Abstract
Background
Airway neutrophils are abundant in some children with severe asthma, but their functions are poorly understood.
Objective
We hypothesized that the inflammatory airway environment of children with neutrophil-predominant severe asthma promotes neutrophil survival and disrupts neutrophil-associated innate immune defenses.
Methods
Sixty seven children with severe asthma refractory to high-dose inhaled corticosteroid (ICS) treatment undergoing bronchoscopy with bronchoalveolar lavage (BAL) for clinical indications were stratified into neutrophil “high” versus “low” groups based on BAL differential counts. Neutrophil activation markers, functional assays and phenotyping studies were performed as well as airway macrophage functional assays. Results were compared to those from children with moderate asthma treated with ICS.
Results
Children with neutrophil-predominant severe asthma had increased markers of neutrophil activation/degranulation and a greater magnitude of airway pro-inflammatory cytokine and chemokine release. Primary neutrophils exposed to BAL of these children exhibited greater phagocytic capability and greater neutrophil extracellular trap formation, but a more impaired respiratory burst. Despite greater abundance of airway TGF-β1 the neutrophils were not more apoptotic. Instead, neutrophils had a highly pro-inflammatory phenotype associated with a number of surface markers that regulate neutrophil activation, recruitment/migration and granule release. Airway macrophages from children with neutrophil-predominant severe asthma were also more pro-inflammatory with impaired phagocytosis and increased apoptosis.
Conclusions
Children with neutrophil-predominant severe asthma have pro-inflammatory neutrophils with enhanced survival. Airway macrophages are also pro-inflammatory and dysfunctional and may contribute to global innate immune impairment. Therapies that target neutrophils and related inflammation may be warranted in this subset of children.
Keywords: Severe asthma, Asthma phenotype, Asthma endotype, Neutrophil activation, Neutrophil extracellular trap, Respiratory burst, Airway macrophage, Phagocytosis, Non-Type 2 inflammation, Pediatric asthma patients, Healthy adults
INTRODUCTION
Patients with severe, corticosteroid-refractory asthma are a heterogeneous group necessitating a “personalized” versus a “one-size fits-all” treatment approach.1 Whereas eosinophils are the hallmark Type-2 inflammatory cell and the target of a number of emerging biologic therapies for severe asthma, neutrophils are much less understood. In adults, abundant airway neutrophils have been identified in some patients with severe asthma2-4, but their role is controversial5 and others have argued that neutrophil elevations may simply result from intensive corticosteroid treatment, which can alter neutrophil survival.6, 7 Studies in children are even more limited. In the few phenotypic8-10 and bronchoscopic11, 12 pediatric studies that have been performed, the majority of children with severe asthma studied had an eosinophil-predominant pattern of Type-2 airway inflammation, raising questions about the relevance of neutrophils to pediatric disease.13
We have previously demonstrated that neutrophil elevations are present in the airways of a subpopulation of children with severe asthma and are associated with elevations in C-X-C Motif Chemokine Ligand (CXCL) and C-C Motif Chemokine Ligand (CCL) proteins responsible for neutrophil activation and chemotaxis, even in the absence of pathogenic bacteria or fungi.11 Other recent studies outside of our laboratory suggest that neutrophil elevations may be present in up to 30% of the entire population of children with severe asthma.14, 15 Furthermore, others have also noted increased expression of interleukin (IL)-17 receptor A, which promotes neutrophil maturation,16 and largely undetectable IL-4, IL-5, and IL-13 concentrations in the airways of children with severe asthma.12 suggesting that the inflammation in these children is not isolated to eosinophils alone.
Despite the potential importance of neutrophils in subsets of children with severe asthma, studies of neutrophil biology are lacking in this population. Neutrophils play a fundamental role in the innate immune response through killing of invading microbes and are, by design, highly reactive cells that release proteolytic enzymes from their granules and secretory vesicles and kill pathogens using bursts of reactive oxygen species. However, unlike eosinophils or T cells, neutrophils are less sensitive to the effects of corticosteroids17, 18 and thus their presence in the airways in large numbers cannot be benign. We hypothesized that the inflammatory airway environment of children with neutrophil-predominant severe asthma refractory to high doses of inhaled corticosteroids (ICS) promotes neutrophil survival and disrupts innate immune defenses. To this end, we characterized airway neutrophil activation markers and key functions of phagocytosis, neutrophil extracellular trap (NET) formation, respiratory burst and apoptosis in primary neutrophils exposed to airway fluid from these children. Furthermore, since airway macrophages are the key instigator of host defense and can assume differing immune phenotypes based on the airway cytokine milieu,19 a secondary objective was to characterize airway macrophage inflammatory patterns and functions in these same patients.
METHODS
Participants.
Children and adolescents age 6-21 years with physician-diagnosed asthma and a history of ≥12% relative change in forced expiratory volume in one second (FEV1) after bronchodilator administration undergoing bronchoscopy with bronchoalveolar lavage (BAL) for clinical indications. Children with severe asthma met published criteria for diagnosis20 and were treated with high-dose inhaled fluticasone equivalent (>800 mcg daily if ≥12 years or >400 mcg daily if <12 years of age) plus an additional asthma controller medication (i.e., long-acting beta agonist or montelukast). These participants underwent bronchoscopy for wheezing unresponsive to recommended treatment. For the purpose of comparison, children and adolescents with moderate persistent asthma treated with ICS for at least 6 consecutive months and undergoing bronchoscopy with BAL for clinical indications (suspected foreign body, n = 3; suspected aspiration, n = 5; abnormal chest radiograph, n = 5) were also recruited. Exclusion criteria for all asthmatic participants included birth prior to 35 weeks gestation, an asthma exacerbation treated with systemic corticosteroid within the previous 4 weeks, or a positive BAL bacterial, viral or fungal culture. Informed consent and assent to share the BAL fluid between the clinical and research laboratories was obtained.
Clinical procedures.
Spirometry was performed with a portable spirometer (KoKo® Legend, Ferraris, Louisville, CO). The best of three forced vital capacity (FVC) maneuvers was interpreted according to population reference standards.21 Exhaled nitric oxide was quantified using a commercial device (NIOX, Aerocrine, Solna, Sweden).22 Aeroallergen sensitization was defined as a positive skin prick test or a specific aeroallergen immunoglobulin E (IgE) test >0.35 kU/L. Venipuncture was performed at the time of bronchoscopy for total serum IgE, which was quantified by a local laboratory (Children’s Healthcare of Atlanta, Atlanta, Georgia). Bronchoscopy was performed as described previously using 0.9% sodium chloride warmed to room temperature.11 BAL samples were subjected to standard culture and sensitivity testing for bacteria, viruses and fungus (Children’s Healthcare of Atlanta).
Laboratory procedures.
Laboratory procedures are detailed in the Online Supplement. BAL was centrifuged at 1200 rpm for 7 minutes at 4° C within 1 hour of collection and the cell pellet was resuspended in 10 mL of 1:1 Dulbecco’s modified Eagle’s medium/Ham’s F-12 solution containing 2% FBS, L-glutamine, 15 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, penicillin (10,000 U), streptomycin (10,000 mg/mL), amphotericin (25 mg/mL), and gentamicin (4 μg/mL). Total cell counts were performed with a hemocytometer and cellular differentials were determined from 300 consecutive cells after Kwik-Diff® staining (Thermo Shandon Limited, Runcorn, UK).
Enzyme-linked Immunosorbent Assays (ELISA).
IL-1β, IL-6, IL-7, IL-8 (CXCL8), IL-10, IL-12p70, tumor necrosis factor alpha (TNFα), CXCL1 (growth-related oncogene), CXCL10 (human interferon-inducible protein 10), CCL2 (monocyte chemoattractant protein-1), CCL3 (macrophage inflammatory protein-1α), CCL4 (macrophage inflammatory protein-1β), and CCL22 (macrophage-derived chemokine) were quantified with bead-based multiplex assays (HSCYTO-60SK and MPXHCYTO-60K, Millipore, Billerica, MA). IL-33 (R&D Systems, Minneapolis, MN), transforming growth factor beta-1 (TGF-β1) (Promega, Madison, WI), neutrophil elastase (Abcam, Cambridge, MA), myeloperoxidase (R&D Systems) and lactoferrin (Abcam) ELISA were performed according to manufacturer instructions. Concentrations were determined with a BioTek (Winooski, VT) Synergy HT 96-well plate reader using manufacture recommended wavelengths and standard curve fit.
Cell culture experiments.
HL-60 cells (Sigma-Aldrich, St. Louis, MO) were cultured and differentiated as described in the Online Supplement. Primary human neutrophils were isolated from healthy donor blood using the EasySep Direct Human Neutrophil Isolation Kit (Stemcell Technologies, Vancouver, BC) per the manufacturer’s instructions. Neutrophil purity was confirmed with Kwik-Diff (Thermo Shandon) staining. Cells were cultured at 37°C, 5% CO2 in Roswell Park Memorial Institute-1640 media with L-glutamine supplemented with 10% heat-inactivated Fetal Calf Serum, 1% penicillin/streptomycin, 50 mg/ml gentamicin and 5ng/ml recombinant human TGF-β1 (BioLegend) for 16 hours. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sigma) was added at 100ng/ml as indicated for 30 minutes prior to harvesting.
Neutrophil experiments.
Neutrophil phagocytosis was assessed after overnight culture in equal parts media and BAL supernatant, followed by addition of phorbol 12-myristate 13-acetate (PMA) and pHrodo Staphylococcus aureus BioParticles (Molecular Probes, Eugene,OR). Kinetic measurements of neutrophil extracellular trap (NET) release were taken per established protocol23 and apoptosis was assessed with phycoerythrin-conjugated Cleaved-Caspase-3 antibody (Cell Signaling Technology, Danvers, MA) quantified by flow cytometry (FACSCalibur, BD Biosciences). Respiratory burst was assessed with Dihydrorodamine123 after PMA stimulation using flow cytometry (FACSCalibur). Neutrophil surface staining was performed with CD66b-PerCPCy5.5, CD62L-BV421, CD11b-AF488, CD11c-PECy7, CD16-APCCy7 (BioLegend); cells were permeabilized and then stained intracellularly with CCL2-AF647 (BioLegend) and analyzed with a Cytoflex Flow Cytometer (Beckman-Coulter, Brea, CA). Expression data were analyzed using FlowJo version 10 software (Version 10, FlowJo LLC).
Airway macrophage experiments.
Airway macrophages (100,000 cells) were isolated from the BAL cell suspension and incubated with and without 1 mg/mL lipopolysaccharide as previously described.24 Protein expression of TGF-β1 and poly [ADP-ribose] polymerase 1 (PARP-1) cleavage was analyzed by fluorescent microscopy in cytospins using anti-TGFβ1 and PARP-1 antibodies at a 1:100 dilution (sc-31609 and sc-8007, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Phagocytosis of fluorescein isothiocyanate–conjugated, inactivated Staphylococcus aureus (Invitrogen, Carlsbad, CA) was assessed with an Olympus confocal microscope (model BX51; Olympus America, Inc) with quantitative digital fluorescence imaging software (Olympus FluoView 300, Version 4.3). The phagocytic index of S. aureus (Relative fluorescent units (RFU) × percentage of positive cells) were determined for each phagocytic cell at 50% of the cell depth as described previously.24
Statistical analyses.
Statistical analyses were performed with IBM SPSS Statistics software (Version 24; SPSS, Chicago, Ill). Skewed variables were logarithmically transformed before analysis. Chi-square tests and t tests were used to compare baseline clinical features between groups. Experimental differences were assessed by ANOVA with Tukey post-hoc tests. A 2-tailed probability of 0.05 or less was the threshold of significance for all comparisons.
RESULTS
Seventy one children with severe asthma were approached for the study. Four children had positive bacterial BAL cultures (Haemophilus influenzae, n = 2; Streptococcus pneumoniae, n = 2) and were excluded from further analysis, resulting in a final sample of 67 children. BAL neutrophil percentages varied widely among children with severe asthma, with a median of 5.0% (minimum, 0.66; maximum, 19.80%). By contrast, BAL neutrophil percentages in children with moderate asthma treated with ICS were all below 5%. Children with severe asthma were therefore stratified into neutrophil “high” versus “low” groups using an arbitrary <5.0% and ≥5.0% cut-point as shown in Figure 1A.
Figure 1.
(A) Airway neutrophil percentages and concentrations of (B) myeloperoxidase, (C) elastase and (D) lactoferrin in bronchoalveolar lavage fluid from children with moderate asthma treated with ICS (“MA”), children with neutrophil “low” severe asthma (“NLSA”), and children with neutrophil “high” severe asthma (“NHSA”). Whiskers represent minimum and maximum values; individual participants are shown as dots. *p<0.05
Features of the groups are shown in Table 1. Prior healthcare utilization, medication requirements and exhaled nitric concentrations were significantly greater and lung function values were significantly lower in children with severe versus moderate asthma. There were no differences in clinical characteristics between the two severe groups, aside from lower monocyte/macrophage percentages and higher neutrophil percentages (by definition).
Table 1.
Features of the participants. Data represent the mean ± SEM or the number of participants (%).
| Feature | Moderate asthma treated with ICS N = 13 |
Neutrophil “low” severe asthma N = 29 |
Neutrophil “high” severe asthma N = 38 |
|---|---|---|---|
| Age (years) | 18.0 ± 2.2 | 14.7 ± 2.4 | 13.0 ± 1.8 |
| Males | 6 (46.2) | 11 (37.9) † | 21 (55.3) † |
| Race: White Black |
8 (61.5) 5 (38.5) |
17 (58.6) 12 (41.4) |
24 (63.2) 14 (36.8) |
| Hispanic ethnicity | 1 (7.7) | 2 (6.9) | 2 (7.9) |
| Obese (body mass index ≥95 percentile) | 6 (46.2) | 9 (31.0) | 14 (36.8) |
| Aeroallergen sensitization | 4 (30.8) | 18 (62.1) | 22 (57.9) |
| Emergency department visit for asthma (prior year) | 1 (7.7) | 14 (48.3) † | 21 (55.3) † |
| Hospitalization for asthma (prior year) | 0 | 13 (44.8) † | 17 (44.7) † |
| Intubation for asthma (ever in lifetime) | 0 | 2 (6.9) | 5 (13.2) |
| Daily medication use ICS monotherapy ICS + LABA or LTRA Daily ICS dose (fluticasone equivalent) |
7 (53.8) 6 (46.2) 300.3 ± 49.6 |
0 † 29 (100)† 749.8 ± 66.9 † |
0 † 38 (100) † 705.2 ± 56.9 † |
| Serum IgE (kU/L)1 | 109.5 ± 46.5 | 294.3 ± 105.4 | 419.9 ± 148.2 |
| Exhaled nitric oxide (ppb)1 | 28.2 ± 12.7 | 42.0 ± 9.2 | 51.9 ± 9.4 † |
| Lung function values FVC (% predicted) FEV1 (% predicted) FEV1/FVC FEF25-75 (% predicted) FEV1 bronchodilator reversibility (relative % change from baseline) |
95.7 ± 6.2 92.0 ± 5.4 0.84 ± 0.02 86.3 ± 8.7 5.0 ± 1.2 |
89.8 ± 3.7 80.3 ± 4.8 0.81 ± 0.02 74.3 ± 5.9 15.0 ± 3.2 † |
90.9 ± 3.1 83.6 ± 3.5 0.81 ± 0.02 77.5 ± 6.8 16.3 ± 3.0 † |
| Airway lavage characteristics Protein (mcg/mL) Cell count (x 106 cells) Macrophages/monocytes (%) Neutrophils (%) Eosinophils (%) Lymphocytes (%) |
144.4 ± 27.2 3.99 ± 0.69 91.2 ± 0.8 2.4 ± 0.3 1.4 ± 0.5 4.3 ± 0.5 |
182.88 ± 18.2 3.67 ± 0.50 91.0 ± 0.8 2.7 ± 0.2 1.4 ± 0.3 4.5 ± 0.5 |
192.9 ± 17.9 3.93 ± 0.48 85.9 ± 1.0 †‡ 8.0 ± 0.7 †‡ 1.5 ± 0.5 4.5 ± 0.5 |
p< 0.05 vs. moderate asthma treated with ICS
p < 0.05 vs. neutrophil low severe asthma
Log-transformed for significance testing
Defined as (post value in liters – baseline value in liters)/baseline value in liters
ICS = inhaled corticosteroid; IgE = immunoglobulin E; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; FEF25-75 = forced expiratory flow at 25-75% of vital capacity; LABA = long-acting beta agonist; LTRA = leukotriene receptor antagonist
Neutrophil activation.
Neutrophil activation, reflected by release of myeloperoxidase, elastase and lactoferrin protein in the BAL fluid, was significantly greater in children with neutrophil “high” versus neutrophil “low” severe asthma (Figure 1B-D) and was accompanied by increased concentrations of IL-1β IL-6, Il-33, CXCL1, CXCL8, CXCL10, CCL2, CCL3, CCL4 and CCL22 protein (Table 2). IL-7, IL-12 and TNFα were also elevated in the BAL fluid of children with neutrophil “high” severe asthma but were similar to concentrations in the neutrophil “low” group (Table 2). Normalization of the neutrophil activation markers and cytokine and chemokine concentrations to BAL protein levels did not significantly alter these results (Table E1).
Table 2.
Concentrations of cytokines and chemokines in the bronchoalveolar lavage fluid. Data represent the mean ± SEM.
| Analyte | Minimal detectable concentration |
Moderate asthma treated with ICS N = 13 |
Neutrophil “low” severe asthma N = 29 |
Neutrophil “high” severe asthma N = 38 |
|---|---|---|---|---|
| IL-1β (pg/mL) | 0.06 | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.26 ± 0.05 †‡ |
| IL-6 (pg/mL) | 0.10 | 7.17 ± 2.57 | 7.22 ± 1.23 | 26.44 ± 6.12 †‡ |
| IL-7 (pg/mL) | 0.12 | 2.18 ± 0.65 | 2.46 ± 0.58 | 2.47 ± 0.38 |
| IL-12 (pg/mL) | 0.11 | 0.29 ± 0.12 | 0.32 ± 0.08 | 0.33 ± 0.04 |
| IL-33 (pg/mL) | 0.07 | 1.96 ± 0.73 | 8.52 ± 4.05 | 21.01 ± 4.39 †‡ |
| TNFα (pg/mL) | 0.05 | 0.33 ± 0.07 | 0.91 ± 0.41 | 1.53 ± 0.69 |
| CXCL1 (pg/mL) | 10.10 | 587.85 ± 229.11 | 2133.19 ± 364.66 † | 2790.68 ± 263.42 †‡ |
| CXCL8 (pg/mL) | 0.11 | 42.19 ± 18.64 | 40.33 ± 6.80 | 314.51 ± 125.61 †‡ |
| CXCL10 (pg/mL) | 1.20 | 173.67 ± 38.20 | 444.55 ± 63.87 † | 1067.77 ± 224.52 †‡ |
| CCL2 (pg/mL) | 0.90 | 26.06 ± 10.49 | 35.61 ± 4.95 | 55.59 ± 8.79 †‡ |
| CCL3 (pg/mL) | 3.50 | 4.44 ± 0.63 | 6.20 ± 0.69 | 15.30 ± 4.66 †‡ |
| CCL4 (pg/mL) | 4.50 | 11.02 ± 3.87 | 22.93 ± 4.10 † | 51.81 ± 14.16 †‡ |
| CCL22 (pg/mL) | 3.70 | 63.03 ± 19.69 | 114.59 ± 20.25 † | 165.45 ± 35.33 † |
p< 0.05 vs. moderate asthma treated with ICS
p < 0.05 vs. neutrophil low severe asthma
CCL = chemokine (C-C motif) ligand; CXCL = chemokine (C-X-C motif) ligand; IL = interleukin; TNF = tumor necrosis factor
Neutrophil functional assays.
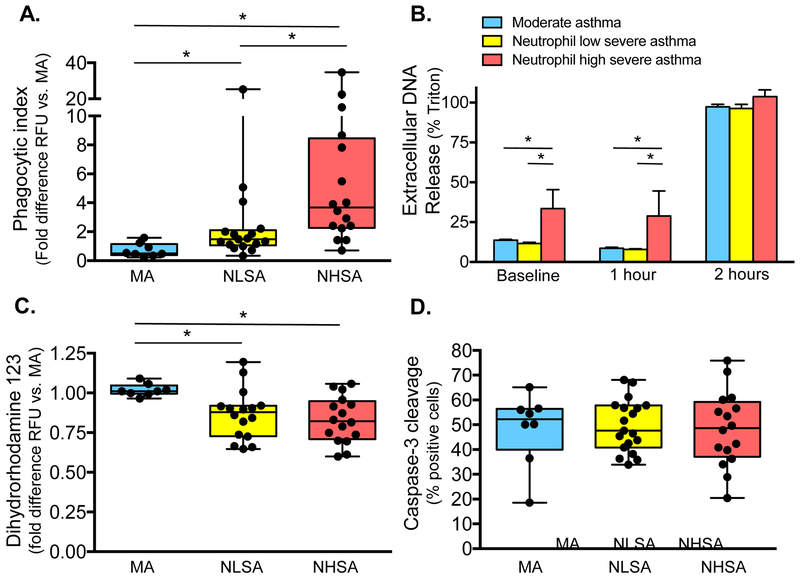
Neutrophil functions were assessed in primary neutrophils exposed to BAL fluid from each of the patient groups. Neutrophil elastase concentrations in the media after culture were similar to those noted in the BAL fluid from each of the patient groups (Figure E1). Neutrophil phagocytic function was enhanced in neutrophils exposed to BAL fluid from children with neutrophil “high” severe asthma compared to the other groups (Figure 2A). NET formation (i.e., extracellular DNA release) was also significantly higher in neutrophils exposed to BAL fluid from children with neutrophil “high” severe asthma at baseline (Figure 2B) 60 minutes, and 80 minutes (Figure E2). In contrast, the neutrophil oxidative burst reflected by dihydrorhodamine-123 expression in response to PMA stimulation was significantly less than that observed in neutrophils exposed to BAL fluid from children with moderate asthma treated with ICS (Figure 2C). Neutrophil apoptosis, evidenced by cleavage of caspase-3, was not significantly different (Figure 2D).
Figure 2.
(A) Neutrophil phagocytic index, (B) extracellular DNA release (mean ± SEM), (C) oxidative burst, and (D) apoptosis in children with moderate asthma treated with ICS (“MA”), children with neutrophil “low” severe asthma (“NLSA”), and children with neutrophil “high” severe asthma (“NHSA”). Whiskers represent minimum and maximum values; dots reflect individual participants. RFU = relative fluorescent units. *p<0.05
Neutrophil phenotyping studies.
In a cell culture model system, TGF-β1 inhibited apoptosis in HL60-derived neutrophils in a time dependent-manner (Figure E3A-B). Prolonged (i.e., overnight) TGF-β1 exposure also enhanced phagocytosis and increased NET formation in HL60-derived neutrophils treated with dexamethasone (Figure E3C-D). In primary neutrophils primed with GM-CSF, prolonged TGF-β1 exposure also reduced expression of the L-selectin, CD62L (Figure E4A), reduced intracellular CCL2 (Figure E4B), increased expression of the integrins CD11b and CD11c (Figure E4C,D) and increased expression of the phagocytosis receptor, CD16 (Figure E4E). No differences in CD66b expression were noted (Figure E4F).
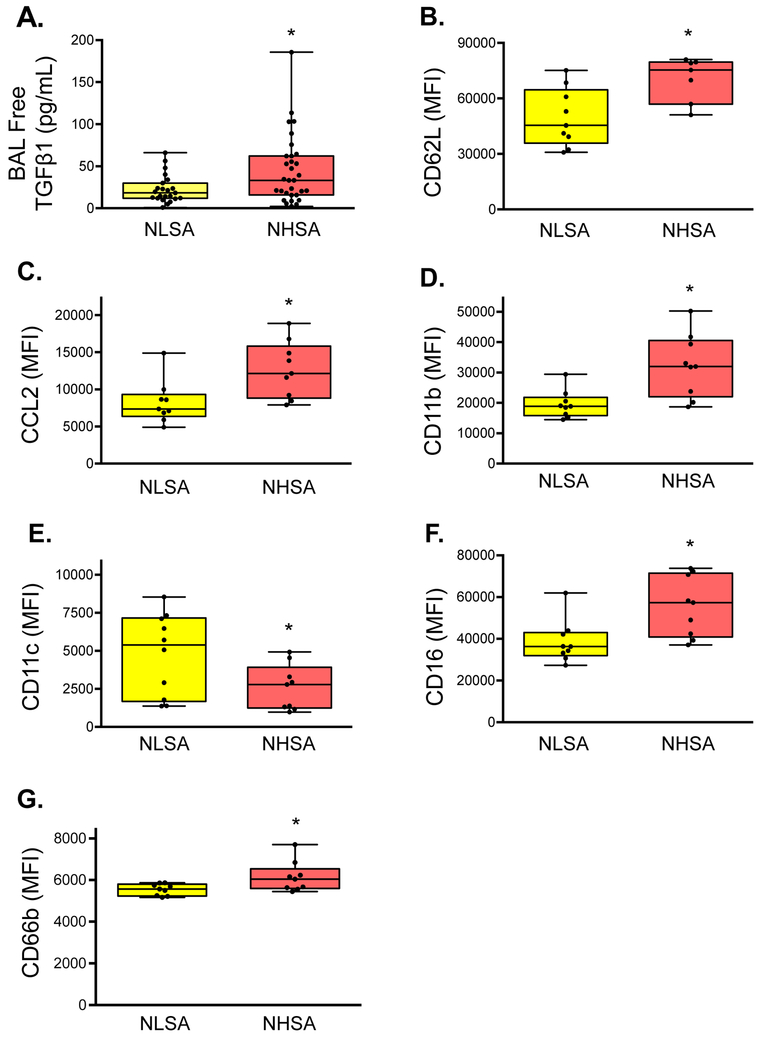
Children with neutrophil “high” severe asthma had higher concentrations of free TGF-β1 in the BAL fluid than children with neutrophil “low” severe asthma (Figure 3A). But despite increased abundance of BAL TGF-β1, primary neutrophils exposed to BAL fluid from these children exhibited a more pro-inflammatory phenotype characterized by increased CD62L, increased CCL2, increased CD11b, decreased CD11c, increased CD16, and increased CD66b expression (Figure 3B-G).
Figure 3.
(A) TGF-β1 concentrations in the bronchoalveolar lavage (BAL) fluid and mean fluorescent intensity (MFI) of (B) CD62L, (C) CCL2, (D) CD11b, (E) CD11c, (F) CD16, and (G) CD66 in primary neutrophils exposed to BAL fluid from children with neutrophil “low” (“NLSA”) and neutrophil “high” severe asthma (“NHSA”). Whiskers represent minimum and maximum values. *p<0.05.
Children with neutrophil “high” severe asthma also had higher TGF-β1 protein expression in airway macrophages (Figure 4A) as well as increased macrophage concentrations of IL-10, CXCL1, CXCL8, CXCL10, and CCL2 (Figure 4B-F). Airway macrophage phagocytic function was also significantly impaired at baseline in children with neutrophil “high” severe asthma and did not improve with lipopolysaccharide stimulation (Figure 4G). Moreover, airway macrophage apoptosis, assessed by cleavage of PARP-1, was also increased in children with neutrophil “high” severe asthma and increased further in response to lipopolysaccharide (Figure 4F).
Figure 4.
(A) Airway macrophage TGF-β1 protein expression, (B) protein-normalized concentrations of IL-10, (C) CXCL1, (D) CXCL8, (E) CXCL10, and (F) CCL2, (G) phagocytosis, and (F) apoptosis in children with neutrophil “low” (“NLSA”) and neutrophil “high” severe asthma (“NHSA”). Whiskers represent minimum and maximum values. *p<0.05. RFU = relative fluorescent unit, LPS = lipopolysaccharide
DISCUSSION
The role of airway neutrophils in children with severe asthma is not understood and the existence of a unique “endotype” of neutrophilic severe asthma in school-age children has been debated.13 We hypothesized that abundant airway neutrophils in children with severe asthma are not benign and that the airway environment of children with neutrophil-predominant severe asthma promotes neutrophil survival and disrupts innate immune defenses. Our findings suggest that airway neutrophils in children with neutrophil-predominant severe asthma are highly dysfunctional and characterized by greater release of pro-inflammatory mediators and granules, greater phagocytic capability and NET formation, but more impaired respiratory burst. Despite a highly immunosuppressive airway TGF-β1 environment, neutrophils exposed to the airway environment of these children were not more apoptotic and displayed a number of surface markers associated with activation, including increased expression of CD62L, an adhesion molecule that promotes endothelial attachment for the purpose of migration, and increased expression of CCL2, which mediates neutrophil recruitment. Although airway macrophages provide some defense against activated neutrophils, airway macrophages from children with neutrophil-predominant severe asthma were also highly pro-inflammatory with disrupted innate immune defenses (i.e., impaired phagocytosis and increased apoptosis) and may perpetuate a sustained environment of neutrophil-mediated inflammation rendering children more susceptible to lower respiratory infections.
Severe asthma is clearly heterogeneous, with a number of different endotypes that have been classically defined by neutrophil and eosinophil percentages in the airways.25 In adults, airway neutrophil numbers tend to increase in difficult-to-treat populations2, 26-28 in association with increased IL-17 levels 29 and other inflammatory cytokines and chemokines. 30-32 Airway neutrophil numbers may also increase further during acute exacerbations.33 However, little is known about neutrophil functions in patients with severe asthma. A few studies suggest that systemic neutrophils may be more activated in non-eosinophilic asthma patients, with greater CXCL8 release34 and greater NET formation,35 which may be augmented after migration to the airways. However, other studies of asthma patients suggest that the process of neutrophil activation may occur in the airways themselves and lead to an activated and degranulated phenotype36 with increased NET formation as assessed by antimicrobial proteins.37 Furthermore, whereas circulating neutrophils are conventionally thought to have a 6- to 8-hour limited life span,38 more recent work suggests that neutrophils can survive up to several days and return to the systemic circulation by reverse migration.39, 40 Indeed, Uddin et al. similarly noted delayed airway neutrophil apoptosis in patients with severe asthma, with the greatest neutrophil survival in patients with the greatest airway neutrophil counts.41
Recent work suggests that neutrophils, like macrophages, are not terminally differentiated and have functional plasticity with cytokine-driven polarization.42 In tumors, neutrophils can acquire an anti-tumorigenic “N1” phenotype characterized by increased cytotoxic activity with more superoxide and hydrogen peroxide release and promotion of CD8+ T cell recruitment and activation through increased proinflammatory cytokine and chemokine release. These same neutrophils can be modulated toward a pro-tumorigenic “N2” phenotype by TGF-β1, which inhibits neutrophil degranulation and reactive mediator release and promotes neutrophil accumulation in the tumor.43, 44 Although global classification of patient neutrophil populations as either “N1” or “N2” is an oversimplification, our results suggest that the neutrophils present in airways of children with neutrophil-predominant severe asthma are largely pro-inflammatory despite a highly imunosuppressive TGF-β1-rich environment, evidenced by a number of “N1”-like features that may provide an opportunity for future therapeutic intervention. Indeed, while a hyperinflammatory response may result in damage to the host epithelium and endothelium (and increased mucosal permeability and leakage), modulation of the “N1” neutrophil phenotype could reduce some collateral damage from reactive oxygen species and granulocyte/protease release without compromising antimicrobial functions.45 In contrast, a predominance of “N2” neutrophils may compromise antimicrobial functions and result in bacterial co-infections.45
To our knowledge, this is the first study to comprehensively examine neutrophil predominance, neutrophil-associated markers of inflammation, neutrophil functions and associated airway macrophage functions in children with severe asthma. However, there are limitations. First, severe asthma by definition necessitates treatment with high doses of ICS;20 therefore it is unclear how intensive corticosteroid treatment may have impacted our results. Nonetheless, we still observed significant differences in neutrophil and macrophage functions between children with neutrophil “high” and “low” asthma despite equivalent ICS treatment. Furthermore, unlike eosinophils or T cells, neutrophils are relatively insensitive to corticosteroids.17, 18 Second, we were unable to perform neutrophil functional experiments on freshly isolated airway neutrophils given the relatively low neutrophil numbers (in comparison with macrophages) and the need to share samples with the clinical laboratory. We therefore do not expect that all of our primary neutrophil surface markers will replicate perfectly in an airway environment, as these could change during the process of recruitment and migration.46 Indeed, a prior study of induced sputum from asthmatic patients noted that airway surface marker expression was not specific for asthmatic airway inflammation or asthma disease activity, and was instead related to tissue migration.47 Likewise, CD62L, which was higher in children with neutrophil “high” severe asthma in the present study, is typically shed during migration and is lower in airway versus systemic compartments.36 However, other surface markers such as CD11b (which is associated with neutrophil granules and secretory vesicles) were also more highly expressed in children with neutrophil “high” severe asthma in the present study and would likely replicate in airway analyses, given our other observations of greater neutrophil-associated granule release (i.e., lactoferrin, myeloperoxidase, elastase) in the BAL fluid of these same children. Finally, our sample sizes were relatively small and limited to children undergoing bronchoscopy with BAL for clinical purposes; this prohibited inclusion of healthy children.
In summary, these results demonstrate that neutrophil abundance in children with severe asthma is not benign. Instead, neutrophils are highly activated and display a number of functional alterations including greater inflammatory mediator and granule release and greater NET formation without an accompanying increase in apoptosis. Children with neutrophil “high” severe asthma therefore exhibit a paradox of immune activation with dysfunctional respiratory burst capacity and prolonged survival despite an immunosuppressive environment. Macrophage defenses were also paradoxically impaired in these same children. Together, these findings may ultimately promote an environment of sustained airway inflammation and structural damage in children with neutrophil “high” severe asthma refractory to treatment with high doses of ICS. Novel therapeutic strategies targeting neutrophil activation such as prostaglandin E3/E4 inhibitors, protein kinase inhibitors, and other emerging treatment strategies may be beneficial in these patients.
Supplementary Material
Highlights Box
What is already known about this topic?
Eosinophils are the hallmark Type-2 inflammatory cell targeted by emerging biologic therapies for severe asthma. However, neutrophils are also abundant in a subset of children with severe asthma, yet their associated functions are poorly understood.
What does this article add to our knowledge?
Children with neutrophil-predominant severe asthma have pro-inflammatory neutrophils with altered function and enhanced survival, as well as pro-inflammatory airway macrophages with impaired innate immune functions that may perpetuate airway inflammation and susceptibility to respiratory infection.
How does this study impact current management guidelines?
Therapeutic strategies targeting neutrophils and neutrophil activation pathways may be beneficial in some children with severe asthma refractory to standard treatment approaches.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Children’s Healthcare of Atlanta and Emory University Pediatric Flow Cytometry Core for the provision of flow cytometry equipment utilized for this study.
ABBREVIATIONS
- BAL
Bronchoalveolar lavage
- CCL
Chemokine (C-C motif) ligand
- CXCL
Chemokine (C-X-C motif) ligand
- ELISA
Enzyme-linked immunosorbent assay
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- ICS
Inhaled corticosteroid
- IgE
Immunoglobulin E
- IL
Interleukin
- LPS
Lipopolysaccharide
- NET
Neutrophil extracellular trap
- PARP-1
Poly [ADP-ribose] polymerase 1
- PMA
Phorbol 12-myristate 13-acetate
- RFU
Relative fluorescent unit
- TGF-β1
Transforming growth factor beta-1
- TNFα
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and Organizational Disclosures:
Jocelyn R. Grunwell: Nothing to disclose.
Susan T. Stephenson: Nothing to disclose
Rabindra Tirouvanziam: Nothing to disclose
Lou Ann S. Brown: Nothing to disclose
Milton R. Brown: Nothing to disclose
Anne M. Fitzpatrick: Nothing to disclose
REFERENCES
- 1.Opina MT, Moore WC. Phenotype-Driven Therapeutics in Severe Asthma. Curr Allergy Asthma Rep 2017; 17:10. [DOI] [PubMed] [Google Scholar]
- 2.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133:1557–63 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi S, Hoffman EA, Wenzel SE, Castro M, Fain S, Jarjour N, et al. Quantitative computed tomographic imaging-based clustering differentiates asthmatic subgroups with distinctive clinical phenotypes. J Allergy Clin Immunol 2017; 140:690–700 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010; 125:1028–36 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruijnzeel PL, Uddin M, Koenderman L. Targeting neutrophilic inflammation in severe neutrophilic asthma: can we target the disease-relevant neutrophil phenotype? J Leukoc Biol 2015; 98:549–56. [DOI] [PubMed] [Google Scholar]
- 6.Cox G Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol 1995; 154:4719–25. [PubMed] [Google Scholar]
- 7.Zhang X, Moilanen E, Kankaanranta H. Beclomethasone, budesonide and fluticasone propionate inhibit human neutrophil apoptosis. Eur J Pharmacol 2001; 431:365–71. [DOI] [PubMed] [Google Scholar]
- 8.Fleming L, Murray C, Bansal AT, Hashimoto S, Bisgaard H, Bush A, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J 2015; 46:1322–33. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart L, Blood Institute Severe Asthma Research P. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol 2006; 118:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011; 127:382–9 e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG, National Institutes of Health/National Heart L, et al. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol 2010; 125:851–7 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol 2012; 129:974–82 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saglani S, Lloyd CM. Eosinophils in the pathogenesis of paediatric severe asthma. Curr Opin Allergy Clin Immunol 2014; 14:143–8. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien CE, Tsirilakis K, Santiago MT, Goldman DL, Vicencio AG. Heterogeneity of lower airway inflammation in children with severe-persistent asthma. Pediatr Pulmonol 2015; 50:1200–4. [DOI] [PubMed] [Google Scholar]
- 15.Guiddir T, Saint-Pierre P, Purenne-Denis E, Lambert N, Laoudi Y, Couderc R, et al. Neutrophilic Steroid-Refractory Recurrent Wheeze and Eosinophilic Steroid-Refractory Asthma in Children. J Allergy Clin Immunol Pract 2017; 5:1351–61 e2. [DOI] [PubMed] [Google Scholar]
- 16.Andersson CK, Adams A, Nagakumar P, Bossley C, Gupta A, De Vries D, et al. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol 2017; 139:1819–29 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belvisi MG. Regulation of inflammatory cell function by corticosteroids. Proc Am Thorac Soc 2004; 1:207–14. [DOI] [PubMed] [Google Scholar]
- 18.Schleimer RP. Effects of glucocorticosteroids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respir Dis 1990; 141:S59–69. [PubMed] [Google Scholar]
- 19.Grunwell JR, Yeligar SM, Stephenson S, Ping XD, Gauthier TW, Fitzpatrick AM, et al. TGF-beta1 Suppresses the Type I IFN Response and Induces Mitochondrial Dysfunction in Alveolar Macrophages. J Immunol 2018; 200:2115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184:602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sil P, Yoo DG, Floyd M, Gingerich A, Rada B. High Throughput Measurement of Extracellular DNA Release and Quantitative NET Formation in Human Neutrophils In Vitro. J Vis Exp 2016; June 18:112 Doi: 10.3791/52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 2008; 121:1372–8, 8 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Israel E, Reddel HK. Severe and Difficult-to-Treat Asthma in Adults. N Engl J Med 2017; 377:965–76. [DOI] [PubMed] [Google Scholar]
- 26.Moore WC, Fitzpatrick AM, Li X, Hastie AT, Li H, Meyers DA, et al. Clinical heterogeneity in the severe asthma research program. Ann Am Thorac Soc 2013; 10 Suppl:S118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol 2016; 138:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray A, Kolls JK. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol 2017; 38:942–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hastie AT, Steele C, Dunaway CW, Moore WC, Rector BM, Ampleford E, et al. Complex association patterns for inflammatory mediators in induced sputum from subjects with asthma. Clin Exp Allergy 2018; 48:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasaian MT, Lee J, Brennan A, Danto SI, Black KE, Fitz L, et al. Proteomic analysis of serum and sputum analytes distinguishes controlled and poorly-controlled asthmatics. Clin Exp Allergy 2018; 48:814–24. [DOI] [PubMed] [Google Scholar]
- 32.Fu JJ, Baines KJ, Wood LG, Gibson PG. Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. OMICS 2013; 17:187–99. [DOI] [PubMed] [Google Scholar]
- 33.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 1995; 95:843–52. [DOI] [PubMed] [Google Scholar]
- 34.Baines KJ, Simpson JL, Bowden NA, Scott RJ, Gibson PG. Differential gene expression and cytokine production from neutrophils in asthma phenotypes. Eur Respir J 2010; 35:522–31. [DOI] [PubMed] [Google Scholar]
- 35.Pham DL, Ban GY, Kim SH, Shin YS, Ye YM, Chwae YJ, et al. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin Exp Allergy 2017; 47:57–70. [DOI] [PubMed] [Google Scholar]
- 36.Tak T, Hilvering B, Tesselaar K, Koenderman L. Similar activation state of neutrophils in sputum of asthma patients irrespective of sputum eosinophilia. Clin Exp Immunol 2015; 182:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright TK, Gibson PG, Simpson JL, McDonald VM, Wood LG, Baines KJ. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016; 21:467–75. [DOI] [PubMed] [Google Scholar]
- 38.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood 2001; 98:1226–30. [DOI] [PubMed] [Google Scholar]
- 39.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 2006; 79:303–11. [DOI] [PubMed] [Google Scholar]
- 40.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010; 116:625–7. [DOI] [PubMed] [Google Scholar]
- 41.Uddin M, Nong G, Ward J, Seumois G, Prince LR, Wilson SJ, et al. Prosurvival activity for airway neutrophils in severe asthma. Thorax 2010; 65:684–9. [DOI] [PubMed] [Google Scholar]
- 42.Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol 2017; 102:343–9. [DOI] [PubMed] [Google Scholar]
- 43.Shaul ME, Levy L, Sun J, Mishalian I, Singhal S, Kapoor V, et al. Tumor-associated neutrophils display a distinct N1 profile following TGFbeta modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 2016; 5:e1232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009; 16:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004; 21:215–26. [DOI] [PubMed] [Google Scholar]
- 46.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 47.in "t Veen JC, Grootendorst DC, Bel EH, Smits HH, Van Der Keur M, Sterk PJ, et al. CD11b and L-selectin expression on eosinophils and neutrophils in blood and induced sputum of patients with asthma compared with normal subjects. Clin Exp Allergy 1998; 28:606–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.