Abstract
Hematopoietic Stem Cell Transplantation (HSCT) activity was evaluated in the African (AFR)/EMRO region and compared to the global activity for the years 2006–2013.
Data were obtained from 1570 teams in the 6 WHO continental regions. Of these, 29 (1.85%) of all teams were active in 12 of the 68 AFR/EMRO countries. They reported 2.331 (3.3%) of the worldwide 71.036 HSCT, and a median transplant rate of 32.8 (TR; HSCT/10 million inhabitants; worldwide 128.5). This reflects still the lowest regional TR despite an increase of 90% since 2006. HSCT activity in AFR/EMRO countries was characterized by a higher use of allogeneic compared to autologous HSCT, an almost exclusive use of family donors, including haploidentical family donors. These findings contrast with the prevalence of autologous over allogeneic HSCT, and a higher frequency of unrelated HSCT in other parts of the world. Of note, the increase by 200% in HSCT for hemoglobinopathies from 2006 to 2013 (72/year) in the AFR/EMRO region. This reflects the specific role of HSCT for these disease categories with high prevalence and incidence in the AFR/EMRO region.
This report provides information for the competent authorities to foster adequate infrastructure. It urges transplant organization to optimize their cooperation.
Keywords: Hematopoietic stem cell transplantation (HSCT), Global perspective, Worldwide Network for Blood and Marrow Transplantation (WBMT), Eastern Mediterranean Regional Organization (EMRO), WHO, Non-Governmental Organization (NGO), transplant rates, haploidentical transplants, trends, emerging technologies
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) has evolved from the beginnings of experimental bone marrow transplantation 60 years ago to a globally accepted successful therapy.1 Transplantation of hematopoietic stem cells and tissues has extended the lifespan and enhanced the quality of life of hundreds of thousands of patients worldwide. Over the last two decades, global HSCT has seen a constant evolution in technology, a decrease in transplant related mortality and rapid expansion. HSCT is no longer limited to younger patients or to patients with a matched donor. Novel conditioning regimens with lower intensity and new techniques have expanded the use of HSCT to older patients, to patients with comorbidities and to patients with haploidentical donors1,2. Today, HSCT is an established but still complex form of therapy for patients with chemo- and immune-sensitive diseases, for the replacement of deficient cells or cellular components and other life-threatening disorders. Its use has also been extended to severe inherited or acquired disorders of the hematopoietic system.
However, HSCT is still associated with significant morbidity and mortality and remains an example of high-cost and highly specialized medicine. It requires extensive experience, significant infrastructure and a network of specialists from all fields of medicine. It also requires the ability to provide specialist continued follow-up and management of patients. The accumulated experience covering a range of past errors and successes should therefore be used to improve access for all patients in need and serve as a model for the application of medical products of human origin in general.
Information on indications, the use of specific technologies and trends in HSCT is essential for correct patient counseling and to allow health care agencies to prepare the necessary infrastructure and to avoid errors in planning. In addition, the guiding principles of the World Health Organization (WHO; www.who.org) declare the transplantation of organs, cells and tissues to be a global task, with the collection of activity data being one of the prime prerequisites3. The Worldwide Network for Blood and Marrow Transplantation (WBMT; www.wbmt.org), as an umbrella organization in the field of HSCT and a Non-Governmental Organization (NGO) in working relation with the WHO, has taken up the challenge of collecting and disseminating worldwide HSCT data on a regular basis. The first report was based on the global HSCT activity in 20064, and was followed by a report on the data available in 2010 and thereafter a retrospective view of the first one million HSCTs in the year 20155,6. The fourth report was focused on the HSCT activity in 2012 and looked at major trends since 20067. The report included a SWOT analysis of the current WBMT policy by key personnel in the field, to identify the strengths, weaknesses, opportunities and threats of the current perspectives in HSCT.
In the current study we analyzed the global HSCT activities reported between 2006 and 2013 concentrating on the AFR/EMRO region in the global context regarding frequency, indication, donor type and geographical distribution in 2013.
PATIENTS AND METHODS
Study design
This was a retrospective survey involving all HSCT teams known to the investigators, organized by WBMT through established international and/or regional organizations. No individual patient data were used thus no ethics committee approval was mandated.
Participating Groups, Continents, Countries and Teams
In 2013, 29 teams in 12 of the 68 countries of the AFR/EMRO region reported their activities. In contrast, the global survey included reports from 1570 teams in 78 reporting countries over 6 WHO continental regions [(www.who.int/about/regions/en/) America (AMR/PAH; WHO regions North-, Middle and South America and Canada); Asia (SEAR/WPR; WHO regions South East Asia and Western Pacific Region which includes Australia and New Zealand); Europe (EUR; includes Turkey and Israel) and AFR/EMRO (WHO regions Eastern Mediterranean and Africa)]. Analyses were performed as previously described7.
Data were provided by the Australasian Bone Marrow Transplant Recipient Registry ABMTRR (www.abmtrr.org), the African Blood and Marrow Transplant Group AfBMT, the Asian Pacific Blood and Marrow Transplant Group APBMT (www.apbmt.org), the Canadian Blood and Marrow Transplant Group CBMTG (www.cbmtg.org), the Center for International Blood and Marrow Transplantation CIBMTR (www.cibmtr.org), the Eastern Mediterranean Blood and Marrow Transplant Group EMBMT (www.embmt.org), the European Group for Blood and Marrow Transplantation EBMT (www.ebmt.org) and the Latin American Blood and Marrow Transplantation Group LABMT (LABMT@wbmt.org).
Collection system and data validation were obtained as previously described7. Data were validated by different independent systems; through confirmation by the reporting teams, following receipt of a computer printout of the entered data, by selective comparison with MED-A/TED data sets in the EBMT or CIBMTR data system or by crosschecking with National Registries. On-site visits to selected teams were part of the quality control program within CIBMTR and EBMT teams. Based on quality controls and contacts with regulatory agencies or national offices, the response rate for allogeneic HSCT was estimated to be >95% and for autologous HSCT 80–90%. The survey focuses on the numbers of patients treated for the first time with HSCT. Transplant rates (TR) were computed as the number of HSCT per 10 million inhabitants not corrected for population age. Population data were obtained from the US census office (http://www.census.gov).
RESULTS
Transplant activities in the AFR/EMRO region from 2006 to 2013
Up to 34 centers from the AFR/EMRO region were noted to have HSCT activity during the period from 2006 to 2013 with fluctuations reflecting challenges in some centers that resulted in inconsistent activity over the years (Table 1 and Figures 1 and 2). In 2013, a total of 29 teams from 12 countries in the AFR/EMRO region provided activity data on a total of 2331 patients. The TR in active countries ranged from 0.1 to 109 for allogeneic HSCT compared to 0 to 172 for autologous HSCT. Several countries had no HSCT activity (Figure 1). Allogeneic TR ranged between 0–44 with the exception of Oman, Saudi Arabia, Jordan and Lebanon (69.8, 96.1, 101.1 and 108.8 TR, respectively). For autologous HSCT, the TR ranged between 0 and 37 with the exception of Tunisia, Jordan and Lebanon (52.6, 61.0 and 172.8 TR, respectively). These figures reflect the limited availability of transplant teams and limited logistic support in many countries of the region.
Table 1:
Trends of HSCT worldwide and in the AFRICAN/EMRO region in 2006 and 2013
| African/EMRO region | Worldwide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2013 | Δ | 2006 | 2013 | Δ | |||||
| 2006–2013 | 2006–2013 | |||||||||
| Allogeneic HSCT | ||||||||||
| total | related | unrelated | total | total | related | unrelated | total | |||
| Leukemia’s | 492 | 670 | 50 | 720 | 46% | 14392 | 11424 | 12912 | 24336 | 69% |
| AML | 186 | 336 | 26 | 362 | 95% | 6611 | 5592 | 6334 | 11926 | 80% |
| ALL | 132 | 226 | 16 | 242 | 83% | 3488 | 2926 | 2753 | 5679 | 63% |
| CML | 139 | 48 | 0 | 48 | -65% | 1334 | 554 | 496 | 1050 | -21% |
| MDS/MPS | 33 | 52 | 6 | 58 | 76% | 2268 | 1957 | 2745 | 4702 | 107% |
| CLL | 2 | 8 | 2 | 10 | 400% | 556 | 298 | 476 | 774 | 39% |
| Other leukemia | 0 | 0 | 0 | 0 | 0 | 135 | 97 | 108 | 205 | 52% |
| Lymphoproliferative disorders | 28 | 53 | 7 | 60 | 114% | 3219 | 2111 | 2471 | 4582 | 42% |
| Plasma cell disorders | 2 | 17 | 0 | 17 | 750% | 773 | 450 | 445 | 895 | 16% |
| lymphomas | 26 | 36 | 17 | 43 | 65% | 2446 | 1639 | 2006 | 3645 | 49% |
| Other lymphoma/Type unknown | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 20 | 42 | 0 |
| Solid tumors | 1 | 0 | 0 | 0 | -100% | 150 | 85 | 45 | 130 | -13% |
| Non malignant disorders | 277 | 531 | 38 | 569 | 105% | 2360 | 2624 | 1756 | 4380 | 86% |
| Bone Marrow Failures | 154 | 234 | 8 | 242 | 57% | 1292 | 1316 | 766 | 2082 | 61% |
| Hemoglobinopathies | 72 | 213 | 3 | 216 | 200% | 392 | 805 | 228 | 1033 | 164% |
| Immune deficiencies | 41 | 74 | 16 | 90 | 120% | 445 | 381 | 470 | 851 | 91% |
| Inherited diseases of metabolism | 10 | 10 | 11 | 21 | 110% | 177 | 63 | 154 | 217 | 23% |
| Autoimmune disorders | 0 | 0 | 0 | 0 | 0 | 16 | 18 | 22 | 40 | 150% |
| Other Non malignant disorders | 0 | 0 | 0 | 0 | 0 | 38 | 41 | 116 | 157 | 313% |
| Others | 5 | 3 | 2 | 5 | 0 | 212 | 61 | 83 | 144 | -32% |
| Total | 803 | 1257 | 97 | 1354 (58,0%) | 69% | 20333 | 16305 | 17267 | 33572 | 65% |
| 48.6% | 51.4% | 47.3% | ||||||||
| Autologous HSCT | ||||||||||
| Leukemias | 58 | n.a. | n.a. | 34 | -41% | 1726 | n.a. | n.a. | 910 | -47% |
| Lymphoproliferative disorders | 338 | n.a. | n.a. | 840 | 148% | 21655 | n.a. | n.a. | 33447 | 65% |
| Plasma cell disorders | 127 | n.a. | n.a. | 404 | 218% | 10675 | n.a. | n.a. | 18766 | 76% |
| Lymphomas | 211 | n.a. | n.a. | 436 | 107% | 10980 | n.a. | n.a. | 14681 | 34% |
| Solid tumors | 29 | n.a. | n.a. | 101 | 248% | 2560 | n.a. | n.a. | 2779 | 9% |
| Non malignant disorders | 2 | n.a. | n.a. | 2 | 0 | 193 | n.a. | n.a. | 290 | 50% |
| Others | 0 | n.a. | n.a. | 0 | 96 | n.a. | n.a. | 38 | -60% | |
| Total | 427 | n.a. | n.a. | 977 (41,9%) | 129% | 26230 | n.a. | n.a. | 37464 | 43% |
| 52.7% | ||||||||||
| Total all HSCT | 1230 | n.a. | n.a. | 2331 | 90% | 46563 | n.a. | n.a. | 71036 | 53% |
n.a.; not applicable
Figure 1:
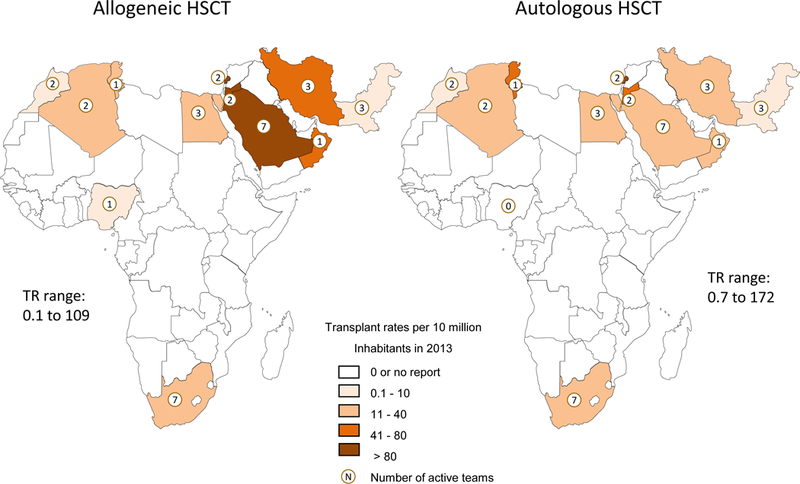
Transplant rates for allogeneic and autologous Hematopoietic Stem Cell Transplantation (HSCT) and number of national transplant centers in the African/EMRO region in 2013
Figure 2:

Worldwide trends 2006 – 2013 in numbers of allogeneic and autologous hematopoietic stem cell transplantations (HSCT) and number of reporting teams in the African/EMRO region and worldwide (upper panel). Numbers of median HSCT/team in the African/EMRO region and worldwide (lower panel)
In contrast to other world regions, more allogeneic (58%) than autologous (42%) HSCT were noted in the region (Figure 2). The AFR/EMRO region showed overall 90% increase in HSCT activity from 2006 to 2013 (1230 to 2331 transplants/year, respectively) with 69% increase in allogeneic HSCT and 129% increase in autologous HSCT (Table 1). Furthermore 93% of patients undergoing allogeneic HSCT had a family donor and received PBSC (67%) or BM (33%) as stem cell source. Cord blood transplants were in the range of 4% of all allogeneic HSCT. Major Increases in allogeneic HSCT were seen for hemoglobinopathies (200%), lymphoproliferative diseases (114%), non-malignant disorders in general (105%) and acute leukemias (89%; Table 1). Autologous HSCT was performed almost exclusively with PBSC and increased from 2006 in the African/EMRO region more than it did worldwide for patients with solid tumors (248% vs 9% respectively), PCD (218% vs. 76%, respectively) and lymphomas (107% vs.34%, respectively). As in the global survey, decreases were observed only in the use of autologous HSCT for leukemia (−41%). Interestingly, the median HSCT per center/year was higher in Africa/EMRO than global (80 vs. 45 HSCT/center/year, respectively; see Figure 2). In comparison to the global survey, differences were noted in indications and donor types. The most striking differences include a more frequent use of allogeneic HSCT for Bone Marrow Failure (BMF; 17.9% of all allogeneic HSCT in Africa/EMRO as compared to 3.0% globally) and in autologous HSCT for Hodgkin/NHL (44.6% of all autologous HSCT in Africa/EMRO as compared to 39.2% globally) and almost exclusive use of family donors in allogeneic HSCT. Centers in the AFR/EMRO region more frequently used haploidentical donors rather than cord blood to perform alternative donor HSCT (Figure 3). The proportion of HSCT using haploidentical donors as graft source started rising from 2009 (n=41) to 2010 (n=60), fell marginally in 2011 yet increased again in 2012 to peak in 2013 (n=74). This was in contrast to the global activity, in which the use of haploidentical HSCT overtook the use of unrelated CB HSCT in 2012. Use of unrelated cord blood remained limited with 40 and 49 CB transplants in 2009 and 2013, respectively.
Figure 3:
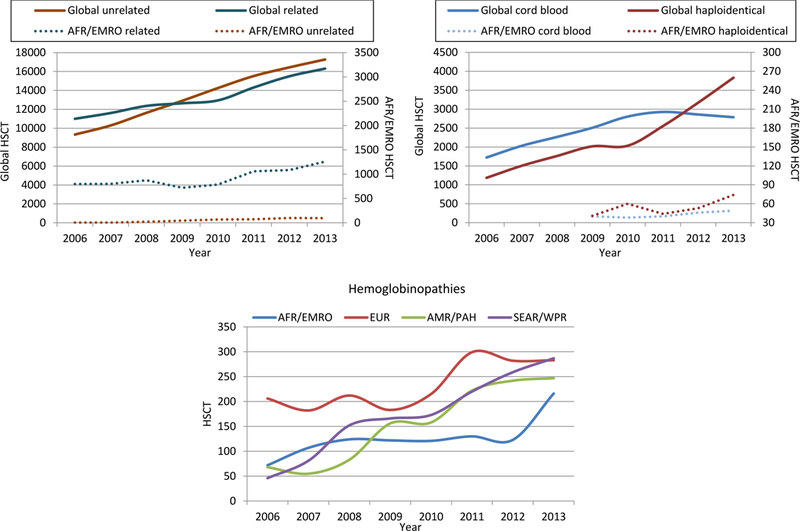
Frequencies of allogeneic Hematopoietic Stem Cell Transplantation (HSCT) according to related or unrelated donors and frequencies of alternative HSCT in the AFR/EMRO region and worldwide (upper panel). In the lower panel HSCT for hemoglobinopathies in the four WHO regions.
Global HSCT activity, main indications and donor type in 2013.
The global HSCT activity in 2013 reached a new high of 71,036 transplants (Table 1). Most of the HSCT in 2013 were performed in Europe, followed by AMR/PAH, SEA/WPR and AFR/EMRO (47%, 30%, 20% and 3% respectively) with a steady and constant increase in activity in all 4 WHO regions. There was a slightly higher frequency of autologous (52.7%) compared to allogeneic (47.3%) HSCT. The main indications were lymphoproliferative disorders (n=38.029; 53%) of which 88% (n=33,447) were autologous and 12% (n=4,582) allogeneic HSCT; leukemias (n=25,246; 35%) of which 96% (n=24,336) were allogeneic and 4% (n=910) autologous HSCT; solid tumors (n=2,909; 4%) of which 4% (n=130) were allogeneic and 96% (n=2,779) autologous HSCT; non-malignant disorders (n=4,670; 7%) of which 94% (n=4,380) were allogeneic and 6% (n=290) autologous HSCT.
The median TR for all HSCT in 2013 fluctuated between regions and between participating countries from 390.9 in Europe (median; range 1.0 to 735.7), 204.4 in Asia (median; range 0.7 to 610.5), 126.2 in the Americas (median; range 17.8 to 538.9) and 81.9 in AFR/EMRO (median; range 0.1 to 281.6). Transplant rates for allogeneic HSCT ranged from 0.1 in Nigeria to 450.8 in Israel (median 104.4) and transplant rates for autologous HSCT from 0.1 in the Philippines to 437.0 in Italy (median 159.7).
The annual HSCT activity increased from 2006 to 2013 by 52.6% (Table 1) while the number of reporting centers increased by 17.3% and plateaued in the years 2012 – 2013 (Figure 2). More importantly, HSCT per center increased from 35.1 to 45.2, confirming increased activity per center (Figure 2). Activity increased primarily for allogeneic HSCT (+65% from 2006) and, among these, especially for non-malignant disorders (+86%), acute leukemias, MDS/MPS (+80%) and lymphoproliferative disorders (+42%; Table 1). Of all allogeneic HSCT, 51.4% were performed using an unrelated donor. Frequencies of autologous HSCT increased (+43%) most markedly in the PCD (+76%), non-malignant disorders (+50%) and lymphoma (+34%) subgroups. Decreased activity was reported only in the use of autologous HSCT for leukemias (−47%).
Special attention was given to hemoglobinopathies as indication for HSCT in the AFR/EMRO region and worldwide. A total of 1033 HSCT were performed for these indications worldwide in 2013, of which 216 (21%) were reported from the AFR/EMRO region representing a considerable increase from the 123 HSCT in 2012. HSCT were performed in SEAR/WPR (n=287), Europe (n=283) and Americas (n=247; Figure 3).
Two major changes were observed during the period 2006–2013. Firstly, HSCT from unrelated donors became more frequent than from related donors (Figure 3). Secondly, mismatched family or “haploidentical” donors increased from 1.186 (6% of allogeneic HSCT) in 2006 to 3.830 (11.8% of allogeneic HSCT) in 2013. Worldwide, the use of haploidentical transplants became more common than that of unrelated cord blood in 2012 (Figure 3).
DISCUSSION
The current analysis is based on information on more than 464,100 HSCT reported to the WBMT over an 8 year period and explores distribution, donor choices and HSCT techniques over time in the AFR/EMRO Region and worldwide. The results allow several important conclusions.
Firstly, HSCT activity has been steadily increasing since 2006 in all world regions (even in high HSCT performance regions) but to different degrees. For the first time, the number of HSCT per year exceeded 70,000, while there continue to be significant differences in transplant rates between the world regions. The relative increase was more prominent in countries with restricted resources, including AFR/EMRO, than in those with a high transplant rate, but the TR still remains the lowest rate worldwide. Many countries, especially in central Africa do not have an active HSCT program.
Second, there is no indication of a plateau in activity. This continuing lack of saturation indicates underuse and implies that more patients would have been treated by HSCT had they had access and had donors been available. Obviously this is more notable for hemoglobinopathies which are highly prevalent in AFR/EMRO regions.
Third, the activity increase worldwide is mainly caused by augmented team activity with the median number of HSCT per team increasing from 29 to 41 between 2006 and 2013. Interestingly, the existing centers in the AFR/EMRO region have a median transplant activity higher than the corresponding global level, indicating the need for more HSCT centers. However, there was no increase in HSCT/center in the analyzed region after 2007. This is probably a reflection of the low density of centers and a possible saturation for the center activity. The increase is only in part a consequence of increased reporting from 2006 to 2013. The proportion of uncaptured data, estimated to be less than 5% for allogeneic and less than 15% for autologous HSCT, also varied between the regions and was estimated to be lowest in the regions with the highest absolute numbers. Despite these limitations, it is unlikely that missing data would alter the overall interpretation.
Significant changes were observed during the study period regarding donor type and graft source. Overall, unrelated HSCT exceeded related donor HSCT worldwide, particularly in Europe8, while related HSCT remained the almost exclusive graft source in the AFR/EMRO region. Also, the incidence of haploidentical related HSCT was consistently higher than cord blood HSCT in the AFR/EMRO region, while globally this was observed only in 2012. From 2012 onwards, worldwide interest in the use of unrelated CB seems to have started decreasing, while use of haploidentical donors is consistently increasing over this period with a steep rise after 2012. This may reflect a learning curve with some teams reducing the use of cord blood in favor of adopting the newer haploidentical transplant strategies. With fine tuning of haploidentical transplant techniques, this may have been perceived as a more cost effective and logistically simpler strategy by some teams in this region. Double cord blood transplant, commonly used for adults, is associated with extra cost. This may also be a reflection of lack of unrelated donor registries and public cord blood banks in most of the AFR/EMRO region. Interestingly, the prominent use of allogeneic HSCT in comparison to autologous HSCT is a characteristic of the AFR/EMRO region.
Overall, only 1033 HSCT were performed worldwide for hemoglobinopathies, of which only 216 were in the AFR/EMRO region. Considering the high prevalence and incidence of thalassemia (40.000 born annually and 80 million carriers; Modell, WHO Bulletin 2008) and of sickle cell anemia (e.g. 91,011 in Nigeria and 39,743 in Congo) in the AFR/EMRO region, this is clearly still lagging behind demand despite the recent increase.12
Ten years after its formation, WBMT has established itself as a global umbrella organization for HSCT. It has accomplished one of the prime prerequisites of WHO guiding principles on cell, organ and tissue transplants: to collect, analyze and disseminate information on global transplantation activity3. More than 1500 teams from 78 countries over all 6 continents contributed to the survey. Lack of a regional organization in some regions remains a clear limitation to the global survey and is an area in which support and expertise from the societies of the WBMT has helped since the establishment of the organization. In fact, two major societies were founded with the help of the WBMT: the LABMT and the AfBMT. While the LABMT has already published their first survey13, the AFR/EMRO region is publishing its first report. Still many countries are without HSCT activities and this publication may aid in the development of further activity in the region.
Initiatives of WBMT involve not only registries, but also regional WBMT workshops such as the most recent workshop organized at King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia in January 2017, under the auspices of the WHO. Telemedicine approaches have been tested for their efficacy in supporting program initiation and are now ready to be used on a larger scale. The WBMT has also been involved in counseling African countries planning to start an HSCT program.
The special position the WBMT has come to occupy as coordinator of global information in HSCT, has created a unique opportunity to build up a truly comprehensive world-wide network of standardized and quality controlled data collection and analysis. Further activities aim to collect outcome data on a global level, representing a major step towards the realization of the WHO principle that “data collection and data analysis remain integral parts of the therapy”. WBMT will continue to interact with competent regulatory authorities to pursue the goal that costs and reimbursement for HSCT should include a component for comprehensive data and quality management. Obviously, in the AFR/EMRO region there is a need for more HSCT centers in addition to progressive growth of existing centers.
ACKNOWLEDGEMENTS
Supported by the Australasian Bone Marrow Transplant Recipient Registry ABMTRR, Asia Pacific Blood and Marrow Transplantation Group (APBMT), the African Blood and Marrow Transplantation Group (AfBMT), the Canadian Blood and Marrow Transplant Group (CBMTG), the Center for International Blood and Marrow Transplantation (CIBMTR), the European Blood and Marrow Transplantation Group (EBMT), the East-Mediterranean Latin American Bone Marrow Transplant Group (EMBMT), the Latin American Blood and Marrow Transplantation Group (LABMT), and the Carreras Foundation Germany.
LIST OF PARTICIPATING CENTERS
AFBMT/EMBMT
Algeria, Alger Pierre and Marie Currie Center, Oran University Hospital EHU 1er November; Egypt, Cairo National Cancer Institute of Cairo University; Iran, Shiraz Namazi Shiraz University of Medicine, Tehran Mahak Pediatric Cancer Treatment & Research Center (peds), Tehran University of Medical Sciences; Jordan, Amman King Hussain Medical Center, Queen Rania Children’s’ Hospital (peds); Lebanon, Beirut American University of Beirut Medical Center, Makassed General Hospital; Morocco, Casablanca, Hospital du 20 Aout, Marrakesh CHU Mohamed VI; Nigeria, Benin University Hospital Benin City; Oman, Muscat Sultan Qaboos University Hospital; Pakistan, Karachi, Aga Khan University Hospital, Islamabad Pakistan Institute of Medical Sciences PIMS Children’s’ Hospital (peds), Rawalpindi Armed Forces Bone Marrow Transplant Center; Saudi Arabia, Dammam King Fahad Specialist Hospital, Jeddah King Faisal Specialist Hospital and Research Center (ads), King Faisal Specialist Hospital and Research Center (peds), Riyadh King Faisal Specialist Hospital and Research Center (ads), KING FAISAL Specialist Hospital and Research Center (peds); South Africa, Cape Town Constantiaberg Medical Clinic, Groot Schuur Hospital, University of Cape Town Private Academic Hospital; Durban, Albert Luthuli Hospital; Johannesburg, Flora Private Clinic; Pretoria; Albert Cellular Therapy, East Hospital; Tunisia, Tunis Centre National de Greffe de Moelle
APBMT
ABBMTR
Australia, Adelaide: Adelaide Cancer Centre, Flinders Medical Centre, Royal Adelaide Hospital, The Queen Elizabeth Hospital, Women’s & Children’s Hospital. Brisbane: Greenslopes Private Hospital, Cilento Children’s Hospital, Mater Medical Centre, Mater Misericordiae Public Hospital, Princess Alexandra Hospital, Royal Brisbane & Women’s Hospital, Wesley Medical Centre. Canberra: The Canberra Hospital. Fremantle: Fremantle Hospital. Geelong: Geelong Hospital. Gosford: Gosford Hospital. Hobart: Royal Hobart Hospital. Melbourne: Alfred Hospital, Austin Hospital, Box Hill Hospital, Peter MacCallum Cancer Institute, Royal Children’s Hospital, Royal Melbourne Hospital, St Vincent’s Hospital Melbourne. Newcastle: Calvary Mater Newcastle, John Hunter Children’s Hospital. Perth: Fiona Stanley Hospital, Princess Margaret Hospital, Royal Perth Hospital, Sir Charles Gairdner Hospital. Southport: Gold Coast University Hospital. Sydney: Concord Hospital, Liverpool Hospital, Nepean Hospital, Prince of Wales Hospital, Royal North Shore Hospital, Royal Prince Alfred Hospital, St George Hospital, St Vincent’s Hospital, Sydney Children’s Hospital, The Children’s Hospital at Westmead, Westmead Hospital. Townsville: Townsville General Hospital. Wollongong: Wollongong Hospital. New Zealand, Auckland: Auckland City Hospital, Starship Hospital. Christchurch: Christchurch Hospital. Hamilton: Waikato Hospital. Palmerston North: Palmerston North Hospital. Wellington: Wellington Hospital.
CBMTG
CIBMTR
EBMT
Austria, Graz: Universitäts Kinderklinik, University of Graz, Innsbruck: University Hospital, Klagenfurt: Klinikum Klagenfurt, Linz: AO Krankenhaus, AOKH der Elisabethinen, Salzburg: LKA Salzburg, Vienna: Donauspital, Hanusch- Krankenhaus, St. Anna Kinderspital, Universitätsklinik fur Innere Medizin-AKH, Wilhelminenspital. Azerbaijan, Baku: Central Clinic Hospital. Belarus, Minsk: Belorussian Center. Belgium, Antwerp: Stuivenberg ZH, University Antwerpen, Brugge: A.Z. St. Jan, Brussels: Clinique Universitaire St. Luc, Institut Jules Bordet, Children’s Hospital, U.L.B. Hôpital Erasme, University Hospital, Charleroi: CHU Charleroi, Grand Hôpital de Charleroi Notre-Dame, Gent: University Hospita, Haine St. Paul: Hôpital de Jolimont, Hasselt: Jessa Ziekenhuis, Leuven: University Hospital Gasthuisberg, Liège: CHR-Citadelle, University Hospital Sart-Tilman, Roeselare: H. Hart Ziekenhuis, Turnhout: AZ Turnhout, Wilrijk: Sint Agustinos GVA, : Clinique universitaire de Mont-Godinne. Bosnia and Herzegovina, Sarajevo: Clinical Centre, Tuzla: University Clinical Centre. Bulgaria, Sofia: National Centre of Hematology, Pediatric Hospital for Oncohematology and BMT. Croatia, Zagreb: Hospital Merkur, University Hospital Dubrava, University Hospital Rebro. Cyprus, Limassol: General Hospital, Nicosia: Nicosia General Hospital. Czech Republic, Brno: Masaryk University Hospital, Hradec Kralové: Charles University, Olomouc: University Hospital, Ostrava: University Hospital Ostrava. Pilsen: Charles Hospital, Prague: Charles University, Charles University Vinohrady, Institute of Hematology and Blood Transfusion, University Hospital Motol. Denmark, Aalborg: Aalborg Hospital, Aarhus: Amtssygehus, Copenhagen: Herlev Hospital, Rigshospitalet. Estonia, Tallinn: North Estonia Medical Centre, Tartu: University Hospital. Finland, Helsinki: Children’s Hospital, University Central Hospital, Oncology University Hospital, Kuopio: University Hospital, Oys Oulu: University Central Hospital, Tampere: University Hospital, Turku: University Central Hospital. France, Amiens: CHU d’Amiens, Angers : Centre Hospitalier, Bayonne : C.H. De la Cote Basque, Besancon : Hôpital Jean Minjoz and St.Jacques, Bordeaux : CHU Hospitalier Pellegrin- Enfants, Boulogne sur Mer : CHU Hôpital Duchenne, Brest : Hôpital Morvan, CHU de Brest, Caen : Centre Reg. Francois Baclesse, CHU Caen Institut d’hématologie de Basse-Normandie, Clermont Ferrand : CRCTCP CHU Estaing, Colmar : Hôpital Civils, Corbeil Essonne: Hopital Gilles de Corbeil, Créteil : Hôpital H. Mondor, Hôpital Henri Mondor, Dijon : Hôpital des Enfants, Dunkerque : Centre Hospitalier, Grenoble: CHU Grenoble, La Réunion : CHU Felix Guyon. Le Chesnay: C.H. De Versailles, Lille: Centre Hospitalier Saint Vincent, Centre Oscar Lambret, Hôpital Claude Huriez, Hôpital Jeanne de Flandre, Limoges : CHU Dupuytren, Lyon : Centre Léon Bérand, Hôpital Edouard Herriot, Institue d’Hématologie et d’Oncologie Pédiatrique, Marseille : Hôpital Timone Enfants, Institute Paoli I. Calmettes, Marseille Bouches du Rhone : Centre Hospitalier Universitaire La Conception, Meaux: CHU de Meaux, Metz : CHR de Metz-Thionville, Montpellier : CHR Lapeyronie, Mulhouse : Hôpital du Hasenrain, Nantes : Hotel Dieu, CHU Nantes, Nice : Centre Antoine Lacassagne, Hôpital de l’Archet 1, Orleans : CHR Orléans, Hôpital de la Source, Paris : Clarmart, Hôpital d’Instruction des Armées Percy, Hôpital d’enfants Armand Trousseau, Hôpital Cochin, Hôpital Neckar des enfants malades, Hôpital Necker, Hôpital Pitié Salpétière, Hôpital Robert Debré, Hôpital St. Antoine, Hôpital St. Louis, Hôpital Tenon, Pessac: Hôpital du Haut Leveque, CHU Bordeaux, Pierre Benite : Centre Hospitalier Lyon-Sud, Poitiers : CHU de Poitiers, Hôpital La Miletrie, Pontoise : Hôpital René Dubos, Reims : Hôpital Robert Debré, Rennes: Clinique Médical Infantile, CHRU, Hôpital Pontchaillou, Roubaix: Hôpital V. Provo, Rouen: Centre Henri Becquerel, Hôpital Charles Nicolle, Saint Priest en Jarez: Institue Lucien Neuwirth, ‘Institute de Cancérologie Lucien Neuwirth, Saint Quentin: Centre Hôpital De Saint Quentin, St. Cloud: Institut Curie, Hôpital René Huguenin, Strasbourg: Hôpital de Hautepierre, Toulouse: Hôpital de Purpan, Tours: Hôpital Bretonneau, Vandoeuvre-les-Nancy: CHU de Brabois, Hôpital d’Enfants, Villejuif :Gustave Roussy Cancer Campus, Hôpital Paul Brousse, Institut G. Roussy. Germany, Aachen: Universitätsklinikum, Augsburg: Klinikum Augsburg, Bayreuth: Klinikum Bayreuth, Berg: Schön Klinik Starnberger See, Berlin: Charite Campus Virchow Klinikum, Charité, Campus Benjamin Franklin, Charité, Campus Virchow Klinikum, HELIOS Klinikum Berlin Buch, Vivantes Klinikum Neukoellen, Bochum: Knappschaftskrankenhaus, Bonn: Universitätsklinikum, Braunschweig: Städtisches Klinikum, Bremen: Evangelistisch Diakonie-Krankenhaus GmbH, Klinikum Bremen-Mitte, Chemnitz: Klinikum Chemnitz GmbH, Cottbus: Carl-Thiem-Klinikum, Dresden: Universitätsklinikum Carl Gustav Carus, Duisburg: HELIOS Klinikum, Düsseldorf: Universitätsklinikum. Erlangen: Universitäts-Klinik für Kinder und Jugendliche, Universitätsklinikum. Essen: Evangelisches Krankenhaus Essen-Werden GmbH, Universitätsklinikum, West German Cancer Center, Flensburg: St. Franziskus Hospital, Frankfurt: J. W. Goethe Universität, KH Bethanien, KH Nordwest, Klinikum Frankfurt Oder, Universitätsklinikum d. J.W.Goethe. Freiburg: Universitatsklinikum. Giessen: Universitätsklinikum, Göttingen: Universitätsklinikum, Greifswald: Universitätsklinikum, Hagen: St. Marien Hospital, Halle: Universitätsklinikum, Hamburg: Asklepios Klinik Altona, Asklepios Klinik St. Georg, Universitätsklinikum Eppendorf, Universitätsklinikum Eppendorf - Onkologisches Zentrum, Hameln: Sana Klinikum Hameln-Pyrmont, Hamm: Evangelisches Krankenhaus, St. Marien Hospital, Hannover: Klinikum Region Siloah, Medizinische Hochschule. Heidelberg: Angelika Lautenschläger-Klinik, Universitätsklinikum, Homburg/Saar: Universität des Saarlandes, Jena: FS Universitäts-Kinderklinik, Universitätklinikum. Kaiserslautern: Westpfalzklinikum, Karlsruhe: Städtische Klinik, Kassel: Klinikum Kassel, Kiel: Universitätsklinikum Schleswig-Holstein, Koblenz: Stiftungsklinikum Mittelrhein, Köln: Universitätsklinikum. Leipzig: Universitätsklinikum, Lemgo: Klinikum Lippe, Lübeck: Sana Klinikum, Universitätsklinikum Schleswig Holstein, Universitätsklinikum Schleswig Holstein, Ludwigshafen: klinikum der Stadt. Magdeburg: Universitätsklinikum. Mainz: Universitätsklinikum, Mannheim: Universitätsklinikum, Marburg: Universitätsklinikum, Minden/Westfalen: Klinikum Minden, Möchengladbach: Klinikum Maria Hilf ll, KH St. Franziska, Munich: KH München Schwabing, Kinderklinikum Universität München Grosshadern, Klinikum Innenstadt der LMU, Klinikum Rechts der Isar, Klinikum Schwabing, SKH Munchen- Harlaching, Universitätsklinikum Grosshadern, Münster: Universitätklinikum Münster, W.-W Universitätklinikum. Nürnberg: Klinikum Nord, Oldenburg: Universitätsklinikum, Osnabrück: Klinikum Osnabrück, Potsdam: Klinikum Ernst von Bergmann, Regensburg: Universitätsklinikum. Rostock: Universitätsklinikum. Rotenburg-Wümme: Diakoniekrankenhaus. Siegen: St. Marien-Krankenhaus, Stuttgart: DiakonissenKH, Klinikum Stuttgart, Katharinenhospital, Robert Bosch Krankenhaus, Universitätsklinikum, Tübingen: Universitätsklinikum, Ulm: Kinderklinik Universitätsklinikum, Villingen: Schwarzwald-Baar Klinikum, Westerstede: Ammerland Klinik, Wiesbaden: Deutsche Klinik für Diagnostik, Dr. Horst Schmidt Klinikum, Würzburg: Universitätsklinikum. Greece, Alexandroupolis: Thrace University Med. School, Athens: Aghia Sophia Children’s Hospital, Alexandra Hospital, University of Athens, Athens Medical Center, Diagnostic & Therapeutic Center ‘Hygeia’, Evanghelismos Hospital, Hellenic Cancer Institute St. Savas, Laikon General Hospital, University of Athens, Crete: University Hospital, Patras: University Medical School Hospital, Thessaloniki: The George Papanicolaou General Hospital. Hungary, Budapest: St Istvan & St. Lazlo Hospitals, Szent Laszlo Hospital, Debrecen: University of Debrecen, Miskolc: GYEK, Postgraduate Medical School, Pécs: University of Pécs. Iceland, Reykjavik: National University Hospital. Ireland, Dublin: Our Lady’s Hospital of Sick Children, Crumlin, St. Vincent’s Hospital, St. James Hospital. Galway: University College Hospital. Israel, Haifa: Rambam Medical Center, Jerusalem: Hadassah University Hospital, Petach-Tikva: Beilinson Hospital, Children’s Medical center, Revohot: Kaplan Hospital, Tel Aviv: Dana-Dwek Children’s Hospital, Sourasky Medical Centre, Sourasky Medical Center, Tel Hashomer: Chaim Sheba Medical Center, Sheba Medical Center. Italy, Alessandria: S.S. Antonio e Biagio e C. Arrigo. Ancona: Ancona University, Azienda Ospedale Salesi Riuniti, Ascoli Piceno: Mazzoni Hospital, Avellino: A.O.S. Guiseppe Moscati, Aviano: CRO Aviano, Bari: Universitá degli Studi di Bari. Bergamo: ASST Papa Giovanni XXIII, Bologna: Instituto Ortopedico Rizzoli, Poli. S. Orsola, San Orsola-Malpighi Hospital, Bolzano : Ospedale S. Maurizio, Brescia: Azienda Spedali Civili, Azienda Spedali Civili, Universitá degli Studi di Brescia, Brindisi: Perrino Hospital, Busto Arsizio: Ospedale di circolo di Busto Arsizio, Cagliari: Binaghi BMT Centre, Ospedale A. Businco, Ospedale per le Microcitemie . Catania: Ospedale Ferrarotto, University of Catania, Ospedale Ferrarotto, University of Catania. Civitanove Marche: Ospedale di Civitanova Marche, Cremona: U.O. Ematologia CTMO, Cuneo: Azienda Ospedale “S. Croce e Carle”, Ferrara: University of Ferrara, Florence: Ospedaliera Universitaria di Careggi, Foggia: Azienda Ospedaliero Universitaria, Genova: Istituto Giannina Gaslini, Ospedale San Martino, Ospedale San Martino, Latina: Ospedale S. Maria Goretti, Lecce: Opsedale Vito Fazzi de Lecce, Medola: Istituto Scientifico Romagnolo, IRST, Messina: Policlinico Universitario Messina. Milan: 1st. Clinico Humanitas, Instituto Europeo di Oncologia, Ist. Nazionale Tumori di Milano, Istituto Scientifico H.S. Raffaele, Ospedale di Niguarda, S. Carlo Borromeo Hospital , University of Milan IRCCS, Mirano: Ospedale Civile di Mirano, Modena: University of Modena, Monza: Ospedale San Gerardo, Ospedale San Gerardo, Universita Di Milano-Bicocca, Napels: Cardarelli Hospital , Frederico ll University, Hospital Pausilipon, National Cancer Institute IRCCS, Novara: Ospedale Maggiore della Carita. Nuoro: Ospedale San Francesco, Padova: Centro Leucemie Infantili, Istituto Oncologia Veneto IOV-IRCCS, Padua: University Hospital, Pagani: Hospital A. Tortora, Palermo: ARNAS Civico Di Cristina, Ospedale die Bambini, Ospedale ‘La Maddalena’, Ospedale V. Cervello- USL 60, Uni delgi Studi di Palermo, Parma: Università degli studi, Pavia: Fondazione Clinica del Lavoro, S. Maugeri, IRCCS Policlinico S. Matteo, Policlinico IRCCS St. Matteo, Perugia: Ospedale Santa Maria della Misericordia, Pesaro: Ospedale San Salvatore, Pescara: Ospedale Civile, Piacenza: Hospital Guglielmo da Saliceto, Pisa: University of Pisa, Potenza: San Carlo Hospital, Ravenna: Ospedale Civile, Reggio di Calabria: Grande Ospedale Metropolitano, Bianchi Melacrino Morelli, Reggio Emilia: Arcispedale S. Maria Nuova, Rimini : Ospedale Infermi Rimini, Rionero in Vulture: IRCCS/CROB, Rome: IRRCS Ospedale Bambino Gesu, Ospedale S. Camillo, Policlinico Tor Vergata, Rome Transplant Network, Università “ La Sapienza”, Università Cattolica , Salerno: AOU San Giovanni di Dio e Ruggi D’Aragona, San Giovanni Rotondo: Hospital Casa Sollievo Sofferenza, Sassari: Universita Di Sassari, Siena: Ospedale Sclavo, Taranto: Institute of Haematologie, Ospedale Nord, Torino: Ospedale Magg. S. G. Battista, Ospedale Ordine Mauriziano Umberto I, University Hospital, Treviso: Presidio Ospedaliero Treviso, Tricase (Lecce): Hospital C. Panico, Istituto per I’Infanzia, IRCCS Burlo Garofolo. Udine: Policlinico Universitario & General Hospital. Varese: Ospedale di Circolo e Fondazione Macchi, Venice: Ospedale dell’Angelo, Verbania Pallanza: Ospedale di Verbania, Vercelli: S. Andrea Hospital , Verona: Policlinico G. B. Rossi, Vicenza: Ospedale S. Bortolo. Kazakhstan, Astana: National Research Center for Oncology and Transplantology. Latvia, Riga: Clinic Linezers. Lithuania, Vilnius: Santariskiu Klinikos. Lithuania, Vilnius: University Children’s Hospital. Luxemburg, Luxemburg, Center Hospitalier. Macedonia, Skopje: Medical Faculty. Netherlands, Amsterdam: Academic Med Centre, Antoni Van Leeuwenhoek Cancer Institute, VU University Medical Center. Enschede: Medisch Spectrum Twente. Groningen: University Hospital. Leiden: University Hospital. Maastrich: University Hospital. Nieuwegein: St. Antonius Hospital. Nijmegen: University Hospital. Rotterdam: Dr. Daniel den Hoed Cancer Center, Sophia Children’s Hospital. The Hague: Haga Hospital. Utrecht: University Hospital for Children, WKZ, Utrecht University Hospital UMCU. Zwolle: Isala klinieken. Norway, Bergen: Haukelands Sjukhus. Oslo: Oslo University Hospital, Rikshospitalet-Radiumhospitalet. Tromso: University Hospital North Norway. Trondheim: St. Olavs Hospital. Poland, Bydgoszcz: Nicolaus Copernicus University. Cracow: University Hospital CMUJ, University Children’s Hospital JUMC. Gdansk: Medical University. Gliwice: Maria Curie Memorial Cancer Centre. Katowice: Silesian Medical Academy. Lodz: Medical University of Lodz. Lublin: Children’s University Hospital, University Medical School. Poznan: Institute of Pediatrics, Pozan Medical Academy. Warsaw: Central Clinical Hospital, Central Hospital Military Medical Academy, Institute of Haematology and Blood Transfusion, Marie Curie Institute. Wroclaw: Lower Silesian Center / BM Donor Registry, Medical Academy, University of Medicine. Portugal, Coimbra: University Hospital. Lisbon: H. St. Antonio dos Capuchos, Hospital de Santa Maria, Instituto Portugues de Oncologia. Porto: Hospital St. Joao, Instituto Portugues de Oncologia. Romania, Bucharest: Coltea Clinical Hospital, Fundeni Clinical Institute. Targu-Mures: Sectia Clinica de Hematologie. Timisoara: Children’s Hospital Louis Turcanu. Russia, Ekaterinburg: Regional Hospital No. 1. Moscow: Burnasyan Fed. Med. Biophysical Centre, Cancer Research Center, Cancer Research Centre, Federal Research Center for Pediatric Hematology, Main Military Clinical Hospital, National Pirogov Medical Centre, Research Haematology Center of RAS, The Russian Children’s Research Hospital. St. Petersburg: Federal Centre V.A. Almazov, First State Pavlov Medical University, Russian Scientific and Research Institute of Haematology. Serbia, Belgrade: Clinical Center of Serbia, Military Medical Academy, Mother and Child Health Institute. Slovak Republic, Banská Bystrica: Roosevelt Hospital. Bratislava: National Cancer Institute, University Hospital. Kosice: University Hospital. Slovenia, Ljublijana: University Medical Centre. Spain, Alicante: Hospital General. Barakaldo Vizcaya: Hospital de Cruces. Barcelona: Hospital Clinic, Hospital General Vall d’Hebron, Hospital Germans Trias i Pujol , Hospital M. Infantil, Vall d’Hebron, Hospital Mutua de Terrassa, Hospital Sant Joan de Deu, Institute Catala d’Oncologia, Hospital Duran i Reynals, Santa Creu i Sant Pau, Santa Creu i San Pau. Caceres: Hospital San Pedro de Alcantara. Cadiz: Hospital de Jérez, Hospital Universitario Puerta del Mar. Castellon de La Plana: Hospital General de Castellon. Cordoba: Hospital Reina Sofia. Galdakao: Hospital de Galdakao. Girona: Institut Catala d’Oncologia, Josep Trueta. Granada: Hospital Virgen de la Nieves. Jaen: Hospital Cuidad de Jaen. La Coruna : Complexo Hosp. Uni. A Coruna. La Laguna, Tenerife: University Hospital Canary Isles, Hospital N. S. De la Candelaria. Las Palmas Canary Isles: Hospital Insular, Hospital Materno Infantil, Hospital Universitario de Gran Canaria ‘Dr. Negrin’. Leon: Hospital Universitario de Leon. Lleida: Hospital Arnau de Vilanova. Logrono: Hospital San Pedro, La Rioja. Lugo: Hospital Lucus Augusti. Madrid: Clinica Moncloa, Fundacion Jimenez Diaz, Hospital de la Princesa, Hospital Doce de Octubre, Hospital General Universitario Gregorio Maranon, Hospital La Paz, Hospital Niño Jesus, Hospital Principe Asturias, Alcala de Henares, Hospital Quiron, Hospital Ramon y Cajal, Hospital Severo Ochoa, Leganés, Hospital Univeristario Materno Infantil Gregorio Maranon, Hospital Universitario de Getafe, Hospital Universitario Puerta de Hierro, Hospital Universitario San Carlos. Malaga: Carlos Haya Hospital. Murcia: Hospital General Universitario Morales Meseguer, Hospital Virgen de la Arrixaca. Orense: Com. Hospital Cristal-Pinor. Oviedo: Hospital Covadonga, Central Asturias. Palma de Mallorca: Hospital son Llatzer, Hospital Universitario Son Espases (Son Dureta). Pamplona: Clinica Universitaria de Navarra, Hospital de Navarra. Pontevedra: Hospital Montecelo. Salamanca: Complejo Hospital. San Sebastian: Hospital Universitario Donostia. Santander: Hospital Universitario M. de Valdecilla. Santiago de Compostela: Hospital Clinico Universitario. Sevilla: Hospital Universitario Virgen del Rocio. Valencia: Hospital Arnau de Vilanova de Valencia, Hospital Clinico Universitario, Hospital Doctor Peset, Hospital Universitario La Fe, Instituto Valenciano de Oncologia. Valladolid: Hospital Rio Hortega. Vigo: CHUVI Hospital Alvaro Cunqueiro. Zaragoza: Clinico Universitario Lozano Blesa, Hospital Miguel Servet. Sweden, Goteborg: CHECT Sahlgrenska University Hospital. Linköping: University Hospital. Lund: University Hospital. Örebro: Medical Center Hospital. Stockholm: Karolinska University Hospital. Umea: University Hospital. Uppsala: University Hospital. Switzerland, Aarau: Kantonsspital Aarau. Basel: University Hospital Basel. Bellinzona: Ospedale San Giovanni. Bern: Inselspital. Geneva: Hôpital Cantonal Universitaire. Lausanne: Centre Hospitalier Universitaire Vaudois. St. Gallen: Kantonsspital. Zurich: University Hospital, Universitäts Kinderklinik. Turkey, Adana: Baskent University Adana, Cukurova University Balcali Hospital. Ankara: Ankara Bayindir Hospital, Ankara University Faculty of Medicine, Dikimevi, Ankara Children’s Hospital, GATA BMT Center, Etlik, Gazi University Medical School, Besevler, Gazi University Medical School, Besevler, Hacettepe University Medical School, Sihhiye, Ihsan Dogramaci Children’s Hospital (Hacettepe), Numune Education and Research Hospital, Private Medicana International Hospital, University Faculty of Medicine, Cebeci, University of Ankara, Cebeci. Antalya: Akdeniz University School of Medicine, Medical Park Antalya Hospital, Lara, Medical Park Hospitals, Medstar Antalya Hospital, Cakirlar. Atakum Samsun: Medicalpark Hospital. Aydin: Adnan Menderes University Medical Faculty. Bursa: Uludag University Scool of Medicine. Eskisehir: Osmangazi University. Gaziantep: University Medical School. Istanbul: Acibadem Altunizade Hospital, Acibadem University Atakent Hospital, Bahcelievler Medical Park Hospital, Cerrahpasa Medical Faculty, Florence Nightingale Sisli Hospital, Istanbul Medipol University, Medical Park Goztepe, University of Istanbul, Hisar Intercontinental Hospital Izmir: Dokuz Eylul University, Ege University Medical Faculty, Bornova, Tepecik Research and Educational Hospital. Kayseri: Erciyes University Faculty of Medicine, Erciyes University Hospital. Kocaeli: Anadolu Medical Center Hospital. Konya: Necmettin Erbakan, Meram University Medical Hospital. Malatya: Inonu University Targut Özal Medical Centre, Samsun: Ondokuz Mayis University. Trabzon: Karadeniz Technical University. Ukraine, Kiev: City BMT Center, National Pediatric Specialized Hospital, OHMATDYT. United Kingdom, Aberdeen: The Royal Infirmary. Bangor: Ysbyty Gwynedd. Bath: Royal United Hospital. Belfast: Belfast City Hospital Trust. Birmingham: Heartlands Hospital, Queen Elizabeth Hospital, The Birmingham Children’s Hospital. Blackpool: Victoria Hospital. Bournemouth: oyal Bournemouth Hospital. Bristol: Avon and Royal Hospital for Sick Children. Cambridge: Addenbrooke’s Hospital. Cardiff/Swansea: University Hospital of Wales. Cheltenham: Cheltenham General Hospital. Coventry: University Hospital & Warwickshire NHS Trust. Dudley: Dudley NHS Trust. Dundee: Ninewells Hospital. Edinburgh: The Western General Hospital. Exeter: Royal Devon and Exeter Hospital. Glasgow: Beatson, West of Scotland Cancer Centre, Royal Hospital for Sick Children: Ipswich: Ipswich Hospital. Leeds: Leeds Teaching Hospitals Trust. Leicester: Royal Infirmary Hospital. Liverpool: Alder Hay Children’s Hospital, Royal Liverpool University Hospital. London: Great Ormond, Street Hospital, Hammersmith Hospitals NHS Trust, King’s College Hospital, Parkside Hospital, London, Royal Marsden Hospital, Mary’s Hospital, St. Bartholomew’s and the Royal London Hospital, St. George’s Hospital, The Harley Street Clinic, The London Clinic, The Royal Free Hospital, University College Hospital. Manchester: Central Manchester NHS Trust, Christie Hospital, The Royal Infirmary. Newcastle upon Tyne: Freeman Hospital. Norwich: Norfolk and Norwich Hospital. Nottingham: Nottingham City Hospital. Churchill, Stoke Manderville Hospitals. Plymouth: Plymouth Hospitals NHS Trust. Poole: Poole Hospital NHS Foundation Trust. Salisbury: Salisbury NHS Foundation Trust. Sheffield: Teaching Hospitals NHS Trust, Children’s Hospital. Southampton: CRC Wessex. Stoke-on-Trent: University Hospital of North Staffordshire. Swindon: Great Western Hospital. Taunton: Taunton and Somerset Hospital.
LABMT
Argentina, Buenos Aires: Hospital Universitario Fundacion Favaloro, Hospital Italiano de Buenos Aires, FUNDALEU, Hospital de Clinicas J de San Martin, Hospital Aleman, Instituto Alexander Fleming, Sanatorio Anchorena , Hospital Britànico de Buenos Aires, Hospital Rodolfo Rossi, Sagrado Corazón, Hospital Italiano de La Plata, Hospital San Martín de La Plata, CEMIC. Córdoba: Sanatorio Allende, Hospital Privado de Còrdoba. Pilar: Hospital Universitario Austral, El Hospital de Niños Sor María Ludovica (HNSML). Santa Fe: CETRAMOR. Brazil, Salvador: Monte Tabor Centro Ítalo Brasileiro de Promoção Sanitário - Hospital São Rafael, Hospital das Clinicas UFBA. Fortaleza: Hospital Universitário Walter Cantídeo - UFC – SAMEAC. Belo Horizonte: Hospital Socor. Juiz de Fora: Hospital Universitário da Juiz da Fora UFJF. Curitiba: Hospital das Clinicas Universitário Fed. Do Parana. Niteroi: Hospital Das Clinicas de Niteroi. Rio de Janiero: Centre de transplante de Medula Ossea INCA, Hospital Universitário Clementino Fraga Filho, Hospital Universitário Pedro Ernesto HUPE UERJ. Porto Alegre: Hospital das Clinicas de Porto Algra UFRG, Hospital das Clinicas de Porto Algra UFRG peds. Barretos: Hospital de Câncer de Barretos - Fundação Pio XII. Campinas: Centro de Hematologia e Hemoterapia de Campinas (Hemocentro) da Universidade Estadual de Campinas (Unicamp), Centro Infantil de Investigações Hematológicas Dr.Domingos A. Boldrini, Real Soc. Beneficência Portuguesa de Campinas, Hospital Vera Cruz S/A. Jau: Hospital Amaral Carvalho - Fundação Dr. Amaral Carvalho. Ribeirao Preto: Hospital de Clínicas FAEPA Ribeirão Preto FMRP USP, Hospital São Lucas de Ribeirão Preto. São Paulo: Fundação Antônio Prudente Hospital A C Camargo, Instituto de Oncologia Pediátrica – GRAACC – UNIFESP, Hospital Israelita Albert Einstein, Hospital Sírio Libanês, Hospital das Clínicas São Paulo - HCFMUSP, Instituto Brasileiro de Controle do Cancer IBCC, Sociedade Hospital Samaritano, HTEJZ - Hospital Brigadeiro - SES (Hospital Dr. Euryclides de Jesus Zerbini), Hospital Santa Helena - Hemome, Hospital Santa Paula, Hospital Bandeirantes, Hospital Nove de Julho, FCM Santa Casa de Misericórdia de São Paulo. Sorocaba: Hospital Unimed Sorocaba. Chile, Santiago: Hospital Del Salvador de Chile adults, Catholic University of Chile Adults, Catholic University of Chile Pediatrics, Clinica Santa Maria Adults and Pediatrics. Colombia, Bogota: Clinica de Marly. Cali: Center Medico Imbanaco. Floridablanca: UNAB FOSCAL. Medellin: Clinicas las Americas IDC, Hospital Pablo Tobin Uribe . Costa Rica, San Jose: Hospital Mexico CCSS, Hospital San Juan de Dios Adults. Ecuador, Guayaquil: OMNI HOSPITAL Guayaquil, SOLCA Guayaquil. Quito: Hospital Metropolitano Quito. Mexico, Guadalajara: Hospital Civil de Pediatria Guadalajara, Juan I Menchaca, Hospital Civil de Guadalajra Frai Antonio Alcalde. Mexico City: Hospital Infantil de Mexico Federico Gomez HIMFG, Hospital Especialidades Centro Medico Nacional Siglo XXI, Instituto National de Cancerologia INCAN, Instituto National de Pediatria. Monterrey: Hospital Uni Antonoma de Nuevo Leon UANL. Puebla: Center de Hematologia y Medicina Interna -Clinica Ruiz, Hospital de Especialidades IMSS. Xalapa: Centro Estatal de Onco (CECAN) Dr. Miguel Dorantes Mesa. Panama, Panama City: Instituto Oncologico Nacional, CHAAM Complejo Hospitalario Metropolitano Dr. Arnulfo Arias Madrid, Hospital del Nino. Paraguay, Asuncion: Instituto de Prevision Social IPS. Peru, Chiclayo: Nacional Almanzor Aguinaga Asenjo . Lima: Hospital Rebagliati, Hospital Almenara, Nacional de Salud del Ninos, Clinica San Borja SANNA. Uruguay, Montevideo: Asociacion Espanola lo de Socorros Mutuos Fundación Pérez-Scremini/Hospital Pereira Rossell, Asociacion Espanola lo de Socorros Mutuos , Hospital Británico , Servicio Médico Integral-SMI, Hospital Maciel. Venezuela : Caracas, Hospital de Clínicas de Caracas. Valencia : Cuidad Hospital Dr. Enrique Tejera CHET.
Footnotes
Conflict of interest statement
There are no conflicts of interests.
REFERENCES
- 1.Thomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE, et al. Bone-marrow transplantation (second of two parts). The New England journal of medicine 1975;292:895–902. [DOI] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror Boeckh M,et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine 2010;363:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO guiding principles on human cell, tissue and organ transplantation. Transplantation 2010;90:229–33. [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al. Hematopoietic stem cell transplantation: a global perspective. Jama 2010;303:1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gratwohl A, Baldomero H, Gratwohl M, Aljurf M, Bouzas LF, Horowitz M et al. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a Global Observational Study. Haematologica 2013;98:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L et al. One million haemopoietic stem-cell transplants: a retrospective observational study. The Lancet Haematology 2015;2:e91–100. [DOI] [PubMed] [Google Scholar]
- 7.Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone marrow transplantation 2016;51:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone marrow transplantation 2015;50:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aljurf MD, Zaidi SZ, El Solh H, Hussain F, Ghavamzadeh A, Mahmoud HK, et al. Special issues related to hematopoietic SCT in the Eastern Mediterranean region and the first regional activity report. Bone marrow transplantation 2009;43:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aljurf M, Zaidi SZ, Hussain F, Ghavamzadeh A, Alimoghaddam K, Jahani M et al. Status of hematopoietic stem cell transplantation in the WHO Eastern Mediterranean Region (EMRO). Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 2010;42:169–75. [DOI] [PubMed] [Google Scholar]
- 11.Aljurf M, Nassar A, Hamidieh AA, Elhaddad A, Hamladji RM, Bazarbachi A et al. Hematopoietic stem cell transplantation in the Eastern Mediterranean Region (EMRO) 2011–2012: A comprehensive report on behalf of the Eastern Mediterranean Blood and Marrow Transplantation group (EMBMT). Hematology/oncology and stem cell therapy 2015;8:167–75. [DOI] [PubMed] [Google Scholar]
- 12.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions. Osrin D, ed. PLoS Medicine. 2013;10(7):e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaimovich G, Martinez Rolon J, Baldomero H, Rivas M, Hanesman I, Bouzas L et al. Latin America: the next region for haematopoietic transplant progress. Bone Marrow Transplantation (2017) 52, 671–677 [DOI] [PubMed] [Google Scholar]


