Abstract
Estuaries are dynamic ecosystems which vary widely in loading of the contaminant methylmercury (MeHg), and in environmental factors which control MeHg exposure to the estuarine foodweb. Inputs of organic carbon and rates of primary production are important influences on MeHg loading and bioaccumulation, and are predicted to increase with changes in climate and land use pressures. To further understand these influences on MeHg levels in estuarine biota, we used a field study approach in sites across different temperature regions, and with varying organic carbon levels. In paired comparisons of sites with high vs. low organic carbon, fish had lower MeHg bioaccumulation factors (normalized to water concentrations) in high carbon sites, particularly subsites with large coastal wetlands and large variability in dissolved organic carbon levels in the water column. Across sites, MeHg level in the water column was strongly tied to dissolved organic carbon, and was the major driver of MeHg concentrations in fish and invertebrates. Higher primary productivity (chlorophyll-a) was associated with increased MeHg partitioning to suspended particulates, but not to the biota. These findings suggest that increased inputs of MeHg and loss of wetlands associated with climate change and anthropogenic land use pressure will increase MeHg concentrations in estuarine food webs.
Capsule
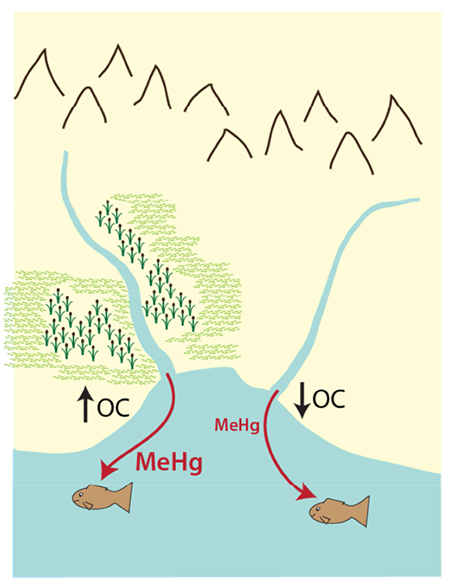
Wetland areas temper the bioaccumulation of methylmercury in estuaries, yet increased watershed inputs of methylmercury associated with climate change and land disturbances are predicted to increase mercury levels in fish.
Introduction
Pathways of methylmercury (MeHg) bioaccumulation in fish and the environmental factors that control them are not sufficiently understood in estuarine and coastal ecosystems (Baumann et al., 2017; Eagles-Smith et al., 2018; Schartup et al., 2018) where much of the seafood consumed by humans is harvested (Sheehan et al., 2014; Sunderland, 2007). Estuaries are critical habitats supporting a multitude of benthic and pelagic species, including both resident and transient fish (Deegan et al., 2000; Kneib, 1997). Nearshore estuarine waters can receive elevated levels of MeHg from watershed sources (Buckman et al., 2017; Eagles-Smith and Ackerman, 2014), and can also support in situ formation of MeHg in the sediments (Balcom et al., 2004) and the water column (Schartup et al., 2015a). Concentrations of MeHg in the water column control uptake of MeHg at the base of the food web and drive concentrations of MeHg in fish (Buckman et al., 2017; Chen et al., 2014). Estuarine fish can act as a conduit of MeHg transfer to larger estuarine and coastal fish by trophic nekton relay and bioadvection (Kneib, 1997). Investigations in these environments are important to understanding pathways and processes controlling MeHg bioaccumulation in coastal ecosystems, particularly under increasing climate and land use pressures.
Atmospheric deposition of Hg has decreased in the Northeastern US since the 1970s (Fitzgerald et al., 2018), however, loading of Hg and organic matter to aquatic ecosystems is predicted to increase under global climate change (Lorey and Driscoll, 1999; Pinkney et al., 2015). Increases in dissolved organic carbon (DOC) loading from rivers to the Gulf of Maine have been observed over the past 80 years, with large increases during wet years (Balch et al., 2016). Inputs of nutrients to estuaries have also been predicted (Cloern, 2001; Driscoll et al., 2012), which may support phytoplankton blooms. In addition, rapid increases in temperature have been observed in the North Atlantic over the last fifteen years (Pershing et al., 2015), and may cause shifts in primary productivity. These ecosystem changes have unpredictable effects on MeHg bioaccumulation.
While watershed inputs of terrestrial DOC have been associated with transport of MeHg to estuarine ecosystems (Conaway et al., 2003), complexation of MeHg to DOC decreases its availability for uptake at the base of the foodweb (Lee and Fisher, 2017; Luengen et al., 2012). In addition, increased inputs of terrestrial DOC may also flocculate during estuarine mixing, becoming a sink for MeHg (Soerensen et al., 2017), and higher DOC will decrease photodemethylation, which requires light penetration in the water column (DiMento and Mason, 2017). Primary productivity is intrinsically tied to the uptake of MeHg at the base of the foodweb (Chen and Folt, 2005; Driscoll et al., 2007), but high algal densities can decrease MeHg bioaccumulation in higher trophic levels by bloom dilution (Pickhardt et al., 2002). Productivity in estuaries may therefore decrease bioaccumulation due to bloom dilution (Driscoll et al., 2012 ; Luengen and Flegal, 2009) or increase MeHg in the biota due to higher MeHg production (Soerensen et al., 2016; Stoichev et al., 2016). Past studies have shown that latitudinal or temperature differences influence MeHg bioaccumulation: higher MeHg concentrations in estuarine fish have been linked with more northern latitudes (Baumann et al., 2017), where Hg biomagnification is highest (Lavoie et al., 2013). Under controlled conditions, increased temperature has been found to increase MeHg bioaccumulation in estuarine fish (Dijkstra et al., 2013).
Predictive models (Driscoll et al., 2012; Stoichev et al., 2016) and mesocosm experiments (Dijkstra et al., 2013; Jonsson et al., 2017; Jonsson et al., 2014; Kim et al., 2008; Van et al., 2016) have enhanced understanding of processes and environmental pressures that affect MeHg bioavailability, however, field studies provide invaluable context and understanding of actual responses to environmental parameters across complex estuarine environments. In this field study, we investigated MeHg bioaccumulation in the intertidal zone at three different latitudes (and temperature regions) in the Northeast USA, with the objective of evaluating the effects of differences in organic carbon, primary productivity and MeHg loading on MeHg accumulation. This approach investigated different subsites, or areas within a site, having low or high organic content in the substrate, to isolate effects of site organic carbon from regional variability in natural populations of microbes and primary producers. We hypothesize that 1) bioaccumulation factors are higher in subsites with low site organic carbon content in the substrate, 2) MeHg concentrations in benthic and pelagic organisms are driven by water column MeHg, which is linked with DOC concentrations and 3) higher rates of primary production lead to higher MeHg uptake at the base of the estuarine food web.
Methods
Field study design:
To evaluate the controls on MeHg bioavailability and bioaccumulation within and across estuarine sites, we collected abiotic and biotic samples at paired sub-sites across three latitudes (Mount Desert Island, ME (44° N); Long Island Sound, CT (41° N), and upper Chesapeake Bay, MD (38° N); (Figure 1 and supplemental material Table 1S)) during the summer months (June – Aug). Growing season average surface water temperature varied by ~10°C between latitudes (ME: 54-63°C, CT: 61-68°C, and MD: 72-84°C). At each latitude, two sites were sampled: Northeast Creek (NC) and Bass Harbor (BH) in Mount Desert Island; Barn Island (BI) and Goshen Cove (GC) from Long Island Sound; and Parker’s Creek (PC) and Jefferson Patterson (JP) in Chesapeake Bay. Within each site, low and high organic carbon subsites (LOC and HOC, respectively) were designated according to substrate consistency (sandy vs. muddy), for a total of 12 sub-sites. All sites were chosen in areas with no known point sources of Hg, although anthropogenic pressures were higher in the southern sites. The sites at each latitude differed in their watersheds with BH, BI, PC all being associated with very large protected coastal marsh areas in Acadia National Park (BH, Maine), the Barn Island Wildlife Management Area (BI, Connecticut) and Parker’s Creek Preserve (PC, Maryland). All sub-sites were at least 0.5 km apart (generally much further via salt water creeks), with the exception of the JP sites, which were in close proximity (0.05 km) but separated by a narrow channel.
Figure 1).
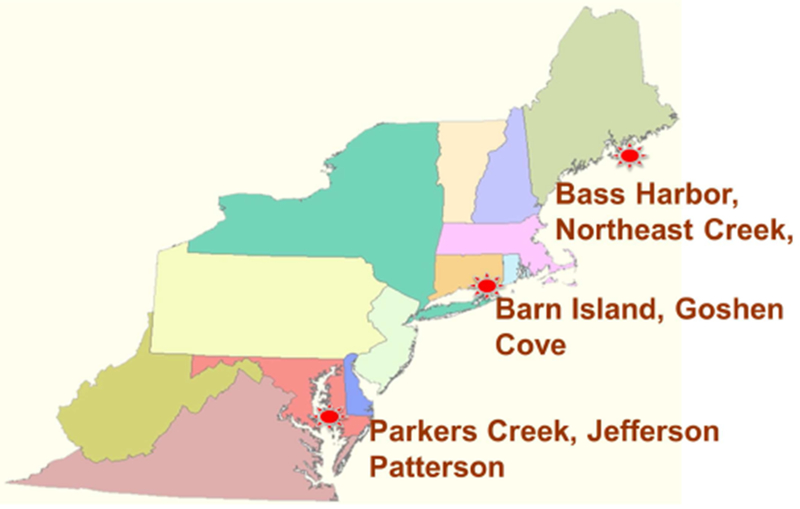
Locations of field sites in this study.
Sampling:
Biotic samples included ribbed and blue mussels (Geukensia demissa and Mytilus edulis), common periwinkle (Littorina littorea), grass and sand shrimp (Palaemonetes spp. and Crangon spp.), blue crab (Callinectes sapidus), green crab (Carcinus maenas), Atlantic silversides (Menidia menidia), mummichog (Fundulus heteroclitus) and striped killifish (Fundulus majalis). Individuals (~10) of each species were collected from the intertidal zone at each subsite where available. Larger numbers of mummichog and silversides (~30 samples) were collected at two sub-sites (for each species) to investigate the relationship between fish size and MeHg concentration.
Sediment samples (top 4 cm) were collected at each subsite by hand using acid-cleaned polycarbonate coring tubes. Samples were frozen in acid-washed, screw cap plastic cups until analyses, which included total Hg (THg) and MeHg concentrations, and bulk organic carbon content as loss on ignition (%LOI).
Surface water samples for Hg speciation were collected at a depth of ~ 0.5m at both high tide and low tide from an inflatable boat using trace-metal clean techniques. Water samples were collected directly into 2L Teflon (FEP) bottles, and stored in double zip-bags inside iced coolers. Water for Hg speciation analysis was filtered within 12 hours after collection using acid-washed Nalgene plastic vacuum filtration units (47 mm 0.45 pm quartz fiber filters, Advantec, Matthews, North Carolina) inside a portable laminar flow hood. Filtrate was acidified (0.5% trace metal grade HCl) and refrigerated, and particulate filters were frozen inside acid-washed plastic dishes. Water filtrations for TSS and DOC were done using the same filtration units and filters; samples for Chl-a and phaeopigment were filtered on 25 mm glass fiber filters (Advantec) using a glass filtration apparatus.
Analysis:
Biota samples were analyzed at the Dartmouth Trace Element Analysis Core, and water and sediment analyses were performed at the Mason lab at UConn. Biota samples were freeze-dried, then analyzed for MeHg and inorganic Hg by species-specific isotope dilution ICP-MS using a MERX-M automated methylmercury analyzer coupled to an Element 2 ICP-MS (Taylor et al., 2011; Taylor et al., 2008). Portions of sample (50 mg) were weighed into 7 mL glass vials, then spiked with an appropriate amount of 201MeHg or 199Hg, and digested overnight in 3 mL 25% tetramethylammonium hydroxide (TMAOH). A 50 μL subsample of the extract was then added to 40 mL of water in an acid-washed brown glass septa vial, along with 0.3 mL citrate buffer and 40 μL of ethylating reagent. Where sample was available, a second sample of the biota was analyzed for total Hg (THg) using a Direct Mercury Analyzer (DMA-80; Milestone Inc., Shelton CT) (U.S. EPA, 1998). The DMA was calibrated using standard reference materials (NIST 1515 Apple leaves, NIST 2976 Mussel, NIST 2709a San Joaquin Soil (National Institute of Standards and Technology; Gaithersburg, MD), NRCC TORT-2, and NRCC DORM 4 (National Research Council of Canada, Ottawa, Canada). Calibration was repeated as needed based on daily performance checks.
Recoveries of MeHg for certified reference materials were 95±4% for NRCC DORM-4 (354 ng/g; n=14); 100±7%for NIST 2976 Mussel (28 ng/g; n=11); 110±4 %for NRCC TORT-2 (152 ng/g; n=4). Recoveries of THg were 98±5% for NRCC DORM-4 (410 ng/g; n=14); 100±10%for NIST 2976 Mussel (61 ng/g; n=11); 100±6 %for NRCC TORT-2 (270 ng/g; n=4).
THg in water was measured in both filtered and particulate fractions using cold vapor atomic fluorescence spectrometry (CVAFS). Analytical methods for analysis of THg in these phases are detailed elsewhere (Bloom and Fitzgerald, 1988; Bloom and Crecelius, 1983; Gill and Fitzgerald, 1979). In brief, 50–100 mL aliquots of filtered (0.45 μm) water were digested chemically with bromine monochloride (BrCl) and subsequently neutralized with hydroxylamine hydrochloride (NH2OHṀHCl). Sample Hg was reduced to Hg0 with stannous chloride (SnCl2) in a sparging vessel, purged with nitrogen, and trapped and analyzed by dual gold-amalgamation. The results were calibrated against an Hg0 standard and an aqueous Hg standard traceable to the US National Institute of Standards and Technology (NIST). Particulate THg was determined by acid-leaching material on quartz fiber filters with 4N HNO3 (Hammerschmidt and Fitzgerald, 2001) and analyzed as described above. Sediments were freeze-dried and analyzed for THg using the Milestone DMA-80 (U.S. EPA, 1998). Sample results were corrected for field and preparation blanks as appropriate. The mean ± standard error (SE) relative percent differences (RPD) for preparation and analytical duplicates were 12 ± 6 % and 8 ± 2 %, respectively, for water and particulate samples. Mean aqueous THg standard recoveries were 97 ± 6 %. Mean RPD was 5 ± 1 % for sediment analytical duplicates, and mean marine sediment standard reference material recoveries were 95 ± 2 % for NRCC PACS-2 (3.04 mg/kg) and 94 ± 1 % for NRCC MESS-2 (0.092 mg/kg). THg method detection limits (MDLs) were 0.05 ng/L for water analyses, 0.21 ng/g for particulate analyses, and 1.8 ng/g for sediment analyses.
MeHg in 0.45 μm filtered water, particulate matter, and sediment was determined by purge and trap (tenax) gas chromatographic CVAFS following ethylation (Hammerschmidt and Fitzgerald, 2001; Tseng et al., 2004). MeHg in all samples was separated by distillation with dilute sulfuric acid (H2SO4) and potassium chloride (KCl). Sample MeHg was measured after calibration with an aqueous standard solution traceable to NIST. Sample results were corrected for field and preparation blanks as appropriate. The mean ± (SE) RPD for distillation and analytical duplicates were 14 ± 3 % and 9 ± 2 %, respectively, for water and particulate samples. Distillation MeHg standard spike and aqueous MeHg standard recoveries were 115 ± 4 % and 103 ± 2 %, respectively, for water and particulate samples. Sediment MeHg mean distillation spike and aqueous MeHg standard recoveries were 96 ± 6 % and 95 ± 2 %, respectively. The mean RPD for sediment MeHg distillation and analytical duplicates were 16 ± 2 % and 11 ± 3 %, respectively. MDLs were 0.01 ng/L for water analyses, 0.012 ng/g for particulate analyses, and 0.004 ng/g for sediment analyses.
Additional physicochemical properties of ambient water measured in situ included temperature, salinity and dissolved oxygen using a sonde, total suspended solids (TSS) and dissolved organic carbon (DOC). Chlorophyll-a (Chl-a) and phaeopigment were analyzed for particulate matter samples using a Trilogy fluorometer following extraction with acetone. Sediment loss-on-ignition (LOI; a proxy for organic matter content) was measured by overnight combustion at 5500C.
Data were analyzed using JMP Pro 14 software and were log transformed as needed to the assumptions of parametric tests. Correlations between transformed variables were assessed by Pearson’s correlation coefficents. Bioaccumulation Factors (BAFs) were calculated as concentrations of MeHg in the biota divided by the dissolved water column concentration. Within and between site comparisons of biotic concentrations and BAFs, were evaluated by nested ANOVA, with site and subsite designated as fixed effects. Models were fit using least squares, and Post hoc Tukey’s Honestly Significant Difference test was used to assign significant differences between sub-sites. Partition coefficients (Kd [l/kg]) of MeHg in the water column were calculated as [solid]/[dissolved]. Akaike’s Information Criterion adjusted for small sample sizes (AlCc) was used to assess the best a priori model for describing concentrations of MeHg in estuarine fish. In these models, MeHg in the water column, suspended particulates, and sediment, as well as DOC and Chl-a were chosen a priori as factors that influence MeHg bioaccumulation.
Results
Abiotic and biotic characterization at paired sites within region:
A large range of Chl-a (2.25 to 19.3 μg/L), DOC (1.5 to 34 mg/L), and %LOI (0.7 to 26%) were captured across sites (Fig. 2A to C; all abiotic data is presented in Supplemental Table 2S). The highest concentrations of %LOI and DOC were found at the Maine sites; it should be noted that the LOC and HOC designation is consistent among sub-sites, but not sites (eg. BH LOC has higher %LOI than JP HOC). Across regions, Chl-a, an indicator of net phytoplankton production, was higher in the Connecticut and Maryland sites than in Maine. Large tidal differences in chl-a were evident at both BI sites; at BI chl-a was more elevated at low tide, whereas at all other sites, Chl-a was highest at high tide. Concentrations of DOC in the water column were higher in paired sub-sites with higher %LOI, although the between-subsite difference was small (< 1 mg/L) at the two Maryland sites, GC in Connecticut, and at the NE site in Maine, and tidal variation in DOC was high at the Maine sites (Fig. 2B).
Figure 2).
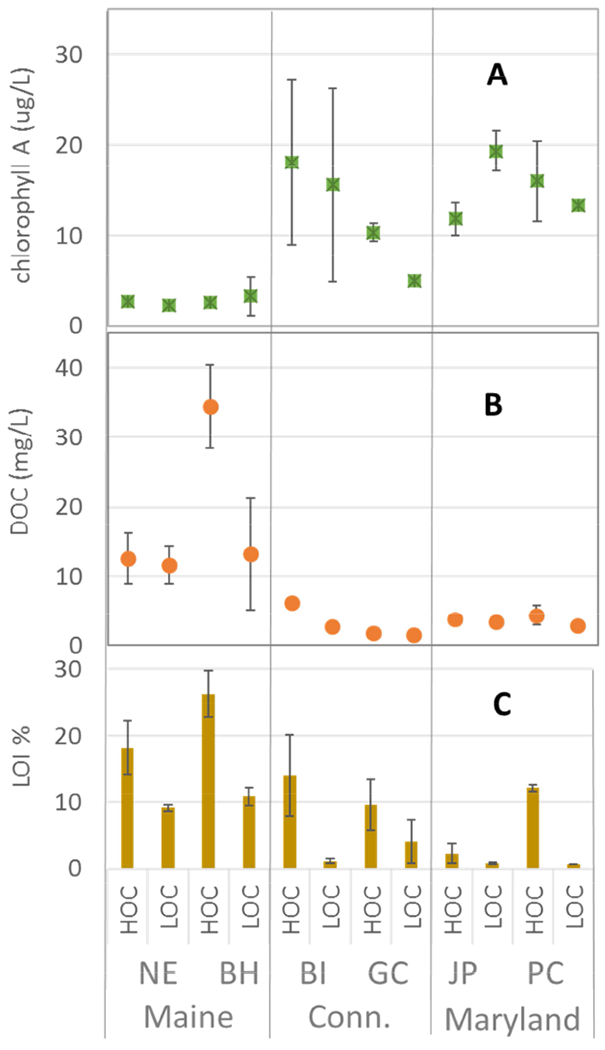
Concentrations of A) Chl-a (μg/L) B) DOC (mg/L) and C) LOI (%) in estuarine water across subsites. Error bars represent low and high tide values of Chl-a and DOC, and min/max values of sediment LOI .
Levels of THg in sediments were consistently higher at subsites with higher sediment organic carbon (Supplemental Table 2S), and correlated strongly with %LOI (r2 = 0.70, p < 0.001). Higher sediment MeHg concentrations did not coincide with HOC subsites (Fig. 3A), and there was no correlation between MeHg and %LOI or THg in sediment. There were no correlations between MeHg in the sediment and the water column. Water column levels of dissolved and particulate MeHg were elevated, and more variable between tides, at the Maine sites and BI (Connecticut) (Fig. 3B). Concentrations of MeHg in the dissolved phase was correlated with MeHg in the particulate phase (log MeHg diss. against log MeHg part r2 = 0.66, p = 0.001 at low tide, and r2 = 0.61, p = 0.004 at high tide). The proportion of MeHg/THg (%MeHg) in the dissolved phase was also much higher in the Maine sites (16 to 34%), relative to the Maryland sites (3-11%).
Fig. 3A).
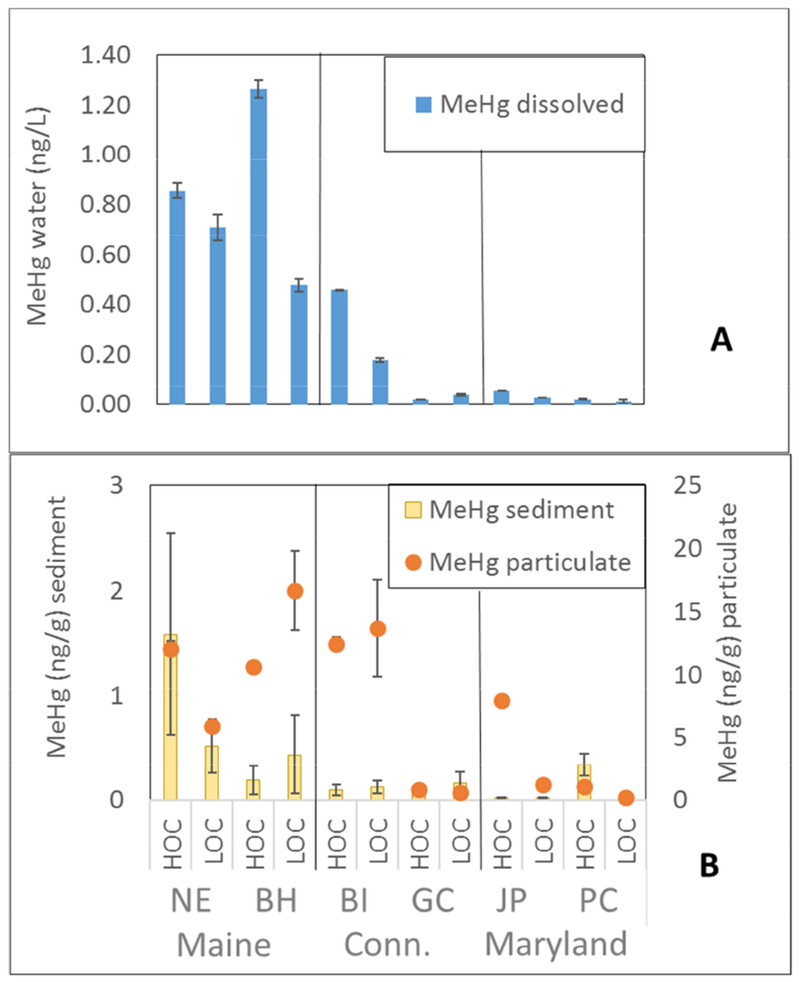
Concentrations of dissolved MeHg (ng/L) in the water column, and B) Concentrations of MeHg (ng/g) in suspended particulates and in sediment, across subsites. Error bars represent low and high tide values of MeHg in the water column, and min/max values of MeHg in sediment.
MeHg concentrations in the biota:
Mean concentrations of MeHg in fish and invertebrates ranged by ~1 order of magnitude across sites (Table 1). There was a strong correlation between log MeHg levels and length for mummichog assessed at two sites where large size distributions of fish were collected: BH HOC (r2 = 0.29; p < 0.001; n = 40) and JP HOC (r2 = 0.36; p < 0.001; n = 27); therefore, log MeHg concentrations were normalized to mean length (5.8 cm) across all sites, according to Eaglesmith and Ackerman (2014). There was no correlation between MeHg and length in silversides within sites (PC HOC (n = 17) or BH LOC (n = 22)); mean MeHg concentrations calculated for each sub-site included all sizes. Higher MeHg concentrations were found in the Maine sites than the Maryland sites for silversides, mummichog, mussels and periwinkle, corresponding with higher MeHg in the water column at these sites. Concentrations of MeHg in biota were not consistently higher in the LOC or HOC subsites; at BH, silversides had higher MeHg concentrations at HOC, whereas mummichog were higher at LOC. At BI, MeHg in shrimp was higher at the HOC site whereas mussels MeHg was more elevated at LOC, and no significant difference between subsites was evident in silversides. At JC, striped killifish had slightly higher MeHg at the HOC subsite, whereas silversides, mummichog and periwinkle did not differ significantly in MeHg between subsites. No trends between sites were evident in either crab species, both of which are highly omnivorous.
Table 1.
Mean MeHg concentrations +/−SD (n) in biota (ng/g) across sites, and letters designate differences in least square means by Tukey’s HSD. Mummichog MeHg concentrations were normalized to length (see text). Subsites not connected by the same letter are significantly different.
| State | Site | Carbon | Silversides | Mummichog | Striped killifish | Shrimp | Mussel | Periwinkle | Blue crab | Green crab |
|---|---|---|---|---|---|---|---|---|---|---|
| Maine | NC | HOC LOC |
92 ± 28 (10) BCD | 124 ± 48 (5) AB 88 ± 49 (10) BC |
43 ± 20 (10) C | 136 ± 32 (10) A | 63 ± 22 (10) A | 64 ± 60 (10) A | ||
| BH | HOC LOC |
179 ±42 (15) A 113 ± 32 (23) B |
66 ± 28 (40) CD 140 ± 65 (12) A |
37 ± 16 (25) C | 155 ± 47 (10) A | 39 ± 6 (10) B | 88 ± 70 (10) A | |||
| Connecticut | BI | HOC LOC |
154 ± 29 (10) A 157 ± 40 (10) A |
36 ± 12 (10) DE | 13 ± 1 (10) B | 122 ± 34 (10) B 293 ± 186 (5) A |
81 ± 16 (10) B 28 ± 10 (10) C |
129 ± 53 (7) A 95 ± 98(10) A |
||
| GC | HOC LOC |
74 ± 18 (10) CD 93 ± 25 (10) BC |
15 ± 6 (10) E | 16 ± 5 (10) AB 14 ± 8 (10) B |
21 ± 13 (8) C 61 ± 30 (10) BC |
55 ± 22 (4) AB 65 ± 26 (5) A |
||||
| Maryland | JP | HOC LOC |
45 ± 45 (8) DE 50 ± 28 (12) DE |
23 ± 13 (27) E 23 ± 8 (19) E |
15 ± 13 (20) B 24 ± 8 (16) A |
15 ± 2 (10) D | 15 ± 4 (9) C 16 ± 6 (10) C |
18 ± 7 (11) AB 28 ± 47 (14) AB |
22 ± 0 (1) A | |
| PC | HOC LOC |
32 ± 23 (17) E 26± 4 (10) E |
13 ± 2 (5) E 20 ± 3 (4) DE |
18 ± 1 (5) AB | 12 ± 7 (2) BC | 19 ± 4 (10) C | 14 ± 0 (1) AB 14 ± 4 (10) A |
Comparison of Bioaccumulation Factors between paired subsites:
Bioaccumulation factors (BAF’s), calculated from the average high and low tide dissolved MeHg concentrations, are shown across sites for silversides and mummichog in Fig. 4. Within sites, significantly higher BAF’s in silversides were observed at the LOC relative to HOC subsites at 4 out of 5 sites. Similarly, BAF’s for mummichog, were significantly higher at 3 of the 4 LOC sites. Three of the sites where lower BAF’s were observed at HOC subsites were those associated with large coastal marsh preserves (BH, BI, and PC), and include sites with the largest differences in water column DOC concentration (BH, BI). Because tidal differences have a large influence on BAF calculation at some subsites, the BAF’s based on low and high tide dissolved MeHg concentrations are shown in Supplemental Figure 1S. Tide did cause a differences in BAF between subsites, as indicated in Figure 4: using low tide dissolved MeHg concentrations, the differences in BAF between subsites for both fish species become insignificant at PC, although mean BAF at the LOC site are still higher than the HOC site. The difference in BAF at BH for silversides was also insignificant at low tide, and the low tide BAF at GC is significantly higher at the HOC subsite. At high tide, the BAFs for both fish at HOC were higher at the JP site.
Figure 4).
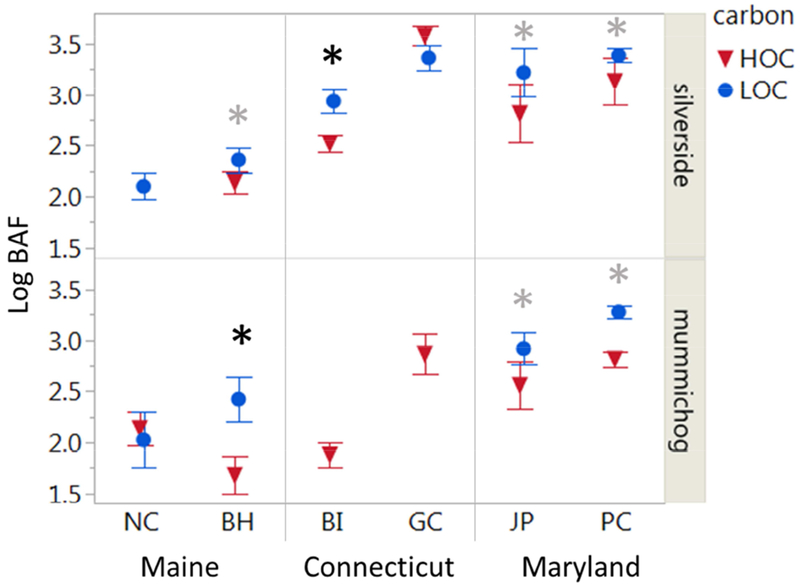
Log Bioaccumulation Factors (BAF) of MeHg in biota vs. water column (dissolved) in fish across subsites (mean ± SD). Asterisks (*) indicates significantly higher BAF (p < 0.05) in the low OC subsite. Black asterisks indicate this relationship was significant using water concentrations across tides; grey asterisks indicate the relationship was not significant using low or high tide water concentrations alone.
Striped silversides and invertebrates were only found across one or two paired subsites; BAFs of these species are shown in the Supplemental Figure 2S. Like the other fish species, striped killifish had higher BAFs at the LOC subsite at JP, but had higher BAF’s at the HOC site at GC. Shrimp had higher BAFs at the LOC site at BI, but also showed no differences at GC. Periwinkle had no significant differences between sites and mussels had slightly higher BAFs at the HOC site.
Factors controlling MeHg in fish across sites
Accumulation of MeHg in fish was investigated by comparing models, first by including average concentrations of MeHg (particulate and dissolved), DOC and Chl-a in the water column, as well as MeHg in the sediment, then further by including high and low tide concentrations of the water column parameters. Correlations between abiotic parameters across sites were first identified, to evaluate collinearity. Concentrations of dissolved MeHg in the water column were positively related to DOC concentrations across sites, with stronger correlations at low tide (Fig. 5). The relationship between Chl-a and MeHg in the water column was more confounding; there was no correlation between MeHg in the suspended particulate with increased productivity in the water column, whereas the MeHg partition coefficients (KD) was positively correlated with Chl-a, which was only significant at high tide (Fig. 6).
Figure 5).
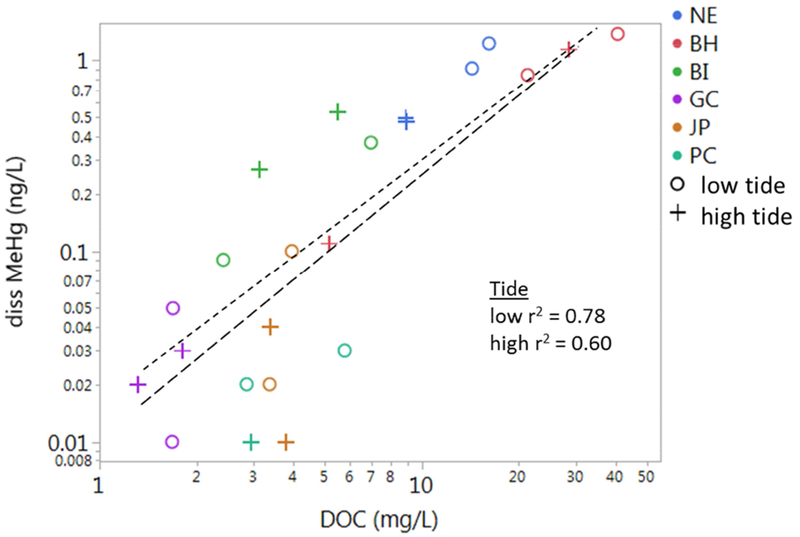
Dissolved MeHg (ng/L) vs. DOC (mg/L) across sites (colors) at low (o; short dashed line) and high tide (+; long dashed line). Correlations of log MeHg vs. log DOC are given for low and high tides.
Figure 6).
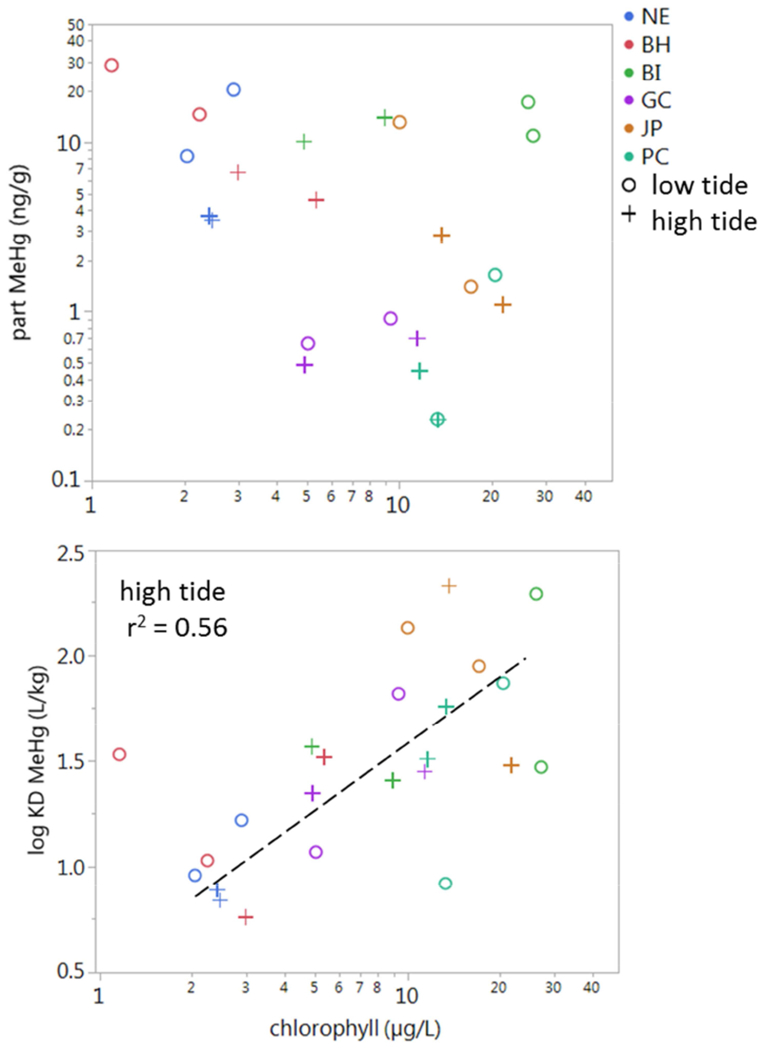
Relationship between A) particulate MeHg (ng/g) and Chl-a (μg/L), and B) log KD (MeHg) vs. Chl-a across sites (colors) at low (o; short dashed line) and high tide (+). Correlations of log KD (MeHg) vs. Chl-a are significant at low tide.
The best model describing MeHg concentrations in silversides was dissolved MeHg – DOC, where both MeHg and DOC were average of high and low tide concentrations (Table 2). Investigations of tidal differences also revealed a strong correlation between MeHg in silversides and high tide dissolved MeHg concentrations (r2 = 0.73 ; r2adjj = 0.69; p = 0.0017), whereas high tide DOC concentrations were not significant in the model. Concentrations of MeHg in mummichog were best described by low tide concentrations of dissolve MeHg. No significant predictors of MeHg in striped killifish or the invertebrate species were identified, likely because these species were found at fewer sites than the other fish.
Table 2.
Regression models of log transformed MeHg body burdens in silversides and mummichog.
| Silversides | RSquare | RSquare Adj | AICc |
| 0.845 | 0.806 | −3.17 | |
| Parameter | Estimate | Prob>F | |
| Intercept | 2.784 | ||
| log MeHg diss AVG | 0.548 | 0.0004 | |
| log DOC AVG | −0.498 | 0.0189 | |
| Mummichog | RSquare | RSquare Adj | AICc |
| 0.819 | 0.797 | 0.588 | |
| Parameter | Estimate | Prob>F | |
| Intercept | 1.883 | ||
| log MeHg diss LOW | 0.398 | 0.0003 |
The relationships between dissolved MeHg, DOC and concentrations of MeHg in fish were further explored in Figure 7. While DOC is linearly correlated with MeHg in mummichog, the relationship is much stronger at low tide. In contrast, there is no significant relationship between MeHg in silversides and DOC. Differences in MeHg concentrations in silversides and mummichog are evident between sites, relative to MeHg and DOC in the water column. In particular, higher concentrations of MeHg were found in silversides than in mummichog at GC and BI sites.
Figure 7).
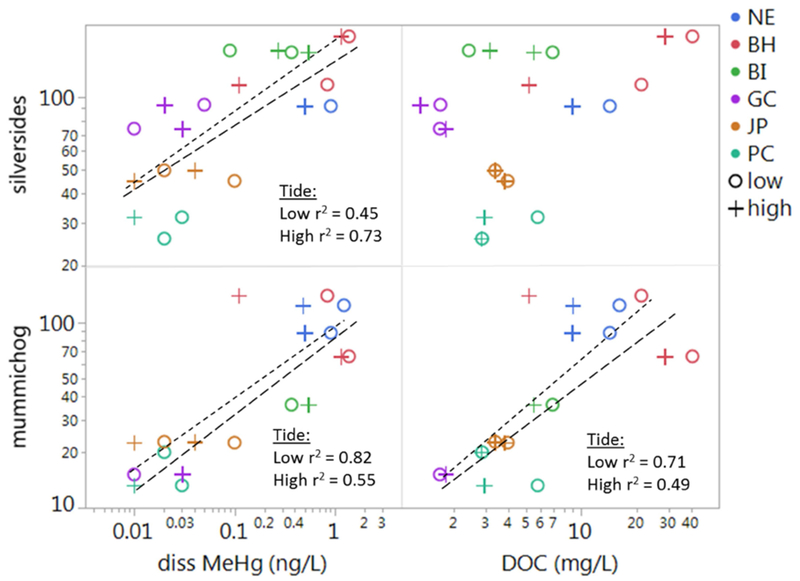
Mean MeHg (ng/g) in silversides (top row) and mummichog (bottom row) vs. dissolved MeHg (ng/L; left column) and DOC (mg/L; right column). Sites are designated by color; low tides are shown as (o; short dashed line) and high tide (+; long dashed line).
Discussion
A wide range of MeHg and organic carbon levels were captured in estuarine sediments and the water column, with concentrations varying across regions and between sites and subsites. In the water column, higher MeHg and DOC were evident at the Maine sites, whereas phytoplankton productivity (as Chl-a) was higher at BI and in warmer, southern regions. The highest concentrations of MeHg in several species of biota, including mummichog, silversides, mussels and periwinkles, were found at the Maine sites. These sites are located within (BH) or adjacent to (NE) Acadia National Park, a known hotspot for MeHg in freshwater (Bank et al., 2007; Loftin et al., 2012). The high MeHg concentrations are likely specific to this area rather than representing a regional or temperature trend, as our previous studies of other sites along the Maine coast (Balcom et al., 2015; Chen et al., 2014) have shown much lower MeHg loading.
Differences in MeHg in the biota were discernible between regions, but differences between sites and subsites were less apparent: only one pair of subsites had significantly different MeHg concentrations in fish (BH), and the two sites in Connecticut (GC and BI), had differences in silverside MeHg concentration. This is in contrast to a study of sites within San Francisco estuary, where large differences in Hg concentration in fish were found at sites within close proximity (Eagles-Smith and Ackerman, 2014). The influence of site chemistry, including sediment and water MeHg loading, were not measured in the other study, so it cannot be ascertained whether there were larger differences in water chemistry between sites.
Biotic-water BAFs provided a means of investigating differences in bioaccumulation between subsites with different MeHg loading, and for the intertidal fish, areas with higher organic carbon sediment were associated with an overall effect of decreasing MeHg uptake. While BAFs provide an effective means of comparing within-site differences, they can be problematic deciphering mechanistic relationships between factors controlling bioaccumulation across sites, and require careful interpretation (Pollman and Axelrad, 2014). Differences in fish MeHg concentrations are much smaller than variability in MeHg in the water column (Chen et al., 2009), such that effects of the denominator on BAF values are large. In addition, water analyses were taken at a single time point, or across a tidal cycle, and temporal variation at some sites contributes to variance in BAF values. Differences in BAFs calculated from high and low tide MeHg concentrations were large at some sites. However, while differences between subsites were not always significant across high and low tide, the mean BAF value were consistently higher at LOC sites for both fish species, with the exception of the JP site. This site had low concentrations of organic carbon in the sediment and water column at both subsites, and the smallest differences in %LOI between subsites. In addition, the subsites were in very close proximity to one another. Further support of the difference in bioaccumulation between subsites comes from both mummichog, which are a detrital-feeding resident species (McMahon et al., 2005), and Atlantic silversides, which are more transient and pelagic feeders, having lower mean BAF’s at HOC subsites, across latitudes. The trend was not evident in invertebrates, likely due to their different feeding strategies than fish.
Differences in BAF were most evident at sites associated with large coastal marsh areas (BH, BI, PC) and those having the largest differences in DOC concentration (BH and BI). While DOC increases the solubility and stability of MeHg in the water column in estuaries (Bergamaschi et al., 2011; Mitchell et al., 2012), it also decreases the bioavailability of MeHg at the base of the foodweb (Lee and Fisher, 2017; Luengen et al., 2012). Differences in DOC quality have been suggested to effect MeHg bioavailability, where humic fractions of terrestrial or wetland-derived DOC have been found to form DOC-MeHg complexes with low bioavailability (Schartup et al., 2015b). Labile, marine sourced DOC has been found to be more persistent in the water column (Soerensen et al., 2017) and any MeHg associated with it more bioavailable (Schartup et al., 2015b). Differences in DOC concentration and source therefore likely play a role in influencing BAF between subsites. These different factors influencing BAF add to our understanding from studies where wetland area has been associated with higher MeHg concentrations in estuarine fish (Buckman et al., 2017; Eagles-Smith and Ackerman, 2014), due to increased MeHg production in wetlands (Mitchell and Gilmour, 2008), and suggests these environments are also suppressing MeHg uptake to the biota.
Further investigation of the factors controlling bioaccumulation in estuaries come from evaluating the relationships between water column chemistry and MeHg in the water column and in fish. Across sites, levels of MeHg in the water column, but not the sediment, were found to be predictive of MeHg in the biota. In this study, MeHg in the dissolved fraction, not the particulate fraction, of the water column was more strongly associated with MeHg in the biota, whereas prior studies (Buckman et al., 2017; Chen et al., 2014) found MeHg in suspended particulate to be correlated with fish MeHg concentrations. In this study, a larger range and higher concentrations of dissolved MeHg (0.01 to 1.26 ng/L) were captured than in (Chen et al., 2014) (0.001 to 0.025 ng/L), compared with a slightly smaller range of particulate MeHg in this study (0.2 to 16.7 ng/g) than the previous study (0.14 to 20.1 ng/g).
Differences in water column chemistry (DOC, Chl-a) across tides affected both MeHg concentrations in the water column, and bioaccumulation in the two fish species. Concentrations of dissolved MeHg in the water column were related across sites with DOC, with a stronger correlation at low tide (Fig. 4), suggesting DOC and MeHg inputs were from the watershed. There are few studies of MeHg and DOC across a wide range of estuarine systems. In streams, concentrations of MeHg have been found to correlate with DOC across a wide range of sites (Tsui and Finlay, 2011), although the relationship is less clear across time points because MeHg concentrations vary temporally (Chasar et al., 2009). MeHg concentrations in estuaries also vary temporally throughout the year (Mitchell et al., 2012), but have been found to associate across tidal cycles (Bergamaschi et al., 2011). While MeHg and DOC were correlated in intertidal waters, differences in watershed and marine inputs, and the extent of mixing between sites contribute to changing correlations between tides.
Phytoplankton productivity was also related to MeHg in the water column, where at high tide, MeHg partitioned to the particulate phase with increasing Chl-a (Fig. 6b). This corresponds with higher densities of marine phytoplankton (as Chl-a) at high tides, which can bioconcentrate MeHg by about 105 times relative to seawater (Lee and Fisher, 2017; Schartup et al., 2018). The differences in Chl-a across regions, where phytoplankton productivity is higher in the southern sites, makes its relationship with MeHg concentrations difficult to isolate from other factors in estuarine systems. In freshwater systems, algal blooms have been associated with decreased MeHg levels in plankton due to rapid increases in biomass relative to available MeHg (Chen and Folt, 2005; Pickhardt et al., 2002). Bloom dilution has also been inferred in an undisturbed estuarine system (Luengen and Flegal, 2009), but modelled growth of marine zooplankton suggests blooms in open water marine environments are insufficient to dilute MeHg (Schartup et al., 2018). In this study, the effects of phytoplankton density on MeHg cycling in the water column and on bioaccumulation in fish are difficult to separate from other drivers, and have the strongest influence at high tide.
Across sites, uptake of MeHg into the two fish species differed with their feeding preferences, which corresponded with different water column conditions across tides. Silverside MeHg concentrations were more strongly correlated with high tide MeHg levels in the water column (Fig. 7); this reflects the more selective feeding practices of this species, which prefer to eat zooplankton, found in marine waters. In contrast, mummichog, which correlated more strongly with low tide MeHg water concentrations, are resident in near shore areas where they feed omnivorously from benthic and pelagic sources. The bioaccumulation of MeHg in both killifish and silversides is almost exclusively due to dietary uptake (Dutton and Fisher, 2010, 2014) although 10% of MeHg in fish has been associated with assimilation from water (Hrenchuk et al., 2012), and decreases in MeHg uptake from the water column have been observed in association with higher concentrations of DOC (Dutton and Fisher, 2012). Deciphering specific uptake pathways from the abiotic compartments to higher trophic levels is complex at estuarine sites, because primary producers and consumers, at the base of the foodweb, are associated with the largest Hg bioaccumulation factors, but are difficult and often impossible to isolate from the other seston. Particulate samples from the water column consist of a mixture of suspended sediment, detrital material, and phytoplankton, with each of these fractions likely containing different relative amounts of MeHg, with different bioavailability. In this study, the dissolved fraction of MeHg better represented concentrations in the phytoplankton portion of the suspended particulate, whereas MeHg concentrations in the particulate likely varied between sites because of differences in its overall composition.
The role of organic carbon across sites is also complex, and collinearity between MeHg and DOC makes their individual roles in the MeHg accumulation model difficult to interpret. Organic carbon plays a complex role in MeHg bioavailability, where uptake of MeHg at the base of the foodweb has in some instances been positively correlated with DOC (Pickhardt and Fisher, 2007), but negatively correlated in others (Gorski et al., 2008; Luengen et al., 2012). Other studies have shown a non-linear relationship with increasing MeHg bioaccumulation at low DOC and decreasing at high DOC (Chaves-Ulloa et al., 2016; Driscoll et al., 1995). Recent modeling suggests that bioaccumulation into marine plankton is highest under low DOC concentrations (Schartup et al., 2018). In the nearshore environments of this study, concentrations of MeHg and DOC in the water column are much higher than in open water studies. Mummichog MeHg concentrations correlated with DOC in the water column, which may reflect the association between MeHg and DOC in the water column. By contrast, DOC had an inverse relationship with MeHg in silversides, which may reflect its restricting effect on bioavailability to phytoplankton, at the base of the pelagic foodweb. The non-linearity of the relationship between DOC and MeHg in silversides also infers different sources of carbon between sites, where bioavailability of MeHg in the water column appears to be higher at the Connecticut sites (BI and GC) than the Maine sites (BH and NC). This may be due to more terrestrial DOC at the Maine sites, and more labile, marine DOC at the Connecticut sites. Despite the apparent suppressing effect of DOC on MeHg accumulation in silversides, MeHg levels in both fish species increased with large increases in MeHg in the water column, which were in turn related to DOC in the water column. These findings suggest increased watershed inputs of MeHg and DOC will have an overall impact of increasing MeHg accumulation in estuarine fish.
Conclusion
This study demonstrates that, as hypothesized, within sites, the bioaccumulation of MeHg varies between subsites with different organic carbon content in the sediment, particularly in areas surrounded by coastal marsh and with large differences in DOC in the water column. Across sites, the loading of MeHg to the water column is a strong predictor of the concentrations of MeHg in biota, across a wide range of environmental conditions. Additionally, the water column concentration is not directly related to the levels of MeHg in the bulk local sediments. Furthermore, much larger differences in aqueous MeHg concentrations across sites, which are associated with DOC inputs, provide evidence that increased watershed inputs of DOC and MeHg are driving the higher concentrations in the biota. There is some suggestion that increased productivity will result in increased partitioning of MeHg into phytoplankton and the estuarine foodweb. Furthermore, increased watershed inputs of organic carbon and MeHg associated increase MeHg levels in estuarine fish, despite the within-site effects of organic carbon reducing MeHg bioaccumulation.
Supplementary Material
Highlights.
Dissolved organic carbon drives dissolved methylmercury levels in the intertidal zone
Fish levels of methylmercury are positively associated with water column concentrations
Methylmercury bioaccumulation is lower in environments with high organic carbon content in sediment
Increased water column methylmercury from watersheds will increase levels in fish
Acknowledgements
This paper was supported by funds from the National Institute of Environmental Health Sciences P42ES007373 and RO1ES021950. Its contents are solely the responsibility of the grantees and do not necessarily represent the official views of the National Institutes of Health. Thanks to Veronica Tanguay, Kathleen Gosnell and Brian DiMento for help in the field and with DOC and Chl-a measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Literature cited
- Balch W, Huntington T, Aiken G, Drapeau D, Bowler B, Lubelczyk L, Butler K, 2016. Toward a quantitative and empirical dissolved organic carbon budget for the Gulf of Maine, a semienclosed shelf sea. Global Biogeochemical Cycles 30, 268–292. [Google Scholar]
- Balcom PH, Fitzgerald WF, Vandal GM, Lamborg CH, Rolfllus KR, Langer CS, Hammerschmidt CR, 2004. Mercury sources and cycling in the Connecticut River and Long Island Sound. Marine Chemistry 90, 53–74. [Google Scholar]
- Balcom PH, Schartup AT, Mason RP, Chen CY, 2015. Sources of water column methylmercury across multiple estuaries in the Northeast US. Marine Chemistry 177, 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank MS, Burgess JR, Evers DC, Loftin CS, 2007. Mercury contamination of biota from Acadia National Park, Maine: A review. Environmental Monitoring and Assessment 126, 105–115. [DOI] [PubMed] [Google Scholar]
- Baumann Z, Mason RP, Conover DO, Balcom P, Chen CY, Buckman KL, Fisher NS, Baumann H, 2017. Mercury bioaccumulation increases with latitude in a coastal marine fish (Atlantic silverside, Menidia menidia). Canadian Journal of Fisheries and Aquatic Sciences 74, 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi BA, Fleck JA, Downing BD, Boss E, Pellerin B, Ganju NK, Schoellhamer DH, Byington AA, Heim WA, Stephenson M, Fujii R, 2011. Methyl mercury dynamics in a tidal wetland quantified using in situ optical measurements. Limnology and Oceanography 56, 1355–1371. [Google Scholar]
- Bloom N, Fitzgerald WF, 1988. Determination of Volatile Mercury Species at the Picogram Level by Low-Temperature Gas-Chromatography with Cold-Vapor Atomic Fluorescence Detection. Analytica Chimica Acta 208, 151–161. [Google Scholar]
- Bloom NS, Crecelius EA, 1983. Determination of Mercury in Seawater at Sub-Nanogram Per Liter Levels. Marine Chemistry 14, 49–59. [Google Scholar]
- Buckman K, Taylor V, Broadley H, Hocking D, Balcom P, Mason R, Nislow K, Chen C, 2017. Methylmercury Bioaccumulation in an Urban Estuary: Delaware River, USA. Estuaries and Coasts 40, 1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasar LC, Scudder BC, Stewart AR, Bell AH, Aiken GR, 2009. Mercury Cycling in Stream Ecosystems. 3. Trophic Dynamics and Methylmercury Bioaccumulation. Environmental Science & Technology 43, 2733–2739. [DOI] [PubMed] [Google Scholar]
- Chaves-Ulloa R, Taylor BW, Broadley HJ, Cottingham KL, Baer IA, Weathers KC, Ewing HA, Chen CY, 2016. Dissolved organic carbon modulates mercury concentrations in insect subsidies from streams to terrestrial consumers. Ecological Applications 26, 1771–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Borsuk ME, Bugge DM, Hollweg T, Balcom PH, Ward DM, Williams J, Mason RP, 2014. Benthic and Pelagic Pathways of Methylmercury Bioaccumulation in Estuarine Food Webs of the Northeast United States. Plos One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP, 2009. Mercury Bioavailability and Bioaccumulation in Estuarine Food Webs in the Gulf of Maine. Environmental Science & Technology 43, 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Folt CL, 2005. High plankton densities reduce mercury biomagnification. Environmental Science & Technology 39, 115–121. [PubMed] [Google Scholar]
- Cloern JE, 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210, 223–253. [Google Scholar]
- Conaway CH, Squire S, Mason RP, Flegal AR, 2003. Mercury speciation in the San Francisco Bay estuary. Marine Chemistry 80, 199–225. [Google Scholar]
- Deegan LA, Hughes JE, Rountree RA, 2000. Salt marsh eco-system support of marine transient species., in: Weinstein MP, Kreeger DA (Eds.), Concepts and Controversies in Tidal Marsh Ecology. Kluwer Academic Publishers, Dordrecht, pp. 333–365. [Google Scholar]
- Dijkstra JA, Buckman KL, Ward D, Evans DW, Dionne M, Chen CY, 2013. Experimental and Natural Warming Elevates Mercury Concentrations in Estuarine Fish. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMento BP, Mason RP, 2017. Factors controlling the photochemical degradation of methylmercury in coastal and oceanic waters. Marine Chemistry 196, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CT, Blette V, Yan C, Schofield CL, Munson R, Holsapple J, 1995. The role of dissolved organic-carbon in the chemistry and bioavailability of mercury in remote adirondack lakes. Water Air and Soil Pollution 80, 499–508. [Google Scholar]
- Driscoll CT, Chen CY, Hammerschmidt CR, Mason RP, Gilmour CC, Sunderland EM, Greenfield BK, Buckman KL, Lamborg CH, 2012. Nutrient supply and mercury dynamics in marine ecosystems: A conceptual model. Environmental Research 119, 118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK, 2007. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience 57, 17–28. [Google Scholar]
- Dutton J, Fisher NS, 2010. Intraspecific comparisons of metal bioaccumulation in the juvenile Atlantic silverside Menidia menidia. Aquatic Biology 10, 211–226. [Google Scholar]
- Dutton J, Fisher NS, 2012. Influence of humic acid on the uptake of aqueous metals by the killifish Fundulus heteroclitus. Environmental Toxicology and Chemistry 31, 2225–2232. [DOI] [PubMed] [Google Scholar]
- Dutton J, Fisher NS, 2014. Modeling metal bioaccumulation and tissue distribution in killifish (fundulus heteroclitus) in three contaminated estuaries. Environmental Toxicology and Chemistry 33, 89–101. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, 2014. Mercury bioaccumulation in estuarine wetland fishes: Evaluating habitats and risk to coastal wildlife. Environmental Pollution 193, 147–155. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Silbergeld EK, Basu N, Bustamante P, Diaz-Barriga F, Hopkins WA, Kidd KA, Nyland JF, 2018. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47, 170–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald WF, Engstrom DR, Hammerschmidt CR, Lamborg CH, Balcom PH, Lima-Braun AL, Bothner MH, Reddy CM, 2018. Global and Local Sources of Mercury Deposition in Coastal New England Reconstructed from a Multiproxy, High-Resolution, Estuarine Sediment Record. Environmental Science & Technology. [DOI] [PubMed] [Google Scholar]
- Gill GA, Fitzgerald WF, 1979. Mercury Geochemistry of Long Island Sound - Analytical and Field-Study. Abstracts of Papers of the American Chemical Society, 103–&. [Google Scholar]
- Gorski PR, Armstrong DE, Hurley JP, Krabbenhoft DP, 2008. Influence of natural dissolved organic carbon on the bioavailability of mercury to a freshwater alga. Environmental Pollution 154, 116–123. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, 2001. Formation of artifact methylmercury during extraction from a sediment reference material. Analytical Chemistry 73, 5930–5936. [DOI] [PubMed] [Google Scholar]
- Hrenchuk LE, Blanchfield PJ, Paterson MJ, Hintelmann HH, 2012. Dietary and Waterborne Mercury Accumulation by Yellow Perch: A Field Experiment. Environmental Science & Technology 46, 509–516. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Andersson A, Nilsson MB, Skyllberg U, Lundberg E, Schaefer JK, Akerblom S, Bjorn E, 2017. Terrestrial discharges mediate trophic shifts and enhance methylmercury accumulation in estuarine biota. Science Advances 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Skyllberg U, Nilsson MB, Lundberg E, Andersson A, Bjorn E, 2014. Differentiated availability of geochemical mercury pools controls methylmercury levels in estuarine sediment and biota. Nature Communications 5. [DOI] [PubMed] [Google Scholar]
- Kim E, Mason RP, Bergeron CM, 2008. A modeling study on methylmercury bioaccumulation and its controlling factors. Ecological Modelling 218, 267–289. [Google Scholar]
- Kneib RT, 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanogr. Mar. Biol. Annu. Rev. 35, 163–330. [Google Scholar]
- Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM, 2013. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environmental Science & Technology 47, 13385–13394. [DOI] [PubMed] [Google Scholar]
- Lee CS, Fisher NS, 2017. Bioaccumulation of methylmercury in a marine diatom and the influence of dissolved organic matter. Marine Chemistry 197, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftin CS, Calhoun AJK, Nelson SJ, Elskus AA, Simon K, 2012. Mercury Bioaccumulation in Wood Frogs Developing in Seasonal Pools. Northeastern Naturalist 19, 579–600. [Google Scholar]
- Lorey P, Driscoll CT, 1999. Historical trends of mercury deposition in Adirondack lakes. Environmental Science & Technology 33, 718–722. [Google Scholar]
- Luengen AC, Fisher NS, Bergamaschi BA, 2012. Dissolved organic matter reduces algal accumulation of methylmercury. Environmental Toxicology and Chemistry 31, 1712–1719. [DOI] [PubMed] [Google Scholar]
- Luengen AC, Flegal AR, 2009. Role of phytoplankton in mercury cycling in the San Francisco Bay estuary. Limnology and Oceanography 54, 23–40. [Google Scholar]
- McMahon KW, Johnson BJ, Ambrose WG, 2005. Diet and movement of the killifish, Fundulus heteroclitus, in a Maine salt marsh assessed using gut contents and stable isotope analyses. Estuaries 28, 966–973. [Google Scholar]
- Mitchell CPJ, Gilmour CC, 2008. Methylmercury production in a Chesapeake Bay salt marsh. Journal of Geophysical Research-Biogeosciences 113. [Google Scholar]
- Mitchell CPJ, Jordan TE, Heyes A, Gilmour CC, 2012. Tidal exchange of total mercury and methylmercury between a salt marsh and a Chesapeake Bay sub-estuary. Biogeochemistry 111, 583–600. [Google Scholar]
- Pershing AJ, Alexander MA, Hernandez CM, Kerr LA, Le Bris A, Mills KE, Nye JA, Record NR, Scannell HA, Scott JD, Sherwood GD, Thomass AC, 2015. Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science 350, 809–812. [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, Fisher NS, 2007. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environmental Science & Technology 41, 125–131. [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD, 2002. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proceedings of the National Academy of Sciences of the United States of America 99, 4419–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkney AE, Driscoll CT, Evers DC, Hooper MJ, Horan J, Jones JW, Lazarus RS, Marshall HG, Milliken A, Rattner BA, Schmerfeld J, Sparling DW, 2015. Interactive Effects of Climate Change with Nutrients, Mercury, and Freshwater Acidification on Key Taxa in the North Atlantic Landscape Conservation Cooperative Region. Integrated Environmental Assessment and Management 11, 355–369. [DOI] [PubMed] [Google Scholar]
- Pollman CD, Axelrad DM, 2014. Mercury bioaccumulation factors and spurious correlations. Science of the Total Environment 496, Vi–Xii. [DOI] [PubMed] [Google Scholar]
- Schartup AT, Balcom PH, Soerensen AL, Gosnell KJ, Calder RSD, Mason RP, Sunderland EM, 2015a. Freshwater discharges drive high levels of methylmercury in Arctic marine biota. Proceedings of the National Academy of Sciences of the United States of America 112, 11789–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartup AT, Ndu U, Balcom PH, Mason RP, Sunderland EM, 2015b. Contrasting Effects of Marine and Terrestrially Derived Dissolved Organic Matter on Mercury Speciation and Bioavailability in Seawater. Environmental Science & Technology 49, 5965–5972. [DOI] [PubMed] [Google Scholar]
- Schartup AT, Qureshi A, Dassuncao C, Thackray CP, Harding G, Sunderland EM, 2018. A Model for Methylmercury Uptake and Trophic Transfer by Marine Plankton. Environmental Science & Technology 52, 654–662. [DOI] [PubMed] [Google Scholar]
- Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA, 2014. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bulletin of the World Health Organization 92, 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen AL, Schartup AT, Gustafsson E, Gustafsson BG, Undeman E, Bjorn E, 2016. Eutrophication Increases Phytoplankton Methylmercury Concentrations in a Coastal Sea-A Baltic Sea Case Study. Environmental Science & Technology 50, 11787–11796. [DOI] [PubMed] [Google Scholar]
- Soerensen AL, Schartup AT, Skrobonja A, Bjorn E, 2017. Organic matter drives high interannual variability in methylmercury concentrations in a subarctic coastal sea. Environmental Pollution 229, 531–538. [DOI] [PubMed] [Google Scholar]
- Stoichev T, Tessier E, Amouroux D, Almeida CM, Basto MCP, Vasconcelos VM, 2016. Multiple regression analysis to assess the role of plankton on the distribution and speciation of mercury in water of a contaminated lagoon. Journal of Hazardous Materials 318, 711–722. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, 2007. Mercury exposure from domestic and imported estuarine and marine fish in the US seafood market. Environmental Health Perspectives 115, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VF, Carter A, Davies C, Jackson BP, 2011. Trace-level automated mercury speciation analysis. Analytical Methods 3, 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VF, Jackson BP, Chen CY, 2008. Mercury speciation and total trace element determination of low-biomass biological samples. Analytical and Bioanalytical Chemistry 392, 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CM, Hammerschmidt CR, Fitzgerald WF, 2004. Determination of methylmercury in environmental matrixes by on-line flow injection and atomic fluorescence spectrometry. Analytical Chemistry 76, 7131–7136. [DOI] [PubMed] [Google Scholar]
- Tsui MT, Finlay JC, 2011. Influence of dissolved organic carbon on methylmercury bioavailability across Minnesota stream ecosystems. Environmental Science & Technology 45, 5981–5987. [DOI] [PubMed] [Google Scholar]
- U.S. EPA, E.P.A., 1998. Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, in: Agency, U.S.E.P. (Ed.), Washington, D.C. [Google Scholar]
- Van LN, Jonsson S, Skyllberg U, Nilsson MB, Andersson A, Lundberg E, Bjorn E, 2016. Effects of Nutrient Loading and Mercury Chemical Speciation on the Formation and Degradation of Methylmercury in Estuarine Sediment. Environmental Science & Technology 50, 6983–6990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


