Abstract
Background
Venous thromboembolism is responsible for a significant number of hospital readmissions each year, particularly among post-surgical cohorts. Because early and indiscriminate VTE prophylaxis carries catastrophic consequences in post-craniotomy cohorts, identifying factors associated with a high risk for thromboembolic complications is important for guiding postoperative management.
Objective
To determine VTE incidence in patients undergoing non-emergent craniotomy and to evaluate for factors, which predict 30- and 90-day readmission with VTE.
Methods
The 2010–2014 cohorts of the Nationwide Readmissions Database were employed to generate a large heterogeneous craniotomy sample.
Results
There were 89,450 non-emergent craniotomies that met inclusion criteria. Within 30-days, 1513 patients (1.69%) were readmitted with VTE diagnoses; among them 678 (44.8%) had a diagnosis of DVT alone, 450 (29.7%) had PE alone and 385 (25.4%) had both. The corresponding 30-day DVT and PE incidences were 1.19% and 0.93%, respectively. In multivariate analysis, several factors were significantly associated with VTE readmission namely, craniotomy for tumor, corticosteroids, advanced age, greater length of stay and discharge to institutional care.
Conclusions
Craniotomies for tumor, corticosteroids, advanced age, prolonged length of stay and discharge to institutional care are significant predictors of VTE readmission. The implication of steroids, coupled with their ubiquity in neurosurgery, makes them a potentially modifiable risk factor and a prime target for VTE reduction in craniotomy cohorts. Furthermore, the fact that dose is proportional to VTE risk in the literature suggests that careful consideration should be given towards lowering regimens in situations where use of a lower dose might prove equally sufficient.
Keywords: venous thromboembolism (VTE), pulmonary embolism (PE), deep vein thrombosis (DVT), craniotomy, nationwide database, readmission, adult cohort
Introduction
Venous thromboembolism (VTE) refers to the pathologic entity encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE). Approximately 550,000 patients are affected in the United States each year, with an economic toll as high as $39.3 billion dollars 1,2. VTEs carry substantial patient-related morbidity, as evident from the need for long-term anticoagulation and its numerous attendant complications, longer inpatient stays, higher 30-day hospital readmission rates and the potential to delay adjunctive therapies that influence long-term survival (e.g. chemotherapy, radiation) 3. VTEs also result in higher overall mortality; for example, the risk of death within 30 days after being diagnosed with DVT and PE are estimated at 6% and 12%, respectively 4. VTEs are the second most common cause of prolonged inpatient stays and the third most common cause of excess mortality in hospitalized patients 5,6. Their perceived preventability 7,8, coupled with the aforementioned standings, have made them a target for quality improvement by the Centers for Medicare & Medicaid Services, such that pay-for-performance measures now link hospital reimbursement to VTE incidence.
Among surgical patients, those undergoing neurosurgery are thought to be particularly susceptible to thromboembolic phenomena 9,10. The risks are further increased by operations undertaken for malignancy, for those of prolonged duration or that have a resulting deficit with limb paresis or plegia 11. VTE rates in neurosurgery are highly variable, complicating anywhere from 1.7 to 34% of procedures 12–14. Despite early postoperative mobilization and widespread support for mechanical and chemical prophylaxis, VTEs remain a significant concern in neurosurgery. Part of the challenge is that chemoprophylaxis in patients who have undergone craniotomy is encumbered by concerns for catastrophic intracranial hemorrhage (ICH), particularly if administered within the first several postoperative days, a time when patients are most likely to remain bedridden and at high risk for VTE. Although several systematic reviews in neurosurgical cohorts have deemed chemoprophylaxis both safe and effective at reducing thromboembolic phenomena without added ICH-related morbidity 15–17, there are others which provide cautionary reminders that the risk-benefit profile is only slightly favorable 18. Studies evaluating VTE risk factors in craniotomy cohorts are therefore essential for identifying the most susceptible groups when balancing thromboembolic against hemorrhagic complications.
Here we employed the newly available Nationwide Readmissions Database (NRD) to characterize VTE readmission trends in patients who underwent non-emergent craniotomy. NRD is a pooled database of hospital admissions from numerous contributing states, in which patients are assigned unique linkage numbers that effectively allows them to be tracked for subsequent readmission. It has previously been employed in the literature to analyze readmission patterns for various neurosurgical pathologies 19–22. The goals of this investigation were: (1) to estimate VTE incidence among patients undergoing non-emergent craniotomy, and (2) to identify patient and hospital factors associated with VTE in order to establish high-risk features that could warrant early anticoagulation use.
Methods
Data Source
We used the 2010–2014 cohorts of the NRD for this study. This database represents all hospital discharges from 20–27 participating states, constituting roughly half of all discharges in the United States. NRD assigns anonymized identifiers to patients, which enables their tracking within a given state over the course of a calendar year.
Study Cohort
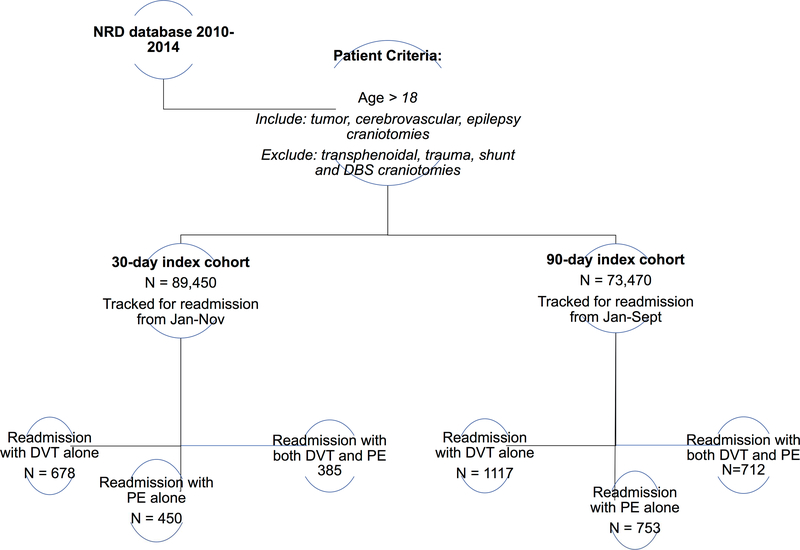
All adults > 18 years who underwent non-emergent craniotomy were included. Craniotomies for ventricular shunting, deep brain stimulation (DBS) and transphenoidal approaches were excluded because their cranial and dural openings are significantly smaller compared to standard neurosurgical operations that require more invasive access to pathology. Craniotomies for trauma were also excluded to isolate our cohort to controlled craniotomies where associated factors such as systemic injury, emergent treatment, and immobilization would not create a confounded and heterogeneous cohort. The final group comprised craniotomies for primarily three diagnoses: tumor, cerebrovascular conditions and epilepsy. Patients were extracted using a combination of International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9CM) diagnosis and procedure codes (Table 1).
Table 1.
Combination of International Classification of Diseases, 9th edition, diagnosis and procedures codes used to extract index cohort
| Craniotomy cohort | ICD-9 Diagnosis code | ICD-9 Procedure code | |
|---|---|---|---|
| Epilepsy | 345.x | 1.53, 1.52 | |
| Tumor | Benign | 192.1, 225.2, 237.6 | 1.51 |
| 225.1 | 4.01 | ||
| 237.0 | 7.61, 7.64 | ||
| 225.0 | 1.59 | ||
| Malignant | 191.0–191.9 | 1.53, 1.59 | |
| 198.3 | 1.59 | ||
| Vascular | Aneurysm | 430, 437.3 | 39.51 |
| AVM | 747.81 | 1.59 | |
| Moya-Moya disease | 437.5 | 39.28 | |
| Cerebroocclusive disease | 433.00, 433.10, 433.20, 433.30, 433.80, 433.90, 434.10, 434.90, 437.0, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 435.x, 437.1 | 39.28 | |
Patients with a personal history of thromboembolism (453.7x, 453.5x, V12.51, V12.55) or a diagnosis of VTE on index presentation were excluded from the original cohort. Additional exclusion criteria include those who died or were missing relevant data (e.g. length of stay or time to procedure). Based on this cohort, we determined readmission trends for VTE (451.1, 451.1x, 451.2, 451.81, 451.83, 451.9, 453.2, 453.3, 453.4x, 453.6, 453.8x, 453.9, 415.1, 415.1x) at both 30- and 90-days from index hospitalization.
Because the NRD only permits tracking patients within a single calendar year, appropriate cutoffs were designated to allow sufficient time to capture the relevant study parameters. As such, 30-day readmissions include only patients discharged from January to November while 90-day readmissions include January through September.
Figure 1 demonstrates study design.
Figure 1.
Schematic of Nationwide readmissions database (NRD) study design
Patient and Hospital Demographics
Numerous patient and hospital characteristics were evaluated for readmission with VTE. Hospital factors included: teaching status, bed size and annual craniotomy volume (high volume being defined as at or above 90th percentile). Patient factors included: age, gender, insurance payer, underlying comorbidities, length of stay and disposition on discharge. Age is a continuous variable in the NRD and was categorized into the following four cohorts for final analysis: 18–44, 45–59, 60–74 and ≥75. Additional variables of interest, chosen for their previously noted associations with VTE in the literature, were also evaluated, namely obesity (278.0, V85.3-V85.4), tobacco use (305.1, V15.82), diabetes (250.xx), chronic steroid use (V58.65), SIRS criteria (995.9x, 785.52), ventilator dependence (V46.1x), history of chemotherapy (V58.11, V87.41), and chronic lung disease.
Statistical Analysis
The clinical outcomes of this investigation were readmission for VTE after non-emergent craniotomy within 30- and 90-days. The NRD was queried using standard HCUP methodology for extracting readmission data. If multiple readmissions were identified for a single patient, only the first readmission was captured in data analysis. Multivariable models were built using two-level mixed-effects modeling accounting for clustering. Data were reported as odds ratios with 95% confidence intervals. All statistical analysis was performed using SAS 9.4 (Cary, NC). Significance was defined as p < 0.05.
Results
Patient and hospital baseline characteristics
Using the 2010–2014 cohorts of the NRD we identified 89,450 non-emergent craniotomies. The majority of surgeries were for tumor (81%, n = 72,571) followed by cerebrovascular conditions (18%, n = 15,937) and epilepsy (1%, n = 942). The median length of stay for all patients on index hospitalization was 5 days with a median cost of $98,063. There were slightly more females (56%, n = 50,451) than males (n = 44%, n = 38,999) in the index cohort. Most patients were between 45 and 74 years (69%, n = 62,382) and had at least one or more underlying comorbidities based on the Elixhauser index23 (68%). The majority had private (48%) or Medicare insurance (33%) and received care at hospitals designated with a teaching status (82%) or large bedsize (80%). See Table 2 for a summary of key demographic data.
Table 2.
Demographics of patients readmitted within 30 days of index craniotomy.
| Variables | N | % | |
|---|---|---|---|
| Diagnosis | Malignant Tumor | 960 | 63.45 |
| Benign Tumor | 409 | 27.03 | |
| Vascular | 139 | 9.19 | |
| Epilepsy | DS | DS | |
| Total | 1513 | 1.7 | |
| Age | 18–44 | 181 | 11.96 |
| 45–59 | 466 | 30.8 | |
| 60–74 | 637 | 42.1 | |
| >=75 | 229 | 15.14 | |
| Gender | Male | 744 | 49.17 |
| Female | 769 | 50.83 | |
| Primary insurance | Medicare | 630 | 41.64 |
| Medicaid | 182 | 12.03 | |
| Private insurance | 596 | 39.39 | |
| Self-pay | 43 | 2.84 | |
| No charge | DS | DS | |
| Other | 53 | 3.5 | |
| Hospital bedsize | Small | 87 | 5.75 |
| Medium | 209 | 13.81 | |
| Large | 1217 | 80.44 | |
| Teaching status | Teaching | 1233 | 81.49 |
| Non-Teaching | 280 | 18.51 | |
| Disposition | Routine | 723 | 47.79 |
| Short-term Hospital | 49 | 3.24 | |
| Transfer Other | 399 | 26.37 | |
| Home Health Care | 342 | 22.6 | |
| Against Medical Advice | DS | DS | |
| Volume | Above 90th percentile | 757 | 50.03 |
| <= 90th percentile (115 / year) | 756 | 49.97 | |
| Elixhauser comorbidity | Yes | 1154 | 76.27 |
| No | 359 | 23.73 | |
| Medical complication | Yes | 84 | 5.55 |
| No | 1429 | 94.45 | |
| Neurological complication | Yes | 329 | 21.74 |
| No | 1184 | 78.26 | |
| Obesity | Yes | DS | DS |
| No | 1510 | 99.8 | |
| Tobacco | Yes | 481 | 31.79 |
| No | 1032 | 68.21 | |
| Diabetes | Yes | 262 | 17.32 |
| No | 1251 | 82.68 | |
| Hypercoagulable state | Yes | DS | DS |
| No | 1510 | 99.8 | |
| Index Length of stay | 0–3 days | 303 | 20.03 |
| 4–5 days | 236 | 15.6 | |
| 6–10 days | 416 | 27.5 | |
| >=11 days | 558 | 36.88 | |
| Median household income for patient’s ZIP code, based on current year | 0–25 percentile | 350 | 23.13 |
| 26–50 percentile | 335 | 22.14 | |
| 51–75 percentile | 391 | 25.84 | |
| 76–100 percentile | 408 | 26.97 | |
| Steroid use | Yes | 43 | 2.84 |
| No | 1470 | 97.16 | |
| SIRS criteria | Yes | 26 | 1.72 |
| No | 1487 | 98.28 | |
| Hx of chemotherapy | Yes | 69 | 4.56 |
| No | 1444 | 95.44 | |
| Ventilator dependence | Yes | DS | DS |
| No | 1506 | 99.54 | |
| Chronic lung disease | Yes | 218 | 14.41 |
| No | 1295 | 85.59 | |
DS = Data suppressed in accordance with HCUP/NRD guidelines.
VTE incidence, readmission demographics
1513 patients were readmitted within 30 days of index craniotomy with a diagnosis of new VTE, corresponding to an incidence of 1.69%. Of those with VTE, 678 (44.8%) patients were diagnosed with DVT alone, 450 (29.7%) with PE alone and 385 (25.4%) with both DVT and PE. The 30-day DVT and PE incidences were thus determined to be 1.19% and 0.93%, respectively. The median time to 30-day readmission was 13 days with a median readmission cost of $44,527.
Within 90 days, there were 2582 readmissions with a diagnosis of VTE, corresponding to an incidence of 3.51%. There were 1117 (43.3%) patients diagnosed with DVT alone, 753 (29.2%) with PE alone and 712 (27.6%) with both. The 90-day DVT and PE incidences were therefore 2.49% and 1.99%, respectively. The median time to 90-day readmission was 32 days with a median readmission cost of $43,092.
See Table 3 for breakdown of VTE incidence by craniotomy cohort.
Table 3.
Breakdown of VTE events by indication for craniotomy
| 30-day readmission | 90-day readmission | |||
|---|---|---|---|---|
| Study Cohort | DVT (# events/rate) | PE (# events/rate) | DVT (# events/rate) | PE (# events/rate) |
| Vascular | 96 (0.60%) | 65 (0.41%) | 114 (0.87%) | 74 (0.57%) |
| Benign tumor | 287 (1.08%) | 239 (0.90%) | 362 (1.65%) | 265 (1.21%) |
| Malignant tumor | 675 (1.47%) | 529 (1.15%) | 1347 (3.57%) | 1124 (2.98%) |
Factors associated with VTE within 30- and 90 days
In multivariate analysis, several factors were associated with readmission for VTE at both 30-and 90 days. Relative to cerebrovascular operations, craniotomies for tumor had increased risk for VTE at both 30 days (OR 2.01–2.43, p < 0.0001) and 90 days (OR 2.08–4.36, p < 0.0001). Increased age correlated with higher VTE risk, with the elderly (≥ 75 years) having close to, or twice, the odds of a thromboembolic complication compared to those aged 18–44 years (OR 1.69–2.03, p < 0.0001). Other patient-related characteristics predictive of VTE include: male gender, length of stay on index hospitalization and non-routine hospital disposition (e.g. transfer to another short-term hospital or skilled nursing facility).
Steroid use was a significant predictor of VTE at 30 days (OR 1.41, p = 0.03), however, the relationship was not significant at 90 days. In like manner, presence of one or more underlying comorbidities as determined by the Elixhauser index had variable association with VTE risk based on time, being significant at 90 days only (OR 1.25, p < 0.0001).
See Tables 4 and 5 for details.
Table 4.
Predictors of 30-day readmissions for VTE by multivariate analysis using surveyadjusted logistic regression
| Variables | Odds Ratio |
95% CI | p-value | ||
|---|---|---|---|---|---|
| Craniotomy cohort | Malignant Tumor | 2.431 | 2.021 | 2.923 | <.0001 |
| Benign Tumor | 2.015 | 1.654 | 2.454 | <.0001 | |
| Vascular | Ref | ||||
| Epilepsy | 0.863 | 0.352 | 2.117 | 0.7468 | |
| Age | 18–44 | Ref | |||
| 45–59 | 1.364 | 1.146 | 1.622 | 0.0005 | |
| 60–74 | 1.613 | 1.36 | 1.913 | <.0001 | |
| >=75 | 1.69 | 1.375 | 2.077 | <.0001 | |
| Gender | Male | 1.15 | 1.037 | 1.276 | 0.0082 |
| Female | Ref | ||||
| Disposition | Routine | Ref | |||
| Short-term Hospital | 2.623 | 1.939 | 3.549 | <.0001 | |
| Transfer Other | 1.736 | 1.506 | 2 | <.0001 | |
| Home Health Care | 1.327 | 1.155 | 1.524 | <.0001 | |
| Against Medical Advice | * | ||||
| Index Length of stay | 0–3 days | Ref | |||
| 4–5 days | 1.232 | 1.037 | 1.465 | 0.0178 | |
| 6–11 days | 1.526 | 1.305 | 1.784 | <.0001 | |
| >=12 days | 1.959 | 1.674 | 2.291 | <.0001 | |
| Steroid use | Yes | 1.408 | 1.034 | 1.917 | 0.03 |
| No | Ref | ||||
OR cannot be computed due to small sample size
Table 5.
Predictors of 90-day readmissions for VTE by multivariate analysis using surveyadjusted logistic regression
| Variables | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Craniotomy cohort | Malignant Tumor | 4.357 | 3.683 | 5.156 | <.0001 |
| Benign Tumor | 2.083 | 1.733 | 2.503 | <.0001 | |
| Vascular | Ref | ||||
| Epilepsy | 1.007 | 0.443 | 2.29 | 0.9872 | |
| Age | 18–44 | Ref | |||
| 45–59 | 1.531 | 1.325 | 1.77 | <.0001 | |
| 60–74 | 1.895 | 1.64 | 2.188 | <.0001 | |
| >=75 | 2.027 | 1.709 | 2.405 | <.0001 | |
| Gender | Male | 1.111 | 1.025 | 1.205 | 0.0103 |
| Female | Ref | ||||
| Disposition | Routine | Ref | |||
| Short-term Hospital | 1.836 | 1.398 | 2.411 | <.0001 | |
| Transfer Other | 1.438 | 1.284 | 1.61 | <.0001 | |
| Home Health Care | 1.218 | 1.095 | 1.356 | 0.0003 | |
| Against Medical Advice | 0.61 | 0.149 | 2.487 | 0.4903 | |
| Elixhauser comorbidity | Yes | 1.254 | 1.13 | 1.391 | <.0001 |
| No | Ref | ||||
| Index Length of stay | 0–3 days | Ref | |||
| 4–5 days | 1.293 | 1.134 | 1.475 | 0.0001 | |
| 6–11 days | 1.512 | 1.341 | 1.705 | <.0001 | |
| >=12 days | 1.85 | 1.635 | 2.094 | <.0001 | |
| Chronic lung disease | Yes | 0.865 | 0.772 | 0.969 | 0.0121 |
| No | Ref | ||||
Discussion
Venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE), accounts for a significant number of hospital readmissions each year. Their assumed preventability, potential for high mortality and exorbitant costs have resulted in a widespread campaign for primary prevention and risk reduction, particularly within the perioperative window. Consistent with this effort, the Surgical Care Improvement Project (SCIP) was implemented in 2006 in the hopes of reducing the incidence of preventable complications like VTEs. This has dramatically transformed the healthcare landscape so much so that hospital reimbursement is increasingly being tied to certain quality of care metrics, one of which corresponds to VTE rates. Because institutional referral streams and their ultimate financial viability depend on meeting these imposed measures, hospitals have devised surgical protocols addressing VTE prophylaxis. However, no guidelines exist for risk stratification, particularly in neurosurgical populations where indiscriminate chemoprophylaxis can have catastrophic hemorrhagic consequences. Here we sought to identify modifiable risk factors associated with the development of postoperative VTE in cranial neurosurgery in order to optimize risk profiling for susceptible groups.
VTE rates are known to be higher in surgical cohorts due to venous stasis from general anesthesia, intraoperative immobilization, limited postoperative mobility and the inevitable activation of inflammatory and coagulation pathways that occurs in response to tissue injury 24. This propensity for thromboembolism is purported to be even higher in patients undergoing neurosurgery 10,11,25. A retrospective study of 38,058 spine and cranial neurosurgery patients from the 2006–2011 NSQIP database found an overall VTE incidence of 1.7% within 30 days of follow-up 14. Another investigation of 10,477 craniotomy patients from 2011–2012 reported a VTE incidence of 3.2%, with rates of DVT and PE of 2.4% and 1.3%, respectively 26. Routine DVT surveillance studies would seem to corroborate these numbers: of 1277 consecutive neurosurgical patients who underwent routine admission and weekly lower-extremity venous duplex ultrasonography surveillance, the overall DVT incidence was 2.8% 27. In our study, the overall VTE rate at 30 days was 1.7%, which is in line with aforementioned values. The rates of DVT and PE at 30 days were 1.19 % and 0.93%, respectively. Although our DVT rates were slightly lower than some of these reported values, this discrepancy likely has to do with differences in study methodology (e.g. length of follow-up, composition of patient cohort, DVT detection by routine surveillance versus workup triggered by symptomatology, among others). Alternately, it is also possible that our exclusion of VTE diagnoses during the index hospitalization contributed to this underestimation of VTE incidence.
Patients who underwent craniotomy for tumor resection had twice the likelihood of a VTE diagnosis on readmission compared with operations for cerebrovascular disorders (Table 3). The literature is replete with reports, which similarly document postoperative VTE rates as being markedly higher in craniotomy for tumor relative to craniotomy for non-neoplastic disease 28–36. In fact, the link between thromboembolic phenomenon and neoplasia is well-established. Among patients with neoplasms, those with malignant gliomas have one of the highest lifetime risk for VTE, second only to pancreatic cancer 37,38. The pathophysiologic basis for this is multifactorial. One possibility is chronic activation of the coagulation cascade through tumor secretion of prothrombotic factors 39–41. For example, Sartori et al found that patients with glioblastoma multiforme harbored higher levels of circulating microparticles (MP) relative to healthy subjects 42 MPs are membrane vesicles shed from tumor cells following their activation or apoptosis, which when coupled to tissue factor can initiate clotting 43,44. Interestingly, circulating levels of MPs have been linked to both tumor burden and subsequent likelihood for VTE development 42,45,46. Other circulating markers have been implicated as well. Brain tumors have increased levels of circulating factor IX, diminished tissue plasminogen activator activity and elevated levels of plasminogen activator inhibitor-1, all of which enhance thrombogenicity 47,48. Altogether, these data suggest that tumor-related factors modulate coagulation, a process exacerbated in the context of surgery due to further release of procoagulants into the circulation 42.
Other reasons cited for the correlation between neoplasia and thromboembolism include chemotherapy and underlying limb paresis/plegia, which both predispose to thromboembolism 30,39. It is also possible that the perioperative management inherent to tumor surgery has some contribution to increased VTE likelihood. In other words: were tumor patients started on chemical prophylaxis later due to hemorrhagic concerns in the wake of resection? Or were they more likely to be administered medications (e.g. steroids) with the potential to unduly influence VTE risk? However, the methodology of research derived from administrative databases such as the NRD cannot answer these questions. Nonetheless, the finding that tumor operations carry higher VTE risk is concerning not only because of the resultant morbidity, but because such a diagnosis may hinder or delay adjunctive therapies (e.g. chemotherapy, radiation) with the potential to influence long-term survival. Aggressive postoperative mobilization as well as timely administration of chemical prophylaxis is therefore imperative in this cohort, especially in patients diagnosed with malignant tumors.
Corticosteroids are widely prescribed throughout neurosurgery for their anti-inflammatory and immunosuppressant effects as well as their benefits on blood-brain barrier integrity. In multivariate analysis, steroids proved to be a significant predictor of 30-day readmission with VTE even after accounting for collinearity with potential variables such as neurologic deficit. The association between VTE and exogenous steroids has previously been noted 49,50. In a prospective population-based case-control study from Denmark, glucocorticoid administration led to an increased risk of VTE, particularly PE. Patients who were recently started on steroids (< 90 days) had a 3-fold increased risk of VTE 49. Moreover, VTE risk was temporally associated with steroid administration, being highest at the onset of therapy and tapering off with increasing duration of use. Thromboembolic risk was also proportional to dose: whereas low-dose prednisolone (<5 mg) had a two-fold higher risk of PE, high-dose regimens (prednisolone >30 mg) carried a 10-fold risk of PE 51. A putative explanation for steroid-induced hypercoagulability is that it increases clotting factor and fibrinogen levels 52. In addition, in vitro studies suggest that there is increased synthesis and secretion of von Willebrand factor and plasminogen activator inhibitor-1, which in effect promote coagulation and inhibit fibrinolysis 53,54.
The relationship between steroids and VTE has also been documented in neurosurgery cohorts 14,55. In one of the largest studies to date, Lieber et al 55 found that corticosteroids carried a higher risk of both PE (OR 1.47, p = 0.004) and DVT (OR 1.55, p < 0.001). Because of their ubiquity, steroids are therefore a prime target for risk modification in patients undergoing craniotomy. While it is understandable that their administration is often times necessary, the VTE risks incurred, at the very least, warrant careful consideration towards a lower dose in the perioperative setting. Interestingly, while steroid use was a significant predictor at 30 days in our study, the relationship lost significance at 90 days. One explanation for this observation is that the majority of craniotomies in our cohort (81%) were for tumor and steroids tend to be discontinued in the wake of surgery addressing any underlying pathology. Hence, by 90 days any steroid-related effect would naturally have been lost from drug discontinuation, paralleling the temporal effects of steroid use noted in the Denmark population-based study above.
In multivariate analysis, advanced age correlated with VTE risk. Patients aged ≥75 years had nearly twice the odds of VTE compared to those 18–44-years old. Throughout the literature, age has been one of the most commonly implicated risk factors in venous thromboembolism 56,57. The reasons for this relationship are not well understood but likely feature some combination of waning mobility and accruing medical comorbidities. Prolonged length of stay (≥ 12 days) and eventual discharge to institutional care (e.g. skilled nursing facility) also proved to be independent predictors of VTE after accounting for collinearity within final analyses. Others have similarly reported on these variables and their associations with VTE 26,29,55,58,59. It seems plausible that patients with a prolonged hospital course and those eventually discharged to institutional care are not at their functional baseline and thus have some degree of restricted mobility. This might explain their higher VTE rates, but as we are unable to adjust for neurologic or dependent functional status within the NRD, we are unable to verify this claim.
Our study is subject to several limitations. As is typical with nationwide database investigations, estimates are susceptible to coding errors at the time of data entry. This may have influenced reported values in an indeterminate direction. Because the NRD does not afford tracking across state lines or calendar years, VTE readmissions may have been undercounted, thereby skewing determined incidences. Additionally, as we excluded VTE diagnoses on the index hospitalization to minimize confounding from preexisting events, this may have underestimated VTE incidence. Other notable limitations include the absence of data on socioeconomic determinants of healthcare, diagnostic workup and perioperative management. Knowledge on heparin prophylaxis strategies, for instance, would have proven useful for contextualizing our derived rates vis-à-vis other published figures in neurosurgery. Another important consideration is that there are studies which suggest a subset of patients may already have VTE events present by the time of their index admission 60. Subsequent detection would thus lead to them erroneously being counted as hospital-acquired VTE events, when in fact they are not. We are unable to adjust for any such potential confounding, as we are limited to formal diagnoses captured through ICD-9 coding after admission.
Conclusion
We employed the 2010–2014 cohorts of the NRD to estimate venous thromboembolism rates after non-emergent craniotomy and to identify potentially modifiable risk factors associated with increased risk. The 30-day VTE incidence was determined to be 1.69%, with corresponding DVT and PE rates of 1.19% and 0.93%, respectively. The 90-day VTE incidence was 3.51%, with corresponding DVT and PE rates 2.49% and 1.99%, respectively. In multivariate analysis, several factors were predictive of VTE, namely advanced age, length of stay, discharge to institutional care, craniotomy for tumor and corticosteroids. These results advocate for increased VTE vigilance and early chemical prophylaxis in the postoperative period, particularly for patients with malignant brain tumors who are currently on steroids. Because VTE risk is known to be proportional to steroid dose, consideration should therefore be given towards lowering medication regimens in cases where a smaller dose might prove equally sufficient.
Acknowledgments
Disclosure of Funding: None
Abbreviations
- NRD
Nationwide Readmissions Database
- NSQIP
National Surgical Quality Improvement Project
- ICD-9CM
International Classification of Diseases, Ninth Edition, Clinical Modification
- VTE
venous thromboembolism
- DVT
deep vein thrombosis
- PE
pulmonary embolism
- ICH
intracranial hemorrhage
- SNF
skilled nursing facility
- LTAC
long-term acute care
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease C, Prevention. Venous thromboembolism in adult hospitalizations -United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22):401–404. [PubMed] [Google Scholar]
- 2.Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291–302. [DOI] [PubMed] [Google Scholar]
- 3.Shah MN, Stoev IT, Sanford DE, et al. Are readmission rates on a neurosurgical service indicators of quality of care? J Neurosurg. 2013;119(4):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. [DOI] [PubMed] [Google Scholar]
- 5.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S–453S. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez MM, Hogue S, Preblick R, Kwong WJ. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res. 2015;7:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495–501. [DOI] [PubMed] [Google Scholar]
- 8.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–1617. [DOI] [PubMed] [Google Scholar]
- 9.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):338S–400S. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton MG, Hull RD, Pineo GF. Venous thromboembolism in neurosurgery and neurology patients: a review. Neurosurgery. 1994;34(2):280–296; discussion 296. [DOI] [PubMed] [Google Scholar]
- 11.Browd SR, Ragel BT, Davis GE, Scott AM, Skalabrin EJ, Couldwell WT. Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurg Focus. 2004;17(4):E1. [DOI] [PubMed] [Google Scholar]
- 12.Cerrato D, Ariano C, Fiacchino F. Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg. 1978;49(3):378–381. [DOI] [PubMed] [Google Scholar]
- 13.Powers SK, Edwards MS. Prophylaxis of thromboembolism in the neurosurgical patient: a review. Neurosurgery. 1982;10(4):509–513. [DOI] [PubMed] [Google Scholar]
- 14.Rolston JD, Han SJ, Bloch O, Parsa AT. What clinical factors predict the incidence of deep venous thrombosis and pulmonary embolism in neurosurgical patients? J Neurosurg. 2014;121(4):908–918. [DOI] [PubMed] [Google Scholar]
- 15.Khan NR, Patel PG, Sharpe JP, Lee SL, Sorenson J. Chemical venous thromboembolism prophylaxis in neurosurgical patients: an updated systematic review and meta-analysis. J Neurosurg. 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 16.Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med. 2000;160(15):2327–2332. [DOI] [PubMed] [Google Scholar]
- 17.Collen JF, Jackson JL, Shorr AF, Moores LK. Prevention of venous thromboembolism in neurosurgery: a metaanalysis. Chest. 2008;134(2):237–249. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton MG, Yee WH, Hull RD, Ghali WA. Venous thromboembolism prophylaxis in patients undergoing cranial neurosurgery: a systematic review and meta-analysis. Neurosurgery. 2011;68(3):571–581. [DOI] [PubMed] [Google Scholar]
- 19.Rumalla K, Smith KA, Arnold PM. National Rates, Causes, Risk Factors, and Outcomes Associated With 30-Day and 90-Day Readmissions Following Degenerative Posterior Cervical Spine Surgery Utilizing the Nationwide Readmissions Database. Neurosurgery. 2017;81(5):740–751. [DOI] [PubMed] [Google Scholar]
- 20.Donoho DA, Wen T, Babadjouni RM, et al. Predictors of 30- and 90-day readmission following craniotomy for malignant brain tumors: analysis of nationwide data. J Neurooncol. 2018;136(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vahidy FS, Donnelly JP, McCullough LD, et al. Nationwide Estimates of 30-Day Readmission in Patients With Ischemic Stroke. Stroke. 2017;48(5):1386–1388. [DOI] [PubMed] [Google Scholar]
- 22.Rumalla K, Smith KA, Arnold PM, Mittal MK. Subarachnoid Hemorrhage and Readmissions: National Rates, Causes, Risk Factors, and Outcomes in 16,001 Hospitalized Patients. World Neurosurg. 2018;110:e100–e111. [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 24.Agnelli G Prevention of venous thromboembolism in surgical patients. Circulation. 2004;110(24 Suppl 1):IV4–12. [DOI] [PubMed] [Google Scholar]
- 25.Farray D, Carman TL, Fernandez BB Jr. The treatment and prevention of deep vein thrombosis in the preoperative management of patients who have neurologic diseases. Neurol Clin. 2004;22(2):423–439. [DOI] [PubMed] [Google Scholar]
- 26.Algattas H, Kimmell KT, Vates GE, Jahromi BS. Analysis of Venous Thromboembolism Risk in Patients Undergoing Craniotomy. World Neurosurg. 2015;84(5):1372–1379. [DOI] [PubMed] [Google Scholar]
- 27.Patel AP, Koltz MT, Sansur CA, Gulati M, Hamilton DK. An analysis of deep vein thrombosis in 1277 consecutive neurosurgical patients undergoing routine weekly ultrasonography. J Neurosurg. 2013;118(3):505–509. [DOI] [PubMed] [Google Scholar]
- 28.Kimmell KT, Walter KA. Risk factors for venous thromboembolism in patients undergoing craniotomy for neoplastic disease. J Neurooncol. 2014;120(3):567–573. [DOI] [PubMed] [Google Scholar]
- 29.Kimmell KT, Jahromi BS. Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J Neurosurg. 2015;122(5):1004–1011. [DOI] [PubMed] [Google Scholar]
- 30.Quevedo JF, Buckner JC, Schmidt JL, Dinapoli RP, O’Fallon JR. Thromboembolism in patients with high-grade glioma. Mayo Clin Proc. 1994;69(4):329–332. [DOI] [PubMed] [Google Scholar]
- 31.Qian C, Yan H, Hu X, Zhang W, Liu H. Increased risk of venous thromboembolism in patients with brain tumors: A systematic review and meta-analysis. Thromb Res. 2016;137:58–63. [DOI] [PubMed] [Google Scholar]
- 32.Smith TR, Nanney AD, 3rd, Lall RR, et al. Development of venous thromboembolism (VTE) in patients undergoing surgery for brain tumors: results from a single center over a 10 year period. J Clin Neurosci. 2015;22(3):519–525. [DOI] [PubMed] [Google Scholar]
- 33.Smith TR, Lall RR, Graham RB, et al. Venous thromboembolism in high grade glioma among surgical patients: results from a single center over a 10 year period. J Neurooncol. 2014;120(2):347–352. [DOI] [PubMed] [Google Scholar]
- 34.Hoefnagel D, Kwee LE, van Putten EH, Kros JM, Dirven CM, Dammers R. The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin Neurol Neurosurg. 2014;123:150–154. [DOI] [PubMed] [Google Scholar]
- 35.Brandes AA, Scelzi E, Salmistraro G, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33(10):1592–1596. [DOI] [PubMed] [Google Scholar]
- 36.Senders JT, Goldhaber NH, Cote DJ, et al. Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: a National Surgical Quality Improvement Program analysis. J Neurooncol. 2018;136(1):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119(1):60–68. [DOI] [PubMed] [Google Scholar]
- 38.Petterson TM, Marks RS, Ashrani AA, Bailey KR, Heit JA. Risk of site-specific cancer in incident venous thromboembolism: a population-based study. Thromb Res. 2015;135(3):472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bick RL. Cancer-associated thrombosis. N Engl J Med. 2003;349(2):109–111. [DOI] [PubMed] [Google Scholar]
- 40.Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3(1):27–34. [DOI] [PubMed] [Google Scholar]
- 41.Sciacca FL, Ciusani E, Silvani A, et al. Genetic and plasma markers of venous thromboembolism in patients with high grade glioma. Clin Cancer Res. 2004;10(4):1312–1317. [DOI] [PubMed] [Google Scholar]
- 42.Sartori MT, Della Puppa A, Ballin A, et al. Prothrombotic state in glioblastoma multiforme: an evaluation of the procoagulant activity of circulating microparticles. J Neurooncol. 2011;104(1):225–231. [DOI] [PubMed] [Google Scholar]
- 43.Zwicker JI, Liebman HA, Neuberg D, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15(22):6830–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5(3):520–527. [DOI] [PubMed] [Google Scholar]
- 45.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7(8):1421–1423. [DOI] [PubMed] [Google Scholar]
- 46.Manly DA, Wang J, Glover SL, et al. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125(6):511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawaya R, Glas-Greenwalt P. Postoperative venous thromboembolism and brain tumors: Part II. Hemostatic profile. J Neurooncol. 1992;14(2):127–134. [DOI] [PubMed] [Google Scholar]
- 48.Iberti TJ, Miller M, Abalos A, et al. Abnormal coagulation profile in brain tumor patients during surgery. Neurosurgery. 1994;34(3):389–394; discussion 394–385. [DOI] [PubMed] [Google Scholar]
- 49.Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743–752. [DOI] [PubMed] [Google Scholar]
- 50.Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935–943. [DOI] [PubMed] [Google Scholar]
- 51.Stuijver DJF, Majoor CJ, van Zaane B, et al. Use of oral glucocorticoids and the risk of pulmonary embolism: a population-based case-control study. Chest. 2013;143(5):1337–1342. [DOI] [PubMed] [Google Scholar]
- 52.Brotman DJ, Girod JP, Posch A, et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res. 2006;118(2):247–252. [DOI] [PubMed] [Google Scholar]
- 53.Heaton JH, Nebes VL, O’Dell LG, Morris SM, Jr., Gelehrter TD. Glucocorticoid and cyclic nucleotide regulation of plasminogen activator and plasminogen activator-inhibitor gene expression in primary cultures of rat hepatocytes. Mol Endocrinol. 1989;3(1):185–192. [DOI] [PubMed] [Google Scholar]
- 54.Morange PE, Aubert J, Peiretti F, et al. Glucocorticoids and insulin promote plasminogen activator inhibitor 1 production by human adipose tissue. Diabetes. 1999;48(4):890–895. [DOI] [PubMed] [Google Scholar]
- 55.Lieber BA, Han J, Appelboom G, et al. Association of Steroid Use with Deep Venous Thrombosis and Pulmonary Embolism in Neurosurgical Patients: A National Database Analysis. World Neurosurg. 2016;89:126–132. [DOI] [PubMed] [Google Scholar]
- 56.Cote DJ, Smith TR. Venous thromboembolism in brain tumor patients. J Clin Neurosci. 2016;25:13–18. [DOI] [PubMed] [Google Scholar]
- 57.Stein PD, Hull RD, Kayali F, Ghali WA, Alshab AK, Olson RE. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med. 2004;164(20):2260–2265. [DOI] [PubMed] [Google Scholar]
- 58.Kshettry VR, Rosenbaum BP, Seicean A, Kelly ML, Schiltz NK, Weil RJ. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21(2):282–286. [DOI] [PubMed] [Google Scholar]
- 59.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. [DOI] [PubMed] [Google Scholar]
- 60.Houchens RL, Elixhauser A, Romano PS. How often are potential patient safety events present on admission? Jt Comm J Qual Patient Saf. 2008;34(3):154–163. [DOI] [PubMed] [Google Scholar]