Abstract
Introduction
Radiofrequency (RF) and Cryo ablation are routinely used to treat arrhythmias, but the extent and time course of edema associated with the two different modalities is unknown. Our goal was to follow the lesion maturation and edema formation after RF and Cryo ablation using serial magnetic resonance imaging (MRI).
Methods and Results
Ventricular ablation was performed in a canine model (n=11) using a Cryo or an irrigated RF catheter. T2-weighted (T2w) edema imaging and late gadolinium enhancement (LGE)-MRI were done immediately (0 day: acute), 1 to 2 weeks (sub-acute), and 8 to 12 weeks (chronic) after ablation. After the final MRI, excised hearts underwent pathological evaluation. As a result, forty-five ventricular lesions (Cryo-group: 20, RF-group: 25) were evaluated. Acute LGE volume was not significantly different but acute edema volume in Cryo-group was significantly smaller (1225.0±263.5 vs 1855.2±520.5 mm3, p=0.01). One week after ablation, edema still existed in both group but was similar in size. Two weeks after ablation there was no edema in either of the groups. In the chronic phase, the lesion volume for Cryo and RF in LGE-MRI (296.7±156.4 vs 281.6±140.8 mm3, p=0.73) and pathology (243.3±125.9 vs 214.5±148.6 mm3, p=0.49) as well as depth was comparable.
Conclusions
When comparing Cryo and RF lesions of similar chronic size, acute edema is larger for RF lesions. Edema resolves in both Cryo and RF lesions in 1 to 2 weeks.
Keywords: Catheter ablation, Radiofrequency, Cryoablation, Magnetic resonance imaging, Late gadolinium enhancement
Introduction
Cryo and radiofrequency (RF) ablation are routinely used to treat cardiac arrhythmias1.
Ablation success depends on creating durable lesions. Despite best efforts, arrhythmia recurrence following catheter ablation is commonly observed2, 3. Prior studies have shown that there can be significant edema formation during ablation4 and some of the recurrence has been attributed to the creation of this edema and as a result, reversible block5. Deeper and bigger lesions can be made by using higher contact force, but higher contact force is also associated with more complications like steam pops as well as edema6, 7. We have also recently reported the large extent of edema that is associated with RF ablation and that it resolves over the span of few weeks8. However, the differences between the creation and timeline of edema associated with Cryo versus RF are still unknown.
Late gadolinium enhancement magnetic resonance imaging (LGE-MRI) has been used to estimate myocardial scar after catheter ablation and T2 weighted (T2w) MRI has been used to estimate edema9–11. Our goal in this study was to study the timeline and differences between edema and lesion maturation between Cryo and RF ablation by using serial MR imaging for acute and chronic ablation lesions of similar size.
Methods
Electrophysiology Study and Ablation
This study was performed in a canine model (n=11). The study protocol was approved by Institutional Animal Care and Use Committee in University of Utah and conforms to the Guide for the Care and Use of Laboratory Animals. Animals were anesthetized using propofol and then intubated for general anesthesia with 1 to 3% isoflurane. Bi-femoral vein access was obtained percutaneously and a 5Fr sheath was also inserted in a femoral artery for continuous arterial blood pressure monitoring. Through the left femoral vein, an intra-cardiac echocardiogram (ICE) catheter (Cypress, Siemens Healthcare, Erlangen, Germany) was inserted to the right atrium. Through the right femoral vein, we placed an 8.5 Fr SL0 sheath. The SL0 sheath was used to go transseptal with a BRK-1 needle (St Jude Medical, Minnetonka, USA) and get left atrial access under ICE and fluoroscopy guidance. Ablation lesions were made in the left and right ventricle. Cryoablation was performed using an 8 mm tip cryothermal ablation catheter (Freezor MAX; Medtronic, Minneapolis, USA). Cryo ablation was performed with a freeze duration of 2 or 4 minutes. RF ablation was done using irrigated catheters (Thermocool SmartTouch™ or Thermocool (Biosense Webster, Diamond Bar, USA)). RF ablation was created using 40 W power for 30 secs under power control mode. During the ablation procedure, a total of 3 to 4 lesions were created within the right and left ventricles. In some animals both Cryo and RF lesions were made but they were done separately with only one type of lesion in the RV or the LV. For lesions made in the RV we only included lesions made in the septum in our analysis as the lesions in the RV free wall were transmural and hence limiting the growth of the edema/lesion size and depth. Animals were transferred to the MRI immediately after the lesion creation and underwent MRI (0day: Acute phase). After the initial ablation and MRI procedure, the animal was extubated and recovered. Each animal underwent additional MRI at 1 or 2 weeks (Sub-acute phase), and 8 to 12 weeks (Chronic phase) following the initial ablation. Following the last MRI, the animals were euthanized and the hearts were excised for pathological lesion assessment.
Lesion Characterization in Gross Pathology:
The hearts were fixed in formalin and then sliced into 2 mm sections. The lesions were matched with the corresponding location in both the chambers and in the MRI. We only evaluated lesions that were distinct and had no overlap with others. Digital images of both sides of each slice were obtained. The area of the lesion (average of the calculated area on the two sides of the slice) was multiplied with the slice thickness to get the lesion volume for each slice. For slices that had lesion only on one edge, the depth and area of the lesion on the surface was used to calculate the lesion volume in that slice assuming a conical shape for the lesion. The volume across all the slices for each lesion was added to get the total lesion volume. For calculating the depth of the lesion, incisions were made through each lesion to measure the maximal depth and it was recorded.
MR Imaging
Following ablation procedure, the animal while intubated was transferred to a 3 Tesla MRI scanner (Verio, Siemens Healthcare, Erlangen, Germany). We obtained both T2 weighted (T2w) images for edema quantification as well as LGE-MRI for lesion size quantification. For non-contrast T2w images we used respiratory navigated, ECG triggered, double inversion recovery (DIR) prepared 2D TSE pulse sequence with echo time (TE)=81 ms, repetition time (TR)=3 cardiac cycles, echo train length=21, fat suppression using SPAIR, in-plane resolution of 1.25×1.25 mm, slice thickness of 4 mm. LGE MRI was acquired 20 mins after injection of Gd-BOPTA (0.15 mmol/kg, Bracco Diagnostic Inc., Princeton, NJ) using respiratory navigated, ECG triggered, inversion recovery prepared 3D GRE sequence with spatial resolution=1.25×1.25×2.5 mm, TR/TE=3.1/1.4 ms, flip angle=14o, and inversion time (TI)=230–330 ms.
Image Processing
The corresponding T2w and LGE images for each lesion were identified. The volume of enhancement for T2w as well as LGE-MRI was calculated using Seg3D image processing software (SCI Institute, University of Utah, USA). The area of enhancement was quantified for each slice and then summed up over all the slices covering that lesion. Figure 1 shows the example of quantification for edema volume and LGE volume. In LGE-MRI, ablated lesion was defined using a pixel intensity threshold algorithm as described in previous reports12, 13. Ablated area was defined as three standard deviations (SDs) above the normal tissue mean pixel intensity. In LGE-MRI acute lesion was characterized by a hypo-intense or micro vascular obstruction (MVO) region in the middle, encircled with a ring of enhancement8. This central MVO region was included in the ablated area measurement, after delineating the ring-like enhanced area using the automated threshold. A similar automated approach was used for edema quantification. A threshold of two standard deviations above the normal wall mean tissue pixel intensity in T2w MR imaging was used. Supplemental figure shows the effect of using different thresholds for edema and outlines the reason for using two SDs used here for edema.
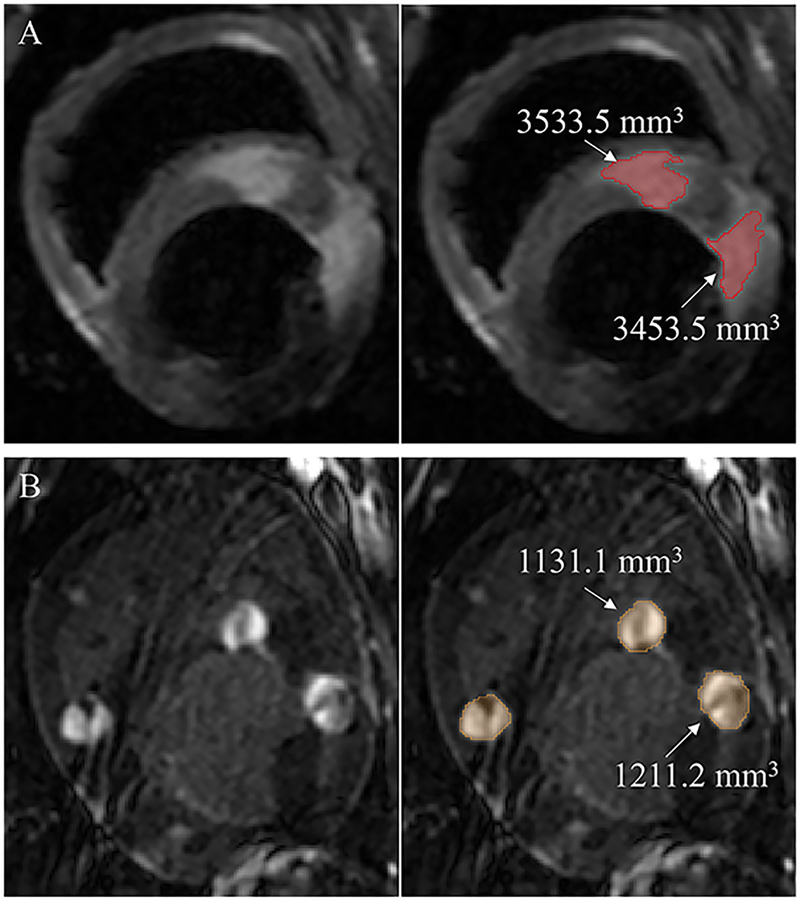
Figure 1.
Edema and LGE MRI volume. (A) Example of edema visualized by acute T2w MRI (left panel) and edema volume quantification by Seg3D (right panel). Edema was defined as two standard deviations above the normal wall mean tissue pixel intensity and is shaded in red. (B) Examples of enhancement in acute LGE-MRI (left panel) and volume quantification by Seg 3D software (right panel). Ablation lesion enhancement was defined as three standard deviations above the normal mean tissue pixel intensity and is shaded in orange.
Statistical Analysis
Statistical analyses were performed with JMP 13 software (SAS Institute Inc., Cary, NC, USA). Categorical data were expressed as frequencies and were compared using chi-square or Fisher’ s exact tests. Continuous data were presented as mean ± SD if normal. Comparison of normally distributed variables between 2 groups was performed by an independent-sample and paired t-test.
To assess the association between acute edema volume and acute LGE volume, a linear regression analysis was performed. Comparing with histology data and lesion size in LGE-MRI, a linear regression analysis was also used to show correlation. A probability (p) value < 0.05 was accepted as indicating statistical significance.
Results
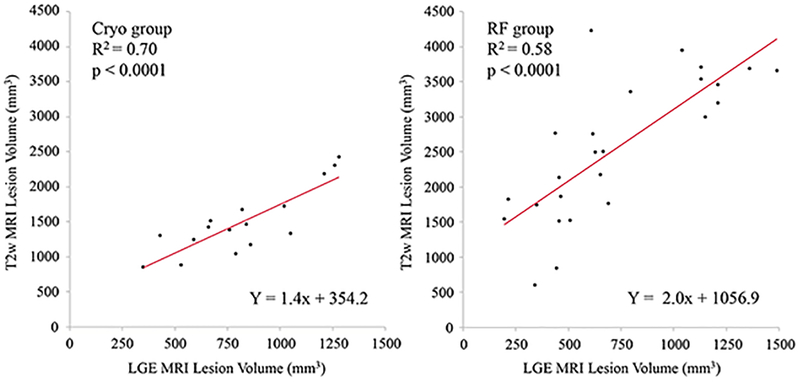
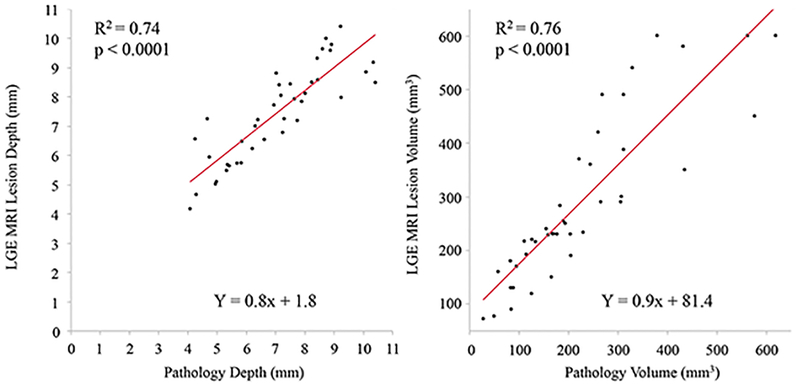
Forty-five (20 Cryo and 25 RF) ventricular lesions in 11 animals were assessed in this study. Figures 2 and 3 show examples of Cryo and RF lesion maturation in MRI acquired at acute phase, 1 week or 2 weeks, and chronic phase and gross pathology. Table 1 describes the lesion location, edema and LGE-MRI volumes in the acute, sub-acute and the chronic phase as well as the pathological volume for Cryo and RF lesions. There was no difference in the lesion location in the two groups. Acute lesion volume in LGE-MRI was comparable between the two groups but acute edema volume in RF group was significantly larger. The T2w imaging for 4 of 45 lesions in the acute phase had poor image quality and hence was not included in the analysis. At one week, both edema and LGE-MRI volume were smaller than the acute phase with no difference between the two groups. After 2 weeks, LGE volume decreased further but more importantly, no enhancement was seen on T2w imaging in both the groups. Figure 4 shows the correlation between LGE-MRI and T2w edema volume in the acute phase (Day 0) for Cryo and RF lesions. While a strong correlation (R2 = 0.70) was observed in Cryo lesion, RF lesion volume had a moderate correlation with edema volume (R2 = 0.58) but it was not significantly different (p = 0.53). Figure 5 shows the correlation between chronic LGE-MRI lesion depth and volume as seen in LGE-MRI and gross pathology. Chronic lesion assessment in LGE-MRI had a strong correlation with pathological findings.
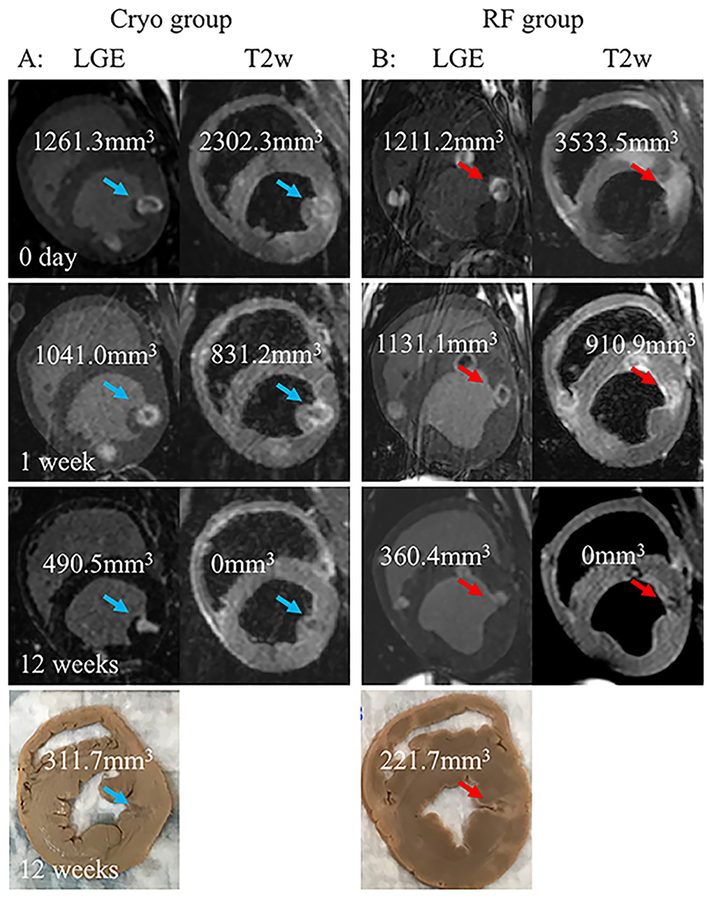
Figure 2.
Acute (0 day), sub-acute (1 week), and chronic (12 weeks) MRI and pathology lesion characterization.
A, LGE and T2w MRI short axis view of a Cryo lesion (blue arrow) acquired acutely (0 day), 1 week and 12 weeks post ablation. The calculated volume for the lesion for the different MRI modalities and pathology is shown.
B, LGE and T2w MRI short axis view of a RF lesion (red arrow) acquired acutely (0 day), 1 week and 12 weeks post ablation. The calculated volume for the lesion for the different MRI modalities and pathology is shown.
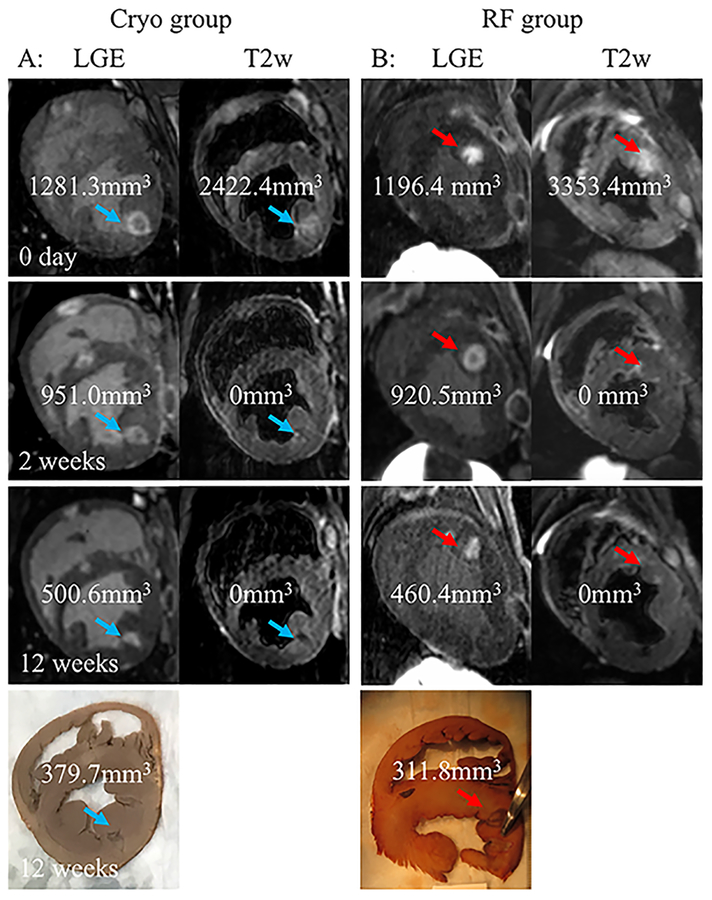
Figure 3.
Acute (0 day), sub-acute (2 week), and chronic (12 weeks) MRI and pathology lesion characterization.
A, LGE and T2w MRI short axis view of a Cryo lesion (blue arrow) acquired acutely (0 day), 2 weeks and 12 weeks post ablation. The calculated volume for the lesion for the different MRI modalities and pathology is shown.
B, LGE and T2w MRI short axis view of a RF lesion (red arrow) acquired acutely (0 day), 2 weeks and 12 weeks post ablation. The calculated volume for the lesion for the different MRI modalities and pathology is shown.
Table 1.
Comparison of Cryo and RF lesion characteristics
| Cryo group | RF group |
p |
n | ||
|---|---|---|---|---|---|
| Number | 20 lesions | 25 lesions | |||
| Location | LV/RV | 12 (60.0)/8 (40.0) | 17 (68.0)/8 (32.0) | 0.58 | |
| Minimum temp, °C | −82.2±1.5 | - | - | ||
| Thaw time, sec | 17.8±17.4 | - | - | ||
| 0 day | Edema Vol, mm3 | 1495.1±496.3 | 2550.2±1003.8 | 0.0008 | 16* vs 25 |
| LGE Vol, mm3 | 817.4±266.8 | 730.7±374.6 | 0.39 | 20 vs 25 | |
| 1 week | Edema Vol, mm3 | 473.2±203.5 | 562.6±292.7 | 0.42 | 15 vs 15 |
| LGE Vol, mm3 | 594.9±217.9 | 708.0±408.9 | 0.35 | 15 vs 15 | |
| 2 weeks | Edema Vol, mm3 | 0 | 0 | - | 5 vs 10 |
| LGE Vol, mm3 | 656.7±270.2 | 463.5±236.9 | 0.18 | 5 vs 10 | |
| 8 to 12 weeks | Edema Vol, mm3 | 0 | 0 | - | 20 vs 25 |
| LGE Vol, mm3 | 296.7±156.4 | 281.6±140.8 | 0.73 | 20 vs 25 | |
| LGE depth, mm | 7.2±1.7 | 7.5±1.5 | 0.56 | 20 vs 25 | |
| Pathology | Depth, mm | 6.8±1.4 | 7.3±1.9 | 0.39 | 20 vs 25 |
| Vol, mm3 | 243.3±125.9 | 214.5±148.6 | 0.49 | 20 vs 25 | |
| Edema, 0 day/LGE, 0 day Vol ratio | 1.9±0.5 | 4.0±1.8 | 0.0001 | 16 vs 25 | |
| LGE, 0 day/LGE, 12 weeks Vol ratio | 2.9±0.6 | 2.6±0.4 | 0.07 | 20 vs 25 | |
| Delay between ablation and T2w MRI, min** | 128.8 ± 42.9 | 172.3 ± 58.6 | 0.02 | 16 vs 25 | |
Four of the T2w MRI had poor image quality and hence not included in the analysis.
Comparison of time gap from ablation to MRIs
RF = radiofrequency, LV = left ventricle, RV = right ventricle, LGE = late gadolinium enhancement, Temp = temperature, vol = volume
Figure 4.
Correlation between acute T2w and LGE MRI volumes for Cryo (left) and RF (right) lesions at day 0.
Figure 5.
Correlation between chronic lesion depth (left panel) and chronic lesion volume (right panel) as seen in LGE MRI and gross pathology.
The delay in time from ablation to MRI was also assessed in both groups. The time delay between lesion creation and T2w imaging was significantly longer for the RF lesions (25 lesions: 172.3 ± 58.6 min) as compared to Cryo lesions (16 lesions: 128.8 ± 42.9 min, p=0.02).
In this study, Cryo group had 2 different freeze cycle times of 120 sec and 240 sec so we also analyzed those two sub groups separately (Table 2). In the 240 sec group, all 9 lesions were made in the LV (p=0.0014). Both acute T2w and LGE volumes in the 240 sec group were significantly larger than those in the 120 sec group. The acute edema/LGE ratio was similar at 1.8 and 2.0 for 240 sec and 120 sec, respectively. In the sub-acute phase, the edema volume had decreased after 1 week and was absent after two weeks. In the chronic phase, lesion depth and volume were significantly larger in the 240 sec group.
Table 2.
Comparison of Cryo lesions
| 120 sec | 240 sec | p | n | ||
|---|---|---|---|---|---|
| N | 11 lesions | 9 lesions | |||
| Location | LV/RV | 3 (27.3)/8 (72.7) | 9 (100)/0 (0) | 0.001 | |
| Minimum temp, °C | −81.7±1.3 | −82.8±1.5 | 0.11 | 11 vs 9 | |
| Thaw time, sec | 12.5±7.4 | 24.3±23.8 | 0.13 | 11 vs 9 | |
| 0 day | Edema Vol, mm3 | 1225.0±263.5 | 1855.2±520.5 | 0.01 | 8 vs 6 |
| LGE Vol, mm3 | 651.1±193.6 | 1020.7±194.1 | 0.0005 | 11 vs 9 | |
| Edema/LGE vol | 2.0±0.6 | 1.8±0.2 | 0.48 | 8 vs 6 | |
| 1 week | Edema Vol, mm3 | 453.0±170.0 | 660.7±524.3 | 0.32 | 8 vs 3 |
| LGE Vol, mm3 | 525.0±207.2 | 734.7±181.4 | 0.08 | 10 vs 5 | |
| 2 weeks | Edema Vol, mm3 | 0 | 0 | - | 1 vs 4 |
| LGE Vol, mm3 | 420.4 | 715.7±272.2 | 0.40 | 1 vs 4 | |
| 8 to 12 weeks | Edema Vol, mm3 | 0 | 0 | 0 | 11 vs 9 |
| LGE Vol, mm3 | 194.4±60.0 | 421.8±147.0 | 0.0002 | 11 vs 9 | |
| LGE depth, mm | 6.0±1.1 | 8.7±1.2 | <0.0001 | 11 vs 9 | |
| Histology | Depth, mm | 5.8±0.8 | 8.1±0.9 | <0.0001 | 11 vs 9 |
| Vol, mm3 | 163.2±70.2 | 341.3±108.9 | 0.0003 | 11 vs 9 |
RF = radiofrequency, LV = left ventricle, RV = right ventricle, LGE = late gadolinium enhancement, Temp = temperature, vol = volume
Discussion
In this study, we report for the first time a comparison of acute lesion formation and maturation between Cryo and RF ablation using serial MR imaging. Some studies have investigated Cryo lesion formation by using intravascular ultrasound14 and MR imaging15, but none of the studies have looked at the time line of edema formation and its resolution by imaging them at different time points. The main result of this study is that the enhancement seen on T2w imaging is present in both Cryo and RF lesions and for lesions of comparable chronic size, acute edema for Cryo lesions is significantly smaller than that for RF lesions. We also show that Cryo lesions with a freeze time of 240 sec result in a larger chronic lesion and edema when compared to lesions with a freeze time of 120 sec.
The mechanism of tissue injury for Cryo lesions is different from that of RF lesions. Cryoablation can cause irreversible alterations of cytoplasmic components and nuclei without disruption of cell membrane leading to cell death by apoptosis and finally scar16. For Cryo lesions, the cell apoptosis rate has been shown to increase progressively for 2 to 8 hours after freezing with a second peak of necrosis observed after 4 days17. For RF lesions, there is direct cell injury from heating and one can expect a larger acute edema immediately after ablation. In our study, the enhancement volume as seen in the T2w MRI scans was significantly smaller for Cryo lesions as compared to the RF lesions for lesions of comparable chronic size. The time delay between lesion creation and the T2w MRI scan done to assess edema was significantly shorter for Cryo as compared to RF lesions in our study. Since we only acquired the T2w MRI at one time point, depending on the rate of increase in edema formation and peak edema we might not have the peak edema volume for either of the two modalities. For clinical relevance, the tissue swelling occurring in the first hour or so acutely after ablation is likely of most significance as that is likely to play a larger role in acute procedural success rate. Further development of edema after few hours is unlikely to make a difference to acute procedural outcomes as these changes will likely be post-procedure. We also show that tissue swelling is still present after a week but is smaller from the acute phase for both Cryo and RF and the enhancement on T2w imaging is mostly resolved by 2 weeks. Time dependence of edema for RF lesions and has been reported by us8 before but a similar course for Cryo lesion based edema recovery is new.
In Cryo group, LGE lesion size was strongly affected by the freeze time. The chronic lesion size in the 240 sec group seen both in MRI and histology was significantly larger than that in 120 sec group, as reported previously18. Despite the larger volume in 240 sec group the acute edema volume/LGE volume ratio was about 2.0 and that was comparable between the 120 sec and the 240 sec groups.
We have recently shown that for RF lesions the acute LGE area of enhancement can be much larger than the chronic lesion size8. The same appears to be true for Cryo lesions. In our study, the acute LGE enhancement for Cryo and RF was 2.6 to 2.7 times larger than the chronic LGE enhancement indicating that LGE enhancement in similar for both types of injury. LGE volume in both groups decreased with time. There was no significant difference in lesion volume reduction in both groups. In our study, RF lesion was made by a 3.5mm catheter tip, whereas Cryo lesions were made by 8.0mm catheter tip. Prior work in RF ablation has shown larger electrode tips lead to larger lesion19. Despite the difference in the electrode tip size between Cryo and RF used in this study, the chronic lesion size was the same, making the two groups quite comparable. Moreover, there is no data about catheter contact force in our study as the contact force has little relevance for Cryo lesions.
The key findings of this research, and their importance to clinical ablation strategies, lie in the relative similarity of the two energy delivery modalities we compared. We created chronic ventricular lesions of similar size and found that the pathway to this chronic state was similar in terms of acute lesion size and the rate of reduction of edema post procedure. The most meaningful difference was that the Cryo ablations generated less initial edema as compared to RF, suggesting the possibility of lower recurrence from swelling induced temporary suppression of excitation.
Limitations
We did not study atrial lesion formation. The study was designed to look at the edema size. Even though the extent of edema in the atrium is of great clinical significance, the thin atrial wall does not lend itself to studies like this where the goal is to look at the edema quantitatively. With the atrial wall being only 2 to 3 mm in thickness, the lesions are transmural and any growth in edema is only going to be sideways and not deep in the wall significantly limiting the extent of the edema. Moreover, the thin atrial wall also makes it difficult to acquire images at a resolution to reliably quantify the extent of these changes. The physics of ablation is not expected to be very different between the atrial and ventricular tissue so we believe that the ventricular findings can easily to translated to atrial ablation.
There was a significant difference in the time delay between lesion creation and edema imaging for the two modalities. A recent study that did serial imaging for about an hour for RF ablation didn’t show any significant change in edema formation, so we believe that by the time the imaging was done in this study most the edema would have formed already8. Nonetheless, further studies with dedicated imaging at the same time points after ablation will be needed to address this though one can argue that any change in edema a few hours after lesion creation will be less relevant clinically.
Conclusions
Acute lesion size and chronic lesion were comparable in Cryo and RF group. On the other hand, when comparing Cryo and RF lesions of similar chronic size, acute edema is smaller for Cryo lesions suggesting the possibility of lower recurrence from swelling induced temporary suppression of excitation. Edema resolves in both Cryo and RF lesions in 1 to 2 weeks.
Supplementary Material
Acknowledgments
The study was partially funded by a research grant from Medtronic Inc to University of Utah with Ravi Ranjan as the PI and K23 HL115084 from NIH to RR. University of Utah also has/recently had research grant from Biosense Webster and Abbott with RR as the PI.
Footnotes
RR is a consultant to Medtronic and Abbott. Other authors: No disclosures.
References
- [1].Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN, Investigators SAC: Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013; 61:1713–1723. [DOI] [PubMed] [Google Scholar]
- [2].Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, Yu R, Vangala S, Tseng CH, Choi EK, Khurshid S, Patel M, Mathuria N, Nakahara S, Tzou WS, Sauer WH, Vakil K, Tedrow U, Burkhardt JD, Tholakanahalli VN, Saliaris A, Dickfeld T, Weiss JP, Bunch TJ, Reddy M, Kanmanthareddy A, Callans DJ, Lakkireddy D, Natale A, Marchlinski F, Stevenson WG, Della Bella P, Shivkumar K: Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm 2015; 12:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH: Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005; 111:127–135. [DOI] [PubMed] [Google Scholar]
- [4].Williams SE, Harrison J, Chubb H, Bloch LØ, Andersen NP, Dam H, Karim R, Whitaker J, Gill J, Cooklin M, Rinaldi CA, Rhode K, Wright M, Schaeffter T, Kim WY, Jensen H, Razavi R, O’Neill MD: The Effect of Contact Force in Atrial Radiofrequency Ablation. JACC: Clinical Electrophysiology 2015; 1:421–431. [DOI] [PubMed] [Google Scholar]
- [5].Ranjan R, Kato R, Zviman MM, Dickfeld TM, Roguin A, Berger RD, Tomaselli GF, Halperin HR: Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI. Circ Arrhythm Electrophysiol 2011; 4:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ikeda A, Nakagawa H, Lambert H, Shah DC, Fonck E, Yulzari A, Sharma T, Pitha JV, Lazzara R, Jackman WM: Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart: electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode-tissue contact force and lesion size. Circ Arrhythm Electrophysiol 2014; 7:1174–1180. [DOI] [PubMed] [Google Scholar]
- [7].Thomas S, Silvernagel J, Angel N, Kholmovski E, Ghafoori E, Hu N, Ashton J, Dosdall DJ, MacLeod R, Ranjan R: Higher contact force during radiofrequency ablation leads to a much larger increase in edema as compared to chronic lesion size. J Cardiovasc Electrophysiol 2018;29:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ghafoori E, Kholmovski EG, Thomas S, Silvernagel J, Angel N, Hu N, Dosdall DJ, MacLeod R, Ranjan R: Characterization of Gadolinium Contrast Enhancement of Radiofrequency Ablation Lesions in Predicting Edema and Chronic Lesion Size. Circ Arrhythm Electrophysiol 2017; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harrison JL, Jensen HK, Peel SA, Chiribiri A, Grondal AK, Bloch LO, Pedersen SF, Bentzon JF, Kolbitsch C, Karim R, Williams SE, Linton NW, Rhode KS, Gill J, Cooklin M, Rinaldi CA, Wright M, Kim WY, Schaeffter T, Razavi RS, O’Neill MD: Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J 2014; 35:1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dickfeld T, Kato R, Zviman M, Lai S, Meininger G, Lardo AC, Roguin A, Blumke D, Berger R, Calkins H, Halperin H: Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2006; 47:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kholmovski EG, Coulombe N, Silvernagel J, Angel N, Parker D, Macleod R, Marrouche N, Ranjan R: Real-Time MRI-Guided Cardiac Cryo-Ablation: A Feasibility Study. J Cardiovasc Electrophysiol 2016; 27:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF: New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol 2008; 52:1263–1271. [DOI] [PubMed] [Google Scholar]
- [13].Ranjan R: Magnetic resonance imaging in clinical cardiac electrophysiology. Crit Rev Biomed Eng 2012; 40:409–426. [DOI] [PubMed] [Google Scholar]
- [14].Baran J, Lewandowski P, Smarz K, Sikorska A, Zaborska B, Kulakowski P: Acute Hemodynamic and Tissue Effects of Cryoballoon Ablation on Pulmonary Vessels: The IVUS-Cryo Study. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khurram IM, Catanzaro JN, Zimmerman S, Zipunnikov V, Berger RD, Cheng A, Sinha S, Dewire J, Marine J, Spragg D, Ashikaga H, Halperin H, Calkins H, Nazarian S: MRI Evaluation of Radiofrequency, Cryothermal, and Laser Left Atrial Lesion Formation in Patients with Atrial Fibrillation. Pacing Clin Electrophysiol 2015; 38:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lustgarten DL, Keane D, Ruskin J: Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog Cardiovasc Dis 1999; 41:481–498. [DOI] [PubMed] [Google Scholar]
- [17].Forest V, Peoc’h M, Campos L, Guyotat D, Vergnon JM: Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology 2005; 50:29–37. [DOI] [PubMed] [Google Scholar]
- [18].Hunt GB, Chard RB, Johnson DC, Ross DL: Comparison of early and late dimensions and arrhythmogenicity of cryolesions in the normothermic canine heart. J Thorac Cardiovasc Surg 1989; 97:313–318. [PubMed] [Google Scholar]
- [19].Khairy P, Rivard L, Guerra PG, Tanguay JF, Mawad W, Roy D, Talajic M, Thibault B, Macle L, Dubuc M: Morphometric ablation lesion characteristics comparing 4, 6, and 8 mm electrode-tip cryocatheters. J Cardiovasc Electrophysiol 2008; 19:1203–1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.