Abstract
Background:
Recorded at the time of transplant and reported to the Organ Procurement and Transplantation Network (OPTN), patient’s functional status is measured using the Karnofsky Performance Score (KPS), ranging 0–100. Functional status analysis may provide insights on candidate listing and posttransplant survival outcomes for deceased-donor kidney transplants (DDKT).
Methods:
The cohort consisted of adult DDKT recipients transplanted beginning January 2007. One-year and 3-year Cox models for posttransplant survival were fitted with current Scientific Registry of Transplant Recipients (SRTR) variables and KPS. Comparative analyses were performed between the SRTR model without KPS and the augmented model with it. Using the augmented model, we examined the impact of Kidney Donor Profile Index (KDPI) on posttransplant survivals for 5 different KPS strata: 10–30, 40–50, 60–70, 80–90, and 100.
Results:
Comparative analyses showed that KPS was a statistically significant predictor for posttransplant survival: it improved model calibration, discrimination, and predictive accuracy. From the augmented model, the survival curves illustrated that recipients with KPS 40–50 and kidneys with KDPI as high as 99 have expected survival probabilities of above 90% in 1 year and above 80% in 3 years. The expected survival probabilities improve as KPS increases. Recipients with KPS 10–30 have the worst survival probability, even if they received high-quality kidneys.
Conclusions:
Insights from the survival analyses recommend possible inclusion of functional status into SRTR’s risk-adjusted models. Moreover, they invite further examination of its use in order to improve current listing and transplantation strategies at transplant centers and potentially reduce deceased-donor kidney discard rate.
Introduction
Over 90 000 patients are currently on the waitlist to receive kidney transplant1, but only about 20 000 patients were transplanted in 2016 with deceased donation rate of kidneys remaining stable in the past 10 years2, so the demand for kidneys is critical. Unfortunately, in the same year, the discard rate of kidneys recovered from deceased adult donors was about 20% while more than 8000 patients were removed from the waitlist due to deteriorating health or death2. Therefore, current listing and transplantation strategies at transplant centers need to be reevaluated in order to decrease the number of unwanted removals and discard rate of deceased-donor kidneys.
These strategies could be improved by considering transplant candidates’ functional statuses. This information is readily available in the Scientific Registry of Transplant Recipients (SRTR) and Organ Procurement and Transplantation Network (OPTN) data and it is measured by the Karnofsky Performance Score (KPS), using an 11-point rating scale ranging from 0 (dead) to 100 (normal). Table 1 summarizes the scores. Developed as an assessment tool in oncology3, KPS gained traction as an independent risk factor for mortality in the renal settings, such as for acute renal failure4, end-stage renal disease (ESRD)5, and dialysis6–8. Including KPS in the current risk-adjusted Cox regression models would improve their accuracies, motivating transplant centers to modify their current transplantation strategies.
Table 1:
Karnofsky Performance Score Index
| Strata | Score | Description |
|---|---|---|
| Normal | 100 | Normal, no complaints, no evidence of disease |
| Able to carry on normal activity; unable to perform physically strenuous activity |
90 | Able to carry on normal activity: minor symptoms of disease |
| 80 | Normal activity with effort: some symptoms of disease | |
| Capable of self-care; unable to carry on normal activity |
70 | Cares for self: unable to carry on normal activity or active work |
| 60 | Requires occasional assistance but is able to care for needs | |
| Require care and assistance | 50 | Requires considerable assistance and frequent medical care |
| 40 | Disabled: requires special care and assistance | |
| Incapable of self-care | 30 | Severely disabled: hospitalization is indicated, death not imminent |
| 20 | Very sick, hospitalization necessary: active treatment necessary |
|
| 10 | Moribund, fatal processes progressing rapidly | |
| Dead | 0 | Dead |
Estimated from the risk-adjusted Cox models, the expected 1-year and 3-year graft and patient survivals9,10 set the performance standards that transplant centers have to satisfy and thus influence their strategies11. The expected survival statistics are provided to the Membership and Professional Standards Committee (MPSC) of the OPTN and then used to evaluate transplant center performances9,12. If a transplant center failed to meet performance standards, it is flagged, thereby undergoing a review process by United Network of Organ Sharing (UNOS) and/or Centers for Medicare and Medicaid Services (CMS). Consequently, the center may face unintended consequences, such as decertification by CMS, decrease in transplant patient volume and candidate referrals, and loss of insurance contracts11. Lack of improvement might shut it down. Therefore, an underperforming center needs to change its listing and transplantation strategies. Schold et al surveyed that low-performing centers were significantly more likely to increase recipient and donor selection criteria13, thereby restricting access to kidney transplantation and significantly linking them with reduced transplant volumes14. To mitigate these unintended consequences, risk-adjusted Cox models must be as accurate as possible in order to prevent transplant centers from being falsely flagged.
Because functional status is not included in any of the current kidney-related risk-adjustment models15, the aim of this study is to analyze its predictive power measured in KPS on 1-year and 3-year posttransplant survival for deceased-donor kidney transplant (DDKT) recipients. In addition, posttransplant survival outcomes are evaluated for patients transplanted with low-quality kidneys across different KPS strata. The improved Cox models could serve as insightful tools that drive efficacious kidney transplantation decisions.
Materials and Methods
Datasets
This study used data from SRTR. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
The dataset was used to perform statistical analyses on DDKT recipients who were at least 18 years old at the time of transplant and who were not subsequently retransplanted. The latest follow-up status date posttransplant in the given cohort was September 2, 2015. From this cohort, 2 datasets were created for 1-year and 3-year survival analyses, respectively.
One dataset consisted of 72 839 DDKT recipients transplanted from January 1, 2007-September 2, 2014. In this dataset, each patient had at least 1-year follow-up. Patients were right-censored if they were lost to follow-up within 1 year posttransplant or they survived past it. This dataset was used for the 1-year survival model.
Another dataset consisted of 53 242 DDKT recipients transplanted from January 1, 2007-September 2, 2012. Each patient had at least 3-year follow-up. Patients were right-censored if they were lost to follow-up within 3 years posttransplant or they survived past it. This dataset was used for the 3-year survival model.
Covariates
KPS at transplant and most of the covariates used in SRTR risk-adjustment models15 for DDKT recipients were already included in the SRTR dataset. Most of the recipients’ information were gathered from the Transplant Candidate Registration (TCR) form and the Transplant Recipient Registration (TRR) form16. TCR records information at the time of listing, such as ethnicity and education; TRR records information at the time of initial transplant admission, such as age, kidney diagnoses, and KPS at transplant. Donor’s information was recorded at the time of organ donation in the Deceased Donor Registration form16. Computed using the available covariates in the SRTR dataset, the only derived covariates were estimated glomerular filtration rate (eGFR)17, kidney donor risk index (KDRI)18, total ESRD time, and donor’s body mass index (BMI). KPS was stratified into 6 strata: 10–30, 40–50, 60–70, 80–90, 100, and “Unknown.” These strata were based on similar definitions and Ma et al’s work19, which mapped KPS to the Eastern Cooperative Oncology Group (ECOG) scores. The stratification is summarized in Table 1. The covariates are summarized in Tables 2 (1-year) and 3 (3-year).
Table 2:
Summary of recipient and donor covariates for deceased-donor kidney recipients with ≥ 1-year follow-up.
| Covariates | N=72839 | |
|---|---|---|
| Donor | Donor Age (mean±SD) | 39.24±15.68 |
| Donor Blood Type A AB B O |
36.80% 3.59% 12.08% 47.53% |
|
| Donor Arginine Vasopressin Positive Unknown/Missing |
57.17% 0.12% |
|
| Donor BUN in mg/dL (mean±SD) Missing |
17.06±11.90 0.44% |
|
| Donor Cigarette Use (> 20 pack years) Ever Positive Unknown |
24.51% 1.38% |
|
| Donor Creatinine (mean±SD) Missing |
1.15±0.99 2.28% |
|
| Donor eGFR Missing |
92.12±39.91 2.28% |
|
| Donor Gender Male Female |
60.36% 39.64% |
|
| Donor Hematocrit (mean±SD) Missing |
31.02±5.88 0.09% |
|
| Donor Hepatitis C Virus Result Positive Unknown/Missing |
2.49% 0.02% |
|
| Donor KDRI (mean±SD) Missing |
1.26±0.39 3.11% |
|
| Kidney Transplanted Locally | 76.22% | |
| Kidney Pumped | 25.40% | |
| Donor- Recipient |
HLA A Mismatches 1 2 0 |
16.28% 36.74% 46.98% |
| Recipient | Age at Transplant (mean±SD) | 53.03±13.09 |
| Latino | 15.18% | |
| Race Caucasian Asian Black Other |
59.14% 6.25% 32.84% 1.77% |
|
| Diabetes Type 1 Type 2 Other |
4.34% 27.39% 4.49% |
|
| Education Grade School/None High School Technical/Some College College Unknown/Missing |
6.79% 41.48% 22.59% 19.90% 9.25% |
|
| HBV Core Antibody Positive Unknown/Missing |
8.46% 13.27% |
|
| HCV Serostatus Positive Unknown/Missing |
5.49% 3.58% |
|
| HIV Serostatus Positive Unknown/Missing |
0.66% 10.74% |
|
| Had Previous Transplant | 14.29% | |
| Kidney Cold Ischemia Time in Hours (mean±SD) Missing |
17.65±9.25 2.21% |
|
| Kidney Primary Diagnosis at Transplant Congenital, Rare Familial, & Metabolic Disorders Diabetes Glomerular Diseases Hypertensive Nephrosclerosis Unknown/Other |
1.79% 27.93% 22.64% 27.80% 19.84% |
|
| Most Recent CPRA (mean±SD) Missing |
16.03±29.24 7.73% |
|
| Peripheral Vascular Disease Positive Unknown |
4.55% 3.05% |
|
| Primary Payment Public Private/Other/Unknown |
74.71% 25.29% |
|
| Total ESRD Time at Transplant in Days (mean±SD) | 1581.65±1127.20 | |
| Total Serum Albumin at Listing (mean±SD) Missing |
3.92±0.58 13.67% |
|
| Karnofsky Performance Score at Transplant 10–30 40–50 60–70 80–90 100 Unknown |
0.15% 3.14% 25.44% 54.23% 13.81% 3.25% |
|
| Death within 1 year | 3.80% (2766) |
BUN: blood urea nitrogen
CPRA: calculated panel reactive antibodies
eGFR: estimated glomerular filtration rate
ESRD: end-stage renal disese
HLA: human leukocyte antigens
HBV: hepatitis B virus
HCV: hepatitis C virus
HIV: human immunodeficiency virus
SD: standard deviation
Missing Data
A considerable number of the covariates used in survival model building had missing data, but no observation was omitted for having “Unknown” or missing values in any of the considered covariates. Missing data were addressed for categorical covariates and numeric covariates separately. If a categorical covariate had at least 1% missing or unknown, these values were treated together as 1 separate category. If it had less than 1% missing or unknown and the other values were “P/N” (positive/negative) or “Y/N” (yes/no), then the missing and unknown values were imputed using multiple imputation by chained equations (MICE)20. MICE was also applied to numeric covariates with missing data. The imputation method used logistic regression for categorical covariates with “P/N” or “Y/N” values and predictive mean matching for numeric covariates. Imputation onto a dataset produced 10 multiply imputed datasets, each with distinct model estimates of the missing values. In total, 20 multiply imputed datasets were obtained, 10 each from the 1-year and 3-year survival datasets. These datasets were used for the statistical analyses.
Statistical Analysis
The statistical analyses were conducted using the R programming language21. Imputation was performed using the mice package20 and survival modeling was performed using the survival package22. For both the 1-year and 3-year survival datasets, log-rank tests were applied to examine pairwise comparison of survival times between KPS strata. For each imputed dataset, 2 multivariate Cox proportional hazard models were fitted: the standard and the augmented models. The standard model was the SRTR risk-adjustment model15, either first-year outcomes (47 total covariates) or 3-year outcomes (61 total covariates), while the augmented model was the same model with 5 additional indicator covariates, each corresponding to a KPS stratum. “Unknown” or missing values that appear at least 1% within a categorical covariate are set as the reference level before fitting the model. Four sets of 10 models were fitted: standard 1-year survival, augmented 1-year survival, standard 3-year survival, and augmented 3-year survival. The coefficients of the models were averaged to obtain the pooled coefficients, which were used to construct the pooled Cox proportional hazard model. The pooled coefficients were recorded in Tables S1–S4.
The log-likelihood, Akaike information criterion (AIC)23, Bayesian information criterion (BIC)24, and Harrell’s c statistic25 were computed using the pooled Cox model. To evaluate model calibration improvement, Meng and Rubin’s method26,27 was used to perform the likelihood ratio test. To compare the differences in AICs, BICs, and c statistics between the standard and augmented models, paired t tests with 9-degrees of freedom were performed.
In order to estimate the expected survival curves conditional on a covariate of interest (eg, KPS), the unique combinations of categorical covariates, excluding the conditioned covariate if categorical, were listed along with their number of frequencies within the corresponding dataset of the pooled Cox model. The list was converted to a dataset, where each observation had its categorical covariates as one of the unique combinations and its numerical covariates as the averages of their respective, nonmissing values within the dataset after splitting the original SRTR dataset and before imputation. Each observation in the newly constructed dataset has the same value for the covariate of interest being conditioned on. For each observation of the new dataset, the pooled Cox model was used to estimate its survival curve. The adjusted expected survival curve was computed as the weighted average of these survival curves using the normalized frequencies as the weights.
Disclaimer
This study was obtained under IRB approval (STU00204041) from Northwestern University Institutional Review Board. All identifiers were removed upon data receipt for the purposes of this study.
Results
Study Cohort Characteristics
The KPS distributions between the 1-year and 3-year survival cohorts were similar. The most prevalent KPS value was 80–90 (approximately 54%), followed by 60–70 (approximately 23–25%), and 100 (approximately 14–15%). Recipients with KPS ≤ 50 comprised approximately 3% of the cohort. In addition, 3% of the cohort reported their KPS values as unknown. Therefore, approximately 94% of the recipients were capable of self-care. The specific percentages of each KPS stratum for each cohort are listed Tables 2 and 3. Among the 1-year survival cohort, 2766/72 839 recipients died (Table 2); among the 3-year survival cohort, 4879/53 242 recipients died (Table 3). Table 4 counts the number of deaths and provides the prevalence rates for each KPS stratum for both the 1-year and 3-year survival datasets. Although the prevalence rates for KPS strata 10–30 and 40–50 are extremely low that they may compromise model accuracy, the numbers of events (or deaths) per independent variables (EPV) for both 1-year and 3-year models exceed 10, ensuring accurate estimation of the regression coefficients28. Specifically, the EPV for the augmented 1-year Cox model is 2766/52 = 55.32 while the EPV for the augmented 3-year Cox model is 4879/66=73.92. Even they exceed 40–50, the threshold that ensures minimal bias in estimating regression coefficients of binary covariates with very low prevalence29.
Table 3:
Summary of recipient and donor covariates for deceased-donor kidney recipients with ≥ 3-year follow-up. This cohort consists of recipients who received transplant between 2007/01/01–2012/09/02.
| Covariate | N=53242 | |
|---|---|---|
| Donor | Donor Age (mean±SD) | 39.30±15.79 |
| Donor Race Caucasian Asian Black Other |
82.77% 2.32% 14.04% 0.88% |
|
| Donor Blood Type A AB B O |
36.71% 3.66% 12.15% 47.49% |
|
| Donor BMI (mean±SD) | 27.46±6.69 | |
| Donor BUN in mg/dL (mean±SD) Missing |
16.42±11.04 0.54% |
|
| Donor Cause of Death Anoxia Cerebrovascular/Stroke Head Trauma Central Nervous System Tumor Other |
23.89% 35.79% 37.12% 0.50% 2.70% |
|
| Donor Clinical Infection of the Lung Positive |
40.58% |
|
| Donor Creatinine (mean±SD) Missing |
1.14±1.02 3.12% |
|
| Donor Diuretics Positive Missing |
58.15% 0.17% |
|
| Donor Drug-Treated Systematic Hypertension Positive Missing |
20.43% 0.20% |
|
| Donor eGFR Missing |
91.68±39.35 3.12% |
|
| Donor Hematocrit (mean±SD) Missing |
31.29±5.86 0.11% |
|
| Donor Hepatitis C Virus Result Positive Unknown/Missing |
2.52% 0.03% |
|
| Donor History of Diabetes Positive Unknown |
7.46% 0.48% |
|
| Donor INR (mean±SD) Missing |
1.39±1.36 1.63% |
|
| Donor KDRI (mean±SD) Missing |
1.26±0.40 3.96% |
|
| Kidney Transplanted Locally | 75.32% | |
| Kidney Pumped | 23.57% | |
| Donor Tattoos Yes Missing |
30.19% 0.40% |
|
| Donor- Recipient |
HLA B Mismatches 0 1 2 |
12.98% 23.71% 63.30% |
| HLA DR Mismatches 0 1 2 |
21.91% 44.16% 33.93% |
|
| Recipient | Recipient Age at Transplant (mean±SD) | 52.74±13.12 |
| Latino | 14.71% | |
| Recipient Race Caucasian Asian Black Other |
59.19% 6.00% 33.09% 1.72% |
|
| BMI (mean±SD) Missing |
28.19±5.57 3.50% |
|
| Diabetes Type 1 Type 2 Other |
4.55% 26.00% 5.16% |
|
| Education Grade School/None High School Technical/Some College College Unknown/Missing |
6.62% 41.50% 21.91% 19.10% 10.87% |
|
| Gender Male Female |
60.57% 39.43% |
|
| HBV Core Antibody Positive Unknown/Missing |
8.43% 14.83% |
|
| HCV Serostatus Positive Unknown/Missing |
5.69% 4.21% |
|
| Had Previous Malignancy Positive Unknown/Missing |
5.17% 2.58% |
|
| Had Previous Transplant | 14.22% | |
| Kidney Cold Ischemia Time in Hours (mean±SD) Missing |
17.84±9.46 2.64% |
|
| Kidney Primary Diagnosis at Transplant Congenital, Rare Familial, & Metabolic Disorders Diabetes Glomerular Diseases Hypertensive Nephrosclerosis Unknown/Other |
1.78% 27.47% 22.84% 28.32% 19.60% |
|
| Most Recent CPRA (mean±SD) Missing |
15.35±28.78 6.19% |
|
| Peripheral Vascular Disease Positive Unknown |
4.38% 3.51% |
|
| Primary Payment Public Private/Other/Unknown |
73.75% 26.25% |
|
| Procedure Type Left Kidney Right Kidney |
47.03% 52.97% |
|
| Total ESRD Time at Transplant in Days (mean±SD) | 1549.52±1124.83 | |
| Total Serum Albumin at Listing (mean±SD) Missing |
3.91±0.58 15.99% |
|
| Karnofsky Score at Transplant 10–30 40–50 60–70 80–90 100 Unknown |
0.16% 3.31% 23.47% 54.63% 14.77% 3.66% |
|
| Death within 3 years | 9.16% (4879) |
BMI: body mass index
BUN: blood urea nitrogen
CPRA: calculated panel reactive antibodies
eGFR: estimated glomerular filtration rate
ESRD: end-stage renal disese
HLA: human leukocyte antigens
HBV: hepatitis B virus
HCV: hepatitis C virus
INR: international normalized ratio
SD: standard deviation
Table 4:
Number of deaths in 1 year and 3 years after transplant for each KPS strata
| Karnofsky Performance Score at Transplant |
Number of Deaths in One Year after Transplant Among Patients Transplanted between 2007/01/01- 2014/09/02 |
Number of Deaths in Three Years after Transplant Among Patients Transplanted between 2007/01/01–2012/09/02 |
|---|---|---|
| 10–30 40–50 60–70 80–90 100 Unknown |
54 (1.95%) 163 (5.89%) 805 (29.10%) 1355 (48.99%) 283 (10.23%) 106 (3.83%) |
47 (0.96%) 257 (5.27%) 1350 (27.67%) 2497 (51.18%) 564 (11.56%) 164 (3.36%) |
| Total | 2766 | 4879 |
One-Year Patient Survival
Table 5 summarizes the statistical results for the SRTR model and the augmented model for the 1-year survival dataset. After including KPS into the SRTR model, the augmented model’s c statistics improved by 0.01. Although incremental, the increase was statistically significant (P < 0.001) according to the paired t test. Furthermore, KPS improved model calibration since the augmented model’s average log-likelihoods using the original coefficients and the pooled coefficients were higher than the SRTR model’s average log-likelihoods. By the likelihood ratio test, the improvement in model calibration was statistically significant (P <0.001), indicating that KPS was a significant predictor of 1-year posttransplant survival. KPS also improved the model’s predictive accuracy as indicated by the decrease in AIC and BIC. The differences in AICs and BICs between the SRTR and the augmented models were significant (P<0.001 for both). More interestingly, because BIC penalized against models with large number of variables, the significant improvement in BIC indicated that including KPS still strengthened the predictive accuracy of the model. Table 6 confirmed specifically that KPS 10–30, KPS 80–90, and KPS 100 were independent predictors of 1-year posttransplant survival. More importantly, it showed that KPS 10–30 has an extremely high hazard ratio (HR) of 11.25, whereas KPS 40–50 has HR 1.28 and the other strata have HR < 1.00.
Table 5:
Statistical results for standard and augmented 1-year posttransplant survival models for DDKT recipients
| Average Log- Likelihood |
Average Pooled Log- Likelihood |
Likelihood Ratio Test P value |
Average Pooled AIC |
Pooled AIC Paired t Test P value |
Average Pooled BIC |
Pooled BIC Paired t Test P value |
Average Pooled c Statistic |
Pooled c Statistic Paired t Test P value |
|
|---|---|---|---|---|---|---|---|---|---|
| Standard: SRTR |
−30191.78 | −30191.96 | 60477.91 | 60756.39 | 0.700 | ||||
| Augmented: SRTR + KPS |
−30061.53 | −30061.71 | <0.001 | 60227.41 | <0.001 | 60535.52 | <0.001 | 0.710 | <0.001 |
Table 6:
Pooled coefficients and hazard ratios of KPS from augmented 1-year posttransplant survival model
| Functional Status | Coefficient | 95% Confidence Interval |
Hazard Ratio |
P Value |
|---|---|---|---|---|
| Unknown 10–30 40–50 60–70 80–90 100 |
Reference 2.42 2.44E-01 −2.06E-01 −3.64E-01 −4.99E-01 |
(2.09, 2.76) (−3.60E-03, 4.91E-01) (−4.12E-01, −1.02E-03) (−5.64E-01, −1.63E-01) (−7.24E-01, −2.73E-01) |
11.25 1.28 0.81 0.69 0.61 |
<0.001 0.05 0.05 <0.001 <0.001 |
Table 7 shows the P values for the pairwise log-rank tests between the KPS strata. The survival differences between any pair of KPS strata are statistically significant (P <0.05 for all pairs). Validating these differences, Figure 1 shows the adjusted expected 1-year survival curves conditional on each KPS stratum at transplant and their 95% confidence intervals estimated using the augmented model with pooled coefficients. According to the figure, DDKT recipients with KPS scores 10–30 have the worst survival with their 1-year expected survival probabilities being below 65%. However, KPS 40–50 recipients have significantly better expected survival with their expected 1-year survival probabilities being approximately 95%. As KPS score increases, the survival probabilities improve.
Table 7:
P values of pairwise log-rank tests between KPS strata for 1-year posttransplant survival data
| KPS 40–50 | KPS 60–70 | KPS 80–90 | KPS 100 | |
|---|---|---|---|---|
| KPS 10–30 |
< 0.001 | <0.001 | <0.001 | <0.001 |
| KPS 40–50 |
<0.001 | <0.001 | <0.001 | |
| KPS 60–70 |
<0.001 | <0.001 | ||
| KPS 80–90 |
0.002 |
KPS – Karnofsky Performance Score
Figure 1:
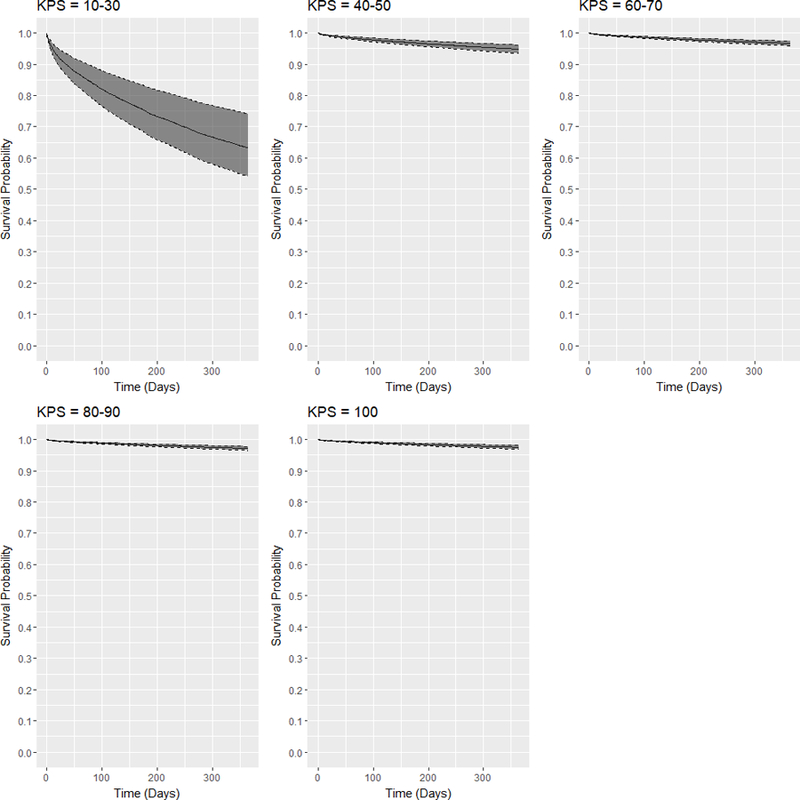
Adjusted expected 1-year posttransplant survival curves and 95% confidence intervals for DDKT recipients of each KPS stratum at transplant.
Figure 2 shows the families of adjusted expected survival curves conditioned on KDRI and on each KPS stratum at transplant. In the figure, KDRI was converted to kidney donor profile index (KDPI)30, where each integer value from 0–100 corresponds to a range of KDRI values. The figure shows that KPS 10–30 recipients have the largest range in expected 1-year survival probability from approximately 76% with the best quality kidneys (KDPI = 0) to approximately 26% with the worst quality kidneys (KDPI = 100). Even if KPS 10–30 recipients were transplanted with the best quality kidneys, their expected survival probabilities are still worse than the probabilities of KPS 40–50 recipients transplanted with the worst quality kidneys. For DDKT recipients with KPS 40–50, their expected 1-year survival probabilities range from above 95% to approximately 85%. Transplanted with KDPI ≤ 99 kidneys, DDKT recipients are expected to live with above 90% survival probability in 1 year. As KPS improves, the minimum and maximum expected 1-year survival probabilities increase and the ranges become narrower.
Figure 2:
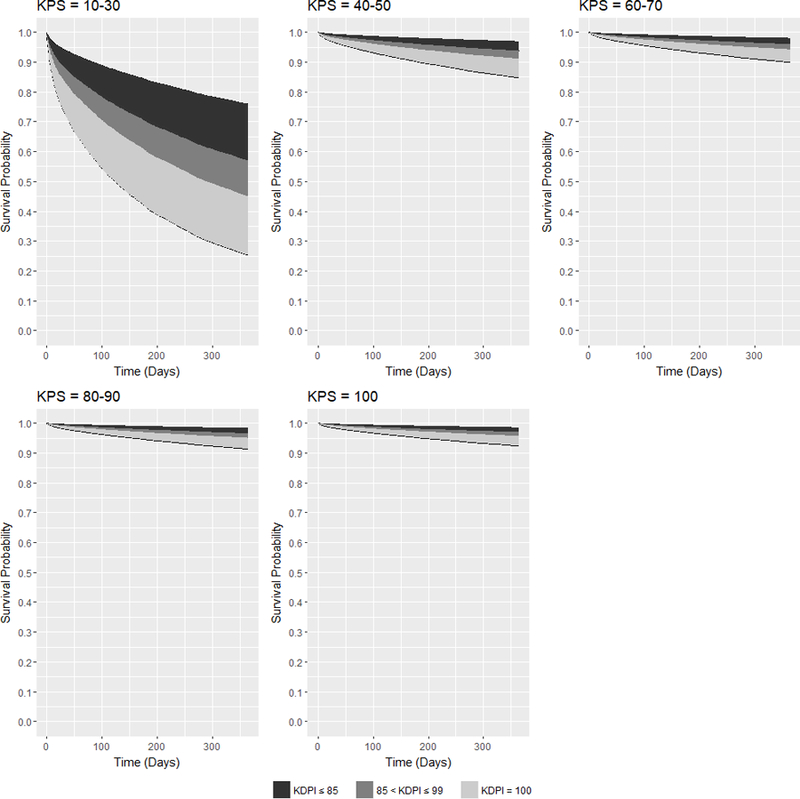
Families of adjusted expected 1-year posttransplant survival curves conditional on KDPI for DDKT recipients of each KPS stratum at transplant.
Three-Year Patient Survival
Table 8 summarizes the statistical results for the SRTR model and the augmented model with the 5 additional KPS indicator covariates for the 3-year survival dataset. Including KPS in the 3-year survival model showed similar improvements as it did for the 1-year survival model. The c statistic of the augmented model improved by 0.005, but paired t test verified its statistical significance (P < 0.001). Despite the incremental improvement in model discrimination, KPS was validated to be a significant predictor of 3-year posttransplant survival. By likelihood ratio test, model calibration improved statistically significantly (P <0.001). Furthermore, predictive accuracy improved as conveyed by the lower AIC and BIC values after including KPS. Improvements in AIC and BIC values were again statistically significant (P < 0.001 for both). Table 9 confirmed specifically that KPS 10–30, KPS 40–50, and KPS 100 are independent predictors of 3-year posttransplant survival. KPS 10–30 has an extremely high HR being 10.38, whereas the other KPS strata have HR < 1.5.
Table 8:
Statistical results for standard and augmented 3-year posttransplant survival models for DDKT recipients
| Average Log- Likelihood |
Average Pooled Log- Likelihood |
Likelihood Ratio Test P value |
Average Pooled AIC |
Pooled AIC Paired t Test P value |
Average Pooled BIC |
Pooled BIC Paired t Test P value |
Average Pooled c Statistic |
Pooled c Statistic Paired t Test P value |
|
|---|---|---|---|---|---|---|---|---|---|
| Standard: SRTR |
−51541.84 | −51541.98 | 103206.0 | 103602.0 | 0.702 | ||||
| Augmented: SRTR + KPS |
−51436.46 | −51436.61 | <0.001 | 103005.2 | <0.001 | 103433.7 | <0.001 | 0.707 | <0.001 |
Table 9:
Pooled coefficients and hazard ratios of KPS from augmented 3-year posttransplant survival model
| Functional Status | Coefficient | 95% Confidence Interval |
Hazard Ratio |
P Value |
|---|---|---|---|---|
| Unknown 10–30 40–50 60–70 80–90 100 |
Reference 2.34 3.95E-01 1.38E-01 −3.08E-02 −1.84E-01 |
(2.01, 2.66) (1.98E-01, 5.93E-01) (−2.58E-02, 3.01E-01) (−1.90E-01, 1.28E-01) (−3.60E-01, −8.79E-03) |
10.38 1.48 1.15 0.97 0.83 |
<0.001 <0.001 0.1 0.7 0.04 |
Table 10 shows the P values for the pairwise log-rank tests between the KPS strata. The survival differences between any pair of KPS strata are statistically significant (P <0.05 for all pairs). Validating these differences, Figure 3 shows the adjusted expected 3-year survival curves conditional on each KPS stratum at transplant and their 95% confidence intervals estimated using the augmented model with pooled coefficients. KPS 10–30 recipients have the worst expected survival probabilities in 3 years, significantly decreasing to below 50% from after 1 year. In the next KPS stratum 40–50, although the expected survival probabilities decrease over time, the rate of decrease in expected survival probabilities is not as severe as for the KPS stratum 10–30. The expected 3-year survival probabilities remain above 85%. As for the other strata, the expected 3-year survival probabilities are above 90%.
Table 10:
P values of pairwise log-rank tests between KPS strata for 3-year posttransplant survival data
| KPS 40–50 | KPS 60–70 | KPS 80–90 | KPS 100 | |
|---|---|---|---|---|
| KPS 10–30 |
< 0.001 | <0.001 | <0.001 | <0.001 |
| KPS 40–50 |
<0.001 | <0.001 | <0.001 | |
| KPS 60–70 |
<0.001 | <0.001 | ||
| KPS 80–90 |
<0.001 |
Figure 3:
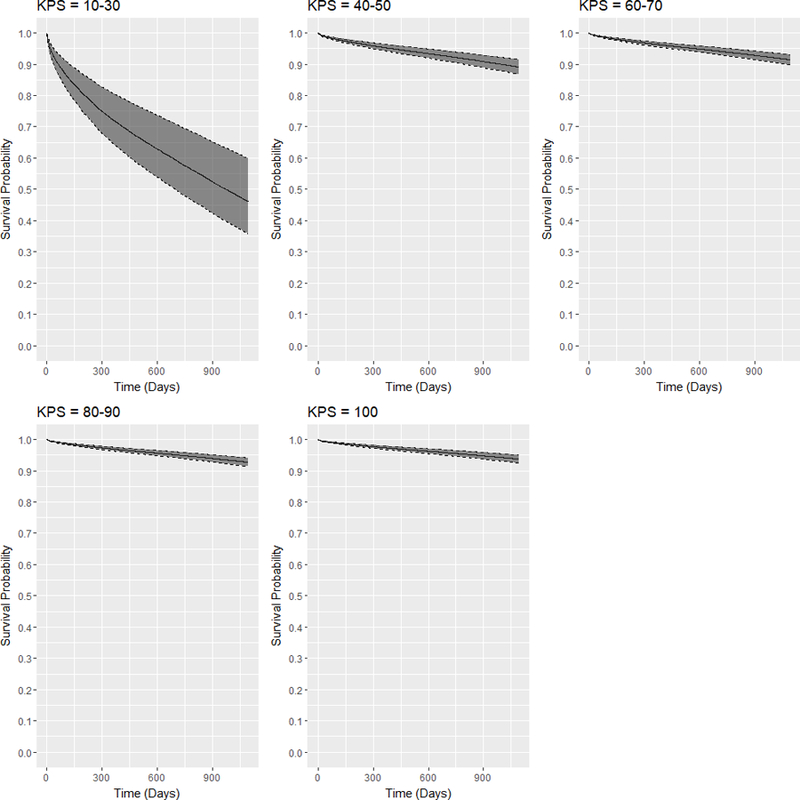
Adjusted expected 3-year posttransplant survival curves and 95% confidence intervals for DDKT recipients of each KPS stratum at transplant.
Figure 4 shows the families of adjusted expected 3-year survival curves conditional on KDRI and on each KPS stratum at transplant. Like in Figure 2, KDRI was converted to KDPI. With the best kidney quality (KDPI = 0), DDKT recipients with KPS 10–30 have at most 60% expected 3-year survival probabilities. In the next stratum KPS 40–50, DDKT recipients’ expected survival probabilities are much better because with the worst quality kidney (KDPI = 100), their expected survival probabilities are as low as 75%. However, if they were transplanted with KDPI ≤ 99 kidneys, their expected survival probabilities are greater than 80%. As for the other strata, expected 3-year survival probabilities are above 80%.
Figure 4:
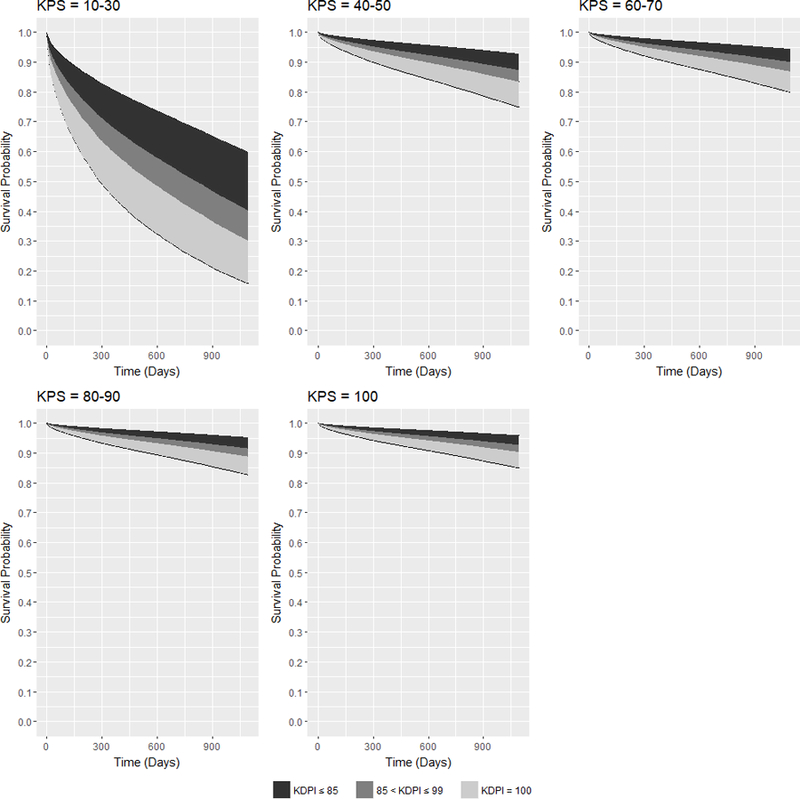
Families of adjusted expected 3-year posttransplant survival curves conditional on KDPI for DDKT recipients of each KPS stratum at transplant.
Discussion
Previous studies have attempted to improve posttransplant graft/patient survival models for DDKT recipients by including additional covariates such as comorbidities not considered by the Scientific Registry of Transplant Recipients (SRTR). Jassal et al31 and Grosso et al32 included comorbidities indices into their risk adjustment models and reported that Charlson Comorbidity Index was a significant independent predictor of kidney posttransplant mortality. Wu et al33 confirmed that the Charlson Comorbidity Index was correlated with graft loss and patient death after renal transplantation. Weinhandl et al34 gathered a list of 31 comorbid conditions, which includes the Elixhauser conditions35 and cardiac arrhythmias, from CMS and incorporated them in the risk-adjustment Cox models for graft survivals trained on 1992–2005 data. Pelletier et al36 focused on incorporating cardiovascular comorbidities and demonstrated improvement in the 1-year posttransplant model for kidney graft survival. Unfortunately, the comorbidity indices and most of the comorbid conditions are unavailable in both the SRTR and OPTN dataset. An alternative covariate that summarizes the overall impact of comorbidities onto a recipient’s health is functional status, which measures the patient’s ability to perform normal daily activities and to maintain health and well-being37.
Kutner et al38 and Reese et al39,40 studied the impact of functional status on posttransplant survival for kidney transplant recipients. In their statistical analyses, functional status was recorded using the Physical Functioning (PF) domain of the Medical Outcomes Study 36-Item Short Form Health Survey41, which was self-reported by the patient instead of physician-reported. Furthermore, the PF scores used in the statistical analyses may not be up-to-date as they were recorded a few months before transplant. Despite these limitations, the authors demonstrated that PF was an independent predictor of posttransplant survival for kidney transplant recipients. However, oPF scores are unavailable in the SRTR and OPTN data.
Unlike the PF scores, KPS is physician-reported and thus more reliable. It was validated by Mor et al42 as an accurate measure for functional status. KPS has been examined as an independent predictor for posttransplant survival for recipients of other solid organs. For example, Dolgin et al stratified KPS into 3 strata and demonstrated that it was a reliable predictor of posttransplant mortality for liver transplant recipients43. Grimm et al examined the impact of KPS onto posttransplant survival outcomes for lung transplant recipients and found it to be a significant independent predictor for 1-year mortality44. In addition, Kilic et al assessed that KPS was a significant predictor of posttransplant survival outcomes for lung retransplant recipients45. Contributing to the KPS literature in transplantation, this study examined the impact of KPS on posttransplant survival outcomes for DDKT recipients and determined whether KPS could help make better transplantation decisions.
According to the cohort analysis, only about 3% of transplant recipients had KPS 40–50 at the time of transplant. This proportion is comparable to the proportion of KPS at listing from April 1, 2005 (the earliest date when KPS was recorded in SRTR46) to September 2, 2014, which is summarized in Table 11. The small proportion suggested that the transplant centers may be risk averse in listing and even transplanting KPS 40–50 patients, but the survival analyses demonstrated that they have a high probability of surviving at least 3 years, even if they were transplanted with low-quality kidneys. Based on the survival curves in Figures 2 and 4, the differences in survival probabilities between recipients with KPS 10–30 and KPS 40–50 could be as large as 50% for 1 year and 60% for 3 years if they were transplanted with the same quality kidneys. On the other hand, the differences in survival probabilities between recipients with KPS 40–50 and other higher KPS stratum were less than 10% for both 1 year and 3 years if transplanted with the same quality kidneys. Although transplanting a KPS 40–50 candidate is riskier than a higher KPS candidate, it is not as severe as transplanting a candidate with KPS 10–30. If more KPS 40–50 patients were listed and transplanted, KPS 10–30 patients could be encouraged to undergo prehabilitation to improve their functional statuses, potentially improving their accessibility to DDKT and bettering their posttransplant survival outcomes.
Table 11:
Proportion of KPS among waitlisted candidates from April 1, 2005-September 2, 2014
| Karnofsky Score at Listing | N=311325 |
|---|---|
| 10–30 | 0.77% |
| 40–50 | 2.62% |
| 60–70 | 22.63% |
| 80–90 | 52.60% |
| 100 | 14.36% |
| Unknown | 7.02% |
For KPS 40–100 recipients, being transplanted with a low-quality kidney can still yield a high survival probability. According to Figures 2 and 4, recipients transplanted with KDPI ≤ 99 kidneys have survival probabilities ≥ 90% for 1 year and ≥ 80% for 3 years. These results could dispel concerns that being transplanted with low-quality kidneys result in low survival probabilities for KPS 40–100 candidates. Among the waitlisted candidates, the willingness to accept a kidney with KDPI > 85 was at 49.9% in 2014, but it decreased to 45.7% in 20162. This behavior may have attributed to the high discard rate of low-quality kidneys. Nearly 60% of kidneys with KDPI > 85 were discarded in 20162, yet these discarded kidneys could have been transplanted to viable candidates, such as those with KPS 40–50.
This study has some limitations. For example, KPS is susceptible to observer bias and its evaluation can vary within and across transplant centers. The second limitation is the missing values in the SRTR data. Finally, KPS was originally developed for oncology patients and it may not be entirely applicable to ESRD patients because disease symptoms and progression differ between them.
Despite these limitations, this study reveals the potential utility of functional status in making decisions of transplanting patients given their physical health and their offered kidney qualities. It shows that functional status provides a more objective measure than the “eyeball test” that may have discriminated against older patients from being listed and transplanted47,48. Furthermore, it contributes to the growing literature that justify functional status as a potential predictor of postsurgical survival and recovery outcomes. Robinson et al demonstrated that functional independence measured using the Katz Index of independence was a significant predictor of 6-month postoperative mortality in elderly patients49. Lawrence et al showed that better functional status nearly always predicted better recovery in shorter time after major abdominal surgery for elderly patients50. In a related study, Broquet et al showed that mobility and physical function for daily living activities were independent predictors of postoperative delirium for elderly patients who underwent major abdominal surgery51. In liver transplantation within the United Kingdom and Ireland patient population, Jacob et al found that functional status measured by ECOG had an impact on posttransplant outcome52.
To refine the analyses of this study, KPS’s recording requires standardization within and across transplant centers and validation of interrater reliability in the ESRD setting, as it has been done for palliative care53,54 and oncology55,56. However, if a more reliable functional status measure, especially for the renal setting, were developed, then similar analyses using this alternative measure can still be performed and be used to assist in making transplantation decisions. Overall, this study encourages further investigation of the use of functional status to help improve current transplant center strategies in transplanting more ESRD patients, maximizing listed patients’ survival outcomes, and reducing the kidney discard rate.
Supplementary Material
Acknowledgments
This research was supported in part through the computational resources and staff contributions provided for the Quest high performance computing facility at Northwestern University, which is jointly supported by the Office of the Provost, the Office for Research, and Northwestern University Information Technology.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
This work is funded by National Institutes for Health award 1R21DK108104-01.
Abbreviations
- AIC
Akaike information criterion
- BIC
Bayesian information criterion
- BMI
body mass index
- BUN
blood urea nitrogen
- CMS
Centers for Medicare and Medicaid Services
- CPRA
calculated panel reactive antibodies
- DDKT
deceased-donor kidney transplant
- ECOG
Eastern Cooperative Oncology Group
- eGFR
estimated glomerular filtration rate
- EPV
number of events per independent variables
- ESRD
end-stage renal disease
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HLA
human leukocyte antigens
- HR
hazard ratio
- INR
international normalized ratio
- KDPI
kidney donor profile index
- KDRI
kidney donor risk index
- KPS
Karnofsky Performance Score
- MICE
multiple imputation by chained equations
- MPSC
Membership and Professional Standards Committee
- OPTN
Organ Procurement and Transplantation Network
- PF
Physical Functioning
- SD
standard deviation
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Authors have no conflicts of interest to disclose.
Developed research design, conducted data analysis, and drafted the article
Reviewed research design and edited article
Reviewed and edited article
Developed research design, supervised data analysis, and edited the article
References
- 1.HRSA. Organ Procurement and Transplantation Network Data Reports https://optn.transplant.hrsa.gov/data/. Published 2018.
- 2.Hart A, Smith J, Skeans M, et al. OPTN/SRTR 2016 Annual Data Report: Kidney. Am J Transplant 2018;18(S1):18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In MacLeod CM, ed. Evaluation of chemotherapeutic agents New York: Columbia University Press; 1949:191–205. [Google Scholar]
- 4.Perez Valdivieso JR, Bes-Rastrollo M, Monedero P, De Irala J, Lavilla FJ. Karnofsky performance score in acute renal failure as a predictor of short‐term survival. Nephrology (Carlton) 2007;12:533–538. [DOI] [PubMed] [Google Scholar]
- 5.McClellan WM, Anson C, Birkeli K, Tuttle E. Functional status and quality of life: Predictors of early mortality among patients entering treatment for end stage renal disease. J Clinl Epidemiol 1991;44:83–89. [DOI] [PubMed] [Google Scholar]
- 6.Ifudu O, Paul HR, Homel P, Friedman EA. Predictive value of functional status for mortality in patients on maintenance hemodialysis. Am J Nephrol 1998;18:109–116. [DOI] [PubMed] [Google Scholar]
- 7.Okada T, Nakao T. Physical functional status and factors contributing to disability in Japanese chronic dialysis patients. Nephrology 1998;4:195–203. [Google Scholar]
- 8.Bossola M, Marino C, Di Napoli A, et al. Functional impairment and risk of mortality in patients on chronic hemodialysis: results of the lazio dialysis registry. J Nephrol 2018:31:593–602. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson D, Shearon T, O’keefe J, et al. SRTR Center‐Specific Reporting Tools: Posttransplant Outcomes. Am J Transplant 2006;6:1198–1211. [DOI] [PubMed] [Google Scholar]
- 10.Snyder JJ, Salkowski N, Kim SJ, et al. Developing statistical models to assess transplant outcomes using national registries: the process in the United States. Transplantation 2016;100:288–294. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Centers for Medicare & Medicaid Services. CMS Conditions of Participation https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Transplant.html. Accessed April 13, 2018.
- 12.Pelletier RP, Henry ML. Program Specific Reports: Friend or Foe?—The Intended and Unintended Consequences of Scientific Registry of Transplant Recipient Program Specific Reports. Curr Transplant Rep 2014;1:86–90. [Google Scholar]
- 13.Schold JD, Arrington CJ, Levine G. Significant alterations in reported clinical practice associated with increased oversight of organ transplant center performance. Prog Transplant 2010;20:279–287. [DOI] [PubMed] [Google Scholar]
- 14.Schold JD, Buccini L, Srinivas T, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant 2013;13:67–75. [DOI] [PubMed] [Google Scholar]
- 15.SRTR. SRTR Risk Adjustment Model Documentation: Waitlist and Posttransplant Outcomes https://www.srtr.org/reports-tools/risk-adjustment-models-posttransplant-outcomes/.
- 16.Massie AB, Kuricka L, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 19.Ma C, Bandukwala S, Burman D, et al. Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer 2010;46:3175–3183. [DOI] [PubMed] [Google Scholar]
- 20.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–68. [Google Scholar]
- 21.Team RC. R: A Language and Environment for Statistical Computing https://www.R-project.org. Pubished 2018.
- 22.Therneau TM, Lumley T. Package ‘survival’. R Package Version 2017:2:41–43. [Google Scholar]
- 23.Collett D Modelling survival data in medical research CRC press; 2015. [Google Scholar]
- 24.Volinsky CT, Raftery AE. Bayesian information criterion for censored survival models. Biometrics 2000;56(1):256–262. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 26.Meng X- L, Rubin DB. Performing likelihood ratio tests with multiply-imputed data sets. Biometrika 1992;79(1):103–111. [Google Scholar]
- 27.Van Buuren S Flexible imputation of missing data New York: CRC press; 2012. [Google Scholar]
- 28.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–1510. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC, Allignol A, Fine JP. The number of primary events per variable affects estimation of the subdistribution hazard competing risks model. J Clin Epidemiol 2017;83:75–84. [DOI] [PubMed] [Google Scholar]
- 30.OPTN, UNOS. KDRI to KDPI Mapping Table https://optn.transplant.hrsa.gov/media/2150/kdpi_mapping_table.pdf. Updated March 9, 2018.
- 31.Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis 2005;46:136–142. [DOI] [PubMed] [Google Scholar]
- 32.Grosso G, Corona D, Mistretta A, et al. Predictive value of the Charlson comorbidity index in kidney transplantation. Paper presented at: Transplantation proceedings 2012. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Evans I, Joseph R, et al. Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol 2005;16:3437–3444. [DOI] [PubMed] [Google Scholar]
- 34.Weinhandl E, Snyder J, Israni A, Kasiske B. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant 2009;9:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier RP, Phillips GS, Rajab A, Pesavento TE, Henry ML. Effects of cardiovascular comorbidity adjustment on SRTR risk-adjusted cox proportional hazard models of graft survival. Transplantation 2014;97:686–693. [DOI] [PubMed] [Google Scholar]
- 37.Leidy NK. Functional status and the forward progress of merry-go-rounds: toward a coherent analytical framework. Nurs Res 1994;43:196–202. [PubMed] [Google Scholar]
- 38.Kutner NG, Zhang R, Bowles T, Painter P. Pretransplant physical functioning and kidney patients’ risk for posttransplantation hospitalization/death: evidence from a national cohort. Clin Jo Am Soc Nephrol 2006;1:837–843. [DOI] [PubMed] [Google Scholar]
- 39.Reese PP, Bloom RD, Shults J, et al. Functional status and survival after kidney transplantation–. Transplantation 2014;97:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reese PP, Shults J, Bloom RD, et al. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kid Dis 2015;66:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 42.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer 1984;53:2002–2007. [DOI] [PubMed] [Google Scholar]
- 43.Dolgin NH, Martins PN, Movahedi B, Lapane KL, Anderson FA, Bozorgzadeh A. Functional status predicts postoperative mortality after liver transplantation. Clin Transplant 2016;30:1403–1410. [DOI] [PubMed] [Google Scholar]
- 44.Grimm JC, Valero V, Kilic A, et al. Preoperative performance status impacts perioperative morbidity and mortality after lung transplantation. Ann Thorac Surg 2015;99:482–489. [DOI] [PubMed] [Google Scholar]
- 45.Kilic A, Beaty CA, Merlo CA, Conte JV, Shah AS. Functional status is highly predictive of outcomes after redo lung transplantation: an analysis of 390 cases in the modern era. Ann Thorac Surg 2013;96:1804–1811. [DOI] [PubMed] [Google Scholar]
- 46.Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segev DL, Kucirka LM, Oberai PC, et al. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol 2009;20:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abecassis M, Bridges N, Clancy C, et al. Solid‐Organ Transplantation in Older Adults: Current Status and Future Research. Am J Transplant 2012;12:2608–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009;250:449–455. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg 2004;199:762–772. [DOI] [PubMed] [Google Scholar]
- 51.Brouquet A, Cudennec T, Benoist S, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg 2010;251:759–765. [DOI] [PubMed] [Google Scholar]
- 52.Jacob M, Copley LP, Lewsey JD, Gimson A, Rela M, van der Meulen JH. Functional status of patients before liver transplantation as a predictor of posttransplant mortality. Transplantation 2005;80:52–57. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann C, Burman D, Bandukwala S, et al. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support Care Cancer 2010;18:609–616. [DOI] [PubMed] [Google Scholar]
- 54.Myers J, Gardiner K, Harris K, et al. Evaluating correlation and interrater reliability for four performance scales in the palliative care setting. J Pain Symptom Manage 2010;39:250–258. [DOI] [PubMed] [Google Scholar]
- 55.Schag CC, Heinrich RL, Ganz P. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–193. [DOI] [PubMed] [Google Scholar]
- 56.Liem BJ, Holland JM, Kang MY, Hoffelt SC, Marquez CM. Karnofsky performance status assessment: resident versus attending. J Cancer Ed 2002;17:138–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


