Abstract
Objective:
To develop a postoperative mortality case mix adjustment model to facilitate assessment of CICU quality of care, and to describe variation in adjusted CICU mortality across hospitals within the Pediatric Cardiac Critical Care Consortium (PC4).
Design:
Observational analysis
Setting:
Multicenter PC4 clinical registry
Participants:
All surgical CICU admissions between August 2014 and May 2016. The analysis included 8,543 admissions from 23 dedicated CICUs.
Interventions:
None.
Measurements and Main Results:
We developed a novel case mix adjustment model to measure postoperative CICU mortality after congenital heart surgery. Multivariable logistic regression was performed to assess preoperative, intraoperative and immediate postoperative severity of illness variables as candidate predictors. We used generalized estimating equations to account for clustering of patients within hospital and obtain robust standard errors. Bootstrap resampling (1000 samples) was used to derive bias-corrected 95% confidence intervals (CI) around each predictor and validate the model. The final model was used to calculate expected mortality at each hospital. We calculated a standardized-mortality-ratio (SMR; observed-to-expected mortality) for each hospital and derived 95% CIs around the SMR estimate. Hospital SMR was considered a statistically significant outlier if the 95% CI did not include 1. Significant preoperative predictors of mortality in the final model included age, chromosomal abnormality/syndrome, previous cardiac surgeries, preoperative mechanical ventilation and surgical complexity. Significant early postoperative risk factors included open sternum, mechanical ventilation, maximum vasoactive inotropic score and extracorporeal membrane oxygenation. The model demonstrated excellent discrimination (C-statistic 0.92) and adequate calibration. Comparison across PC4 hospitals revealed five-fold difference in SMR (0.4 to 1.9). Two hospitals had significantly better-than-expected and two had significantly worse-than-expected mortality.
Conclusions:
For the first time, we have demonstrated that variation in mortality as a quality metric exists across dedicated CICUs. These findings can guide efforts to reduce mortality after cardiac surgery.
Introduction
Survival for patients undergoing congenital heart surgery, particularly for complex lesions, has improved dramatically over recent decades [1]. Despite this success, wide variation in outcomes persists across centers, suggesting opportunities for improvement [2,3]. Data registries are an opportunity to provide benchmarking to enable programs to identify their relative strengths and weaknesses.
Due to variation in complexity of cases between centers, validated case mix adjustment models are vital for accurate benchmarking. The Society of Thoracic Surgeons Congenital Heart Surgery Database (STS CHSD) Mortality Risk Model provides the best current methodology for case mix adjustment in children undergoing cardiac surgery [4,5]. This model predicts mortality based upon the patient status upon entry into the operating room. However, this model does not specifically address quality of cardiac intensive care. Understanding both of these dimensions of quality can facilitate directing discipline specific improvement initiatives. The development of a complimentary case mix adjustment model, which reflects patient condition when care is transferred from the surgical to the CICU team, is needed for assessing CICU quality of care. Variation in CICU postoperative quality of care across hospitals has not been previously reported.
In this context, our primary aim was to assess variation in CICU postoperative quality of care with respect to mortality across hospitals within the PC4. To achieve this, we aimed to develop a validated case mix adjustment model (PC4 CICU Post-Surgical Mortality Model) of CICU care after pediatric heart surgery using the Pediatric Cardiac Critical Care Consortium (PC4) clinical registry for our analyses. Our secondary aim was to determine if center volume impacted risk adjusted CICU postoperative quality of care.
Materials and Methods
Data Source
PC4 is a quality improvement collaborative that collects data on all patients with primary cardiac disease admitted to the CICU service of participating hospitals [6]. PC4 maintains a clinical registry to support research and quality improvement initiatives. At the time of this analysis, 23 hospitals were submitting cases to the PC4 registry.
Each participating center has a trained data manager who has completed a certification exam. The data managers collect and enter data in accordance with the standardized PC4 Data Definitions Manual. The PC4 registry shares common terminology and definitions with applicable data points from the International Pediatric and Congenital Cardiac Code (IPCCC) [7] and the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database [8] as previously described [6]. Participating centers are audited on a regular schedule and audit results suggest complete, accurate and timely submission of data across centers, with the most recent published results demonstrating a major discrepancy rate of 0.6% across 29,476 fields (9). The University of Michigan Institutional Review Board provides oversight for the PC4 Data Coordinating Center; this study was reviewed and approved with waiver of informed consent.
Defining the episode for analysis: inclusion and exclusion criteria
The episode of the analysis was the CICU admission associated with an index cardiovascular operation (as defined by the STS CHSD [8]) that occurred immediately before or during the CICU admission. Surgery included both acquired and congenital heart disease. Episodes of analysis included situations where (1) a patient was first admitted to the CICU prior to the operation and returned to the CICU following surgery or (2) a patient was first admitted to the CICU directly from the operating room and/or the post anesthesia care unit. We excluded CICU admissions where the primary operation was ligation of patent ductus arteriosus in a neonate <2.5 kg or if it could not be classified according to the STS CHSD Mortality Categories (STAT) [4,5]. For patients with two index operations during the same CICU admission, the analysis was based on the first operation. A patient could have more than one qualifying surgical CICU admission in the same hospitalization and both were included in the analysis. Less than 1% of all surgical CICU admissions were readmissions which included an index operation. As these represent high-risk admissions, we elected to retain them in the analytic cohort.
Data Collection: outcome and variable definitions
The primary outcome variable for the case mix adjustment model was mortality prior to CICU discharge during the surgical CICU admission. Candidate predictor variables were chosen by expert panel consensus. Supplemental Table 1 provides the complete list of variables included in the analysis along with their definitions. Extracardiac or chromosomal anomalies and syndromes were defined according to the IPCCC [7]. Weight-for-age z-scores were calculated according to patient age using World Health Organization or Centers for Disease Control standards depending upon age. Preoperative risk factors were those defined within the STS-CHSD [4,5]. Vasoactive-inotropic score (VIS) was measured as previously described [10]. Previous cardiothoracic surgery was defined as cardiac surgery with or without cardiopulmonary bypass.
To measure postoperative illness severity, we explored several variables measured in the first two hours after admission to the CICU from the operating room (Table 1). We felt that these variables indicated the patient’s clinical condition upon transfer of care to the CICU team, and as such they were independent of the postoperative care. Pulmonary hypertension requiring long-term therapy was defined as vasodilator therapy initiated in the first two hours and lasting for greater than 14 days. We could not explore maximum lactate in the first two hours because this data was not routinely collected at some hospitals.
Table 1.
Patient characteristics in the overall study population and univariate analysis (abbreviated version). Complete analysis seen in Supplemental Table 2.
| Candidate Predictors | N (%) | CICU mortality | P value | |
|---|---|---|---|---|
| N=8543 | Yes (203) | No (8340) | ||
| PREOPERATIVE | ||||
| Age at surgery | <.0001 | |||
| Gender, male | 55.1% | 55.2% | 55.1% | 0.9908 |
| Extracardiac anomalies | 15.2% | 24.1% | 14.9% | 0.0003 |
| Chromosomal abnormalities/syndromes | 20.4% | 28.6% | 20.2% | 0.0034 |
| Prenatal diagnosis (neonates) | 58.6% | 67.2% | 57.9% | 0.0419 |
| Weight at surgery < 2.5 kg | 2.8% | 14.0% | 2.5% | <.0001 |
| LOS, days (median, IQR) | 0 (0–3) | 6 (2–12) | 0 (0–3) | <.0001 |
| Readmission to CICU | 4.5% | 10.3% | 4.3% | <.0001 |
| Prior cardiac surgery Yes | 35.1% | 29.0% | 35.2% | 0.0695 |
| No | 64.9% | 70.9% | 64.8% | |
| STS-CHS risk factors | 28.8% | 53.7% | 28.2% | <.0001 |
| Mechanical ventilation | 13.9% | 54.7% | 12.9% | <.0001 |
| Interventional catheterization | 4.4% | 11.8% | 4.2% | <.0001 |
| ECMO | 0.6% | 7.4% | 0.5% | <.0001 |
| Viral infection | 1.7% | 3.5% | 1.7% | 0.0891 |
| CPR | 1.2% | 7.4% | 1.0% | <.0001 |
| Inotropic support at time of surgery | 6.1% | 30.1% | 5.5% | <.0001 |
| Tracheostomy at CICU admission | 1.2% | 1.5% | 1.1% | 0.5065 |
| STAT Score | 0.5(0.3–1.3) | 1.7(0.9–2.5) | 0.5(0.3–1.2) | <.0001 |
| INTRAOPERATIVE | ||||
| CPB Duration (min) | 96(64–145) | 163(111–263) | 95(64143) | <.0001 |
| Aortic XC Duration (min) | 52(30–85) | 67(43–124) | 52(30–85) | <.0001 |
| DHCA/RCP Duration (min) | 31(20–46) | 43(33–59) | 30(20–44) | <.0001 |
| POSTOPERATIVE (FIRST 2 HOURS) | ||||
| Open sternum | 8.9% | 57.1% | 7.7% | <.0001 |
| Inotropic support, VIS | 5(0–8) | 10(5–17) | 5(0–8) | <.0001 |
| Mechanical ventilation | 65.0% | 97.0% | 64.0% | <.0001 |
| Chest tube output > or equal to 5 cc/kg/hour | 10.3% | 39.9% | 9.6% | <.0001 |
| Minimum pulse pressure (median, IQR) | 32(24–41) | 18(8–30) | 33(25–42) | <.0001 |
| Arrhythmia | 8.1% | 18.2% | 7.9% | <.0001 |
| Reoperation for bleeding | 0.3% | 1.5% | 0.2% | 0.0128 |
| Unplanned reoperation, other | 0.2% | 0.5% | 0.2% | 0.3196 |
| Hemothorax | 0.1% | 0% | 0.1% | 0.9999 |
| Pleural effusion | 0.01% | 0% | 0.01% | 0.9999 |
| Cardiac arrest | 0.2% | 0.5% | 0.2% | 0.3516 |
| ECMO | 1.5% | 31.0% | 0.7% | <.0001 |
| PHTN treatment | 0.4% | 7.4% | 0.2% | <.0001 |
LOS, preoperative length of stay; IQR, interquartile range; CICU, cardiac intensive care unit; STS-CHS, Society of Thoracic Surgeons- Congenital Heart Surgeons; ECMO, extracorporeal membrane oxygenation; CPR, cardiopulmonary resuscitation; STAT, Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Risk Categories; CPB, cardiopulmonary bypass; XC, cross clamp; DHCA/RCP, deep hypothermic circulatory arrest/regional cerebral perfusion; VIS, vasoactive infusion score [10]; PHTN, pulmonary hypertension.
Statistical analysis
We first created a mortality case mix adjustment model to account for difference across hospitals in patient characteristics and immediate postoperative severity of illness. This model was used to predict mortality at the population level; it was not intended for prediction of mortality of individual patients. We evaluated all potential predictor variables in univariate analyses using Chi Square, Fisher’s exact test, or Wilcoxon rank sum test as appropriate. Variables associated with CICU mortality on univariate analyses at p<0.1 were included in a multivariable logistic regression model. We used generalized estimating equations to account for clustering of patients within hospitals and obtain robust (sandwich) estimates of standard errors. A fixed effects hospital term was incorporated in the model because our aim was to allow all PC4 hospitals to assess their performance within this distinct cohort of hospitals. All variables associated with CICU mortality at p<0.05 in the final multivariable model were retained. We used 1000 bootstrap resamples to obtain bias-corrected 95% confidence intervals (CI) of the odds ratio for each covariate, and an optimism-corrected C-statistic for the final model [11]. We assessed calibration of the final model using the Hosmer-Lemeshow X2 test (where a p-value > 0.05 demonstrates adequate fit of the model). Variance inflation factor was used to assess multicollinearity. We then explored a secondary analysis of a case mix adjustment model with only pre- and intraoperative variables included in order to assess the value of adding our novel postoperative illness severity measure on model performance.
After determining the final case mix adjustment model, we calculated a standardized mortality ratio (SMR) for each hospital where SMR equals the observed CICU postoperative mortality divided by the expected CICU postoperative mortality. Expected mortality was derived from our final model that included postoperative illness severity variables. Empirical distribution of the SMR was obtained based on the bootstrap resamples, and 95% CIs derived for the SMR. Hospitals whose CIs did not include 1 were considered to have statistically significant better-than-expected (SMR < 1) or worse-than-expected (SMR > 1) performance. We calculated the SMR at 21 of the 23 centers submitting data. Two centers were excluded from these comparative analyses due to evidence at one site of non-consecutive case submission on the internal audit, and the other due to low number of CICU admissions (n = 37). Pearson’s correlation coefficient was calculated to understand the relationship between annual CICU admissions and the SMR.
Finally, we performed two sensitivity analyses to assess if practice pattern variation across centers systematically altered the interpretation of the SMR. We explored whether the SMR rank order would be noticeably altered at sites with high rates of delayed sternal closure and/or early extubation (in the operating room or on arrival to the CICU). Models excluding these two variables were analyzed and we compared the respective hospital rank order by SMR to those from the full model. Analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC) or STATA Version 14 (Stata Corp, College Station, TX), with statistical significance at a p-value of less than 0.05.
Results
Between August 1, 2014 and May 30, 2016 there were 8,696 surgical CICU admissions and 7,996 unique surgical patients in the PC4 registry. Excluded from the analysis were 153 (1.8%) patients whose procedure could not be classified in one of the STAT categories. The final analytic cohort included 8,543 CICU admissions from 23 centers with dedicated CICUs. The overall CICU mortality for surgical patients was 2.4% (n = 203) with a median postoperative time to death of 18.8 days (interquartile range of 6.9 and 49.1 days).
PC4 CICU Post-Surgical Mortality Model
Table 1 shows abbreviated results of the univariate analyses; complete results in Supplemental Table 2. Table 2 shows the results of the multivariable analysis. Significant preoperative variables were similar to those previously reported using the STS-CHSD [4]. We further identified significant intraoperative and immediate postoperative variables including: open sternum, maximum VIS, mechanical ventilation and ECMO. The PC4 CICU Post-Surgical Mortality Model showed excellent discrimination with a C-statistic of 0.92 and adequate calibration with a Hosmer Lemeshow coefficient X2 of 7.9 (p = 0.45). On sensitivity analyses, we found that removing the immediate postoperative variables from the model weakened its performance: the C-statistic decreased to 0.88 and there was poor calibration with a Hosmer Lemeshow coefficient X2 of 19.66 (p = 0.01).
Table 2.
Multivariable Model for the PC4 CICU Post-Surgical Risk Model
| Variable | Odds Ratio |
95% confidence interval |
P Value |
|---|---|---|---|
| Age group | |||
| Reference: child | |||
| Preterm neonate | 4.62 | 2.20 to 9.77 | < 0.0001 |
| Full term neonate | 2.48 | 1.33 to 4.63 | 0.004 |
| Infant | 1.63 | 0.93 to 2.83 | 0.086 |
| Adult | 1.46 | 0.57 to 3.75 | 0.432 |
| Underweight | 1.78 | 1.20 to 2.64 | 0.004 |
| Chromosomal abnormality or syndrome | 1.58 | 1.10 to 2.26 | 0.013 |
| 3 or more cardiothoracic surgeries | 3.05 | 1.70 to 5.47 | < 0.0001 |
| Any STS preoperative risk factor | 2.13 | 1.50 to 3.03 | < 0.0001 |
| Preoperative mechanical ventilation | 2.49 | 1.76 to 3.54 | < 0.0001 |
| STAT score | 1.51 | 1.29 to 1.77 | <0.0001 |
| Open sternum in OR or during the first 2 hours after arrival to CICU | 2.16 | 1.46 to 3.19 | < 0.0001 |
| Maximum VIS score during the first 2 hours after arrival to CICU | 1.02 | 1.01 to 1.03 | < 0.0001 |
| On mechanical ventilation 2 hours after arrival to CICU | 4.57 | 1.60 to 13.01 | 0.004 |
| On ECMO 2 hours after arrival to CICU | 15.88 | 9.77 to 25.83 | < 0.0001 |
STS-CHS, Society for Thoracic Surgeons- Congenital Heart Surgeons; STAT, Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Risk Categories; OR, operating room; CICU, cardiac intensive care unit; VIS, vasoactive infusion score [10]; ECMO, extracorporeal membrane oxygenation.
Although rare (1.5% of cases), ECMO support initiated either in the operating room or immediately upon arrival to the CICU was the strongest predictor of CICU mortality. Mechanical ventilation and open sternum at two hours following arrival in the CICU following index surgery were also important predictors.
PC4 CICU Post-Surgical Mortality Model: variation across hospitals
A five-fold variation in the SMR existed across hospitals ranging from 0.4 to 1.9 (Figure 1A). CICUs with a SMR of less than 1 performed statistically better than expected while those with a SMR of greater than 1 performed statistically worse than expected with regard to mortality. Four hospitals were statistically significant outliers; two had better than expected mortality (SMR 0.4), and two were worse than expected (SMR 1.6 and 1.9). Figure 1B shows SMR by annual CICU admission volume. There was a weak association between higher CICU volume and SMR (Pearson’s r = 0.14; 95% confidence interval of −0.32 to 0.55).
Figure 1.
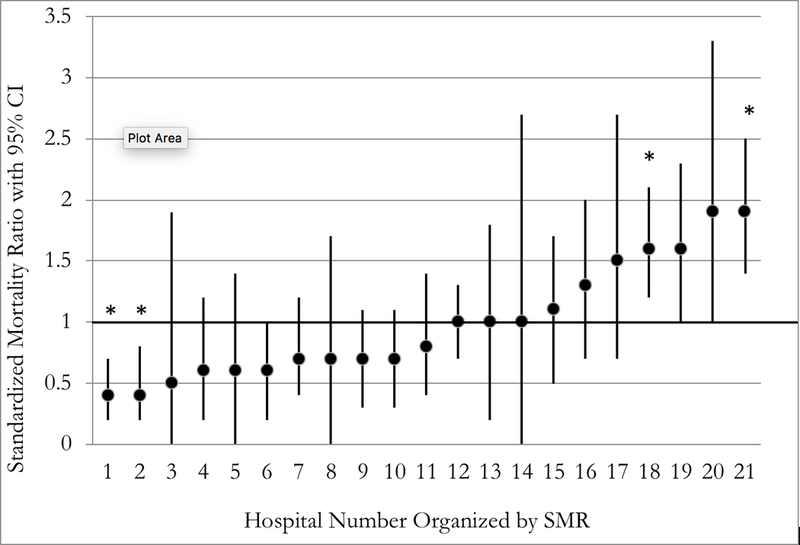
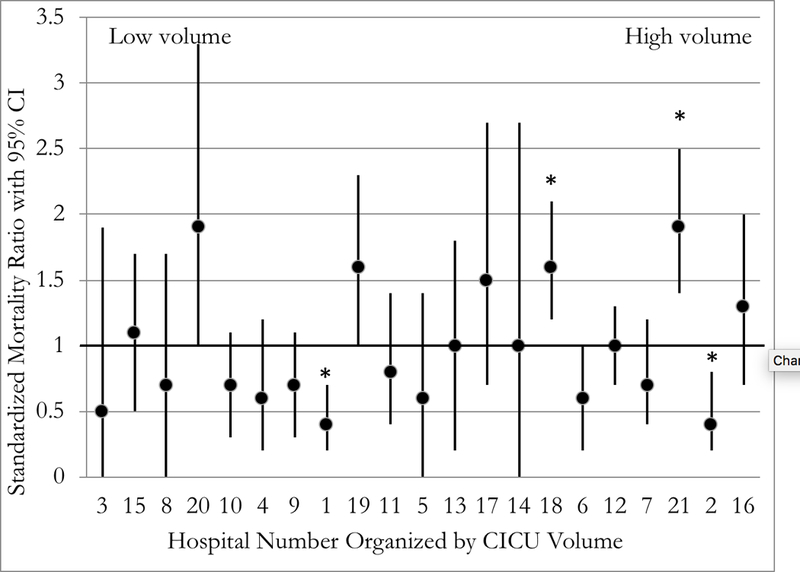
The PC4 CICU Post-Surgical standardized mortality ratio (SMR) with 95% confidence intervals. (*) Indicate statistically significant outliers where the confidence interval does not include one. (A) Organized SMR (low to high); (B) Organized by center CICU volume (low to high).
PC4 CICU Post-Surgical Mortality Model: Sensitivity Analysis
Although we aimed to focus on variables that reflected the patient severity of illness at transition from the surgical to the CICU teams, we were cognizant that differences in center dependent practices may impact these candidate predictors independent of the patient status. For this specific reason, we explored the impact of open sternum and early extubation (occurring prior to CICU postoperative hour 2) on the interpretation of center SMR and outlier status. We calculated hospital SMRs using alternative models which excluded these two variables. The greatest absolute change in any center’s SMR was 0.15 (without mechanical ventilation) and 0.23 (without open sternum). Elimination of either of these variables from the PC4 CICU Post-Surgical Mortality Model had no impact on the hospital rank order by SMR, nor did any hospital shift status from significantly better or worse than expected mortality.
Discussion
Validated case mix adjustment models for accurate benchmarking are necessary due to variation in patient population and complexity of cases between centers [2]. The STS-CHSD Mortality Risk Model represents the current gold standard in pediatric cardiac surgical mortality case mix adjustment. This data driven model accounts for patient characteristics and operative complexity at the time of surgery [4,5] but does not include variables necessary to accurately assess CICU quality of care as it does not reflect the clinical status when the CICU team begins postoperative care.
For example, consider two hypothetical patients with uncomplicated d-Transposition of the Great Arteries who go to the operating room for an arterial switch operation. One patient undergoes a straight-forward coronary transfer and arrives to the CICU on low dose inotropic support and mechanical ventilation; the other patient’s operative course is complicated by several bypass runs to revise the coronary buttons and arrives to the CICU with an open sternum and on ECMO. Clearly, the challenges to the cardiac intensive care team differ significantly for these two patients and yet based upon the STS-CHSD Mortality Risk model their predicted risk of mortality would be identical. Thus, to assess CICU performance, a case mix adjustment model that reflects the physiologic derangements when the CICU team assumes patient management is essential to facilitate benchmarking and collaborative learning to improve CICU quality of care.
We feel it is important to disentangle surgical from cardiac intensive care quality of care. As such, we report the development of a novel case mix adjustment model specifically designed to support the assessment of CICU quality of care. Applying this model to the hypothetical cases above results in significantly different risk stratification for the two patients at the time the CICU assumes care. Thus, the PC4 CICU Post-Surgical Mortality Model is specifically designed to facilitate assessment of CICU quality of care. This model had excellent discrimination and adequate calibration.
An assessment of postoperative CICU quality of care across hospitals has not been previously reported. We demonstrate that variation in quality of care exists across dedicated pediatric CICUs using case mix adjusted mortality following cardiac surgery as the primary outcome. Statistically significant deviation from expected mortality was observed, with some CICUs deviating above and other deviating below the expected mortality. These findings may have important implications for quality improvement. Finally, we observed a weak association between annual CICU admission volume and adjusted mortality.
Previous efforts to provide adjusted metrics of CICU surgical mortality advanced important concepts, but also left significant gaps. Using data from the Virtual Pediatric Intensive Care Unit Systems (VPS, LLC, Los Angeles) database, Jeffries et al developed a Pediatric Index of Cardiac Surgical Intensive Care Mortality (PICSIM) model from 16,574 cardiac surgery patients with an area under the curve of 0.87 and a Hosmer-Lemeshow p value of 0.22 [12]. Different from our model, their analysis included ECMO within 12 hours of surgery. This longer time window may reflect aspects of CICU quality of care rather than patient condition at time of transfer. Further, this model was applied at the time of CICU admission, such that stable patients admitted to the CICU preoperatively could potentially underestimate their immediate postoperative severity of illness. In addition, other analyses from the VPS database over the same time period report exclusion of up to 32% of patients due to no index surgery or STAT category recorded, potentially resulting in an important selection bias [13]. An important strength of the PC4 data registry is sharing of data fields with the STS CHSD, insuring the accuracy of surgical diagnoses and procedures. Thus, our rate of patients without STAT categories (1.8%) is similar to reports from the STS-CHSD. More importantly than creating a slightly different model, we report statistically significant variation in comparative outcomes across the 23 PC4 centers.
Previous studies have demonstrated a volume-outcome relationship with lower surgical mortality at higher volume centers [3, 14]. Our data demonstrated only a weak association between CICU SMR and annual volume of CICU admissions. This may reflect the fact that all of the hospitals in the PC4 registry are mid- to high-volume CICUs (over 250 admissions per year). Potentially, above a certain volume threshold the previously reported volume-outcome relationships are much weaker [14]. In addition, currently available PC4 sample sizes do not provide the granularity to assess volume-outcome relationships within certain subgroups; previous analyses have demonstrated that the volume-outcome association is primarily present for high complexity surgeries [3]. Continued accrual of patients in the PC4 registry will support these analyses in the future.
Clinical registries are an important resource providing data for benchmarking across hospitals and enabling programs to identify their relative strengths and opportunities for improvement. Transparency within registries facilitates integrated learning between centers. Active collaboration between centers has been demonstrated to improve practice and outcomes following cardiac surgery [15] and congenital heart surgery [14]. PC4 is unique in the field of pediatric cardiovascular care in that it provides real-time, transparent (i.e., hospitals are individually identified) benchmarked data to participating CICUs specifically geared toward fostering quality improvement activities. Application of the PC4 Post-Surgical Mortality Model will promote collaborative learning aimed at decreasing postoperative CICU mortality. Using the same methodology, PC4 statistical team will routinely revalidate the model to provide accurate benchmarking to participating centers.
While the aim of the PC4 Post-Surgical Mortality Model is to isolate CICU quality from surgical quality, complete separation is likely impossible. Future models which incorporate more granular data such as residual lesions, systolic and diastolic dysfunction, and arrhythmia burden may further facilitate determination of risk adjusted CICU quality of care.
Limitations to this study include the inherent bias of expert panel selection of risk variables for the model, and sample size limitations that prevent subgroup analyses. Three risk factors in the final model reflect patient level care, for which sensitivity analyses were not performed (vasoactive infusions, pre-operative mechanical ventilation or ECMO), where individual practitioner or institutional practice could increase risk stratification. As the goal of our model was to assess CICU quality of care, mortalities occurring after the post-surgical CICU discharge were not included. In addition, it is also important to acknowledge that we provide a static view of quality in this analysis. As a first step we have analyzed mortality, as it is the most widely accepted quality metric in the field. Future study of other quality metrics including morbidities and longer-term outcomes will also be important. Finally, we did not perform an analysis to understand what CICU processes are associated with case mix adjusted mortality.[9]
Conclusion
In summary, the PC4 Post-Surgical Mortality Model is a novel method for measuring case mix adjusted CICU performance. We provide the first evidence that significant variation exists in CICU quality of care, as defined by mortality, across CICUs participating in PC4. Our analysis suggests that opportunities may exist to improve CICU care. The PC4 collaborative is uniquely positioned to carry out data-driven quality improvement efforts aimed to reduce CICU mortality following pediatric cardiothoracic surgery through shared learning mechanisms. Clinician researchers must gain a deeper understanding of system processes that drive high quality care, and effectively implement these practices to reduce hospital postoperative mortality.
Supplementary Material
Acknowledgments
Acknowledgements.
We would like to acknowledge all members of the PC4 registry who contributed to accurate data collection and the generous donors to the University of Michigan Congenital Heart Center and CHAMPS for Mott for their support of PC4.
Sources of Funding. Dr. Gaies receives support from the National Heart, Lung, and Blood Institute (K08HL116639, Principal Investigator) that indirectly supports this research. His institution received grant support from the NIH (K08 award from NHLBI [PI: Gaies]). Dr. Pasquali receives funding from the Janette Ferrantino Professorship.
Footnotes
Copyright form disclosure: Mr. Zhang disclosed work for hire. Dr. Thiagarajan’s institutionreceived funding from Bristol Myers Squibb and Pfizer. Dr. Dimick received funding fromArborMetrix, Inc. Dr. Gaies received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Disclosures. None.
Addendum:
World Health Organization [www.who.int/childgrowth/standards/weight_for_age/en]
Centers for Disease Control [www.cdc.gov/growthcharts/zscore.htm]
References
- 1.Jacobs JP, He X, Mayer JE Jr, et al. Mortality Trends in Pediatric and Congenital Heart Surgery: An Analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2016; 102(4):1345–1352. [DOI] [PubMed] [Google Scholar]
- 2.Pasquali SK, Wallace AS, Gaynor JW, et al. Congenital Heart Surgery Case Mix Across North American Centers and Impact on Performance Assessment. Ann Thorac Surg. 2016; 102(5):1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquali SK, Li JS, Burstein DS, et al. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics. 2012; 129(2):e370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien SM, Jacobs JP, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 1-Statistical Methodology. Ann Thorac Surg. 2015; 100(3):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs JP, O’Brien SM, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 2-Clinical Application. Ann Thorac Surg. 2015; 100(3):1063–8; discussion 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaies M, Cooper DS, Tabbutt S, et al. Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4). Cardiol Young. 2015; 25(5):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin RC, Jacobs JP, Krogmann ON, et al. Nomenclature for congenital and paediatric cardiac disease: historical perspectives and The International Pediatric and Congenital Cardiac Code. Cardiol Young. 2008; Suppl 2:70–80. www.ipccc.net [DOI] [PubMed] [Google Scholar]
- 8.STS Congenital Heart Surgery Database Data Specifications. Version 3.22. Available at: http://www.sts.org/sites/default/files/documents/CongenitalDataSpecsV3_22.pdf. Accessed March 22, 2017
- 9.Gaies M, Donohue JE, Willis GM, et al. Data integrity of the Pediatric Cardiac Critical Care Consortium (PC4) clinical registry. Cardiol Young. 2016; 26(6):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. 2014; 15(6):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GC, Seaman SR, Wood AM, et al. Correcting for Optimistic Prediction in Small Data Sets. Am J Epidemiol 2014; 180(3):318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffries HE, Soto-Campos G, Katch A, et al. Pediatric Index of Cardiac Surgical Intensive Care Mortality Risk Score for Pediatric Cardiac Critical Care. Pediatr Crit Care Med. 2015; 16(9):846–852. [DOI] [PubMed] [Google Scholar]
- 13.Gupta P, Rettiganti M, Gossett JM, et al. Risk factors for mechanical ventilation and reintubation after pediatric heart surgery. J Thorac Cardiovasc Surg. 2016; 151(2):451–458. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch JC1, Gurney JG, Donohue JE, et al. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pediatr Cardiol. 2008; 29(4):713–717. [DOI] [PubMed] [Google Scholar]
- 15.Cohen ME, Liu Y, Ko CY, et al. Improved Surgical Outcomes for ACS NSQIP Hospitals Over Time: Evaluation of Hospital Cohorts With up to 8 Years of Participation. Ann Surg. 2016; 263(2):267–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


