method of cCMV diagnosis | No (%) |
| Urine | 9 (56.2%) |
| Saliva | 7 (43.7%) |
| Dried blood spot | 4 (25%) |
| Extent of CMV disease | No (%) |
| Thrombocytopenia | 3 (18.7%) |
| IUGR | 4 (25%) |
| Hepatitis | 4 (25%) |
| CNS Involvement | 10 (62.5%) |
| Microcephaly | 2 (12.5%) |
| >2 Symptoms | 5 (31.2%) |
| Neuroimaging results | No (%) |
| Normal | 7 (43.8%) |
| Abnormal | 9 (56.2%) |
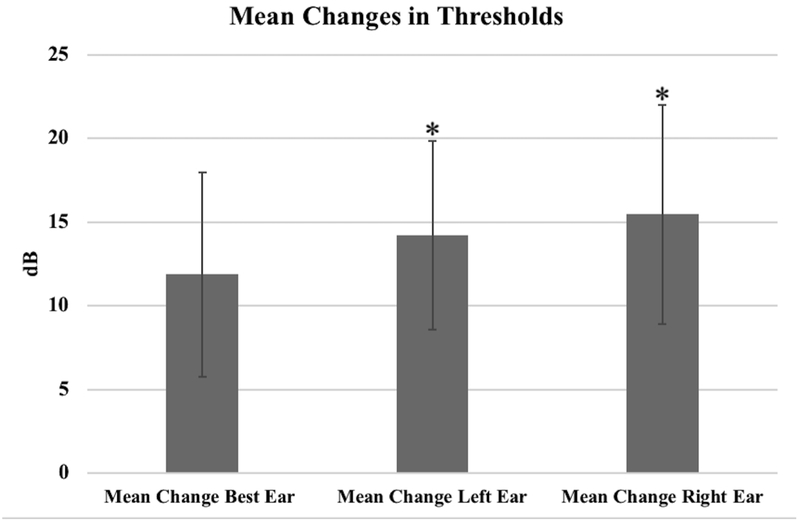
There was a measurable worsening, but not a statistically significant change in the baseline and follow-up best ear hearing scores, where the mean worsening change was 11.9 dB (95% CI: −1.11 – 24.86, p-value=0.070). However, of the 16 patients, 14 (87.5%) were found to have clinically significant worsening in hearing. The change in total hearing scores for the right ear was found to have a mean change of 15.5 dB (95% CI: 1.52 – 29.42, p-value=0.032). The change in total hearing scores for the left ear was found to have a mean change of 14.2 dB (95% CI: 2.25 – 26.19, p-value=0.023). Mean changes for thresholds are shown in Figure 1.
FIGURE 1:
The mean changes for the best ear, left ear, and right ear thresholds are demonstrated. Asterisk indicates statistically significant mean change at 0.05 level.
The mean elapsed time for progression of hearing loss was 2.6 ±0.2 years. The youngest child to develop progressive hearing loss was 5 months of age, while the oldest was 4.3 years of age. Two patients eventually underwent cochlear implantation (CI), although ten more would be considered candidates for CI based on the patients meeting criteria for profound hearing loss. When examining the 11 cases where the better or worse ear could be compared over time, the following results were found: no change (n=2), worsening at the same time (n=3), better ear deteriorated first (n=3), and worse ear deteriorated first (n=3). Stratification based on duration of antiviral therapy revealed that 100% (6/6), 71.4% (5/7), and 100% (3/3) developed worsening hearing thresholds when treated for less than 6 weeks, between 6 weeks and 6 months, and greater than 6 months, respectively. Stratification based on age when therapy was started showed that 100% (8/8) and 75% (6/8) developed worsening hearing thresholds for those who initiated therapy when less than one month of age and for those older than one month of age, respectively. An overview of the individual hearing outcomes of each patient is demonstrated in Table 2.
TABLE 2:
Hearing Outcomes for All Study Participants
| Subject | Overall Mean Change dB |
Mean Change left ear dB |
Mean Change right ear dB |
Cochlear Implant |
Change 2000 Hertz |
Change 4000 Hertz |
Worsening Hearing? |
|---|---|---|---|---|---|---|---|
| 1 | −12.5 | 15.0 | −12.5 | Candidate | 10 | −10 | Yes |
| 2 | 12.5 | 12.5 | 22.5 | Candidate | 10 | 15 | Yes |
| 3 | 20.0 | 0.0 | 20.0 | No | 20 | 0 | Yes |
| 4 | 67.5 | 67.5 | 27.5 | Yes | 70 | 65 | Yes |
| 5 | −20.0 | −22.5 | −17.5 | No | −10 | −25 | No |
| 6 | 25.0 | 25.0 | 0.0 | Yes | 20 | 30 | Yes |
| 7 | 15.0 | 15.0 | 0.0 | Candidate | 15 | 15 | Yes |
| 8 | 0.0 | 10.0 | 0.0 | Candidate | 0 | 0 | Yes |
| 9 | 10.0 | 10.0 | 15.0 | No | 5 | 15 | Yes |
| 10 | −7.5 | −10.0 | −7.5 | Candidate | −95 | −5 | Yes |
| 11 | 57.5 | 57.5 | 45.0 | Candidate | 60 | 55 | Yes |
| 12 | 0.0 | 0.0 | 80.0 | Candidate | 0 | 0 | Yes |
| 13 | 0.0 | 0.0 | 20.0 | Candidate | 0 | 0 | Yes |
| 14 | 7.5 | 7.5 | 25.0 | Candidate | 5 | 10 | Yes |
| 15 | 30.0 | 15.0 | 45.0 | Candidate | 45 | 30 | Yes |
| 16 | −15.0 | 25.0 | −15.0 | No | −15 | −15 | No |
Individual characteristics on hearing scores for all 16 participants. Negative scores indicate improvement of baseline hearing scores to follow-up, while positive scores indicate worsening of baseline hearing scores to follow-up.
4. DISCUSSION:
This study is the first to our knowledge to report follow-up of symptomatic cCMV infected children treated with VGCV beyond two years, with some patients being followed for almost ten years. Results of our study demonstrated that 14 of 16 participants had worsening of their hearing. Our primary outcome of the mean change in hearing scores for the best ear from baseline was not statistically significant. However, the change in total hearing for the right and left ear was found to be statistically significant, suggesting progressive hearing loss despite antiviral therapy.
The natural history of untreated severely affected cCMV children suggests that many will develop progressive loss [9]. Dahle et al. compared the hearing outcomes of 209 symptomatic cCMV infected children to 651 asymptomatic cCMV infected children [10]. SNHL occurred in 48 (7.4%) of asymptomatic and 85 (40.7 %) of symptomatic cCMV infected children. Thirty-seven percent of the symptomatic cCMV infected children developed delayed onset and progressive SNHL. The age of presentation ranged from six months to 16.4 years, which highlights the need for continued and long-term audiologic surveillance of these children.
Studies reporting efficacy of antiviral therapy for CMV induced SNHL have included only symptomatically infected infants with a relatively short follow-up period [7,11]. The National Institute of Allergy and Infectious Disease CASG presented a large, randomized controlled study of six weeks of intravenous ganciclovir compared with no treatment in infants with central nervous system involvement and less than 30 days of age [11]. They found that infants treated with ganciclovir were more likely to have stable hearing or even hearing improvement compared to those infants who received no treatment. None of the treated infants had hearing deterioration at six months compared to 41% of the untreated infants. At the one-year follow-up, 21% infants in the treated group had some hearing deterioration in the better ear compared with 68% in the untreated group. The CASG group recently compared six-month VGC versus six-week therapy [7]. Results showed that a longer duration of antiviral therapy does not further improve hearing function at six months, but improves hearing outcomes at 12 and 24 months as compared with a six weeks therapy (73% vs. 57%, p-value=0.01; 77% vs. 64%, p-value=0.04). At 24 months the six-month group also had better neurodevelopmental scores on the language composite component of the Bayley-III (p-value=0.004). Amir et al. reported their results in 23 infants with symptomatic cCMV treated with ganciclovir for six weeks followed by oral VGCV to age 12 months [12]. None of the 25 children with normal hearing developed worsening of their hearing at one year of age. For the 21 children presenting with hearing loss, twelve (57%) had improved hearing, eight (38%) had no change and one (5%) had worse thresholds at one-year follow-up.
While this study provides valuable information regarding a longer period of hearing outcomes among symptomatic cCMV patients previously treated with VGCV, there are limitations to our study. First, we had a small sample size of 16 patients. More severely affected and treated CMV infected children will need to be evaluated longitudinally. However, it is important to recognize that symptomatic CMV is rare, which limits patient recruitment for this study. Another limitation to our study is that nearly half of the cohort underwent antiviral therapy after they were one month of age. The CASG trials have demonstrated efficacy only for infants under one month of age. Nonetheless, all of the children treated with VGCV before one month of age in our study showed progressive hearing loss. There was some variability in antiviral treatment duration. Some infants underwent shorter than six months of antiviral therapy because that was our institutional standard of care prior to the 2015. After 2015, we started to implement six months of VGCV therapy. Other reasons for such variation in therapy included patient compliance, parent concerns regarding the medication and adverse effects, and rarely because of a persistently low absolute neutrophil count or elevated liver enzymes. Nevertheless, we did not detect a significant difference in outcomes based on duration of therapy. Variation in follow-up is also a limitation, as the follow-up time ranged from 0.26 – 9.98 years. This variation is unfortunately the nature of clinical practice, as some patients may not return for follow-up. We also serve a particularly large group of patients who are from out-of-state, so these families may opt for follow-up that is closer to home. Nonetheless, we may be underestimating the hearing loss progression in those followed for a shorter duration of time. Finally, there was no control group in this study to better characterize the effect VGCV has or does not have on long-term hearing outcomes. Furthermore, since this study evaluates symptomatic patients, there are other factors that could contribute to hearing loss, including neurologic abnormalities. Regardless of these limitations, we can conclude that a significant proportion of symptomatic cCMV infected children are at risk for progressive SNHL despite undergoing antiviral therapy. These results highlight the importance of continued surveillance with audiological testing for at least four and a half years after VGCV therapy, as 4.3 years was the last detected hearing loss among this cohort of patients. While having all patients followed for this duration of time would have strengthened this recommendation, over one-third of patients in this study were followed for at least five years. Furthermore, both ears should be tested since either ear is at risk to worsen. There is also a need for a novel and perhaps longer duration therapy to prevent the long-term effects of cCMV.
5. CONCLUSIONS:
Our study showed a measurable, but not a statistically significant worsening outcome in the best ear change between baseline and follow-up hearing scores. However, our secondary measures showed a statistically significant worsening outcome, including change in total hearing for the right and left ear. The proportion of children with worsening hearing thresholds suggests that VGCV may provide only a short-term improvement in hearing outcomes. These preliminary findings suggest the need for continued close hearing surveillance of these children.
Acknowledgments
Source of Financial Support or Funding: This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764).
Footnotes
Conflicts of Interest: None.
Presented at the American Society of Pediatric Otolaryngology at COSM. National Harbor, MD; April 18-22, 2018. 3rd place recipient of the 2018 Charles F. Ferguson Clinical Research award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK, The “silent” global burden of congenital cytomegalovirus, Clin. Microbiol. Rev 26 (2013) 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cannon MJ, Grosse S, Fowler K, The Epidemiology and Public Health Impact of Congenital Cytomegalovirus Infection, Caister Academic Press, Mainz, 2013. [Google Scholar]
- [3].Trincado DE, Rawlinson WD, Congenital and perinatal infections with cytomegalovirus, Paediatr. Child Health 37 (2001) 187–192. [DOI] [PubMed] [Google Scholar]
- [4].Grosse SD, Ross DS, Dollard SC, Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment, J. Clin. Virol 42 (2008) 57–62. [DOI] [PubMed] [Google Scholar]
- [5].Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review, Pediatrics 134 (2014) 972–982. [DOI] [PubMed] [Google Scholar]
- [6].Schleiss MR. Congenital Cytomegalovirus Infection: Update on Management Strategies, Curr. Treat Options Neurol. 10 (2008) 86–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A et al. , Valganciclovir for symptomatic congenital cytomegalovirus disease, N. Engl. J. Med 372 (2015) 933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Habilitation and Rehabilitation. Little, Brown and Company, Boston, 1976. [Google Scholar]
- [9].H James S, Kimberlin DW, Advances in the prevention and treatment of congenital cytomegalovirus infection, Curr. Opin. Pediatr 28 (2016) 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF, Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J. Am. Acad. Audiol 11 (2000) 283–290. [PubMed] [Google Scholar]
- [11].Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ et al. , Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial, J. Pediatr 143 (2003) 16–25. [DOI] [PubMed] [Google Scholar]
- [12].Amir J, Wolf DG, Levy I, Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur. J. Pediatr 169 (2010) 1061–1067. [DOI] [PubMed] [Google Scholar]