Abstract
Endothelial dysfunction and vascular leak, pathogenic hallmarks of severe dengue disease, are directly triggered by the dengue virus (DENV) nonstructural protein 1 (NS1). Previous studies have shown that immunization with NS1, as well as passive transfer of NS1-immune serum or anti-NS1 monoclonal antibodies, prevent NS1-mediated lethality in vivo. In this study, we evaluated the immunogenicity and protective capacity of recombinant DENV NS1 administered with cyclic dinucleotides (CDNs), potent activators of innate immune pathways and highly immunogenic adjuvants. Using both wild-type C57BL/6 mice and IFN-α/β receptor–deficient (Ifnar−/−) mice, we show that NS1-CDN immunizations elicit serotype-specific and cross-reactive antibody and T-cell responses. Further, NS1-CDN vaccinations conferred significant homotypic and heterotypic protection from DENV2-induced morbidity and mortality. In addition, we demonstrate that high anti-NS1 antibody titers are associated with protection, supporting the role of humoral responses against DENV NS1 as correlates of protection. These findings highlight the potential of CDN-based adjuvants for inducing antibody and T-cell responses and validate NS1 as an important candidate for dengue vaccine development.
Keywords: dengue virus, NS1, cyclic dinucleotide, vaccine, adjuvant
INTRODUCTION
The four dengue virus serotypes (DENV1–4) are transmitted by Aedes aegypti and Ae. albopictus mosquitoes, causing up to an estimated 390 million infections and 96 million cases of dengue annually (1). DENV is an enveloped flavivirus with 3 structural (C, capsid; prM/M, membrane; E, envelope) and 7 non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5) proteins. Human DENV infections can range from asymptomatic to dengue fever (DF) to the potentially lethal dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). During a secondary infection with DENV, antibodies to the E protein may trigger antibody-dependent enhancement (ADE), where they facilitate entry of DENV into Fcγ receptor-bearing cells, leading to increased viral load, immune activation, and more severe disease (2–4). In contrast, since NS1 is not a structural component of the DENV virion, it is reasoned that antibodies to NS1 should not enhance viral uptake.
As an immunogen, formulations based on recombinant NS1 induce strong immune responses and confer protection in rodent models of dengue disease (5–8). Further, passive transfer of polyclonal sera from NS1-immunized mice or anti-NS1 monoclonal antibodies into naïve mice conferred protection against vascular leak disease, likely by promoting lysis of infected cells and/or by blocking the pathogenic effects of secreted NS1 (5, 9, 10). Importantly, NS1 is approximately 64–79% conserved across the four DENV serotypes (11, 12), and immunodominant regions of this protein have been identified in both NS1-immunized and DENV-infected mice, as well as in naturally infected humans (10, 13–16). The role of NS1-specific CD4+ T cells against DENV infection is a topic of active investigation, and studies have provided evidence of the likely protective effect of these cells (17–20). Taken together, these findings support further research of NS1 as an important antigen for dengue vaccine development.
One of the main challenges of nonreplicating vaccines is the use of adjuvants capable of eliciting strong memory T cells and protective antibodies (21, 22). Cyclic dinucleotides (CDNs) are ubiquitous second messengers synthesized by bacteria, which are capable of activating the cytosolic receptor stimulator of interferon genes (STING), resulting in the activation of different immune pathways (23–27). Due to their immunostimulatory properties, CDNs were initially used as vaccine adjuvants to elicit protective antibody and T-cell responses against pathogenic extracellular bacteria (28–30). However, more recent studies have shown that CDN compounds also have a significant capacity to induce potent anti-tumor responses (31–33) and to elicit protective Th1 and Th17 cellular immune responses against Mycobacterium tuberculosis infection (34).
In this study, we evaluated the immunogenicity of DENV NS1 proteins together with CDN compounds in comparison to monophosphoryl lipid A (MPLA) adjuvant, a TLR4 agonist capable of eliciting strong Th1 responses and high antibody titers (35–38) that has been approved for human use in vaccines for hepatitis B virus and human papillomavirus infection (39, 40). Using both wild-type C57BL/6 and IFN-α/β receptor–deficient C57BL/6 (Ifnar−/−) mice, we measured IgG titers and T-cell responses after immunizations with each NS1 from all four DENV serotypes. We found that NS1-CDN vaccinations induced balanced antibody responses against all DENV NS1 serotypes, which were comparable or higher in magnitude to those elicited by NS1-MPLA immunizations. Further, NS1-CDN immunized mice developed serotype-specific and cross-reactive T-cell responses, greater than MPLA-adjuvanted NS1, underscoring the ability of CDN compounds to induce cellular immunity. Finally, using a mouse model of lethal DENV infection, we show that NS1 combined with CDNs confers significant protection against DENV–induced morbidity and mortality.
MATERIALS AND METHODS
Ethics statement.
All experimental procedures involving the use of animals were pre-approved and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of California, Berkeley.
Mice.
Wild-type C57BL/6 (WTB6) and IFN-α/β receptor–deficient C57BL/6 (Ifnar−/−) mice were bred and co-housed in specific pathogen-free conditions at the University of California, Berkeley Animal Facility. Five- to 8-week-old male and female mice were used for all experimental procedures.
Recombinant NS1 proteins.
Recombinant NS1 proteins from DENV serotypes 1 (Nauru/Western Pacific/1974), 2 (Thailand/16681/84), 3 (Sri Lanka D3/H/IMTSSA-SRI/2000/1266) and 4 (Dominica/814669/1981) were purchased from the Native Antigen Company (Oxford, UK).
Viruses.
DENV2 D220 was generated in our laboratory from the parental strain DENV2 PL046 (41). The virus was propagated in Aedes albopictus C6/36 cell line [American Type Culture Collection (ATCC)] and titered by plaque assay on baby hamster kidney cells (BHK21, clone 15). The parental strain DENV2 PL046 was obtained originally from H.-Y. Lei (National Cheng Kung University, Taiwan).
NS1 immunizations.
Mice were immunized subcutaneously 2 times (study days 0 and 21) with 20 μg of NS1 or OVA in combination with 5–15 μg cyclic dinucleotide (CDN) compounds or 1 μg Monophosphoryl Lipid A (MPLA), each formulated in AddaVax (0.5% sorbitan trioleate, 5% squalene, 0.5% Tween-80 in 10 mM sodium citrate buffer). CDN compounds dithio-(Rp,Rp)-[cyclic[G(2’,5’)pA(3’,5’)p]] (mixed linkage dithio cyclic guanosine monophosphate-adenosine monophosphate, or ML-RR-cGAMP), and a derivative of this compound consisting of cyclic [G(2’,5’)pA(3’,5’)p] comprising Rp,Rp-bisphosphorothiolate linkages (ML-RR-cGAMP-D) were produced by Aduro Biotech, Inc. as previously reported (31, 34, 42). MPLA from Salmonella minnesota R595 and AddaVax were acquired from InvivoGen (San Diego, CA).
Anti-NS1 ELISA.
Antibody responses induced by NS1 were evaluated 1 week following the second immunization. Blood samples were collected via submandibular bleed to evaluate polyclonal sera against recombinant NS1 by ELISA. Briefly, MaxiSorp® ELISA plates (Thermo Scientific Nunc) were coated with 50 μl of NS1 (0.5 μg/ml) and incubated overnight at room temperature. After blocking with 1% BSA in PBS (1% BSA-PBS), wells were incubated for 1 hour at room temperature with 100 μl of serial dilutions of polyclonal sera. Plates were then washed and incubated for 1 hour at room temperature with peroxidase-labeled goat anti-mouse secondary antibodies (Jackson ImmunoResearch) at 0.5 μg/ml in 1% BSA-PBS. After a washing step, the assay was developed using an ABTS-HRP substrate kit (KPL), according to the manufacturer’s specifications. Antibody titers are reported as area under the curve, calculated using GraphPad Prism 6 software.
T-cell assays.
T-cell responses in mouse spleen were evaluated 1 week after the second immunization. For ELISpot assays, plate wells were coated with anti-mouse IFN-γ antibody (BD Biosciences) and incubated overnight at 4°C. Plates were then blocked with tissue culture media (RMPI-1640, 10% fetal calf serum and 5% Penicillin-Streptomycin), and single-cell suspensions (2 × 105 cells/well) were incubated with 1 μg/mL NS1 protein, 1 μM OVA CD4 peptide (265TEWTSSNVMEERKIKV280), or 1 μM OVA CD8 peptide (257SIINFEKL264) for a minimum of 18 hours at 37°C. Biotinylated anti-IFN-γ antibody (BD Biosciences) was then added, followed by a washing step and addition of streptavidin–conjugated alkaline phosphatase. The assay was developed with nitro-blue tetrazolium and 5-bromo-4-chloro-3’-indolyphosphate substrate (ThermoFisher Scientific). For intracellular cytokine staining (ICS), 2 × 106 cells were incubated at 37°C for 1 hour with 1 μg/mL DENV NS1 protein, followed by 4 hours in the presence of 1 μg/ml brefeldin A (GolgiPlug, BD Biosciences) and 2 μM monensin (GolgiStop, BD Biosciences). Cells were stained with the viability dye Zombie Green (BioLegend), CD8a-BUV395, CD4-BUV737, Ly6G-FITC, CD90.2 V500 (BD Biosciences), and MHCII-FITC (BioLegend). Cells were then treated with Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences) and stained with IL-2 PE, TNF-α-PEcy7, IFN-γ APC/647 (BioLegend).
In vitro assessment of antibody-dependent enhancement (ADE).
Serial dilutions of polyclonal sera were mixed with DENV2 D220 for 1 hour at 37° C and then mixed with human erythroleukemic K562 cells at an MOI of 1 for 24 hours in a 96-well plate. Cells were then washed with FACS buffer and fixed in 2% paraformaldehyde for 10 min at room temperature. Cells were stained using 2.5 μg/ml 4G2-Alexa 488 (Invitrogen) upon permeabilization with FACS buffer containing 0.1% saponin (Sigma Aldrich). K562 cells were then washed, and the percentage of infection was determined using Guava flow cytometer (EMD Millipore) by gating Alexa 488-positive cells.
Dengue virus challenge experiments.
To evaluate the protective capacity of DENV NS1-CDN vaccinations, immunized Ifnar−/− mice were challenged with 5 μg of 4G2 (anti-Envelope mAb) 20–24 hours prior to infection with 3 × 105 PFU of DENV2 D220. Virus challenge was administered by intravenous (i.v.) injection 4 weeks after the second immunization, and mice were monitored every 12 hours using a morbidity scoring system on a scale of 1 to 5 (41). Mice were immediately euthanized when they became moribund (score of 5).
Statistical analysis.
Data were analyzed and plotted using GraphPad Prism 6 software. Differences in antibody and T-cell responses between two treatment groups were evaluated using a two-sided unpaired Student t test. Comparison of survival rates was conducted using a log-rank (Mantel-Cox) test and graphed as Kaplan-Meier survival curves.
RESULTS
CDN compounds induce robust anti-NS1 antibody and T-cell responses
To characterize immune responses elicited by NS1 vaccination, wild-type C57BL/6 (WTB6) and Ifnar−/− mice were immunized with DENV2 NS1 in combination with the CDN compound ML-RR-cGAMP, a stable, lipophilic derivative of the endogenous mammalian cyclic dinucleotide ML-cGAMP, or MPLA, a TLR4 agonist, each formulated in a squalene-in-water emulsion (AddaVax). As determined by ELISA, WTB6 animals immunized with NS1 in combination with CDN compounds developed higher total IgG responses compared to those vaccinated with MPLA. However, total IgG titers in Ifnar−/− mice were comparable among all NS1 immunized groups, irrespective of the adjuvant used. Characterization of IgG subclass titers revealed higher Th1-associated IgG2b and IgG2c isotype responses in WTB6 animals immunized with NS1 and ML-RR-cGAMP or a ML-RR-cGAMP derivative (ML-RR-cGAMP-D), although MPLA induced higher IgG2b titers in Ifnar−/− mice. Further, the two CDN compounds and MPLA induced comparable anti-DENV2 NS1 IgG1 and IgG3 titers in WTB6 and in Ifnar−/− mice (Fig. 1A, 1D). ELISpot analysis of T-cell responses in spleen showed higher IFN-γ production in mice immunized with NS1 in combination with ML-RR-cGAMP or ML-RR-cGAMP-D as compared to MPLA-NS1-vaccinated animals (Fig. 1B, 1E). A similar trend was observed by ICS analysis, which also revealed higher TNF-α and IL-2 production as a result of CDN-NS1 immunizations, indicating a polyfunctional T-cell activation status (Fig. 1C, 1F). In addition, immunizations with ML-RR-cGAMP or ML-RR-cGAMP-D in combination with OVA induced significantly higher CD4+ and CD8+ T-cell responses in both WTB6 and Ifnar−/− mice (Supplementary Fig. 1).
FIGURE 1. ML-RR-cGAMP and ML-RR-cGAMP-D induce significant antibody and T-cell responses against DENV2 NS1 in WTB6 and Ifnar−/− mice.
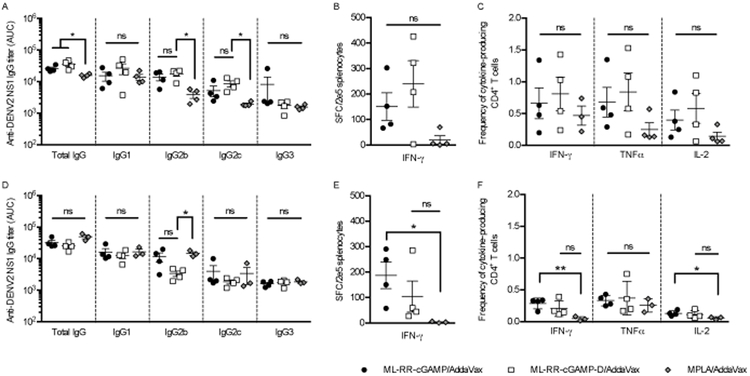
Mice were immunized twice with 20 μg of DENV2 NS1 in combination with ML-RR-cGAMP, ML-RR-cGAMP-D or MPLA, each formulated in AddaVax, and immune responses were evaluated 1 week following the second immunization. Anti-NS1 IgG titers were measured by direct ELISA using sera from WTB6 (A) and Ifnar−/− (D) mice. Calculations of area under the curve (AUC) based on serially diluted serum samples are shown. Antigen-specific IFN-γ responses induced by recombinant DENV2 NS1 were measured by ELISpot with splenocytes from WTB6 (B) and Ifnar−/− (E) mice. Frequencies of DENV2 NS1-specific IFN-γ−, TNF-α− and IL-2-producing CD4+ T cells in the spleen of WTB6 (C) and Ifnar−/− (F) mice were measured by ICS. Data are representative of 2 independent experiments (mean ± SEM; n=3–4; * p<0.05, ** p<0.01).
ML-RR-cGAMP-D elicits balanced anti-NS1 humoral responses against all DENV NS1 serotypes and induces a Th1-associated antibody profile in WTB6 mice
To evaluate the effect of CDNs on the induction of serotype-specific and cross-reactive DENV NS1 antibody responses, mice were immunized with NS1 from DENV serotypes 1–4 in combination with ML-RR-cGAMP-D or MPLA. Antibody responses in polyclonal sera were measured by ELISA using recombinant NS1 from all 4 DENV serotypes. In general, immunization with DENV NS1 in combination with ML-RR-cGAMP-D or MPLA resulted in comparable anti-NS1 responses in both WTB6 and Ifnar−/− mice. However, WTB6 mice immunized with DENV3 NS1 in combination with ML-RR-cGAMP-D developed higher anti-DENV1 NS1 and anti-DENV3 NS1 IgG titers than when MPLA was used as an adjuvant. Similarly, DENV2 NS1-ML-RR-cGAMP-D-immunized WTB6 animals generated higher anti-DENV2 NS1 titers than DENV2 NS1-MPLA-immunized mice (Fig. 2A). In Ifnar−/− mice, vaccination with DENV1 NS1 or DENV4 NS1 adjuvanted with ML-RR-cGAMP-D resulted in higher antibody titers against DENV1 NS1 than those elicited with MPLA formulations. Further, Ifnar−/− mice immunized with DENV4 NS1-ML-RR-cGAMP-D had higher anti-DENV3 NS1 IgG titers than DENV4 NS1-MPLA-immunized mice. In contrast, Ifnar−/− mice vaccinated with DENV3 NS1-MPLA developed higher anti-DENV1 NS1 titers than those immunized with DENV3 NS1-ML-RR-cGAMP-D (Fig. 2B).
FIGURE 2. ML-RR-cGAMP-D induces antibody responses against all four DENV NS1 serotypes and skews immunity toward the Th1 phenotype in WTB6 mice.
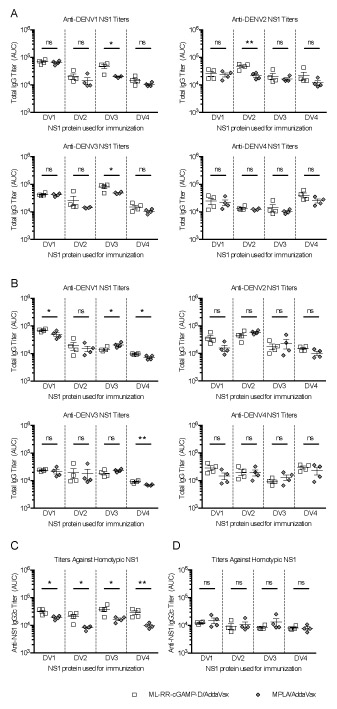
Mice were immunized twice with 20 μg of NS1 from each DENV serotype in combination with ML-RR-cGAMP-D or MPLA formulated in AddaVax. Total IgG titers against NS1 from DENV1–4 were assessed by direct ELISA 1 week after the second immunization in WTB6 (A) and Ifnar−/− (B) mice. Anti-NS1 IgG2c responses in WTB6 (C) and Ifnar−/− (D) mice were used as surrogate markers of Th1 responses. Titers are shown as area under the curve (AUC) based on serially diluted serum samples. Data are representative of 2 independent experiments (mean ± SEM; n=4; * p<0.05, ** p<0.01).
To further characterize humoral responses induced by NS1-ML-RR-cGAMP-D immunization, we quantified anti-NS1 IgG subclass responses. Notably, IgG2c responses, typically associated with skewing of immune responses toward a Th1 phenotype, were significantly higher in WTB6 mice immunized with ML-RR-cGAMP-D and NS1, regardless of the DENV NS1 serotype used for immunization (Fig. 2C). This trend, however, was not observed in Ifnar−/− mice (Fig. 2D). Compared to MPLA-adjuvanted NS1 immunization, IgG1 titers were significantly higher in WTB6 mice vaccinated with DENV2 NS1 or DENV3 NS1 in combination with ML-RR-cGAMP-D, although in Ifnar−/− mice higher IgG1 titers were achieved upon DENV1 NS1- or DENV4 NS1-ML-RR-cGAMP-D vaccination (Supplementary Fig. 2A, 2B). In contrast, MPLA-adjuvanted DENV2 NS1 and DENV3 NS1 induced stronger IgG2b responses than those elicited by ML-RR-cGAMP-D-adjuvanted vaccinations in Ifnar−/− mice, although no significant differences were found in WTB6 animals (Supplementary Fig. 2B, 2E). Finally, IgG3 titers elicited by ML-RR-cGAMP-D or MPLA in combination with NS1 were comparable among all DENV NS1 serotypes, in both WTB6 and Ifnar−/− mice (Supplementary Fig. 2C, 2F).
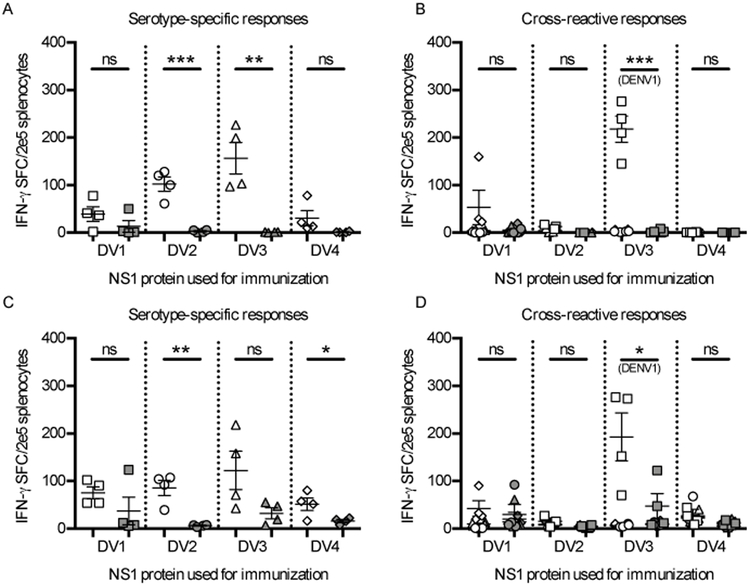
ML-RR-cGAMP-D induces serotype-specific and cross-reactive DENV NS1 T-cell responses
Several studies have established that CDN compounds are effective inducers of T-cell immune responses (reviewed in (27)). To assess the ability of ML-RR-cGAMP-D to elicit cellular immunity against the different DENV serotype NS1 proteins, we measured responses in the spleen of immunized mice using ELISpot assays. In WTB6 mice, immunization with DENV2 NS1 or DENV3 NS1 in combination with ML-RR-cGAMP-D elicited significantly higher IFN-γ serotype-specific production than MPLA-NS1 vaccination (Fig. 3A). A similar trend was observed in Ifnar−/− mice, where DENV2 NS1 and DENV4 NS1 adjuvanted with ML-RR-cGAMP-D induced significantly stronger responses than MPLA-adjuvanted immunization (Fig. 3C). Cross-reactive responses were assessed by stimulating splenocytes with each of the NS1 proteins from the DENV serotypes that were not used for immunization (e.g., splenocytes from mice immunized with DENV1 NS1 were stimulated with NS1 proteins from DENV serotypes 2, 3 and 4). Notably, in both WTB6 and Ifnar−/− mice, immunization with DENV3 NS1 in combination with ML-RR-cGAMP-D resulted in strong IFN-γ responses against DENV1 NS1. However, immunization with NS1 from DENV serotypes 1, 2, and 4 failed to induce considerable cross-reactive responses, and none of the MPLA-adjuvanted immunizations elicited significant IFN-γ production (Fig. 3B, 3D). Finally, ICS analysis of cross-reactive CD4+ T-cell responses from immunized WTB6 mice showed similar results to those from ELISpot assays. Vaccination with DENV2 NS1 or DENV3 NS1 adjuvanted with ML-RR-cGAMP-D elicited significant serotype-specific IFN-γ, TNF-α and IL-2 production, while only DENV3 NS1 immunization resulted in strong cross-reactive responses against DENV1 NS1 (Supplementary Fig. 3).
FIGURE 3. ML-RR-cGAMP-D elicits serotype-specific and cross-reactive anti-NS1 T-cell responses in WTB6 and Ifnar−/− mice.
T-cell responses in the spleen of mice immunized twice with 20 μg of each DENV NS1 serotype in combination with ML-RR-cGAMP-D (white symbols) or MPLA (gray symbols) formulated in AddaVax were characterized by ELISpot assay 1 week after the second immunization. IFN-γ production was measured upon stimulation with NS1 proteins from DENV1 (square), DENV2 (circle), DENV3 (triangle) or DENV4 (diamond). Serotype-specific responses (against the same DENV NS1 serotype used for immunization) and cross-reactive responses (to DENV NS1 serotypes different from the one used for immunization) were measured in WTB6 (A-B) and Ifnar−/− (C-D) mice. Data are representative of 2 independent experiments (mean ± SEM; n=4; * p<0.05, ** p<0.01, *** p<0.001).
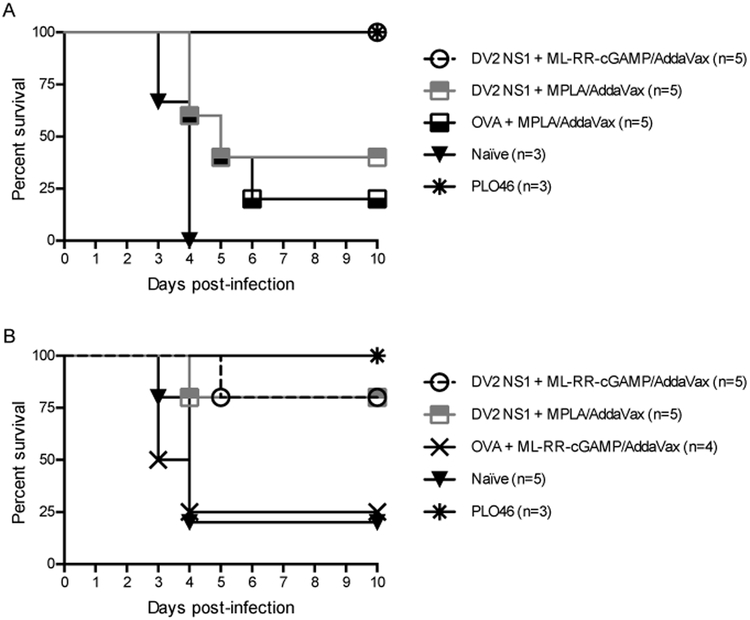
ML-RR-cGAMP-NS1 vaccination confers significant protection against DENV–induced pathogenesis
Given the ability of CDN compounds to induce substantial antibody and T-cell responses, we tested whether immunization with NS1 in combination with ML-RR-cGAMP could prevent lethal DENV disease in Ifnar−/− mice. ML-RR-cGAMP is a potent activator of T-cell immunity, capable of binding all common STING alleles (data not shown). Ifnar−/− mice were immunized two times with 20 μg of DENV1–4 NS1 and ML-RR-cGAMP over a 3-week period (days 0 and 21) and challenged with DENV2 D220 4 weeks after the last immunization (day 49) using antibody-dependent enhancement conditions. NS1-immunized mice showed less signs of DENV-induced morbidity and delayed disease progression compared to OVA-immunized animals and naïve controls (Fig. 4A). Vaccination with DENV1 NS1, DENV2 NS1 or DENV3 NS1 in combination with ML-RR-cGAMP resulted in significant protection compared to OVA-ML-RR-cGAMP-immunized controls or to naïve mice. Indeed, survival among NS1-immunized animals was 60% and 70% for DENV1 NS1 and DENV2 NS1 or DENV3 NS1, respectively, compared to 11% in OVA-immunized mice and 20% among naïve controls. Although only 30% of DENV4 NS1-ML-RR-cGAMP-immunized mice survived lethal DENV infection, mortality was significantly delayed (p < 0.05) compared to OVA-immunized animals. As a positive control, a low-dose (105 PFU) primary homologous infection with the parental DENV2 strain PL046 conferred 100% protection against lethal challenge (Fig. 4B). Finally, in 2 independent experiments, immunizations with ML-RR-cGAMP in combination with DENV2 NS1 conferred similar or greater protection than MPLA-DENV2 NS1 vaccinations against lethal DENV2 D220 infection (Fig. 5).
FIGURE 4. Immunization with DENV NS1 and ML-RR-cGAMP significantly decreases DENV-induced morbidity and mortality in Ifnar−/− mice.
Mice were immunized subcutaneously with 20 μg of each DENV NS1 serotype (n=10) or OVA (n=9) in combination with ML-RR-cGAMP/AddaVax on days 0 and 21, or were infected with a sublethal dose (105 PFU) of DENV2 PL046 (n=6) on day 0. On day 49, mice were challenged intravenously with lethal antibody-enhanced DENV2 D220 infection. (A) Signs of DENV-induced morbidity were assessed daily for 10 days. The percentage of each group of mice displaying the indicated signs is shown. (B) A Kaplan-Meier survival curve is shown. Mice immunized with NS1 were significantly protected compared to OVA controls. Data are pooled from 2 similar experiments (Naïve group, n=10; * p<0.05, ** p<0.01, *** p<0.001).
FIGURE 5. Immunization with DENV2 NS1 and ML-RR-cGAMP confer comparable or greater protection than DENV2 NS1-MPLA vaccination against lethal DENV2 challenge in Ifnar−/− mice.
Mice were immunized subcutaneously with 20 μg of DENV2 NS1 or OVA in combination with ML-RR-cGAMP/AddaVax or MPLA/AddaVax on days 0 and 21. Control mice were infected with a sublethal dose (105 PFU) of DENV2 PL046 on day 0. On day 49, mice were challenged intravenously with 3 × 105 PFU (A) or 1 × 106 PFU (B) of DENV2 D220 using antibody enhancement conditions (5 μg of 4G2 mAb, 20–24 hours prior to infection). Kaplan-Meier survival curves from 2 independent experiments are shown.
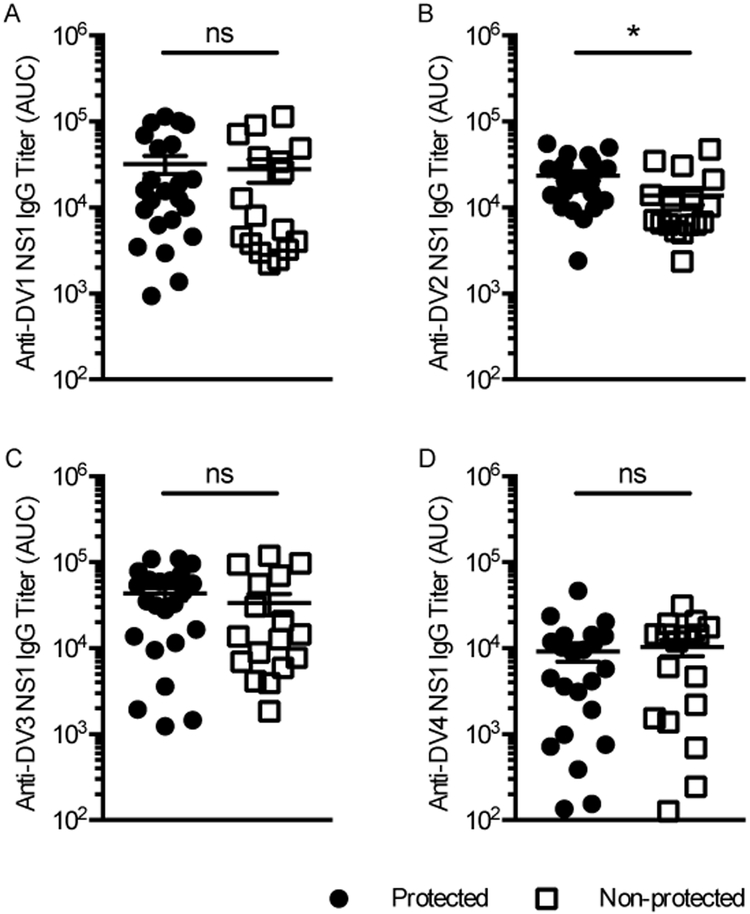
Anti-DENV2 NS1 antibody responses induced by ML-RR-cGAMP-NS1 vaccination are associated with protection against lethal dengue challenge
Previously, we showed that passive transfer of NS1-immune serum and anti-NS1 monoclonal antibodies prevent NS1-induced lethality in vivo (5). To determine if antibody responses in mice immunized with ML-RR-cGAMP-NS1 were associated with protection, serum samples were tested by ELISA against NS1 proteins from the 4 DENV serotypes. Notably, mice that were protected against lethal DENV challenge developed significantly higher anti-DENV2 NS1 IgG titers than those that succumbed to the infection. Antibody titers against DENV1 NS1 and DENV3 NS1 showed a similar trend, albeit differences between protected and non-protected groups were not statistically significantly (Fig. 6).
FIGURE 6. Anti-DENV2 NS1 antibody titers are associated with protection from DENV2-induced mortality.
Sera from mice immunized with each DENV NS1 serotype in combination with ML-RR-cGAMP/AddaVax were tested by direct ELISA against DENV1 NS1 (A), DENV2 NS1 (B), DENV3 NS1 (C) and DENV4 NS1 (D). These are the same mice evaluated for morbidity and mortality in Figure 4. Mice that did not succumb to DENV2 D220 infection developed significantly higher anti-DENV2 NS1 IgG titers than non-protected mice. Data are representative of 2 independent experiments (protected, n=23; non-protected, n=17; * p<0.05).
Sera from mice immunized with ML-RR-cGAMP-NS1 do not enhance DENV infection in vitro
Antibody-dependent enhancement (ADE) is the phenomenon by which weakly neutralizing and/or insufficient amounts of pre-existing anti-DENV antibodies facilitate DENV infection of FcγR-bearing cells. This results in increased viral replication and can potentially lead to more severe disease. Because NS1 is not a structural component of the DENV virion, anti-NS1 antibodies should not enable ADE. To examine whether sera from mice immunized with ML-RR-cGAMP-NS1 could enhance DENV infection in vitro, pooled serum samples were incubated with DENV2 D220 virus and used to infect K562 cells, a human erythroleukemic cell line that expresses FcγRIIA (CD32A) and is non-permissive in the absence of enhancing antibodies. Importantly, sera from mice vaccinated with DENV NS1 in combination with ML-RR-cGAMP did not allow infection of K562 cells. In contrast, sera from mice immunized with PL046 enabled DENV2 D220 infection, with peak enhancement between 1:135 and 1:405 serum dilutions (Supplementary Fig. 4).
DISCUSSION
Earlier research had indicated that NS1 antigenemia in DENV-infected patients is associated with disease severity (43–45); however, it was not shown until recently that NS1 is directly involved in triggering endothelial permeability and vascular leak, and that immune responses against NS1 can prevent lethal DENV infection (5, 46). On the basis of these findings, we sought to characterize immune responses and assess the protective efficacy of immunization with NS1 in combination with the CDN compounds ML-RR-cGAMP or ML-RR-cGAMP-D, which are phosphodiesterase-resistant, highly lipophilic activators of all common human STING alleles (31). Since immunocompetent WTB6 mice are not susceptible to DENV infection, immune responses to NS1-CDN vaccinations were assessed in parallel in Ifnar−/− mice, an in vivo model for mimicking key features of severe human dengue (41).
Compared to DENV2-MPLA vaccination, both WTB6 and Ifnar−/− mice developed similar or higher IgG responses upon immunization with DENV2 NS1 in combination with ML-RR-cGAMP or ML-RR-cGAMP-D. With the exception of IgG2b titers in Ifnar−/− mice, IgG1, IgG2c, and IgG3 subclass titers were also superior or equivalent in mice immunized with NS1-CDNs compared to NS1-MPLA-immunized animals. The ability of MPLA to induce robust antibody responses against clinically-relevant antigens is well documented (47); however, few studies have assessed humoral responses elicited by viral proteins in combination with CDN compounds (48–51). Therefore, these results provide additional evidence demonstrating the ability of mammalian cGAMP homologues to induce robust and protective antibody responses.
In both WTB6 and Ifnar−/− mice, immunization with NS1 in combination with CDNs induced overall more robust T-cell responses than NS1-MPLA vaccination. Similar results were obtained when using OVA as an immunogen, which also allowed us to assess CD8+ T-cell responses by circumventing the lack of cytotoxic T-cell epitopes in DENV NS1 for the C57BL/6 (H-2Kb) background. As expected, immunization of NS1 adjuvanted with ML-RR-cGAMP and ML-RR-cGAMP-D resulted in higher frequencies of cytokine-producing CD4+ and CD8+ T cells than immunization with MPLA. Nonetheless, previous research indicates that MPLA is capable of inducing considerable T-cell responses at higher immunization doses (52, 53). There is a paucity of studies addressing the possible protective role of NS1-specific T cells against DENV infection. However, investigations in DENV-infected patients indicate that CD4+ T cells preferentially target NS1, the envelope and capsid, the same proteins thought to be recognized by B cells (18). In clinical trials, the NIH TV-003 DENV vaccine candidate induced NS1-specific CD4+ and CD8+ T-cell responses comparable in breadth and magnitude to those found upon natural infection (54, 55). Further, a study testing an NS1-based DNA vaccine in mice indicated that both NS1-specific antibodies and CD4+ T cells are critical for protection against DENV infection (17). Thus, these data suggest that the induction of NS1-specific CD4+ T-cell responses by dengue vaccine candidates may significantly contribute to protective efficacy.
Immunization with each DENV NS1 serotype in combination with ML-RR-cGAMP-D or MPLA elicited serotype-specific and cross-reactive antibody responses. As in previous experiments, ML-RR-cGAMP-D-adjuvanted vaccination induced higher or similar IgG titers compared to those elicited by MPLA-NS1. Characterization of IgG subclass responses showed that ML-RR-cGAMP-D-NS1 immunization elicited significantly higher (p<0.05) IgG2c responses than MPLA in WTB6 mice, indicative of skewing toward the Th1 phenotype. However, this was not observed in Ifnar−/− mice, likely due to the importance of type I IFNs to anti-viral Th1 polarization (56). In addition, IgG1, IgG2b and IgG3 titers were comparable between mice immunized with NS1-ML-RR-cGAMP-D or NS1-MPLA, indicating that for these IgG subclasses, both adjuvants induce similar humoral immunity. In both WTB6 and Ifnar−/− mice, ML-RR-cGAMP-D induced stronger serotype-specific responses T-cell responses than vaccination with MPLA. Nonetheless, only immunization with DENV3 NS1 and ML-RR-cGAMP-D elicited significantly higher cross-reactive responses to DENV1 NS1 than MPLA, probably due to the ability of CDNs to induce more robust polyfunctional CD4+ T-cell responses than MPLA (48) and because the NS1 sequence of both serotypes is closely related.
To assess the protective efficacy of NS1-ML-RR-cGAMP immunizations, Ifnar−/− mice were vaccinated twice with NS1 from each DENV serotype and challenged with lethal DENV2 D220 infection. Compared to OVA-ML-RR-cGAMP-immunized Ifnar−/− mice, immunizations with each of the 4 NS1 serotypes combined with ML-RR-cGAMP conferred significant protection against DENV2-induced morbidity and mortality. Importantly, mice immunized with ML-RR-cGAMP in combination with DENV2 NS1 showed comparable or greater protection than animals vaccinated with DENV2 NS1-MPLA/AddaVax, underscoring the substantial immunogenicity of CDN adjuvants. Using NS1-MPLA/AddaVax immunizations, we previously reported higher levels of homotypic and heterotypic protection than those observed here (5); however, in that study, mice were immunized 3 times by i.p. injection. Notwithstanding, the 2-dose vaccination schedule and s.c. immunization route used in this study are more appealing from a vaccine-development perspective, and future research should aim to increase the immunogenicity and protective efficacy of this vaccination regimen. In general, immunocompetent rodent models are better suited for the assessment of vaccine-induced immunity and protective efficacy. However, the use of these models for the evaluation of dengue vaccine candidates is hindered by the inability of DENV to infect immunocompetent mice. DENV NS3 and NS5 proteins trigger degradation of human but not murine STING and STAT2, respectively, which are central components of the IFN signaling pathway (57–59). This enables DENV to suppress the IFN response, replicate, and cause disease in humans but not wild-type mice. Thus, most DENV infection models use mice deficient in the IFN pathway, which display virus tropism similar to humans and a vascular leak syndrome with key features of severe dengue disease (60, 61). Importantly, our data show that Ifnar−/− mice are capable of mounting robust antibody and T-cell responses as a result of adjuvanted protein immunization. Additionally, a previous study demonstrated that cytotoxic responses to DENV infection --including CD8+ T-cell proliferation, degranulation, and cytokine production-- can develop in the absence of IFN-α/β signaling (62).
Protection against DENV challenge among NS1-immunized mice is likely antibody-mediated, and previous studies have demonstrated that passive transfer of anti-NS1 antibodies are capable of preventing DENV-induced mortality (5, 9, 10). Further, antibody responses against the wing domain of DENV NS1 have been associated with protection against DENV challenge in mice and reduced disease severity in humans (15, 16). In this study, we show that the development of high anti-DENV2 NS1 titers is associated with protection from lethal DENV2 challenge, regardless of the NS1 serotype used for immunization. Thus, this provides further evidence that anti-NS1 titers correlate with protection from DENV-induced morbidity and mortality.
An important observation from this study is that Ifnar−/− mice are capable of mounting potent responses as a result of immunization with CDNs. Indeed, although these compounds activate the production of type I IFNs through the activation of the STING pathway, they have also been shown to work in Ifnar−/− mice, which are deficient in IFN-α/β receptor signaling (63). In addition, previous research has also indicated that type I IFN signaling is nonessential for the induction of humoral responses by CDN-adjuvanted subunit vaccines (49, 64). cGAMP can also be delivered through gap junctions to bystander cells, leading to STING activation and subsequent antiviral immunity independent of type I IFN signaling (65). Thus, these data underscore the ability of CDN compounds to induce protective immunity through multiple signaling pathways, even in the absence of autocrine and paracrine type I IFN signaling. The necessity for using Ifnar−/− mice in this infection model may also obscure the actual potency of these CDNs relative to other NF-κB-dependent TLR agonists, such as MPLA. Therefore, MyD88/TRIF-dependent TLR agonists, such as MPLA, may be suboptimal for T-cell priming against infectious disease antigens.
The induction of potent T-cell responses by soluble protein-in-adjuvant formulations has remained a difficult task. CDNs have shown promising results as vaccine adjuvants for intracellular pathogens in animal models, and their immunostimulatory properties can be readily optimized through chemical synthesis (27). Importantly, phase I clinical trials for a synthetic human STING-activating CDN (ADU-S100) as a chemotherapeutic agent alone and in combination with checkpoint inhibition are currently in progress (ClinicalTrials.gov NCT02675439 and NCT03172936). However, to date there are no human studies assessing CDNs as adjuvants against infectious agents.
In conclusion, we demonstrate that NS1-CDN immunizations induce potent antibody and T-cell responses in both WTB6 and Ifnar−/− mice and confer significant protection from lethal DENV infection. We also show that anti-NS1 antibody titers in NS1-immunized mice are associated with protection, adding to previous research indicating that humoral responses against DENV NS1 are potential correlates of protection. Taken together, our results support the inclusion of NS1 in dengue vaccine candidates.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kaycie Hopkins and Kalani Ratnasiri for their early contributions to the project, and Jeffrey Li for technical support with in vivo experiments.
FUNDING
This work was supported in part by grant U19AI109761 (I Lipkin; EH project leader) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. This work was also sponsored by Aduro Biotech, Inc.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, and Hay SI. 2013. The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB, and O’Rourke EJ. 1977. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med 146: 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead SB 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140: 527–533. [DOI] [PubMed] [Google Scholar]
- 4.Kliks SC, Nisalak A, Brandt WE, Wahl L, and Burke DS. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40: 444–451. [DOI] [PubMed] [Google Scholar]
- 5.Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, and Harris E. 2015. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7: 304ra141. [DOI] [PubMed] [Google Scholar]
- 6.Schlesinger JJ, Brandriss MW, and Walsh EE. 1987. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol 68 (Pt 3): 853–857. [DOI] [PubMed] [Google Scholar]
- 7.Falgout B, Bray M, Schlesinger JJ, and Lai CJ. 1990. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol 64: 4356–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amorim JH, Diniz MO, Cariri FA, Rodrigues JF, Bizerra RS, Gonçalves AJ, de Barcelos Alves AM, and de Souza Ferreira LC. 2012. Protective immunity to DENV2 after immunization with a recombinant NS1 protein using a genetically detoxified heat-labile toxin as an adjuvant. Vaccine 30: 837–845. [DOI] [PubMed] [Google Scholar]
- 9.Wan SW, Lu YT, Huang CH, Lin CF, Anderson R, Liu HS, Yeh TM, Yen YT, Wu-Hsieh BA, and Lin YS. 2014. Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS One 9: e92495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henchal EA, Henchal LS, and Schlesinger JJ. 1988. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J Gen Virol 69 (Pt 8): 2101–2107. [DOI] [PubMed] [Google Scholar]
- 11.Twiddy SS, Farrar JJ, Vinh Chau N, Wills B, Gould EA, Gritsun T, Lloyd G, and Holmes EC. 2002. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology 298: 63–72. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Vaughan K, Weiskopf D, Grifoni A, Diamond MS, Sette A, and Peters B. 2016. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falconar AK, Young PR, and Miles MA. 1994. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch Virol 137: 315–326. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Pan Y, Guo Y, Qiu L, Ding X, and Che X. 2010. Comprehensive mapping of immunodominant and conserved serotype- and group-specific B-cell epitopes of nonstructural protein 1 from dengue virus type 1. Virology 398: 290–298. [DOI] [PubMed] [Google Scholar]
- 15.Lai YC, Chuang YC, Liu CC, Ho TS, Lin YS, Anderson R, and Yeh TM. 2017. Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection. Sci Rep 7: 6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertz T, Beatty PR, MacMillen Z, Killingbeck SS, Wang C, and Harris E. 2017. Antibody Epitopes Identified in Critical Regions of Dengue Virus Nonstructural 1 Protein in Mouse Vaccination and Natural Human Infections. J Immunol 198: 4025–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonçalves AJ, Oliveira ER, Costa SM, Paes MV, Silva JF, Azevedo AS, Mantuano-Barradas M, Nogueira AC, Almeida CJ, and Alves AM. 2015. Cooperation between CD4+ T Cells and Humoral Immunity Is Critical for Protection against Dengue Using a DNA Vaccine Based on the NS1 Antigen. PLoS Negl Trop Dis 9: e0004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, Hanson BJ, Smith KG, Bertoletti A, Kemeny DM, and MacAry PA. 2013. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 87: 2693–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambuel Y, Young G, Brewoo JN, Paykel J, Weisgrau KL, Rakasz EG, Haller AA, Royals M, Huang CY, Capuano S, Stinchcomb DT, Partidos CD, and Osorio JE. 2014. A rapid immunization strategy with a live-attenuated tetravalent dengue vaccine elicits protective neutralizing antibody responses in non-human primates. Front Immunol 5: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriques HR, Rampazo EV, Gonçalves AJ, Vicentin EC, Amorim JH, Panatieri RH, Amorim KN, Yamamoto MM, Ferreira LC, Alves AM, and Boscardin SB. 2013. Targeting the non-structural protein 1 from dengue virus to a dendritic cell population confers protective immunity to lethal virus challenge. PLoS Negl Trop Dis 7: e2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foged C, Hansen J, and Agger EM. 2012. License to kill: Formulation requirements for optimal priming of CD8(+) CTL responses with particulate vaccine delivery systems. Eur J Pharm Sci 45: 482–491. [DOI] [PubMed] [Google Scholar]
- 22.Coffman RL, Sher A, and Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, and Vance RE. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdette DL, and Vance RE. 2013. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 14: 19–26. [DOI] [PubMed] [Google Scholar]
- 25.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, Fitzgerald KA, Hayakawa Y, and Vance RE. 2009. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med 206: 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, Xia W, Gu J, Ishikawa H, Gutman D, Barber G, Qin Z, and Jiang Z. 2011. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell 147: 436–446. [DOI] [PubMed] [Google Scholar]
- 27.Dubensky TW, Kanne DB, and Leong ML. 2013. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther Adv Vaccines 1: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu DL, Narita K, Hyodo M, Hayakawa Y, Nakane A, and Karaolis DK. 2009. c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine 27: 4867–4873. [DOI] [PubMed] [Google Scholar]
- 29.Yan H, KuoLee R, Tram K, Qiu H, Zhang J, Patel GB, and Chen W. 2009. 3’,5’-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem Biophys Res Commun 387: 581–584. [DOI] [PubMed] [Google Scholar]
- 30.Ogunniyi AD, Paton JC, Kirby AC, McCullers JA, Cook J, Hyodo M, Hayakawa Y, and Karaolis DK. 2008. c-di-GMP is an effective immunomodulator and vaccine adjuvant against pneumococcal infection. Vaccine 26: 4676–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW, and Gajewski TF. 2015. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep 11: 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, and Gravekamp C. 2014. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW, and Kim Y. 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7: 283ra252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dis E, Sogi KM, Rae CS, Sivick KE, Surh NH, Leong ML, Kanne DB, Metchette K, Leong JJ, Bruml JR, Chen V, Heydari K, Cadieux N, Evans T, McWhirter SM, Dubensky TW, Portnoy DA, and Stanley SA. 2018. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep 23: 1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson HS, Davies ML, Watts MJ, Mann AE, Holding FP, O’Neill T, Beech JT, Thompson SJ, Leesman GD, and Ulrich JT. 1998. Enhanced immunogenicity of a recombinant genital warts vaccine adjuvanted with monophosphoryl lipid A. Vaccine 16: 1993–1999. [DOI] [PubMed] [Google Scholar]
- 36.Thoelen S, Van Damme P, Mathei C, Leroux-Roels G, Desombere I, Safary A, Vandepapeliere P, Slaoui M, and Meheus A. 1998. Safety and immunogenicity of a hepatitis B vaccine formulated with a novel adjuvant system. Vaccine 16: 708–714. [DOI] [PubMed] [Google Scholar]
- 37.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garçon N, Krzych U, and Marchand M. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med 336: 86–91. [DOI] [PubMed] [Google Scholar]
- 38.Moore A, McCarthy L, and Mills KH. 1999. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 17: 2517–2527. [DOI] [PubMed] [Google Scholar]
- 39.Alving CR, Peachman KK, Rao M, and Reed SG. 2012. Adjuvants for human vaccines. Curr Opin Immunol 24: 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casella CR, and Mitchell TC. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci 65: 3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orozco S, Schmid MA, Parameswaran P, Lachica R, Henn MR, Beatty R, and Harris E. 2012. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J Gen Virol 93: 2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaffney BL, and Jones RA. 2012. One-flask synthesis of cyclic diguanosine monophosphate (c-di-GMP). Curr Protoc Nucleic Acid Chem Chapter 14: Unit 14.18.11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, and Rothman AL. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186: 1165–1168. [DOI] [PubMed] [Google Scholar]
- 44.Duyen HT, Ngoc TV, Ha d. T., Hang VT, Kieu NT, Young PR, Farrar JJ, Simmons CP, Wolbers M, and Wills. BA 2011. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis 203: 1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paranavitane SA, Gomes L, Kamaladasa A, Adikari TN, Wickramasinghe N, Jeewandara C, Shyamali NL, Ogg GS, and Malavige GN. 2014. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect Dis 14: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, and Young PR. 2015. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 7: 304ra142. [DOI] [PubMed] [Google Scholar]
- 47.Alving CR, Rao M, Steers NJ, Matyas GR, and Mayorov AV. 2012. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines 11: 733–744. [DOI] [PubMed] [Google Scholar]
- 48.Landi A, Law J, Hockman D, Logan M, Crawford K, Chen C, Kundu J, Ebensen T, Guzman CA, Deschatelets L, Krishnan L, Tyrrell DLJ, and Houghton M. 2017. Superior immunogenicity of HCV envelope glycoproteins when adjuvanted with cyclic-di-AMP, a STING activator or archaeosomes. Vaccine 35: 6949–6956. [DOI] [PubMed] [Google Scholar]
- 49.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, and Irvine DJ. 2015. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest 125: 2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madhun AS, Haaheim LR, Nøstbakken JK, Ebensen T, Chichester J, Yusibov V, Guzman CA, and Cox RJ. 2011. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 29: 4973–4982. [DOI] [PubMed] [Google Scholar]
- 51.Svindland SC, Pedersen GK, Pathirana RD, Bredholt G, Nøstbakken JK, Jul-Larsen Å, Guzmán CA, Montomoli E, Lapini G, Piccirella S, Jabbal-Gill I, Hinchcliffe M, and Cox RJ. 2013. A study of Chitosan and c-di-GMP as mucosal adjuvants for intranasal influenza H5N1 vaccine. Influenza Other Respir Viruses 7: 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poteet E, Lewis P, Li F, Zhang S, Gu J, Chen C, Ho SO, Do T, Chiang S, Fujii G, and Yao Q. 2015. A Novel Prime and Boost Regimen of HIV Virus-Like Particles with TLR4 Adjuvant MPLA Induces Th1 Oriented Immune Responses against HIV. PLoS One 10: e0136862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu X, Liu R, and Zhu N. 2013. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology 218: 1524–1528. [DOI] [PubMed] [Google Scholar]
- 54.Weiskopf D, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, de Silva AD, Lindow JC, Diehl SA, Whitehead S, Durbin A, Kirkpatrick B, and Sette A. 2015. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol 89: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angelo MA, Grifoni A, O’Rourke PH, Sidney J, Paul S, Peters B, de Silva AD, Phillips E, Mallal S, Diehl SA, Kirkpatrick BD, Whitehead SS, Durbin AP, Sette A, and Weiskopf D. 2017. Human CD4 T cell responses to an attenuated tetravalent dengue vaccine parallel those induced by natural infection in magnitude, HLA restriction, and antigen specificity. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, and Steinman RM. 2009. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 206: 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, and Harris E. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol 78: 2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashour J, Morrison J, Laurent-Rolle M, Belicha-Villanueva A, Plumlee CR, Bernal-Rubio D, Williams KL, Harris E, Fernandez-Sesma A, Schindler C, and García-Sastre A. 2010. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe 8: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, Rodriguez-Madoz JR, Mulder LC, Barber GN, and Fernandez-Sesma A. 2012. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog 8: e1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, and Harris E. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol 80: 10208–10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, Beatty PR, and Harris E. 2009. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J Trop Med Hyg 80: 416–424. [PubMed] [Google Scholar]
- 62.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, and Shresta S. 2009. A protective role for dengue virus-specific CD8+ T cells. J Immunol 182: 4865–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Li P, and Wu MX. 2016. Natural STING Agonist as an “Ideal” Adjuvant for Cutaneous Vaccination. J Invest Dermatol 136: 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blaauboer SM, Gabrielle VD, and Jin L. 2014. MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3’−5’)-cyclic-di-guanosine-monophosphate in vivo. J Immunol 192: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, and Hornung V. 2013. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503: 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.