Abstract
Background:
Cervical dystonia is a disabling medical condition that drastically decreases quality of life. Surgical treatment consists of peripheral nerve denervation procedures with or without myectomies or deep brain stimulation (DBS). The current objective was to compare the efficacy of peripheral denervation versus DBS in improving severity of cervical dystonia through a systematic review and meta-analysis.
Methods:
A search of PubMed, MEDLINE, EMBASE and Web of Science electronic databases was conducted in accordance with PRISMA guidelines. Pre- and post-operative Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total scores were used to generate standardized mean differences and 95% confidence intervals, which were combined in a random-effects model. Both mean percentage and absolute reduction in TWSTRS scores were calculated. Absolute reduction was used for forest plots.
Results:
Eighteen studies met inclusion criteria, comprising 870 patients with 180 (21%) undergoing DBS and 690 (79%) undergoing peripheral denervation procedures. Mean follow-up was 31.5 months (range 12–38 months). In assessing efficacy of each intervention, forest plots revealed significant absolute reduction in total post-operative TWSTRS scores for both peripheral denervation (standardized mean difference 1.54; 95% CI 1.42–1.66) and DBS (standardized mean difference 2.07; 95% CI 1.43–2.71). On subgroup analysis, DBS therapy was significantly associated with improvement in post-operative TWSTRS severity (standardized mean difference 2.08; 95% CI 1.66–2.50) and disability (standardized mean difference 2.12; 95% CI 1.57–2.68), but not pain (standardized mean difference 1.18; 95% CI 0.80–1.55).
Conclusions:
Both peripheral denervation and DBS are associated with a significant reduction in absolute TWSTRS total score, with no significant difference in the magnitude of reduction observed between the two treatments. Further comparative data are needed to better evaluate the long-term results of both interventions.
Keywords: DBS, cervical dystonia, selective peripheral degeneration
INTRODUCTION
Cervical dystonia is characterized by abnormal posturing and movement of the head and neck as a result of involuntary, stereotypic contractions of neck and shoulder muscles. Though four primary subtypes exist, including rotational torticollis, antecollis, retrocollis, and laterocollis, presentation is heterogeneous and often is a blend of the subtypes.1 With prevalence estimates ranging from approximately 30 to 180 cases per million people, cervical dystonia is the most common adult-onset focal dystonia.2,3 The disease, often alternatively referred to as spasmodic torticollis, occurs more frequently in women and has its typical onset in the fifth decade of life.1,4–6 Cervical dystonia is associated with significant decrement in quality of life, due to pain, swallowing difficulty, and impairment in the ability to complete activities of daily living.7
To objectively measure impairment, the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) was developed to assess functional impairment secondary to disease progression, by way of the severity, pain and disability subscores.8While botulinum toxin injection has been shown to significantly improve postural symptoms, up to 10% of patients fail to respond to injection therapy; furthermore, medication side effects and need for continued dosing may reduce patient adherence.9,10 Patients that have failed botulinum toxin therapy are classified as either primary (i.e., a response was never observed) or secondary (i.e., the clinical effect was lost following an initial response). Patients with persistent debilitating symptoms despite conservative therapy may be candidates for surgical intervention, which comes in two forms: peripheral denervation with or without myectomies and deep brain stimulation (DBS).
Data comparing the two alternative surgical strategies are lacking and neither clinical superiority of one method over the other nor specific indications for one strategy versus the other are yet to be established. Moreover, studies reporting outcomes associated with each of these procedures have been inconsistent in the method of reporting and the specific rating scales used, which has limited the ability to make direct inter-study comparisons of primary outcomes following both DBS and peripheral denervation. This lack of consensus of superiority of either DBS or peripheral denervation to treat cervical dystonia was the impetus for the current analysis. The objective of this study was to conduct a systematic review and meta-analysis of available studies to compare the efficacy of DBS versus peripheral denervation, utilizing absolute improvement in the total TWSTRS score (i.e., the sum of the severity, pain, and disability subscores) as the primary outcome.
METHODS
Literature search strategy
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, MEDLINE, EMBASE and Web of Science electronic databases were searched from inception until November 2017 for English language studies. The following terms were combined to maximize search strategy: cervical dystonia, deep brain stimulation, peripheral denervation, surgery, torticollis.
Selection criteria
Inclusion criteria for eligible studies were: a) utilization of Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) outcome scores and b) reporting of mean and standard deviations of total post-operative TWSTRS scores and/or post-operative severity, pain and disability scores. Case reports, conference presentations, editorials, reviews, non-English studies, studies that included less than 5 patients and studies that combined both DBS and peripheral denervation surgery sequentially in the same patients were excluded.
Independent screening of all available titles and abstracts was performed by two reviewers using the pre-defined inclusion and exclusion criteria (NGK, SLZ). Full-text review of articles identified in screening was then performed by two reviewers (KR, SLZ), with application of inclusion criteria. Disagreement was resolved through inter-reviewer discussion.
Data extraction
Data extraction from articles was conducted using a standardized form. Information collected included study characteristics, baseline patient characteristics, prior pharmacological treatment and botulinum toxin responder status, procedural details of intervention, pre- and post-operative TWSTRS pain, severity, disability and total scores, procedural complications and medication changes post-intervention. Consultation between senior authors (DJE, TJW) was used to resolve discrepancies in data extraction.
Statistical analysis
For each study, effect size was determined by calculating standardized mean absolute differences in total TWSTRS and 95% confidence intervals. While both percentage change and total change in TWSTRS scores were calculated, absolute change in total TWSTRS score was used to calculate standardized mean differences and corresponding forest plots. An inverse variance-weighted average of standardized mean differences was used to combine study-specific values, using a random effects model. The degree of heterogeneity across studies was quantitatively determined using the I2 statistic. Low, moderate and high degrees of heterogeneity corresponded with I2 values of 25%, 50% and 75%, respectively. Publication bias was assessed via funnel plot asymmetry and the Egger test. All statistical analyses were conducted using STATA 12 (STATA Corp, College Station, TX).
RESULTS
Search strategy
After removal of duplicates, 399 studies were identified following search of electronic databases (Figure 1). After careful evaluation via screening of titles and abstracts, 29 full-texts were assessed for inclusion in the meta-analysis. Following application of inclusion criteria, a total of 18 articles were suitable for inclusion, with baseline study characteristics presented in Table 1. Of the included 18 studies, the primary intervention was peripheral denervation surgery in 3 studies, while the remaining 15 studies utilized DBS. The 18 studies comprised 870 patients; 180 underwent DBS and 690 underwent peripheral denervation. The paradox between number of studies and patients is largely due to a single peripheral denervation study of 648 patients.11 Mean follow-up was 31.5 months (range 12–38 months). Mean percentage reductions in total TWSTRS scores were 53.0% and 39.1% following pooling of DBS and peripheral denervation studies for summary purposes, respectively.
Figure 1.
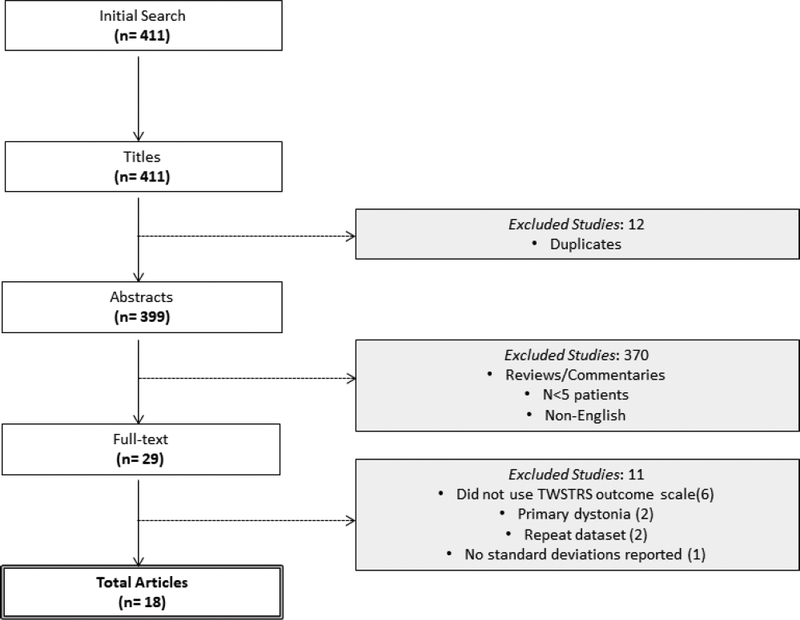
PRISMA flow diagram detailing results of the search and selection of studies
Table 1.
Characteristics of the included 18 studies. Standard deviation detailed where reported.
| First author |
Year | Study design | Intervention | Size | Follow-up duration, months (mean ± SD) | Pre-operative total TWSTRS score (mean ± SD) | Post-operative total TWSTRS score (mean ± SD) | % improvement |
|---|---|---|---|---|---|---|---|---|
| Munchau | 2001 | Prospective cohort study | SPD | 26 | 16.7±12.6 | 47.0±10.0 | 31.0±13.0 | 34 |
| Yianni | 2003 | Retrospective cohort study | DBS | 6 | 18.9 | 57.8±8.2 | 23.0±9.1 | 60 |
| Hung | 2007 | Longitudinal cohort study | DBS | 10 | 31.9±20.9 | 53.7±17.2 | 23.2±11.6 | 57 |
| Kiss | 2007 | Prospective single-blind study |
DBS | 10 | 12 | 14.7±4.2§ | 8.4±441§ | NR* |
| Jeong | 2009 | Consecutive case series | DBS | 6 | 18.7±11.1 | 60.5±3.6 | 15.8±4.3 | 74 |
| Moro | 2009 | Crosssectional study | DBS | 8 | 28.6±19.2 | 24.4±4.1§ | 11.1 ±2.6 § | 57§ |
| Huh | 2010 | Retrospective cohort study | DBS | 24 | 29.5±18.6 | 25.6±8.9§ | 10.03±4.1§ | 38§ |
| Cacciola | 2010 | Consecutive case series | DBS | 10 | 37.6 ±16.9 | 55.7±8.3 | 17.6±13.4 | 68 |
| Yamada | 2013 | Retrospective cohort study | DBS | 7 | 63.5 ±38.2 | 21.3±4.9§ | 6.4±5.7§ | NR* |
| Schjerling | 2013 | Randomized crossover trial | DBS | 6 | 6 | 23.5±5.3§ | 14.5±9.6§ | NR* |
| Sadnicka | 2013 | Case-control study |
DBS | 11 | 26±15 | 50.0±12.2 | 18.0±19.4 | 64 |
| Witt | 2013 | Retrospective cohort study | DBS | 28 | 33.7±25.0 | 53.5±10.3 | 24.2±15 | 55 |
| Walsh | 2013 | Prospective single-blind cohort |
DBS | 10 | 21.5±4.6 | 54.5±12.4 | 29.0±14.4 | 47 |
| Volkmann | 2014 | Randomized sham- controlled trial |
DBS | 32 | 19.9±3.7 | 45.9±9.9 | 27.8±14.5 | 40 |
| Ostrem | 2014 | Prospective cohort study | DBS | 7 | 12 | 36.0±9.7 | 31.8±8.8 | 12 |
| Chung | 2015 | Retrospective cohort study | SPD | 16 | 19.9±11.5 | 24.9±8.0 | 14.9±3.6 | 40 |
| Wang | 2015 | Retrospective cohort study | SPD | 648 | 33.4 | 54.7±18.3 | 31.1±11.6 | 43 |
| Ostrem | 2017 | Prospective cohort study | DBS | 16 | 36 | 41.0±18.9 | 18.8±14.8 | 54 |
TWSTRS severity sub-scores
not reported: percentage improvement unable to calculated from study data
Efficacy of DBS and peripheral denervation
Of 15 included DBS studies, 10 reported total TWSTRS scores. In assessing efficacy of each intervention, forest plots revealed significant improvement in total TWSTRS scores post-operatively for both peripheral denervation (standardized mean difference 1.54; 95% CI 1.42–1.66; p<0.001) and DBS (standardized mean difference 2.07; 95% CI 1.43–2.71; p<0.001) (Figure 2). There was significant heterogeneity amongst pooled studies noted overall (I2=67%, p<0.0001) and DBS studies (I2=74%, p<0.0001), but little heterogeneity amongst peripheral denervation studies, (I2=0%, p=0.826). The known heterogeneity in the disorder of cervical dystonia in conjunction with this statistical heterogeneity thus supports, in part, use of a random effects model compared to a fixed effects model for analysis. In all studies except one, the study effect size was greater than 1.
Figure 2.
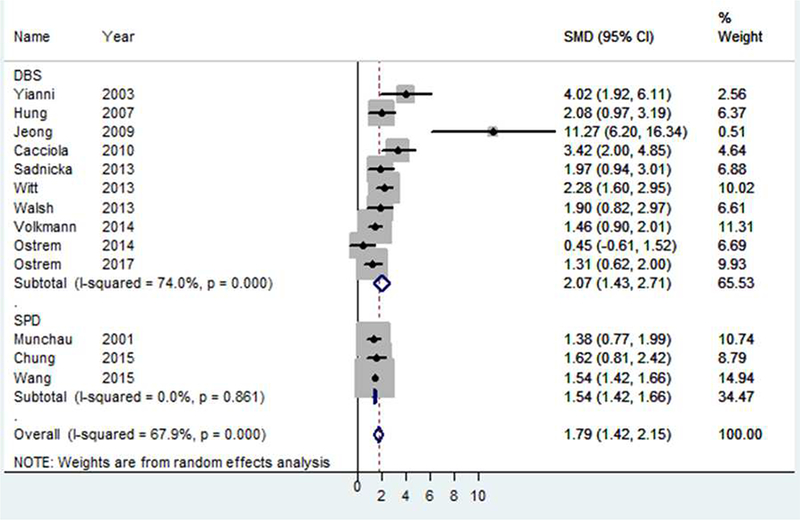
Forest plot demonstrating the effect of DBS and peripheral denervation on total TWSTRS scores.
Effect of DBS on TWSTRS severity subscore
Among the 15 included DBS studies, all reported pre- and post-operative TWSTRS severity subscores. DBS was significantly associated with improvement in post-operative TWSTRS severity subscore (standardized mean difference 2.08; 95% CI 1.66–2.50). Significant heterogeneity was noted (I2=56%, p=0.004) (Figure 3).
Figure 3.
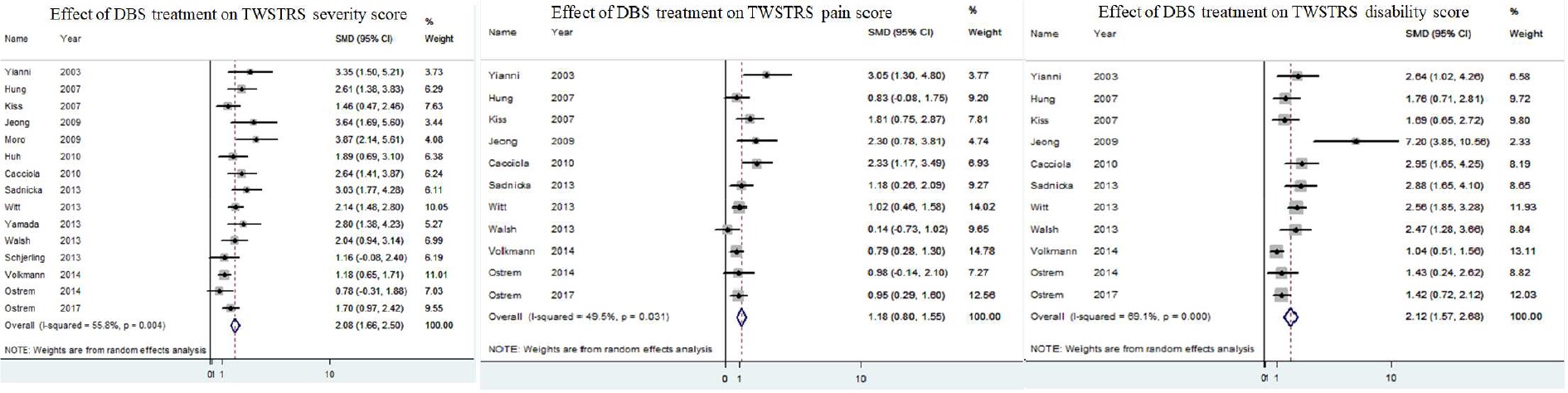
Forest plots demonstrating effect of DBS on TWSTRS severity (A), pain (B) and disability (C) subscores
Effect of DBS on TWSTRS pain subscore
Pre- and post-operative TWSTRS pain subscores were reported in 11 of 15 included DBS studies. DBS therapy was not significantly associated with an improvement in post-operative TWSTRS pain subscore (standardized mean difference 1.18; 95% CI 0.80–1.55, I2=50%, p=0.03 (Figure 3).
Effect of DBS on TWSTRS disability subscore
In 11 of 15 included DBS studies pre- and post-operative TWSTRS disability subscores were reported. DBS therapy was significantly associated with an improvement in post-operative TWSTRS disability subscore (standardized mean difference 2.12; 95% CI 1.57–2.68, I2=69%, p<0.0001) (Figure 3).
Bias assessment
Any potential publication bias in this study was assessed using both the funnel plot and the Egger test. Funnel plots of both DBS-only studies and pooled studies showed asymmetry, and Egger test for publication bias was significant (p=0.025, 2-tailed) (Figure 4).
Figure 4.
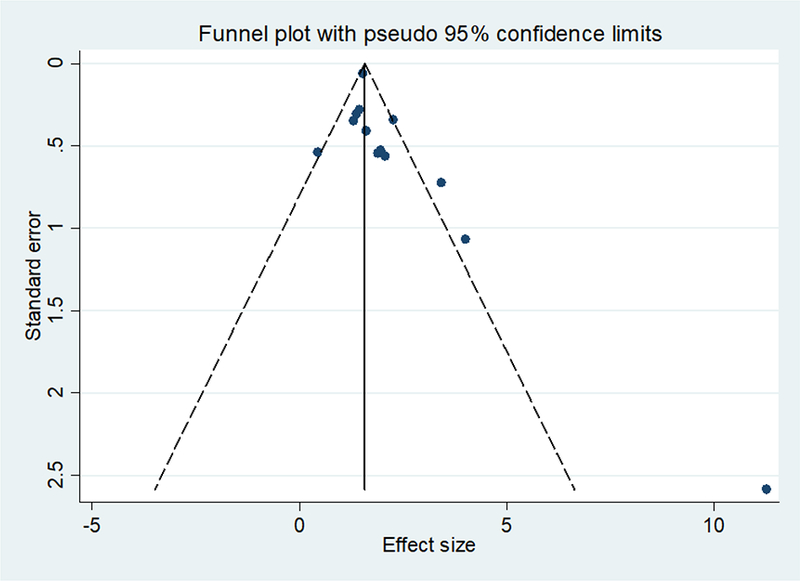
Funnel plot of both DBS-only studies and pooled studies, depicting asymmetry.
DISCUSSION
The current systematic review and meta-analysis sought to assess the relative efficacy of DBS against peripheral denervation in the treatment of cervical dystonia as measured via the absolute reduction in total TWSTRS score. Both DBS and peripheral denervation were associated with post-operative reduction in TWSTRS total score. There was no significant difference in effect size comparing the two treatment modalities. TWSTRS subscores were only able to be analysed for the DBS studies. DBS was associated with a reduction in both severity and disability from the dystonia, but not with decreased pain. These results suggest that both DBS and selective peripheral denervation are effective treatments for cervical dystonia, with neither intervention proving superior to the other.
Originally described at the end of the 19th century, current peripheral denervation techniques are derived from modification of Bertrand and colleagues’ originally described procedure.12,13 Bertrand’s original procedure of extradural denervation has subsequently undergone a variety of modifications. The goal of denervate and/or remove the offending muscles that all of the variations of the procedure is to abnormal posture. The specific muscles to be targeted is guided by the dominant subtype of are contributing to the observed cervical dystonia and the preoperative electromyographic assessment.7 These procedures have shown high short-term efficacy with low morbidity and mortality.11,13,14 The sustainability of clinical effect in the long-term, however, is less definitive. In Bergenheim and co-authors’ long-term (42 months) follow-up of 61 patients who underwent peripheral denervation procedures, the rate of re-innervation was 29%, requiring subsequent re- operation in 26%.14 In another series of 62 patients, the incidence of reinnervation was 25%, with a mean of only 16 months follow-up. In the largest study, Wang and colleagues’ 18-year single-institutional experience with selective peripheral denervation comprising 648 patients (mean follow-up 33.4 months), however, the rate of symptom recurrence was only 4.2%.11
As suggested by Fox and Alterman in their 2015 review, the ability to modulate stimulus to achieve desired clinical effect, reversibility of stimulation and safety of bilateral intervention make DBS an attractive alternative to peripheral denervation. A 2010 meta-regression of published literature on DBS for dystonia identified 67 patients with spasmodic torticollis across 14 studies treated with DBS, in whom mean improvement was 48%, with a standard deviation of 41%.15 The continuing role of selective peripheral denervation for management of botulinum-refractory cervical dystonia has been questioned, in light of these promising results with neurostimulation.16 However, response to DBS therapy is variable. In a 2007 prospective study of bilateral pallidal stimulation in 10 patients, improvement in dystonia severity ranged from 20% to 83%.17 Reduced morbidity due to the reversibility of stimulation, though oft-quoted, is not necessarily the case. In the only controlled trial of DBS for cervical dystonia, comparing pallidal stimulation with sham stimulation, 6 out of 62 patients developed permanent dysarthria as a direct result of electrode placement.18
Recent years have seen DBS emerge as an attractive alternative due to its reversibility and the ability to adjust for clinical effect.12,19–21 Advantages still remain for the use of peripheral denervation, not the least of which is that it avoids an intracranial procedure and the associated risk of intracranial hemorrhage, the need for implanted hardware and additionally the requirement for regular follow-up and repeat operations for battery replacement. We believe that our results support the efficacy of both techniques and with the current state of the literature one technique cannot be considered to be superior over the other. However, a number of important factors could not be analysed. We analysed only the effectiveness of each technique using the TWSTRS score as the outcome. Additional outcomes that need to be compared in order to determine the superiority of one technique over the other include the major and minor complication rate and symptom recurrence rate. Furthermore, it may turn out that there are subsets of patients that would benefit from one technique over the other, but other subsets that are more responsive to the opposite technique. The specific indications for each technique need further clarification. For example, could laterocollis be fundamentally different than retrocollis in response to DBS versus peripheral denervation? Furthermore, it is important to remember that the performance of one surgical technique does not necessarily preclude utilizing the other technique in the case of failure. Some of the remaining questions may be best answered in a randomized clinical trial or a large prospective registry.
Several studies were excluded in the present analysis due to lack of TWSTRS score utilization. Additional commonly used rating scales for assessment of cervical dystonia include the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) and the Tsui score, among others. The BFMDRS is not specific for cervical dystonia and the Tsui score has low sensitivity to detect changes.22 In earlier studies of botulinum toxin therapy for cervical dystonia, the Tsui score was the most commonly used rating scale due to its simplicity.23 According to a Movement Disorders Society task force-commissioned guideline, only the TWSTRS and the Cervical Dystonia Impact Scale (CDIP-58) are recommended rating scales for cervical dystonia.22 TWSTRS has been shown to have strong internal validity and inter-rater reliability. Its main criticisms have been the complexity of subscales and applicability to everyday practice, given its inclusion of a videotape protocol.22 While the TWSTRS score provides a better assessment of functional outcomes, it does not include a component for tremor, unlike the Tsui score, and furthermore, is only available in English. Globally, the ability of these scales to detect small changes in clinical effect is limited. In several consecutive surgical series, subjective non-uniform rating of clinical status has been used. In Cohen-Gadol and colleagues’ 2003 series of 168 patients, clinical improvement was graded by a neurologist as either ‘mild’, ‘moderate’ or ‘severe’, while in Bertrand’s original description of his procedure in 207 patients, improvement was stratified from ‘excellent’ to ‘poor’.13 Only one study across both denervation and DBS studies has formally included a TWSTRS score cut-off as inclusion for intervention.18
The current analysis is limited in several ways. First, a small number of studies was included due to specific inclusion criteria. Second, the included studies largely comprised single arm, retrospective, observational studies. Few comparison studies of both procedures exist. Indeed, only three studies have directly compared outcomes between peripheral denervation and DBS, all of which were weakened by retrospective design and small sample size, identifying a clear need in the literature.24–26 Third, significant imbalance in cases existed between each surgical procedure. Furthermore, limited granularity of data, including lack of TWSTRS score usage and standard deviation reporting, meant that several larger peripheral denervation studies were not eligible for inclusion here.13,14,27 As indicated by both the heterogeneity statistic values and Egger test, there was significant heterogeneity amongst included studies, which is not surprising for meta-analyses of specific surgical procedures. Nonetheless, 6 studies in this analysis had 10 patients or less, amongst which the highest effect sizes were seen. Amongst DBS studies, the highest quantitative effect size was seen with Jeong and coauthors’ study of 6 patients (standardized mean difference 11.27; 95% confidence interval 6.20–16.34), though this study accordingly received the lowest percentage weight. Likely, the very small sample size of this study contributed to its outlier status in the forest plot (Figure 2), amplifying the effect size of DBS treatment. Inclusion of the 2015 peripheral denervation study by Wang et al. further contributed, as this study comprised 648 patients, more than two-thirds of the entire pooled cohort. The ability to perform more rigorous subgroup analysis in order to better ascertain patients more likely to respond to one intervention over the other was thus also limited.
CONCLUSIONS
The results of this study suggest that both DBS and selective peripheral denervation are effective treatments for cervical dystonia. While DBS is effective at reducing symptom severity and disability, its effect on pain reduction is minimal. Though both interventions are associated with clinical advantages and disadvantages, specific sub-populations may preferentially respond to one intervention over the other. As of yet, identification of these patients that may benefit from either DBS or peripheral denervation has not been made. This study thus highlights a need for further studies that may provide greater insight into long-term results of both surgical interventions and better characterize patients more suited for one intervention over the other.
Abbreviations
- DBS:
deep brain stimulation
- SPD:
selective peripheral denervation
- TWSTRS:
Toronto Western Spasmodic Torticollis Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
Disclosure of Funding: none
REFERENCES
- 1.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41(7):1088–1091. [DOI] [PubMed] [Google Scholar]
- 2.Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. The Lancet Neurology. 2004;3(11):673–678. [DOI] [PubMed] [Google Scholar]
- 3.Defazio G, Jankovic J, Giel JL, Papapetropoulos S. Descriptive epidemiology of cervical dystonia. Tremor and other hyperkinetic movements (New York, NY). 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S, Martino D. Cervical dystonia: from pathophysiology to pharmacotherapy. Behavioural neurology. 2013;26(4):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Movement disorders : official journal of the Movement Disorder Society. 2012;27(14):1789–1796. [DOI] [PubMed] [Google Scholar]
- 6.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Movement disorders : official journal of the Movement Disorder Society. 1991;6(2):119–126. [DOI] [PubMed] [Google Scholar]
- 7.Wilson TJ, Spinner RJ. Selective Cervical Denervation for Cervical Dystonia: Modification of the Bertrand Procedure. Operative neurosurgery (Hagerstown, Md). 2018;14(5):546–555. [DOI] [PubMed] [Google Scholar]
- 8.Jost WH, Hefter H, Stenner A, Reichel G. Rating scales for cervical dystonia: a critical evaluation of tools for outcome assessment of botulinum toxin therapy. Journal of neural transmission (Vienna, Austria : 1996). 2013;120(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jankovic J, Schwartz K. Response and immunoresistance to botulinum toxin injections. Neurology. 1995;45(9):1743–1746. [DOI] [PubMed] [Google Scholar]
- 10.Kessler KR, Skutta M, Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. German Dystonia Study Group. Journal of neurology. 1999;246(4):265–274. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Li J, Han L, et al. Selective peripheral denervation for the treatment of spasmodic torticollis: long-term follow-up results from 648 patients. Acta Neurochir (Wien). 2015;157(3):427–433; discussion 433. [DOI] [PubMed] [Google Scholar]
- 12.Bertrand CM. Selective peripheral denervation for spasmodic torticollis: surgical technique, results, and observations in 260 cases. Surg Neurol. 1993;40(2):96–103. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Gadol AA, Ahlskog JE, Matsumoto JY, Swenson MA, McClelland RL, Davis DH. Selective peripheral denervation for the treatment of intractable spasmodic torticollis: experience with 168 patients at the Mayo Clinic. J Neurosurg. 2003;98(6):1247–1254. [DOI] [PubMed] [Google Scholar]
- 14.Bergenheim AT, Nordh E, Larsson E, Hariz MI. Selective peripheral denervation for cervical dystonia: long-term follow-up. Journal of neurology, neurosurgery, and psychiatry. 2015;86(12):1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. Journal of neurology, neurosurgery, and psychiatry. 2010;81(12):1383–1389. [DOI] [PubMed] [Google Scholar]
- 16.Warnke PC. Selective peripheral denervation for cervical dystonia: a historical procedure in the age of deep brain stimulation? Journal of neurology, neurosurgery, and psychiatry. 2015;86(12):1283. [DOI] [PubMed] [Google Scholar]
- 17.Hung SW, Hamani C, Lozano AM, et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68(6):457–459. [DOI] [PubMed] [Google Scholar]
- 18.Volkmann J, Mueller J, Deuschl G, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. The Lancet Neurology. 2014;13(9):875–884. [DOI] [PubMed] [Google Scholar]
- 19.Fox MD, Alterman RL. Brain Stimulation for Torsion Dystonia. JAMA neurology. 2015;72(6):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munchau A, Palmer JD, Dressler D, et al. Prospective study of selective peripheral denervation for botulinum-toxin resistant patients with cervical dystonia. Brain : a journal of neurology. 2001;124(Pt 4):769–783. [DOI] [PubMed] [Google Scholar]
- 21.Ostrem JL, Markun LC, Glass GA, et al. Effect of frequency on subthalamic nucleus deep brain stimulation in primary dystonia. Parkinsonism Relat Disord. 2014;20(4):432–438. [DOI] [PubMed] [Google Scholar]
- 22.Albanese A, Sorbo FD, Comella C, et al. Dystonia rating scales: critique and recommendations. Movement disorders : official journal of the Movement Disorder Society. 2013;28(7):874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarsy D Comparison of clinical rating scales in treatment of cervical dystonia with botulinum toxin. Mov Disord. 1997;12(1):100–102. [DOI] [PubMed] [Google Scholar]
- 24.Contarino MF, Van Den Munckhof P, Tijssen MA, et al. Selective peripheral denervation:comparison with pallidal stimulation and literature review. Journal of neurology. 2014;261(2):300–308. [DOI] [PubMed] [Google Scholar]
- 25.Jeong SG, Lee MK, Kang JY, Jun SM, Lee WH, Ghang CG. Pallidal deep brain stimulation in primary cervical dystonia with phasic type : clinical outcome and postoperative course. Journal of Korean Neurosurgical Society. 2009;46(4):346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung M, Han I, Chung SS, Jang DK, Huh R. Effectiveness of selective peripheral denervation in combination with pallidal deep brain stimulation for the treatment of cervical dystonia. Acta neurochirurgica. 2015;157(3):435–442. [DOI] [PubMed] [Google Scholar]
- 27.Krauss JK, Toups EG, Jankovic J, Grossman RG. Symptomatic and functional outcome of surgical treatment of cervical dystonia. Journal of neurology, neurosurgery, and psychiatry. 1997;63(5):642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]


