Abstract
Heme is an essential cofactor and signaling molecule required for virtually all aerobic life. However, excess heme is cytotoxic. Therefore, heme must be safely transported and trafficked from the site of synthesis in the mitochondria or uptake at the cell surface, to hemoproteins in most subcellular compartments. While heme synthesis and degradation are relatively well characterized, little is known about how heme is trafficked and transported throughout the cell. Herein, we review eukaryotic heme transport, trafficking, and mobilization, with a focus on factors that regulate bioavailable heme. We also highlight the role of gasotransmitters and small molecules in heme mobilization and bioavailability, and heme trafficking at the host-pathogen interface.
Keywords: heme, heme trafficking, heme transport, nitric oxide, hydrogen peroxide, iron, host-pathogen interactions
Graphical abstract
Introduction.
Heme (iron protoporphyrin IX, heme b, or protoheme) is an essential but potentially toxic iron containing cofactor and signaling molecule. In mammals, heme constitutes the most abundant form of iron due to its requirement as a cofactor for the oxygen (O2) carrier hemoglobin (Hb). Indeed, heme iron constitutes 95% of the total iron quota in humans,[1] typically 3–4 g of iron, and erythrocytes accumulate as much as ~80 mM heme or ~5×109 heme molecules per cell.[2] Beyond the requirement for heme in O2 transport, virtually every cell and organism on the planet that metabolizes oxygen requires heme for viability. The Lewis acidity, redox activity, and hydrophobicity of heme makes it essential for many biological functions, including as a cofactor that enables electron transfer, catalysis, and gas sensing and transport, and as a signaling molecule in the regulation of transcription factors, ion channels, kinases, and cell surface receptors.[3, 4] However, the very same properties that make heme essential also render it toxic to the cell. Indeed, heme can inappropriately bind to various biomolecules and alter their structure and function[5, 6] and can catalyze the formation of deleterious reactive oxygen species (ROS).[7, 8] Therefore, the concentration and bioavailability of heme must be tightly controlled. Whether heme is synthesized endogenously or acquired from exogenous sources, it must be safely transported and trafficked to hemoproteins located in virtually every subcellular compartment, including the nucleus, ER, golgi, mitochondria, cytosol, and peroxisomes. Given that hemoproteins span a continuum between being soluble, membrane bound or extracellular, and reside in different pH and redox environments, heme trafficking mechanisms must be attuned to and compatible with diverse biological locations. Moreover, since cells can export and traffic heme between cells in metazoans,[9, 10] there must be mechanisms in place to safely share and regulate this nutrient on a systemic level. In the context of hostpathogen interactions and complex microbial communities,[11, 12] cells must even steal heme to ensure survival in resource-limited settings, necessitating that heme acquisition factors are constantly interacting with and competing against rival species. Despite the tremendous importance of heme transport and trafficking for aerobic life, the molecules and mechanisms underlying intra- and inter-cellular heme mobilization and its dynamics are poorly understood.
Herein, we review the current state of knowledge on eukaryotic heme transport, trafficking, and dynamics, including its import and export from cells, as well as highlight heme homeostatic mechanisms of bacterial and fungal human pathogens that interact with host heme. We begin by describing conceptual paradigms for heme transport and trafficking, review the factors that dictate heme concentration and bioavailability, with a focus on the latter, discuss heme trafficking dynamics and the molecules that may mobilize heme, and consider heme homeostatic mechanisms in human pathogens in order to highlight heme trafficking at the inter-species host-pathogen interface. Altogether, we aim to provide a comprehensive snapshot of the latest advances on the mechanisms underlying the mobilization of heme and highlight open questions.
Conceptual paradigm for heme trafficking and mobilization.
In order to mitigate heme toxicity, cells carefully control the concentration and bioavailability of heme. Heme concentration is regulated by its biosynthesis and degradation, which as discussed below are relatively well-understood processes. Indeed, the mechanisms and atomic resolution structures of all eight enzymes of the heme biosynthetic pathway and the heme-degrading enzyme, heme oxygenase, are known.[3, 4, 13] On the other hand, the molecules and mechanisms that control heme availability are poorly understood.[3, 4, 13] The speciation of heme in complex biological matrices, including in cells and tissues, is important for understanding its transport, trafficking, and mobilization. For reference, “free” or aqueous heme at neutral pH is 5coordinate, with iron coordinated by the four tetrapyrrolic nitrogens, and a 5th water or hydroxo axial ligand.[14] Heme can readily form back-to-back π-π dimers, with dimerization dissociation constants, KDDimer, on the order of 500 nM over a pH range between 6.0 and 9.0.[14] The monomer-dimer equilibria of heme is responsible for the large observed variation in the reduction potential of the “free” heme Fe(III)/Fe(II) redox couple, determined to be as low as −220 mV vs. the normal hydrogen electrode (NHE) in concentrated heme solutions or as high as −50 mV vs. NHE in more dilute heme solutions.[15, 16] Given the reducing environment of the cell, EmCytosol ~ −320 mV vs. NHE,[17] and assuming heme equilibrates with the primary redox buffer in cells, the glutathione (GSH)/ glutathione disulfide (GSSG) system, both monomeric and dimeric “free” heme is expected to be in its reduced state. However, in the cellular milieu, the speciation of aqueous heme would deviate considerably due to the large compendium of biomolecules that can bind and buffer heme. As a consequence, a useful formalism for conceptualizing heme in biology is as the sum of exchange inert and labile complexes.[3, 4]
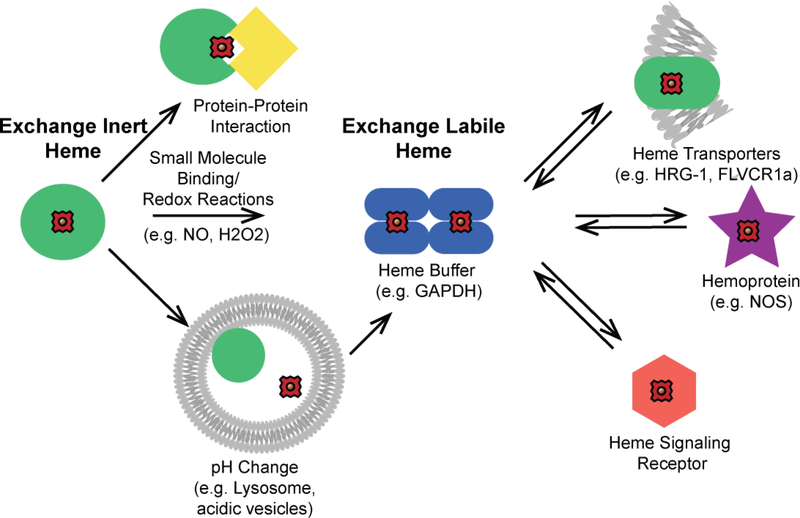
Exchange labile and inert heme corresponds to heme-complexes that can or cannot, respectively, exchange heme with other biomolecules. Exchange inert heme is bound in a non-dissociable manner and/or is kinetically inaccessible by other factors, while labile heme is kinetically accessible and/or dissociable. In this context, the mobilization of heme for intra- or inter-cellular trafficking and/or heme signaling can occur via two primary mechanisms: a. inducing the release or transfer of heme from a stable exchange inert heme-bound biomolecule or b. exchanging labile heme between various buffering factors and downstream clients such as transporters, trafficking factors, apo-hemoproteins, or other receptors down thermodynamic gradients (Figure 1). In terms of mechanism “a”, an otherwise exchange inert heme-complex may transfer heme to a specific client protein via protein-protein interactions that drive the release and transfer of heme. This would be analogous to metallochaperones discovered for iron, e.g. PCBP1,[18] or copper, e.g. CCS1,[19, 20] which specifically bind and deliver the metal to the iron storage protein ferritin or the copper enzyme Cu/Zn Superoxide Dismutase (SOD1), respectively. Alternatively, an exchange inert heme-complex may release its heme to the labile pool due to any number of molecular events that could cause heme dissociation, including protonation of heme coordinating His, Cys, or Tyr residues, oxidation of His or Cys heme binding residues by H2O2 and other ROS, binding of gaseous molecules like NO or H2S to the heme iron that could weaken its interactions with coordinating ligands, allosteric interactions that cause changes in protein structure that weaken the heme affinity, proteolysis, or conditions that promote protein unfolding. In the context of heme signaling, the intact exchange inert hemecomplex itself could be the substrate for a receptor that controls heme-regulated processes.
Figure 1.
Conceptual model for the mobilization of exchange inert and labile heme. Heme is bound by biomolecules that are either exchange inert (green circles) or labile (blue ellipses). Exchange inert heme can be mobilized by specific protein-protein interactions that drive the transfer of heme or by post-translational modifications that can induce the release of heme, e.g. protonation of heme coordinating residues, allosteric regulation, or redox reactions at the heme iron center or amino acid side chains. Labile heme may exchange with downstream clients based on thermodynamic gradients, including with transporters (green ellipse), heme enzymes (purple star), and proteins that are targets of heme-based signaling (orange hexagon).
Trafficking or signaling in the context of exchange labile heme complexes would involve the transfer of heme between various buffering factors to client proteins with greater heme affinity down thermodynamic gradients. Further complicating matters is that exchange inert heme complexes can become labile through various covalent and non-covalent modifications, as previously discussed, or redox events at the heme iron center that lead to the dissociation of heme if the heme affinity of a given molecule is oxidation state dependent. Until recently, the concentration and physiological relevance of labile heme was largely unknown, but often invoked in discussions of heme homeostasis. Recent studies using enzymatic and fluorescent heme reporters in Baker’s yeast and various non-erythroid human cell lines have estimated cytosolic labile heme to be between ~25–300 nM,[21–23] which can be up to ~10% of the total cellular heme concentration. In the malaria parasite, Plasmodium falciparum, which hyperaccumulates heme in a food vacuole, cytosolic labile heme was estimated to be about one order of magnitude higher, ~1.6 μM, using a genetically encoded fluorescent heme sensor.[24] Moreover, it was recently determined that labile heme may be functionally significant. In Saccharomyces cerevisiae, sequestration of cytosolic labile heme due to expression of a high affinity hemoprotein, Cytochrome b562, resulted in the diminution of the activity of a nuclear heme dependent transcription factor, Hap1.[25] However, the nature (speciation, oxidation state, and localization) and dynamics of exchange labile heme pools are not well understood. In cell biological terms, labile heme must be accessed by buffering factors, transporters, chaperones, or lipid vesicles to promote the transport and trafficking of heme to hemoproteins throughout the cell. The identification of heme homeostatic factors have historically relied on biochemical approaches, e.g. identifying proteins that bind heme to hemin agarose or blue sepharose, or forward genetic screens to identify genes that contribute to a heme-related phenotype. [3, 4] More recently, new heme imaging platforms that provide a direct readout for bioavailable heme have been used to identify heme homeostatic factors in reverse genetic screens. [3, 4] The continued utilization of these approaches to identify the molecules that mediate heme transport and trafficking will help inform a better cell biological and biochemical understanding of heme homeostatic mechanisms.
Heme synthesis and degradation.
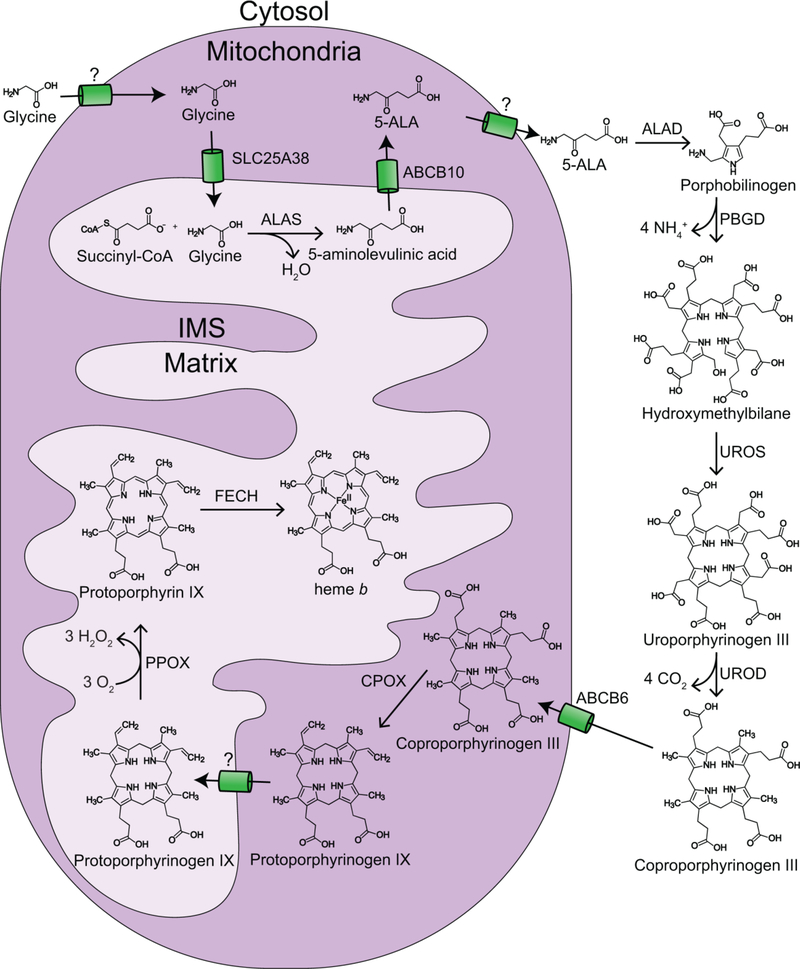
The synthesis and degradation of heme are often thought to be the primary means by which cells control the concentration of heme.[26] With few exceptions, all eukaryotes synthesize heme via the highly conserved Shemin pathway, which involves eight enzymatic steps that are partitioned between the mitochondria and cytosol (Figure 2).[27] The first and rate-limiting step of heme synthesis is the condensation of glycine and succinyl-CoA to form 5-aminolevulinic acid (5-ALA), which is catalyzed by ALA synthase (ALAS) in the mitochondrial matrix. 5-ALA is then transported from the matrix into the cytosol where four cytosolic enzymes convert eight molecules of 5-ALA to coproporphyrinogen III. In the first of the cytosolic biosynthesis steps, two 5-ALA molecules are condensed by ALA dehydratase (ALAD) to form porphobilinogen (PBG). Next, four PBG molecules are condensed into hydroxymethybilane via the cytosolic enzyme PBG deaminase (PBGD). Hydroxymethylbilane is then cyclized by uroporphyrinogen synthase (UROS) to make uroporphyrinogen III. In the final cytosolic biosynthesis step, uroporphyrinogen III is decarboxylated to form coproporphyrinogen III via uroporphyrinogen decarboxylase (UROD). In most eukaryotes, the last three heme synthesis steps from coproporphyrinogen III to heme occur in the mitochondria. Coproporphyrinogen III is trafficked to the intermembrane space (IMS) for oxidation to protoporphyrinogen IX via coproporphyrinogen oxidase (CPOX). CPOX is localized to the IMS in most eukaryotes requiring protoporphyrinogen IX to be transported across the inner mitochondrial membrane for the last two heme biosynthesis steps in the mitochondrial matrix. However, in yeast, CPOX is cytosolic, requiring protoporphyrinogen IX to be transported across both mitochondrial membranes into the matrix.[13] In the matrix, protoporphyrinogen IX is oxidized to protoporphyrin IX via protoporphyrinogen oxidase (PPOX). The final step of heme synthesis occurs on the matrix-side of the mitochondrial inner-membrane with the ferrochelatase (FECH) catalyzed insertion of ferrous iron into protoporphyrin IX. Eukaryotic heme synthesis and its regulation have been previously reviewed comprehensively in the literature[27–31].
Figure 2.
Heme synthesis in eukaryotes. Heme is synthesized in eukaryotes via eight highly conserved enzymes, four in the mitochondria and four in the cytoplasm. The final step of heme biosynthesis is the ferrochelatase catalyzed insertion of ferrous iron into protoporphyrin IX at the matrix side of the mitochondrial inner-membrane. ALAS = 5-aminolevulinic acid (5-ALA) synthase; ALAD = 5-ALA dehydratase; PBGD = porphobilinogen deaminase; UROS = uroporphyrinogen synthase; UROD = uroporphyrinogen decarboxylase; CPOX = coproporphyrinogen oxidase; PPOX = protoporphyrinogen oxidase; FECH = ferrochelatase.
In prokaryotes, it has more recently been discovered that heme synthesis occurs via three different pathways. Variations in the pathways occur during the initial synthesis of 5-ALA, and the final steps from uroporphyrinogen III to heme. In most metazoans, fungi and alphaproteobacteria, 5-ALA is synthesized via the Shemin pathway, which utilizes glycine and succinyl coenzyme A. Most bacteria and archaea, on the other hand, utilize the C5 pathway, which converts the five carbons of glutamate from glutamyl-tRNA to 5-ALA in two steps. From 5-ALA to uroporphyrinogen III, eukaryotes and prokaryotes all share a common pathway utilizing three cytosolic enzymes, with most prokaryotes synthesizing heme from 5-ALA via the canonical pathway that eukaryotes utilize. Actinobacteria and firmicutes, however, both phyla of mostly grampositive bacteria, synthesize heme from uroporphyrinogen III via a noncanonical pathway through the intermediates coproporphyrin and iron-coproheme.[32] The prokaryotic pathways, and their regulation, has been extensively reviewed elsewhere.[33]
Heme is degraded enzymatically by heme oxygenase. Most heme oxygenases degrade heme to biliverdin, CO and free iron,[34] though some bacterial heme oxygenases do not produce CO and biliverdin during heme degradation.[35] In eukaryotes, two isoforms of heme oxygenase are expressed; inducible HO-1, which is induced by heme, metal ions, hormones, and stressors like oxidative stress and heat shock, among others,[36] and the constitutively expressed HO-2, which is most common in the brain and testis.[37] HO-1 degradation of heme is dependent upon an electron donor, such as cytochrome P450 (CYP450), and is co-localized with biliverdin reductase (BVR), which reduces biliverdin to bilirubin preventing inhibition of HO-1. HO-1 is localized to the ER where it degrades cytosolic heme[38], and has also been reported to be localized to the nucleus[39], plasma membrane[40] and mitochondria.[41, 42] While the function of HO-1 outside of the ER is debated, co-localization of HO-1 with CYP450 and BVR in the plasma membrane and mitochondria suggests HO-1 may be able to degrade heme in these locations.[34]
Heme synthesis and degradation within the cell is tightly regulated by heme availability. This regulation is important due to the need to balance the requirement for heme in cellular processes and the cytotoxicity of excess heme. For instance, ALAS transcription and mitochondrial translocation is regulated by heme, and heme induces expression of HO-1.[43] While the synthesis and degradation of heme are well studied, many questions remain, including those relating to the transport of iron, glycine and protoporphyrinogen IX into the mitochondria and the transport of ALA into the cytosol (Figure 2). Additionally, there is still more to be understood about the role of proteinprotein interactions in regulating heme synthesis. For instance, in differentiating erythroid cells, three of the mitochondrial resident heme synthesis enzymes, ALAS2, PPOX, and FECH were found to interact, forming a heme biosynthetic supercomplex, or metabolon, with Abcb10, which has roles in ALA export and iron homeostasis (Figure 2), and Abcb7, which is involved in Fe-S cluster formation.[44] Identification of additional proteins in the complex, the role of this association in regulating heme synthesis and trafficking, and whether the metabolon exists in other cell types remains to be determined.
Trafficking of endogenously synthesized heme.
Once heme is synthesized on the matrix side of the mitochondrial innermembrane (Figure 2), it must then be shuttled for the synthesis of c-type cytochromes or heme a, which are required for respiration, or trafficked to hemoproteins within the mitochondrial matrix or IMS, or to extra-mitochondrial hemoproteins that reside in virtually every subcellular compartment or even outside the cell. The hierarchy of heme distribution, i.e. the preference of certain organelles to acquire heme before others, or the factors that regulate it, are not known. In addition, very little is understood about the transport and trafficking of heme and its spatio-temporal dynamics. This section will focus on what is known about the trafficking of endogenously synthesized heme both within and outside the mitochondria.
Trafficking of synthesized heme within the mitochondria
The rate-limiting step of FECH is the dissociation of heme. As a consequence, there may be chaperones or heme binding proteins that catalyze the dissociation or transfer of heme from FECH in vivo.[45] Indeed, as discussed below, the putative heme chaperone, PGRMC1, was found to interact with FECH in the heme metabolon, and may directly accept heme prior to its trafficking.[46, 47] Second, many matrix or innermembrane localized heme binding proteins, such as cholesterol side-chain cleavage enzyme (also referred to as P450scc[48] or CYP11A1) or cytochrome b of Complex III (cytochrome bc1 complex),[49] which are both localized to the mitochondrial inner membrane may interact directly with FECH to bind newly synthesized heme. Third, mitochondria, which accumulate as much as ~30 μM heme,[50] have an exceedingly low amount of labile heme in the matrix, < 1 nM,[22] suggesting that heme is trafficked from FECH in a manner that limits its dissociation into the labile heme pool. However, the factors governing the trafficking of heme from FECH are still not well understood and need to be further elucidated.
For mitochondrial proteins outside the matrix, heme must traverse the inner membrane to reach soluble hemoproteins within the IMS such as cytochrome c peroxidase (CCP). In principle, the hydrophobic lipophilic nature of heme could enable its diffusion across membranes.[4] However, the ability of heme to catalyze the production of ROS and oxidize lipids,[7, 8] which would lead to the disruption of membrane structure, implies that heme is either transported (discussed below) or ferried across the membrane in a chaperoned manner similar to other redox active metals; albeit the proteins responsible and how this occurs is still largely a mystery. Recently, the progesterone receptor membrane component 1 (PGRMC1), a heme binding protein that spans both the inner and outer membrane of the mitochondria and is found at ER-mitochondrial contact sites, was found to interact with FECH and the heme synthesis metabolon. [46, 47, 51] The preference for PGRMC1 to interact with FECH in the “product release” conformation, and the ability of PGRMC1 to transfer heme to cytochrome b5, provides evidence for its potential role as a heme chaperone that acts as a conduit for newly synthesized heme to reach the ER where it can hemylate a number of ER-localized hemoproteins or be distributed throughout the cell via the ER-golgi network.[46] However, the exact role and molecular mechanisms of PGRMC1 in heme trafficking still need to be determined.
Trafficking of synthesized heme via transporters.
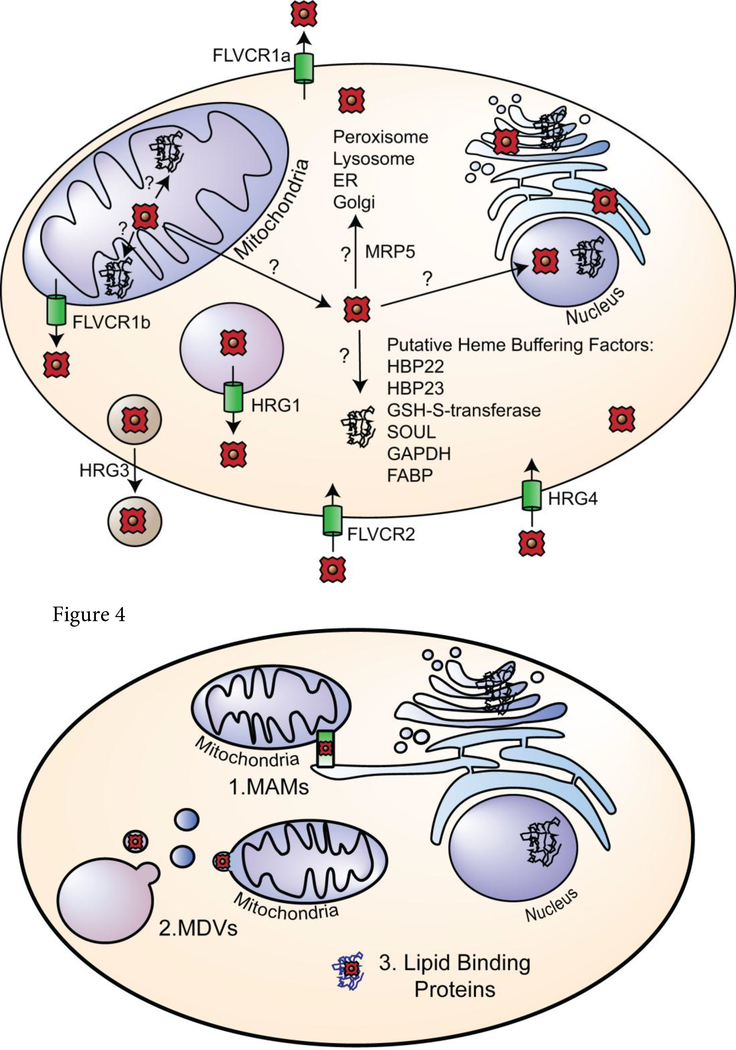
After its synthesis in the mitochondria, heme must be trafficked for incorporation into hemoproteins throughout the cell, and very little is known about the transporters, chaperones and buffering factors responsible (Figure 3). If heme is trafficked sequentially from the mitochondria to the cytosol, then into various compartments, heme transporters likely exist to transport heme from the mitochondria to buffering factors and hemoproteins within the cytosol. Indeed, over the last 15 years, a number of putative heme transporters have been identified, including Feline Leukemia Virus Subgroup C Cellular Receptors (FLVCR), Heme responsive genes (HRG) 1 and 4, and multidrug resistance protein 5 (MRP-5, also called ABCC5) (Figure 3). The FLVCR proteins, which are members of the major facilitator superfamily (MFS) have been implicated in both heme import and export, and though their method of transport is unknown, they do not appear to utilize the bidirectional gradient-dependent transport used by many members of the MFS.[52] The heme exporters FLVCR1a and FLVCR1b are splice variants that are localized to the plasma and mitochondrial membranes, respectively. FLVCR 1a, a ~60kDa protein of ~560 amino acids, is predicted to have twelve hydrophobic transmembrane domains (TMDs), as is common for MFS proteins,[53] while the FLVCR1b isoform is ~28 kDa, with six predicted TMDs.[54] FLVCR1a and b are ubiquitously expressed with high expression in brain, liver, kidney, intestine and bone marrow in mammals.[55] FLVCR1b, which is the only putative mitochondrial heme exporter identified to date, was reported to transport heme from the mitochondria into the cytosol, and has been shown to be necessary for the differentiation of erythroid cells.[54] However, there has been limited biochemical characterization of FLVCR1b, raising many questions about its function and localization. For example, is FLVCR1b located on the inner or outer mitochondrial membrane, or does it span both membranes? Does FLVCR1b interact directly with FECH and the heme metabolon? Interestingly, unlike its plasma membrane isoform FLVCR1a, the mitochondrial associated FLVCR1b lacks the amino acids associated with binding hemopexin (Hx), which increases FLVCR1a heme export across the plasma membrane. Does FLVCR1b also interact with accessory proteins within the mitochondria or cytosol to facilitate heme transport? Additionally, lower eukaryotes, including yeast, do not encode FLVCR homologs, which have only been identified in mammals, suggesting the existence of alternative mechanisms to transport heme out of the mitochondria. Therefore, future studies are needed to determine if there are additional heme transporters or alternative mechanisms of heme export.
Figure 3.
Consensus model of heme transport and trafficking. Heme trafficking from the matrix of the mitochondria to various subcellular locations involve transporters and chaperones. Putative transporters are shown at their predicted location in the cell and denoted via green cylinders. Their direction of transport is noted with arrows. Putative heme buffering factors are listed as well.
Once in the cytosol, heme can be trafficked to cytosolic hemoproteins or into other subcellular compartments. MRP-5 was identified in Caenorhabditis elegans to be necessary for heme export from the intestines.[56] Studies in yeast and mammalian cells suggest MRP-5 is necessary for transport of heme from the cytosol into the secretory pathway.[56] Once in the secretory pathway, heme can be trafficked to hemoproteins within the pathway or other subcellular locations, the plasma membrane or trafficked to the extracellular space. Heme may also be trafficked from the cytosol by binding to heme chaperone proteins that are then translocated into organelles, similar to the twin-arginine translocation (TAT) system in bacteria.[57] In E. coli, hemoproteins acquire heme in the cytoplasm and are then translocated outside of the cell via TAT. The identified hemoprotein, YcdB, can also act as a peroxidase, pointing to a potential for dual enzymatic and chaperone functions of known hemoproteins.[57] Another putative cytosolic heme trafficking factor is BVR, which has been implicated in facilitating the trafficking of heme into the nucleus.[58] The putative heme binding peptide of BVR was found to have a heme dissociation constant of ~240 nM, which is similar to the ~25–300 nM span of labile heme concentrations found in mammalian cells [21–23], suggesting that BVR may sense and acquire heme from the LH pool. While BVR induces HO-1 expression,[58] it has not been directly demonstrated that BVR transports heme into the nucleus and alters nuclear heme concentration. Moreover, the mechanism of trafficking is unknown.
Heme can also be transported from the cytosol across the plasma membrane into the extracellular space. FLVCR1a is localized to the plasma membrane and exports heme from the cell. Heme export via FLVCR1a is necessary to prevent heme toxicity in hepatocytes,[59] enterocytes,[60] erythroid cells,[61] and endothelial cells.[62] FLVCR1a has not been found to be bidirectional and only exports heme when heme binding proteins, such as Hx or albumin are present.[52] Excess heme in the plasma is mostly (~80%) bound via lipoproteins before being transferred to albumin or to Hx for CD91 mediated endocytosis of Hx-heme by hepatocytes.[63] It is not known if the preference of FLVCR1a for Hx is specific, or if are there other unidentified high affinity hemoproteins that would also enhance FLVCR1a transport. Indeed, many questions involving heme export via FLVCR1a remain. Is FLVCR1a export necessary in all cells and is export induced under heme stress? Is the release of heme to Hx a method of preventing free heme in the plasma or is the transport of heme by Hx to hepatocytes important for inter-cellular heme trafficking? More work is needed to determine the role of FLVCR1a in heme homeostasis and trafficking.
Heme buffering factors within the cytosol.
The cytosol contains the greatest repository of labile heme, 20–340 nM [21–23], even more so than the nucleus or mitochondria, < 1 nM.[22] Heme is likely buffered and trafficked within the cytosol in a manner that mitigates the toxicity of heme. Over the years, many heme-binding proteins have been identified based on their affinity for hemin-agarose or blue sepharose and were proposed to have roles in heme homeostasis (Figure 3), including heme binding proteins HBP22[64] and HBP23[65] (also known as peroxiredoxin-1, PRX-1), so named for their molecular weights of 22 and 23 kDa respectively, glutathione S-transferase (GSH-S transferase),[66] HBP-1 (also known as SOUL)[67] and fatty acid binding protein (FABP).[68] These proteins may act to bind and buffer excess heme in the cytosol to prevent toxicity, albeit in vivo evidence for their roles in heme homeostasis is lacking.
An illustrative but interesting case is that of HBP23/PRX1. PRX1 is a thiol peroxidase that was recently found to bind heme with a 1:1 stoichiometry and a dissociation constant of 170 nM [69], which is within the 20–340 nM consensus range of labile heme in cells [21–23]. PRX1, which binds heme using a catalytic Cys, Cys52, exhibits a loss in thiol peroxidase activity upon heme binding. [69] Additionally, heme binding to PRX1 suppresses both the peroxidase activity of heme and the H2O2-mediated degradation of heme. [69] Given the abundance of PRX1 in the cytosol, 20 μM, and ability to bind and potentially detoxify and protect heme from degradation, PRX1 may have dual roles as a peroxide scavenging thiol peroxidase and a heme buffering or storage protein. However, as with many other heme binding proteins proposed to play a role in heme homeostasis, the in vivo function of PRX1 in heme homeostasis has yet to be demonstrated [69].
In contrast, the glycolytic enzyme glyceraldehyde phosphate dehydrogenase (GAPDH) is the only cytosolic protein that has been determined to bind, buffer, and traffic heme to downstream targets in vivo. In mammalian cells, GAPDH was found to be required for heme delivery to nitric oxide synthase (NOS).[70] Additionally, in Baker’s yeast, GAPDH was found to buffer cytosolic heme and regulate its availability to the nuclear heme-dependent transcription factor Hap1.[22] Moreover, it was recently found that a highly conserved His residue, His51, is responsible for heme binding and essential for GAPDH-dependent heme trafficking to NOS and Hap1.[71] The GAPDH heme binding affinity of 24 nM, which is similar to various estimates of the concentration of labile heme in the cytosol, 20–340 nM,[21–23] is consistent with the finding that GAPDH is an important component of the labile heme buffering system. Indeed, deletion of the major GAPDH isoform in yeast, Tdh3, results in a 2-fold increase in labile heme as measured by the fluorescent heme sensor, HS1-M7A,[22] consistent with its role in heme buffering. However, a number of open questions remain. How does GAPDH interact with client hemoproteins to transfer heme? Binding studies and structural models predict that heme is bound in a bis-His fashion via coordination from His51 from two GAPDH monomers at the protomer interface of tetrameric GAPDH.[71] As such, it is unclear how downstream client hemoproteins can acquire heme if it is buried at the oligomer interface. It is possible that GAPDH oligomerization may be dynamic, possibly in a regulated manner via post-translational modifications, to promote heme accessibility to target hemoproteins. Alternatively, there may be adapter proteins that facilitate heme exchange between GAPDH and client proteins. Additionally, in cases where GAPDH traffics heme to other compartments, such as to the nucleus to activate Hap1, it is not known if GAPDH dynamically shuttles between the cytosol and nucleus to directly transfer heme to Hap1 or if there are other mediators that shuttle heme from GAPDH to nuclear Hap1. Apart from GAPDH, the other heme binding proteins listed above may still yet prove to be important for buffering and/or trafficking labile heme.
Trafficking of synthesized heme through membranes and vesicles.
Given the hydrophobic nature of heme, once synthesized, heme may be trafficked via inter-organelle membrane contact points such as mitochondrial-associated membranes (MAMs) or through vesicular trafficking in mitochondrial derived vesicles (MDVs). In yeast, the ER-mitochondria encounter structure (ERMES) has been found to traffic calcium and phospholipids between the mitochondria and the ER (Figure 4).[72] Similarly in mammals, MAMs are associated with import of calcium and phospholipids into the mitochondria, and are important for regulating oxidative phosphorylation.[73] ERMES and MAMs are physical tethers between the ER and mitochondria that could facilitate the trafficking of heme between these organelles. Once in the ER, which forms a continuous network of tubules and cisternae that extend throughout the cell and to many subcellular compartments, as well as communicate with other organelles via vesicular transport and physical contact,[74] heme can gain access to hemoproteins residing in virtually any location. For instance, heme could be incorporated into nascent hemoproteins as they fold and mature prior to their trafficking to other locations via the trans golgi network and secretory pathway. Alternatively, heme could be shuttled in ER or golgi-derived vesicles to awaiting hemoproteins in disparate locations such as the nucleus, where heme regulated transcription factors reside, (e.g. Hap1,[75] p53,[76] Bach1,[77] Reverb[78]), or peroxisomes, which contain catalase. Indeed, a recent study has shown that the secretory pathway may be important for heme trafficking after synthesis in the mitochondria. In HEK293 cells, labile heme was detected by subcellular reporters to repopulate the ER first, then the Golgi and mitochondria, followed by the cytoplasm and nucleus, after heme synthesis was restored.[79] Intriguingly, a number of components of the heme synthesis metabolon, including FECH, Sucla2 and Abcb7[44], which otherwise reside in the matrix-side of the mitochondrial inner-membrane, as well as the heme biosynthetic enzyme CPOX, which is localized in the IMS (Figure 2), have been shown to be localized to MAMs.[80] However, the specific contribution of ERMES/MAMs to cellular heme distribution has never been directly tested and the mechanisms underlying how heme is trafficked to and within the pathway is still unknown.
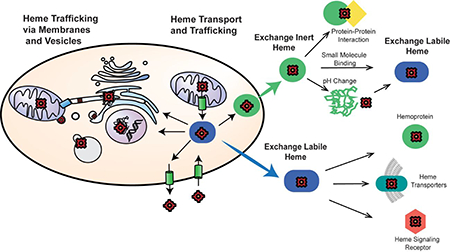
Figure 4.
Trafficking of heme via membranes and vesicles. Heme may be trafficked from the mitochondria via (1) direct inter-organelle contact points such as MAMs, (2) via vesicular mediated trafficking in MDVs, or (3) chaperoned by lipid binding proteins.
For MDV-mediated heme trafficking, newly synthesized heme would be trafficked from the mitochondria to endolysosomes, where it could be exported into the cytosol, bypassing the secretory pathway. Once in the cytosol, heme could be incorporated into any number of heme buffering or trafficking factors, including GAPDH. The recent development of subcellular heme sensors will continue to shed light on the trafficking of heme throughout the cell.[22, 23, 79]
Import and Trafficking of Exogenous Heme
Exogenous heme can be imported into the cell either directly by heme transporters or through the endocytosis of extracellular hemoproteins. Once in the cytosol, exogenous heme may be trafficked to HO-1 for degradation or used intact to activate any number of hemoproteins.[79] Two putative plasma membrane heme importers have been identified in mammals (Figure 3), FLVCR2 and heme carrier protein 1 (HCP-1). FLVCR2, another member of the MFS with 12 predicted TMDs, has been purported to import heme into the cell across the plasma membrane.[81] Little is known of the mechanisms of transport for FLVCR2, including whether it is dependent on heme chaperones or heme binding proteins, and the affinity of FLVCR2 for heme is unknown. HCP-1 has been shown to import heme into the cell, though the role of HCP1 in heme trafficking is debated, as HCP-1 was also identified as the proton-coupled folate transporter (PCFT).[82] HCP-1/PCFT in mice and humans respectively, is a 459 amino acid protein of ~ 50 kDa with nine predicted transmembrane helices.[83, 84] Evidence supports HCP-1 may be a low-affinity heme transporter,[85] and may be involved in heme export from the endosome in macrophages.[86]
The paralogous heme responsive genes HRG-1 and HRG-4 were identified in the heme auxotroph C. elegans, and found to be important for the transport and utilization of exogenous heme.[87] HRG-1 and HRG-4, are ~19 kDa with four predicted TMDs. HRG-1 is localized to membranes of endosomal/lysosomal compartments and transports exogenous heme into the cytosol. HRG-4, which is not conserved in vertebrates, transports exogenous heme across the plasma membrane into the cytosol and is involved in intestinal heme uptake in C. elegans.[87] The human homolog of HRG-1 was found to be necessary for heme recycling by macrophages.[88] Human HRG-1 transports exogenous heme from the phagolysosome into the cytosol during erythrophagocytosis (Figure 3).
In the fission yeast, Schizosaccharomyces pombe, heme uptake is induced under iron starvation, when two iron-regulated plasma membrane proteins designated Shu1 and Str3 transport heme into the yeast cell.[89, 90] Shu1 is a 25 kDa GPI anchored transporter with a measured heme affinity of 2.2 μM.[89, 91] S. pombe utilization of exogenous heme via Shu1 is dependent on the vacuolar transporter Abc3.[91] Str3, which was discovered to uptake heme in a Shu1 knockout, is predicted to be a 12 TMD member of the MFS, the same superfamily as mammalian FLVCR transporters.[90] Str3 has a lower affinity for heme than Shu1 and was unable to rescue S. pombe growth at low heme concentrations, though Str3 is not dependent on Abc3 for exogenous heme utilization.[90]
The internalization of extracellular hemoproteins via endocytosis is another mechanism by which cells acquire heme. Heme and Hb may be released from erythrocytes into blood plasma, especially during hemolytic stress. Excess heme and Hb can damage cells, especially the endothelia and vasculature, through a number of mechanisms, including the lytic action of heme, membrane disruption, heme-catalyzed ROS production, peroxidation of lipids, or promoting inflammation. As a consequence, heme and Hb must be scavenged from the blood. Hx is a serum heme scavenger, exhibiting femtomolar dissociation constants for ferric heme.[63] Heme bound Hx binds the hepatocyte LDL related receptor CD91, where it is endocytosed. Heme is then released from Hx due to protonation of coordinating heme residues in acidic compartments followed by its degradation by HO-1 or possibly its incorporation into the labile heme pool for use. Similarly, Hb is scavenged by haptoglobin (Hp), which binds Hb with picomolar affinity.[92] Hb-Hp can then be internalized by binding to the CD163 receptor, which is expressed in macrophages/monocytes and in neurons,[93] followed by heme dissociation.[92]
Once exogenous heme is imported into the cytosol, either via heme transporters or through endocytosis, heme may be trafficked to the ER for degradation via HO-1, incorporated into the secretory pathway via transporters such as MRP-5, or trafficked throughout the cell and/or incorporated into the labile heme pool. The extent to which exogenous heme is utilized intact is not entirely clear and the very notion of it being used whole has been debated.[26] However, a number of lines of evidence suggest that heme can indeed be used in metabolism without first being degraded. First, heme auxotrophs, which are either genetically manipulated to lack heme biosynthetic enzymes, e.g. S. cerevisiae cells lacking the gene for ALAS, hem1∆, or naturally do not possess the heme biosynthetic machinery, e.g. C. elegans,[94] and the trypanosomatid parasites Leishmania amazonensis, Trypanosoma cruzi, and Trypanosoma brucei,[95, 96] can be rescued by heme supplementation. Second, in cells that can synthesize heme, e.g. human embryonic kidney HEK293 cells, exogenous heme is accessible to a number of subcellular compartments and contributes to the labile heme pool as measured by a peroxidase-based heme sensor.[79] Third, intravenous heme has been successfully used to treat porphyrias, which are diseases that arise from defects in heme synthesis.[97] Finally, heme scavenged by Hx, which is taken up via endocytosis, can affect BACH1 repression,[98] pointing to the possibility of exogenous heme as an inter-cellular signal.
Altogether, cells can both transport exogenous heme and incorporate it intact for use in physiology, albeit the extent to which this happens is still debated.[26] Once inside the cell, it is unclear how it is transported and trafficked for utilization or to HO-1 for degradation. Interestingly, a recent study using peroxidase-based heme sensors found that exogenous and endogenous heme is trafficked to various subcellular compartments differently, suggesting the existence of an alternative set of chaperones and trafficking factors that can distinguish the source of heme.[79]
Inter-cellular heme trafficking and systemic regulation of heme homeostasis.
The dogma in metazoan heme biology is that every cell lives and dies using its own heme. That is to say, if heme is imported, it is degraded and the liberated iron is recycled for new heme synthesis. If the heme concentration exceeds the cellular requirement for it, excess heme is degraded.[26] However, the discovery of heme exporters, e.g. FLVCR1a, inter-cellular heme trafficking factors, e.g. HRG-3, and signals that communicate heme status between cells and organs, e.g. HRG-7, have challenged old ideas and expanded the cell-centric view of heme homeostasis to a broader organism-wide model of systemic heme regulation.
As mentioned previously, FLVCR1a is a heme exporter that was proposed to play an important role in heme detoxification, especially during erythropoiesis when erythroblasts synthesize large amounts of heme for Hb. However, many non-erythroid cells that have a much lower requirement for heme express FLVCR1a, including endothelial cells[62] and enterocytes,[60] raising the interesting question of what the role of FLCVR1a is when cells are not over-loaded with heme. One intriguing possibility is that cells can supply each other with heme when demand is not met by heme synthesis. For instance, nurse macrophages regulate erythroblast development due to the transmission of signals that promote differentiation and supply of essential nutrients.[99, 100] It has been proposed that nurse macrophages can supply both iron and heme to erythroblasts in order to support the sizeable demand for these metabolites during erythropoiesis.[101]
Using the heme auxotroph, C. elegans, which only acquires heme from its diet, an inter-cellular heme chaperone, HRG-3, was found to deliver heme from the mother to embryos. HRG-3 is necessary for embryonic survival but not survival of adult nematodes.[9] HRG-3 is a soluble 8.1 kDa protein that is secreted by intestinal cells into the pseudocoelom, the circulatory system of C. elegans, and transports heme to the extra-intestinal tissues of the worm.[9] The exact mechanisms of heme binding and trafficking by HRG-3 are still unknown. HRG-3 forms a dimer with a hydrophobic pocket, and structural and spectroscopic studies of HRG-3 predict heme to be enclosed within this pocket, instead of direct coordination by heme-binding residues.[102] HRG-3 is one of only a few known heme chaperones in eukaryotes, however HRG-3 homologs have not been identified in vertebrates and it remains to be seen if higher organisms have analogous inter-cellular heme trafficking factors.
If heme can be shuttled between cells, tissues, or organs, there are likely systemic signals that communicate heme status between distant locations, analogous to hepcidin regulation of systemic iron homeostasis[103] or the unknown signal that communicates cardiac copper deficiency to copper storage organs.[104] Indeed, using C. elegans as a model system, HRG-7, a 37 kDa homolog to A1 aspartic proteases, was identified as a secreted signaling factor that communicates intestinal heme deficiency to extra-intestinal tissues.[105] This pathway is regulated through a DBL-1 dependent signal from neurons. Given that many of the components of the HRG-7 dependent pathway for inter-organ signaling of heme are conserved in mammals, it is tempting to speculate that such a mechanism for the regulation of systemic heme homeostasis occurs in humans as well.[105] However, HRG-7 itself is not conserved in vertebrates and it remains to be demonstrated that there are pathways that regulate systemic heme homeostasis in animals that biosynthesize heme. In total, there is emerging evidence that cells and tissues in metazoans can share and signal the availability of heme, challenging the dogma that every cell lives and dies using its own heme.
Heme Signaling.
In addition to its role as a protein cofactor, heme is a versatile signaling molecule that dynamically regulates a number of proteins and pathways. Heme can signal directly through its interactions with transcription factors, kinases, ion channels, and cell surface receptors, or indirectly via heme-derived metabolites such as CO, bilirubin, and biliverdin, which are produced from the HO-catalyzed degradation of heme. [3, 4, 106] Collectively, heme-dependent signaling controls diverse processes that span oxygen sensing, iron homeostasis, the antioxidant stress response, mitochondrial respiration and biogenesis, mitophagy, apoptosis, immune function, circadian rhythms, cell cycle progression, and proliferation.[76, 107–117]
In terms of direct heme signaling, heme must be dynamically mobilized to activate or repress various physiological processes. In yeast, heme binding to the transcription factor, Hap1, controls its function as a promoter or repressor of gene expression in order to facilitate aerobic metabolism, including sterol synthesis and respiration.[75, 106] In mammalian cells, heme binding to the transcription factors p53 [76] and Bach1,[77] promote their ubiquitination and proteasomal degradation, and heme binding to Rev-erb-α, and Rev-erb-β repress the expression of their target genes.[78] Heme can also regulate ion channels.[118, 119] Heme binding to His and Cys residues on cardiac KATP channels result in increased currents in a dose dependent manner, with maximal and half-maximal responses achieved with 500 nM and 100 nM heme, respectively.[119] Another interesting case of heme signaling is the ability of heme to regulate micro RNA (miRNA) processing.[120] Ferric heme, but not ferrous heme, activates DGCR8, an RNA binding protein that cooperates with Drosha to recognize and cleave long primary transcript pri-miRNA into precursor miRNAs (premiRNAs) in the nucleus.
Heme can also act on extracellular signaling targets. Heme derived from lysed erythrocytes, or perhaps even heme exported from FLVRC1a, can bind the cell surface receptor toll like receptor 4 (TLR4), which results in the inflammatory activation of macrophages and endothelial cells, [121, 122] and is necessary for the innate immune response to hemolytic tissue damage.[123–125] Interestingly, heme has the opposite effect on microglia and astrocytes, suppressing immune signaling and inflammation.[126] The difference between the immune signaling functions of heme in macrophages versus resident brain immune cells remains to be determined.
In terms of indirect heme signaling, the heme degradation products, CO, biliverdin, and bilirubin, the last of which is produced by the action of BVR, can themselves act on a number of downstream targets to effect cell signaling and metabolism. For instance, CO regulates vascular tone and has been shown to be both anti-apoptotic and anti-inflammatory.[114] Biliverdin can regulate the inflammatory response in macrophages by inhibiting TLR4 expression,[115] and bilirubin binds the nuclear receptor, peroxisome proliferator-activated receptor-α (PPARα), reducing accumulation of lipids.[127] Therefore, heme can be regarded as a packaged pro-form of CO or biliverdin that can be activated by HO to control downstream signaling pathways.
Dynamics of heme mobilization for trafficking and signaling.
The (on-going) discovery of heme buffering and trafficking factors, including chaperones and transporters, has greatly transformed our understanding of the mechanisms that enable heme bioavailability for hemoproteins and signaling. However, another very important, but much less understood component of heme homeostasis is the dynamics of heme mobilization and how it can be initiated for the hemylation of heme enzymes and proteins, regulation of downstream factors, or HO-mediated heme degradation. One mechanism is that heme synthesis is regulated in a dynamic fashion to provide transient increases in heme for use in hemoproteins or for signaling. That is to say, heme is made on demand. Indeed, in yeast, the activity of heme-regulated transcription factor Hap1 is closely tied to the synthesis of heme; exposure to O2 [75] or switching from fermentative to respiratory metabolism[128] causes a flood of newly biosynthesized heme to activate Hap1 so as to support aerobic metabolism. In addition, the regulation of metabolic cycling in yeast, the periodic oscillation between oxidative, reductive building, and reductive charging phases, is associated with periodic changes in the synthesis and degradation of heme.[129, 130] In mammals, heme regulation of the circadian clock through binding to the nuclear receptors, Rev-erb-α and Rev-erb-β, is associated with fluctuations in heme synthesis via ALAS expression.[108]
Alternatively, there may be active processes that mobilize pre-existing pools of heme for use. One mechanism is through oxidation-reduction reactions at the heme iron center. Given that hemoproteins likely have differential affinities for ferric or ferrous heme,[120, 131–133] oxidation or reduction at the heme iron center may cause heme dissociation and release into the labile or bioavailable heme pool. As a consequence, molecular oxygen, reactive oxygen or nitrogen species, or perturbations in redox poise of the cell may affect the labilization of heme.
Another possibility is that diffusible signaling molecules can mobilize heme via the post-translational modification of hemoproteins. Indeed, both H2O2 and NO have been demonstrated to labilize heme and affect heme transfer between hemoproteins. For instance, H2O2 was found to induce the transfer of heme between cytochrome c peroxidase (CCP) and catalase. Respiration-derived H2O2 oxidizes a heme-coordinating histidine in CCP, causing heme dissociation and subsequent binding by catalase (Figure 5A).[134] The H2O2 mediated transfer of heme from CCP to catalase is a remarkable circuit that links peroxide-mediated protein oxidation to activation of a peroxide scavenging system. While H2O2 was not shown to influence cell-wide labile heme using genetically encoded heme sensors,[22] the finding that a signaling molecule could labilize otherwise exchange-inert heme for utilization by other proteins could be a model for thinking about other signaling molecules that can alter the heme binding properties of hemoproteins, including the gasotransmiters nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S).
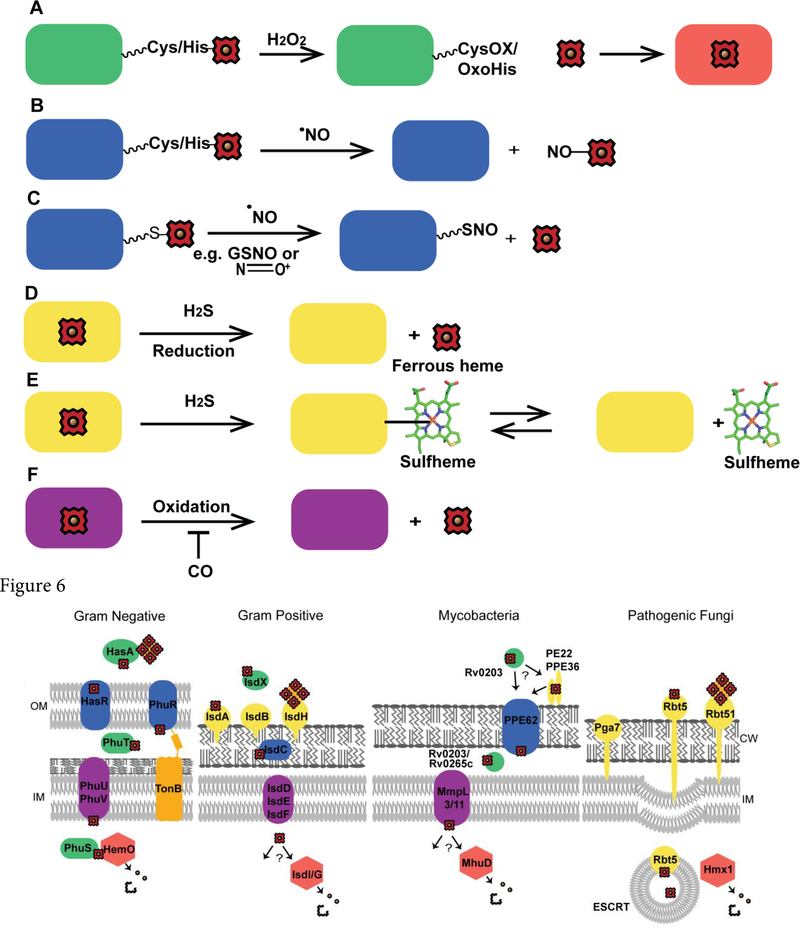
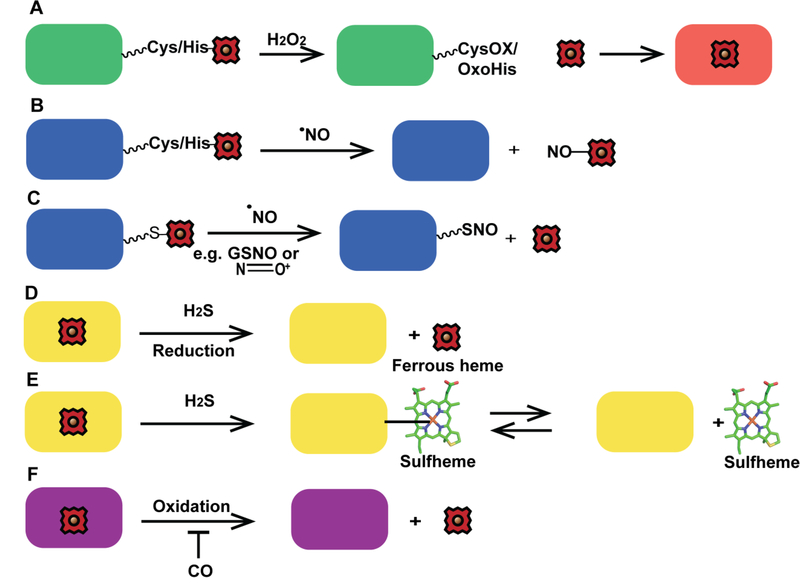
Figure 5.
Schemes for heme mobilization via small molecules. Heme mobilization may be regulated via small molecules. (A) H2O2 can oxidize hemoproteins, including hemecoordinating residues, e.g. Cys or His, and potentially release heme for use by other hemoproteins. CysOX is cysteine sulfenic acid and OxoHis is 2-oxo-histidine. (B) Nitrosylation of heme by NO may cause heme dissociation. (C) S-nitrosation may also initiate the release of heme. (D) H2S can reduce ferric heme, potentially causing the dissociation of ferrous heme. (E) H2S can bind to heme yielding sulfheme, where sulfur is incorporated into one of the porphyrin rings of heme, (structure modified from PDB ID 1YMC) which could cause heme dissociation. (F) CO could potentially inhibit heme mobilization by preventing oxidation of ferrous heme.
Rather paradoxically, NO has been shown to both inhibit heme binding to a number of hemoproteins including NOS, Hb and catalase,[70, 135] as well as increase labile heme.[22] With respect to the former, it was demonstrated that GAPDH regulates heme insertion into iNOS and that S-nitrosation at Cys152 weakens heme−GAPDH interactions and prevents the transfer of heme to target hemoproteins. On the other hand, using genetically encoded heme sensors, NO was found to transiently increase the labile heme pool in the cytosol and nucleus of yeast by 2-fold and more than 100-fold, respectively, over 20 minutes, followed by a slower ~90 min restoration back to initial steady-state levels.[22] Moreover, in endothelial cells, it was proposed that NO induces the release of heme from a number of hemoproteins due to the NO-mediated increase in cellular iron pools and enhanced activity of HO.[136] The NO-dependent release of heme from hemoproteins may be due to nitrosylation of ferrous heme iron centers, which can lead to dissociation of the trans ligand, weakening of the heme affinity and release of heme (Figure 5B),[137, 138] or the S-nitrosation of hemecoordinating cysteine thiols [139] or allosteric Cys sites, which may impact heme binding[70] and promote heme dissociation (Figure 5C).
Despite the wealth of data demonstrating that CO and H2S can interact with heme, there is comparatively little known about the ability of these molecules to affect labile heme and heme trafficking. H2S can reduce ferric heme to ferrous heme in hemoproteins, e.g. cytochrome c oxidase (Figure 5D), or form sulfheme, e.g. Hb and myoglobin (Figure 5E).[140] The interaction between H2S and heme may lead to dissociation of heme into the labile heme pool, but this remains to be demonstrated. Similarly, little is known about the effect of CO on heme trafficking. Given the high affinity of CO for 5- or 6-coordinate ferrous hemoproteins,[141, 142] CO binding may prevent heme oxidation and subsequent dissociation,[143] which would result in less labile or bioavailable heme (Figure 5F).
Overall, the discovery that H2O2 and NO can affect the labilization and bioavailability of heme, and that cellular redox state, CO and H2S may potentially do the same, means that heme mobilization, trafficking, and signaling may be downstream of a number of key physiological processes, including redox metabolism, NOS-dependent NO signaling, NADPH-oxidase mediated H2O2 signaling, sulfur metabolism, and more. Pathological conditions may also mobilize heme. It was recently demonstrated that heavy metal stress, e.g. Pb2+, increases labile heme, potentially contributing to heme toxicity and/or signaling heme to communicate Pb2+ stress.[25] The continued development and application of new heme imaging technologies and heme sensors will help shed light on the dynamics of labile heme and the factors that initiate its mobilization for trafficking and signaling.[22, 23, 79, 144]
Heme at the host-pathogen axis.
During fungal and bacterial infections, invading microbes must acquire host iron for virulence. The most abundant source of iron for these pathogens is heme, which is predominantly bound to hemoproteins such as Hb. As a consequence, host and pathogen heme homeostatic factors inter-mingle and compete for available heme. Bacteria and fungi have evolved numerous systems for heme trafficking. Many bacteria and fungi can both uptake and synthesize heme, while others lack some, or all, of the heme biosynthetic enzymes and are solely dependent on host heme. The pathogenic microbes that assimilate host heme face similar challenges; first they must retrieve heme from the host, transport the heme across the cellular envelope into the cell, and deliver heme to hemoproteins for utilization or heme oxygenases for degradation, all while mitigating heme toxicity.
Microbial pathogens have evolved different heme uptake pathways to match the unique challenges of their environment. During infection, heme within the host may be available as free heme, Hb, heme-Hx or Hb-Hp. Most microbial pathogens utilize cellsurface associated heme binding proteins that can transport free or bound heme or secrete hemophores that sequester free heme or heme binding proteins, such as Hb, and deliver them back to cell surface receptors for internalization. Once taken up, heme trafficking within most microbial pathogens is poorly understood. Herein we will briefly discuss the transport and trafficking of heme within microbial pathogens and how they interact with host heme.
Heme Uptake in Bacteria
Bacteria have developed varied heme uptake systems to overcome their diverse cellular envelope compositions and structures, and host microenvironments (Figure 6). Heme uptake in bacteria is generally divided into direct uptake, hemophore mediated uptake and, at least in the case of Neisseria meningitides, bipartite surface receptor mediated uptake for Hb.[145] Herein we present examples of heme uptake in gram-positive and gram-negative bacteria to point out similarities in uptake and points of divergence as heme uptake in bacteria has been extensively reviewed in the literature.[11, 12, 146, 147]
Figure 6.
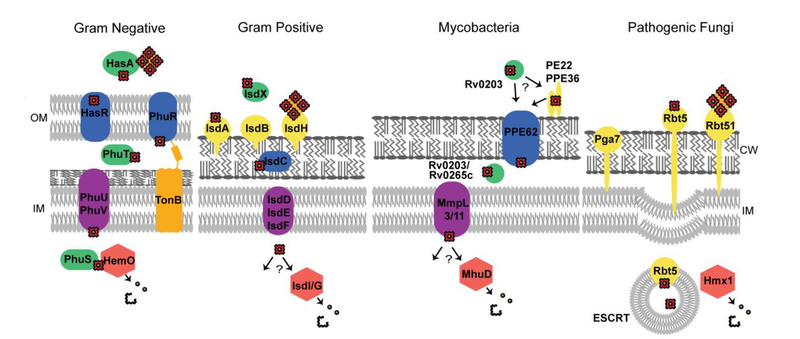
Heme uptake in pathogenic microbes. Representative heme uptake systems for gram negative bacteria (P. aeruginosa), gram positive bacteria (S. aureus), mycobacteria (M. tuberculosis), and fungal pathogens (C. albicans). OM is the outer membrane, IM is inner membrane and CW is the cell wall.
Gram-Positive Bacteria
Arguably the most well characterized heme acquisition system in gram-positive bacteria is the Isd (Iron-regulated surface determinant) system (Figure 6). Both Staphylococcus aureus and Bacillus anthracis utilize Isd proteins to transport heme from the extracellular space, across the cell wall and membrane, and into the cytoplasm. The hallmark of the Isd system is the use of Near Transporter (NEAT) heme binding domains to extract host heme. These NEAT domains are found in cell wall tethered proteins that are attached via sortases in both S. aureus and B. anthracis, [148, 149] and in soluble hemophores in B. anthracis. NEAT domains are necessary for the first step in heme acquisition, which requires retrieving heme from the host. Isd proteins bind free heme or hemoprotein bound heme via NEAT domains,[150] and heme is then transferred to other Isd NEAT proteins, such as IsdC, to transport heme through the cell wall. Finally, heme is transported across the inner membrane into the cytoplasm via the IsdDEF ATP-binding cassette (ABC) transporter complex. Here heme can be trafficked to hemoproteins or to an Isd heme oxygenase within the cytoplasm.[151]
Other gram-positive bacterial heme uptake systems have also been discovered that do not utilize NEAT heme biding domains. Corynebacterium diptheriae utilizes a series of proteins termed the hemin uptake system (Hmu). These proteins do not have NEAT domains and are not attached to the cell surface via sortases.[152] The cell wall associated heme binding proteins of HtaABC of C. diptheriae have an N-terminal secretion signal and a C-terminal transmembrane domain allowing them to be tethered to the cell with an exposed heme binding domain.[153] C. diptheriae have redundant systems to capture and transport heme to the putative ABC transporter HmuTUV. Under low iron conditions, the ChtAB and CirAChrC heme binding proteins are upregulated, and can capture and deliver heme to HmuTUV, to be transported into the cytosol. Recently in Mycobacterium tuberculosis, proteins have been discovered that are necessary for M. tuberculosis to grow utilizing heme as a sole iron source (Figure 6).[154, 155] Though the function of these proteins in heme uptake is still not well understood, heme must be captured from the host, possibly by secreted hemophore Rv0203,[154] or via cell surface heme binding Proline-Proline-Glutamate (PPE) proteins.[155] This heme is then transported into the cell, possibly through Mycobacterial membrane protein large (MMPL) proteins or through PPE transmembrane proteins, where it can be degraded by the IsdG type Mycobacterial heme utilization degrader (MhuD) heme oxygenase to release iron.[35]
Gram-Negative Bacteria
The structure of the cellular envelope of gram-negative bacteria requires heme to be trafficked across the outer membrane, through the periplasm and across the inner membrane before reaching the cytosol. Most gram-negative bacteria accomplish this with TonB dependent import across the outer membrane and, similar to gram-positive bacteria, an ABC transporter at the inner membrane. Shigella dysenteriae utilizes direct transport of heme into the periplasm the via Shigella heme uptake (Shu) heme binding protein, ShuA, which is TonB dependent. Heme is then trafficked through the periplasm via ShuT and delivered to the ABC transporter at the inner membrane, ShuUV. [156–158] Pseudomonas aeruginosa utilizes a similar uptake system to S. dysenteriae, the Pseudomonas heme uptake (Phu) system. This utilizes both direct heme import via PhuRSTUVW[158] and hemophore mediated import via the Heme acquisition system (Has) proteins HasA and HasR (Figure 6).[159]
Heme Uptake in Fungal Pathogens
Fungal pathogens, such as Candida albicans and Cryptococcus neoformans can utilize heme as a sole iron source, though their heme uptake mechanisms are not well understood. The general model is that heme or hemoproteins are bound at the cell surface and transferred across the cell wall for endocytosis at the inner membrane (Figure 6). Heme and Hb binding proteins Rbt5, Rbt51[160] and Pga7[161] have been identified in C. albicans that are anchored within the cell wall and are involved in heme uptake by binding heme or Hb. Transfer of heme across the plasma membrane is most likely via endocytosis as proteins involved in the endosomal sorting complex required for transport (ESCRT) are necessary for heme uptake in both C. albicans[162] and C. neoformans.[163]
Intracellular heme trafficking in Microbial Pathogens
Exogenous Heme
The fate of exogenous heme once it has entered the cytosol of microbial pathogens is not well studied. Some pathogens, such as the heme auxotroph Hemophilus influenzae, must traffic exogenous heme for utilization in all heme dependent proteins. Though how this occurs is unknown. In P. aeruginosa the cytosolic protein PhuS delivers heme to the iron regulated heme oxygenase (Figure 6),[164] and knockout of a homolog in S. dysenteriae, ShuS, increases sensitivity to heme toxicity, suggesting it plays an important role as a heme chaperone.[165] Some bacteria, such as S. aureus and B. anthracis, respond to excess heme by expressing efflux proteins through a heme dependent two-component system, which can alleviate heme toxicity during hemolytic events.[166] In the case of pathogenic fungi like C. albicans, it appears that exogenous heme taken up via endocytosis is mostly, if not entirely, degraded as growth on exogenous heme is dependent on the heme oxygenase CaHMX1.[167] It is not yet known whether heme is degraded in the vacuole or transported to CaHMX1 for degradation.
Endogenous Heme
Endogenously synthesized heme must be trafficked from the site of synthesis to heme dependent proteins throughout the cell. Specialized heme trafficking systems like that involved in cytochrome c biogenesis have been identified. In cytochrome c biogenesis, hemeb is transported to the periplasm of the cell, or in fungi across the mitochondrial inner membrane, and converted to hemec for incorporation into cytochrome c. Heme is trafficked to cytochrome c via three known systems. Each system has unique chaperones and transporters and have been reviewed extensively.[168] Heme must also be trafficked to other hemoproteins, though few heme chaperones in pathogens have been identified.
Microbial pathogens are solely dependent on the host for iron and have developed a number of systems to acquire iron through heme uptake. Fungal and bacterial pathogens must traffic heme across the cell envelope while avoiding heme toxicity. The known heme uptake and trafficking systems utilize heme transport via dedicated transporters, heme trafficking by hemophores and soluble heme chaperones, and heme trafficking via endocytosis. While much has been studied in relation to heme uptake in pathogenic microbes, the systems of some pathogens, such as mycobacterial heme uptake systems, remain to be fully elucidated. Also, as with metazoans, little is known of the fate of heme once inside the cell. Newly developed methods for sensing labile heme may provide some insight on heme trafficking within pathogenic microbes.[22, 23, 79]
Conclusions
The past decade has witnessed a radical transformation of our understanding of heme homeostatic mechanisms, including heme transport, trafficking, and dynamics. At one time, heme was viewed as a static cofactor buried in hemoprotein active sites. However, the development of new heme imaging modalities coupled with integrated biochemical, cell biological, and genetic approaches have revealed that heme is a dynamic molecule that can be mobilized in response to a variety of stimuli, including signaling molecules like NO and H2O2. At one time, metazoan heme homeostasis was viewed from the vantage of individual cells that maintained their own pool of heme, making it or degrading it as needed. No longer! The discovery of heme importers, exporters, inter-cellular trafficking factors, and signals that communicate tissue heme status has created a new paradigm for systemic heme homeostasis. At one time, it was thought that heme is made on demand and any excess is degraded, obviating the need for a heme reserve for use in metabolism. The discovery of a labile heme pool that is highly dynamic has established that a pre-existing supply of bioavailable may be important for heme utilization and signaling. The rapid emergence of new approaches and tools promises to reveal the enigmatic rules that govern heme transport, trafficking, and availability and will no doubt continue to transform and expand our understanding of heme homeostatic mechanisms in cells and animals.
Highlights.
Heme is transported and trafficked within and between cells for use in hemoproteins and for signaling.
The mechanisms underlying heme mobilization, trafficking, and transport, which are not well understood, are reviewed.
A number of signaling molecules, including H2O2 and NO, regulate heme dynamics, bioavailability and trafficking.
The transport and trafficking of heme in most human pathogens is required for virulence.
Acknowledgements:
We acknowledge support from the U.S. National Institutes of Health (ES025661 and GM118744 to A.R.R.), the U.S. National Science Foundation (MCB-1552791 to A.R.R.), the Blanchard Professorship (to A.R.R.) and start-up funding from the Georgia Institute of Technology (to A.R.R.).
Footnotes
Disclosure:
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Hooda J, Shah A, Zhang L, Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes, Nutrients 6(3) (2014) 1080–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aich A, Freundlich M, Vekilov PG, The free heme concentration in healthy human erythrocytes, Blood Cells, Molecules, and Diseases 55(4) (2015) 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanna DA, Martinez-Guzman O, Reddi AR, Heme Gazing: Illuminating Eukaryotic Heme Trafficking, Dynamics, and Signaling with Fluorescent Heme Sensors, Biochemistry 56(13) (2017) 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reddi AR, Hamza I, Heme mobilization in animals: a Metallolipid’s journey, Accounts of chemical research 49(6) (2016) 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Atamna H, Boyle K, Amyloid-β peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease, Proceedings of the National Academy of Sciences of the United States of America 103(9) (2006) 3381–3386 doi 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Atamna H, Frey WH, A role for heme in Alzheimer’s disease: heme binds amyloid β and has altered metabolism, Proceedings of the National Academy of Sciences 101(30) (2004) 11153–11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sassa S, Why heme needs to be degraded to iron, biliverdin IXα, and carbon monoxide?, Antioxidants & redox signaling 6(5) (2004) 819–824. [DOI] [PubMed] [Google Scholar]
- [8].Kumar S, Bandyopadhyay U, Free heme toxicity and its detoxification systems in human, Toxicology letters 157(3) (2005) 175–188. [DOI] [PubMed] [Google Scholar]
- [9].Chen C, Samuel TK, Sinclair J, Dailey HA, Hamza I, An intercellular hemetrafficking protein delivers maternal heme to the embryo during development in C. elegans, Cell 145(5) (2011) 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, A heme export protein is required for red blood cell differentiation and iron homeostasis, Science 319(5864) (2008) 825–828. [DOI] [PubMed] [Google Scholar]
- [11].Choby JE, Skaar EP, Heme Synthesis and Acquisition in Bacterial Pathogens, Journal of Molecular Biology 428(17) (2016) 3408–3428 doi 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Contreras H, Chim N, Credali A, Goulding CW, Heme uptake in bacterial pathogens, Current opinion in chemical biology 19 (2014) 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Severance S, Hamza I, Trafficking of heme and porphyrins in metazoa, Chemical reviews 109(10) (2009) 4596–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Villiers KA, Kaschula CH, Egan TJ, Marques HM, Speciation and structure of ferriprotoporphyrin IX in aqueous solution: spectroscopic and diffusion measurements demonstrate dimerization, but not μ-oxo dimer formation, JBIC Journal of Biological Inorganic Chemistry 12(1) (2007) 101–117. [DOI] [PubMed] [Google Scholar]
- [15].Conant JB, Tongberg C, The Oxidation-Reduction Potentials of Hemin and Related Substances I.The Potentials of Various Hemins and Hematins in the Absence and Presence of Pyridine., Journal of Biological Chemistry 86(2) (1930) 733–741. [Google Scholar]
- [16].Reddi AR, Reedy CJ, Mui S, Gibney BR, Thermodynamic investigation into the mechanisms of proton-coupled electron transfer events in heme protein maquettes, Biochemistry 46(1) (2007) 291–305. [DOI] [PubMed] [Google Scholar]
- [17].Hu J, Dong L, Outten CE, The Redox Environment in the Mitochondrial Intermembrane Space Is Maintained Separately from the Cytosol and Matrix, The Journal of Biological Chemistry 283(43) (2008) 29126–29134 doi 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shi H, Bencze KZ, Stemmler TL, Philpott CC, A cytosolic iron chaperone that delivers iron to ferritin, Science 320(5880) (2008) 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kirby K, Jensen LT, Binnington J, Hilliker AJ, Ulloa J, Culotta VC, Phillips JP, Instability of superoxide dismutase 1 of Drosophila in mutants deficient for its cognate copper chaperone, Journal of Biological Chemistry 283(51) (2008) 35393–35401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rae T, Schmidt P, Pufahl R, Culotta V, O’halloran T, Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase, Science 284(5415) (1999) 805–808. [DOI] [PubMed] [Google Scholar]
- [21].Atamna H, Brahmbhatt M, Atamna W, Shanower GA, Dhahbi JM, ApoHRP-based assay to measure intracellular regulatory heme, Metallomics 7(2) (2015) 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hanna DA, Harvey RM, Martinez-Guzman O, Yuan X, Chandrasekharan B, Raju G, Outten FW, Hamza I, Reddi AR, Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors, Proceedings of the National Academy of Sciences 113(27) (2016) 7539–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Song Y, Yang M, Wegner SV, Zhao J, Zhu R, Wu Y, He C, Chen PR, A genetically encoded FRET sensor for intracellular heme, ACS chemical biology 10(7) (2015) 1610–1615. [DOI] [PubMed] [Google Scholar]
- [24].Abshire JR, Rowlands CJ, Ganesan SM, So PT, Niles JC, Quantification of labile heme in live malaria parasites using a genetically encoded biosensor, Proceedings of the National Academy of Sciences 114(11) (2017) E2068–E2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hanna DA, Hu R, Kim H, Martinez-Guzman O, Torres MP, Reddi AR, Heme bioavailability and signaling in response to stress in yeast cells, Journal of Biological Chemistry (2018) jbc.RA118.002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ponka P, Sheftel AD, English AM, Bohle DS, Garcia-Santos D, Do Mammalian Cells Really Need to Export and Import Heme?, Trends in biochemical sciences 42(5) (2017) 395–406. [DOI] [PubMed] [Google Scholar]
- [27].Heinemann IU, Jahn M, Jahn D, The biochemistry of heme biosynthesis, Archives of Biochemistry and Biophysics 474(2) (2008) 238–251 doi 10.1016/j.abb.2008.02.015. [DOI] [PubMed] [Google Scholar]
- [28].Franken ACW, Lokman BC, Ram AFJ, Punt PJ, van den Hondel CAMJJ, de Weert S, Heme biosynthesis and its regulation: towards understanding and improvement of heme biosynthesis in filamentous fungi, Applied Microbiology and Biotechnology 91(3) (2011) 447–460 doi 10.1007/s00253-011-3391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ajioka RS, Phillips JD, Kushner JP, Biosynthesis of heme in mammals, Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1763(7) (2006) 723–736 doi 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- [30].Hamza I, Dailey HA, One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans, Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1823(9) (2012) 1617–1632 doi 10.1016/j.bbamcr.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barupala DP, Dzul SP, Riggs-Gelasco PJ, Stemmler TL, Synthesis, delivery and regulation of eukaryotic heme and Fe–S cluster cofactors, Archives of Biochemistry and Biophysics 592 (2016) 60–75 doi 10.1016/j.abb.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dailey HA, Gerdes S, Dailey TA, Burch JS, Phillips JD, Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin, Proceedings of the National Academy of Sciences 112(7) (2015) 2210–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, Warren MJ, Prokaryotic heme biosynthesis: multiple pathways to a common essential product, Microbiology and Molecular Biology Reviews 81(1) (2017) e00048–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stocker R, Perrella MA, Heme Oxygenase-1, A Novel Drug Target for Atherosclerotic Diseases? 114(20) (2006) 2178–2189 doi 10.1161/circulationaha.105.598698. [DOI] [PubMed] [Google Scholar]
- [35].Nambu S, Matsui T, Goulding CW, Takahashi S, Ikeda-Saito M, A new way to degrade heme the Mycobacterium tuberculosis enzyme MhuD catalyzes heme degradation without generating CO, Journal of Biological Chemistry 288(14) (2013) 10101–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rowe A, Houghton JD, Mammalian haem oxygenase—A tale of two enzymes, Biochemistry and Molecular Biology Education 25(3) (1997) 118–128. [Google Scholar]
- [37].Maines MD, The heme oxygenase system: a regulator of second messenger gases, Annual review of pharmacology and toxicology 37(1) (1997) 517–554. [DOI] [PubMed] [Google Scholar]
- [38].Gottlieb Y, Truman M, Cohen LA, Leichtmann-Bardoogo Y, Meyron-Holtz EG, Endoplasmic reticulum anchored heme-oxygenase 1 faces the cytosol, Haematologica 97(10) (2012) 1489–1493 doi 10.3324/haematol.2012.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lin Q, Weis S, Yang G, Weng Y-H, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress, Journal of Biological Chemistry 282(28) (2007) 20621–20633. [DOI] [PubMed] [Google Scholar]
- [40].KIM HP, WANG X, GALBIATI F, RYTER SW, CHOI AM, Caveolae compartmentalization of heme oxygenase-1 in endothelial cells, The FASEB Journal 18(10) (2004) 1080–1089. [DOI] [PubMed] [Google Scholar]
- [41].Slebos D-J, Ryter SW, Van Der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke–induced cell death, American journal of respiratory cell and molecular biology 36(4) (2007) 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [42].Converso DP, Taillé C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J, HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism, The FASEB Journal 20(8) (2006) 1236–1238. [DOI] [PubMed] [Google Scholar]
- [43].Tadashi Y, Peter B, Tirza C, M.R. M., S. Shigeki, Human heme oxygenase cDNA and induction of its mRNA by hemin, European Journal of Biochemistry 171(3) (1988) 457–461 doidoi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- [44].Medlock AE, Shiferaw MT, Marcero JR, Vashisht AA, Wohlschlegel JA, Phillips JD, Dailey HA, Identification of the Mitochondrial Heme Metabolism Complex, PLOS ONE 10(8) (2015) e0135896 doi 10.1371/journal.pone.0135896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hoggins M, Dailey H, Hunter C, Reid J, Direct measurement of metal ion chelation in the active site of human ferrochelatase, Biochemistry 46(27) (2007) 8121–8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Piel RB, Shiferaw MT, Vashisht AA, Marcero JR, Praissman JL, Phillips JD, Wohlschlegel JA, Medlock AE, A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): A Partner and Regulator of Ferrochelatase, Biochemistry 55(37) (2016) 5204–5217 doi 10.1021/acs.biochem.6b00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kabe Y, Nakane T, Koike I, Yamamoto T, Sugiura Y, Harada E, Sugase K, Shimamura T, Ohmura M, Muraoka K, Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance, Nature communications 7 (2016) 11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cherradi N, Rossier MF, Vallotton MB, Timberg R, Friedberg I, Orly J, Wang XJ, Stocco DM, Capponi AM, Submitochondrial distribution of three key steroidogenic proteins (steroidogenic acute regulatory protein and cytochrome P450scc and 3β-hydroxysteroid dehydrogenase isomerase enzymes) upon stimulation by intracellular calcium in adrenal glomerulosa cells, Journal of Biological Chemistry 272(12) (1997) 7899–7907. [DOI] [PubMed] [Google Scholar]
- [49].Crofts AR, The cytochrome bc 1 complex: function in the context of structure, Annu. Rev. Physiol. 66 (2004) 689–733. [DOI] [PubMed] [Google Scholar]
- [50].Garber Morales J, Holmes-Hampton GP, Miao R,. Mu nck Guo, Lindahl PA, Biophysical characterization of iron in mitochondria isolated from respiring and fermenting yeast, Biochemistry 49(26) (2010) 5436–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cho I-T, Adelmant G, Lim Y, Marto JA, Cho G, Golden JA, Ascorbate peroxidase proximity labeling coupled with biochemical fractionation identifies promoters of endoplasmic reticulum mitochondrial contacts, Journal of Biological Chemistry (2017) jbc. M117. 795286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yang Z, Philips JD, Doty RT, Giraudi P, Ostrow JD, Tiribelli C, Smith A, Abkowitz JL, Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin, Journal of Biological Chemistry 285(37) (2010) 28874–28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Quigley JG, Burns CC, Anderson MM, Lynch ED, Sabo KM, Overbaugh J, Abkowitz JL, Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia, Blood 95(3) (2000) 1093–1099. [PubMed] [Google Scholar]
- [54].Chiabrando D, Marro S, Mercurio S, Giorgi C, Petrillo S, Vinchi F, Fiorito V, Fagoonee S, Camporeale A, Turco E, The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation, The Journal of clinical investigation 122(12) (2012) 4569–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Khan AA, Quigley JG, Heme and FLVCR-related transporter families SLC48 and SLC49, Molecular aspects of medicine 34(2–3) (2013) 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Korolnek T, Zhang J, Beardsley S, George L Scheffer, I. Hamza, Control of Metazoan Heme Homeostasis by a Conserved Multidrug Resistance Protein, Cell Metabolism 19(6) (2014) 1008–1019 doi 10.1016/j.cmet.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sturm A, Schierhorn A, Lindenstrauss U, Lilie H, Brüser T, YcdB from Escherichia coli reveals a novel class of Tat-dependently translocated hemoproteins, Journal of Biological Chemistry 281(20) (2006) 13972–13978. [DOI] [PubMed] [Google Scholar]
- [58].Tudor C, Lerner-Marmarosh N, Engelborghs Y, Gibbs PEM, Maines MD, Biliverdin Reductase is a Transporter of Heme into the Nucleus and is Essential to regulation of HO-1 Gene Expression by Hematin, The Biochemical journal 413(3) (2008) 405–416 doi 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vinchi F, Ingoglia G, Chiabrando D, Mercurio S, Turco E, Silengo L, Altruda F, Tolosano E, Heme exporter FLVCR1a regulates heme synthesis and degradation and controls activity of cytochromes P450, Gastroenterology 146(5) (2014) 1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fiorito V, Forni M, Silengo L, Altruda F, Tolosano E, Crucial role of Flvcr1a in the maintenance of intestinal heme homeostasis, Antioxidants & redox signaling 23(18) (2015) 1410–1423. [DOI] [PubMed] [Google Scholar]
- [61].Mercurio S, Petrillo S, Chiabrando D, Bassi ZI, Gays D, Camporeale A, Vacaru A, Miniscalco B, Valperga G, Silengo L, The heme exporter Flvcr1 regulates expansion and differentiation of committed erythroid progenitors by controlling intracellular heme accumulation, haematologica 100(6) (2015) 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Petrillo S, Chiabrando D, Genova T, Fiorito V, Ingoglia G, Vinchi F, Mussano F, Carossa S, Silengo L, Altruda F, Heme accumulation in endothelial cells impairs angiogenesis by triggering paraptosis, Cell Death & Differentiation 25(3) (2018) 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M, Hemoglobin and heme scavenging, IUBMB life 57(11) (2005) 749–759. [DOI] [PubMed] [Google Scholar]
- [64].Taketani S, Adachi Y, Kohno H, Ikehara S, Tokunaga R, Ishii T, Molecular characterization of a newly identified heme-binding protein induced during differentiation of urine erythroleukemia cells, Journal of Biological Chemistry 273(47) (1998) 31388–31394. [DOI] [PubMed] [Google Scholar]
- [65].Iwahara S.-i., Satoh H, Song D-X, Webb J, Burlingame AL, Nagae Y, Muller-Eberhard U, Purification, characterization, and cloning of a heme-binding protein (23 kDa) in rat liver cytosol, Biochemistry 34(41) (1995) 13398–13406. [DOI] [PubMed] [Google Scholar]
- [66].Ketley JN, Habig W, Jakoby W, Binding of nonsubstrate ligands to the glutathione S-transferases, Journal of Biological Chemistry 250(22) (1975) 8670–8673. [PubMed] [Google Scholar]
- [67].Zylka MJ, Reppert SM, Discovery of a putative heme-binding protein family (SOUL/HBP) by two-tissue suppression subtractive hybridization and database searches, Molecular brain research 74(1–2) (1999) 175–181. [DOI] [PubMed] [Google Scholar]
- [68].Vincent SH, Muller-Eberhard U, A protein of the Z class of liver cytosolic proteins in the rat that preferentially binds heme, Journal of Biological Chemistry 260(27) (1985) 14521–14528. [PubMed] [Google Scholar]
- [69].Watanabe Y, Ishimori K, Uchida T, Dual role of the active-center cysteine in human peroxiredoxin 1: Peroxidase activity and heme binding, Biochemical and Biophysical Research Communications 483(3) (2017) 930–935 doi 10.1016/j.bbrc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- [70].Chakravarti R, Aulak KS, Fox PL, Stuehr DJ, GAPDH regulates cellular heme insertion into inducible nitric oxide synthase, Proceedings of the National Academy of Sciences 107(42) (2010) 18004–18009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sweeny EA, Singh AB, Chakravarti R, Martinez-Guzman O, Saini A, Haque MM, Garee G, Dans PD, Hannibal L, Reddi AR, and Stuehr DJ, Glyceraldehyde 3-phosphate dehydrogenase is a chaperone that allocates bioavailable heme in cells, J. Biol. Chem. accepted. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P, An ER-mitochondria tethering complex revealed by a synthetic biology screen, Science 325(5939) (2009) 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hayashi T, Rizzuto R, Hajnoczky G, Su T-P, MAM: more than just a housekeeper, Trends in cell biology 19(2) (2009) 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, De Camilli P, Contacts between the endoplasmic reticulum and other membranes in neurons, Proceedings of the National Academy of Sciences 114(24) (2017) E4859–E4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang L, Hach A, Molecular mechanism of heme signaling in yeast: the transcriptional activator Hap1 serves as the key mediator, Cellular and Molecular Life Sciences CMLS 56(5–6) (1999) 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, Li D, Wu Y, Shang Y, Kong X, Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function, Cell reports 7(1) (2014) 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, Igarashi K, Heme induces ubiquitination and degradation of the transcription factor Bach1, Molecular and cellular biology 27(19) (2007) 6962–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Carter EL, Gupta N, Ragsdale SW, High affinity heme binding to a heme regulatory motif on the nuclear receptor Rev-erbβ leads to its degradation and indirectly regulates its interaction with nuclear receptor corepressor, Journal of Biological Chemistry (2015) jbc. M115. 670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yuan X, Rietzschel N, Kwon H, Nuno ABW, Hanna DA, Phillips JD, Raven EL, Reddi AR, Hamza I, Regulation of intracellular heme trafficking revealed by subcellular reporters, Proceedings of the National Academy of Sciences 113(35) (2016) E5144–E5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Poston CN, Duong E, Cao Y, Bazemore-Walker CR, Proteomic analysis of lipid raft-enriched membranes isolated from internal organelles, Biochemical and biophysical research communications 415(2) (2011) 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Duffy SP, Shing J, Saraon P, Berger LC, Eiden MV, Wilde A, Tailor CS, The Fowler syndrome-associated protein FLVCR2 is an importer of heme, Molecular and cellular biology 30(22) (2010) 5318–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Inoue K, Nakai Y, Ueda S, Kamigaso S, K.-y. Ohta, M. Hatakeyama, Y. Hayashi, M. Otagiri, H. Yuasa, Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model, American Journal of Physiology-Gastrointestinal and Liver Physiology 294(3) (2008) G660–G668 doi 10.1152/ajpgi.00309.2007. [DOI] [PubMed] [Google Scholar]
- [83].Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Identification of an intestinal heme transporter, Cell 122(5) (2005) 789–801. [DOI] [PubMed] [Google Scholar]
- [84].Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID, Identification of an Intestinal Folate Transporter and the Molecular Basis for Hereditary Folate Malabsorption, Cell 127(5) (2006) 917–928 doi 10.1016/j.cell.2006.09.041.. [DOI] [PubMed] [Google Scholar]
- [85].Blanc SL, Garrick MD, Arredondo M, Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism, American Journal of Physiology-Cell Physiology 302(12) (2012) C1780–C1785 doi 10.1152/ajpcell.00080.2012. [DOI] [PubMed] [Google Scholar]
- [86].Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ, Heme carrier protein (HCP‐1) spatially interacts with the CD163 hemoglobin uptake pathway and is a target of inflammatory macrophage activation, Journal of Leukocyte Biology 83(2) (2008) 325–333 doidoi: 10.1189/jlb.0407226. [DOI] [PubMed] [Google Scholar]
- [87].Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Krause M, Hamza I, Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins, Nature 453 (2008) 1127 doi 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, Ward DM, Liu P, Fleming MD, Hamza I, HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis, Cell metabolism 17(2) (2013) 261–270 doi 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mourer T, Jacques J-F, Brault A, Bisaillon M, Labbé S, Shu1 is a cell-surface protein involved in iron acquisition from heme in Schizosaccharomyces pombe, Journal of Biological Chemistry (2015) jbc. M115. 642058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Normant V, Mourer T, Labbe S, The major facilitator transporter Str3 is required for low-affinity heme acquisition in Schizosaccharomyces pombe, Journal of Biological Chemistry (2018) jbc. RA118. 002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mourer T, Normant V, Labbé S, Heme Assimilation in Schizosaccharomyces pombe Requires Cell-surface-anchored Protein Shu1 and Vacuolar Transporter Abc3, The Journal of Biological Chemistry 292(12) (2017) 4898–4912 doi 10.1074/jbc.M117.776807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Garton T, Keep RF, Hua Y, Xi G, CD163, a Hemoglobin/Haptoglobin Scavenger Receptor, After Intracerebral Hemorrhage: Functions in Microglia/Macrophages Versus Neurons, Translational Stroke Research 8(6) (2017) 612–616 doi 10.1007/s12975-017-0535-5. [DOI] [PubMed] [Google Scholar]
- [93].Liu R, Cao S, Hua Y, Keep RF, Huang Y, Xi G, CD163 expression in neurons after experimental intracerebral hemorrhage, Stroke 48(5) (2017) 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rao AU, Carta LK, Lesuisse E, Hamza I, Lack of heme synthesis in a free-living eukaryote, Proceedings of the National Academy of Sciences of the United States of America 102(12) (2005) 4270–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tripodi KE, Menendez Bravo SM, Cricco JA, Role of heme and heme-proteins in trypanosomatid essential metabolic pathways, Enzyme research 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cabello‐Donayre M, Malagarie‐Cazenave S, Campos‐Salinas J, Gálvez FJ, Rodríguez‐Martínez A, Pineda‐Molina E, Orrego LM, Martínez‐García M, Sánchez‐Cañete MP, Estévez AM, Trypanosomatid parasites rescue heme from endocytosed hemoglobin through lysosomal HRG transporters, Molecular microbiology 101(6) (2016) 895–908. [DOI] [PubMed] [Google Scholar]
- [97].Puy H, Gouya L, Deybach J-C, Porphyrias, The Lancet 375(9718) (2010) 924–937 doi 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- [98].Hada H, Shiraki T, Watanabe-Matsui M, Igarashi K, Hemopexin-dependent heme uptake via endocytosis regulates the Bach1 transcription repressor and heme oxygenase gene activation, Biochimica et Biophysica Acta (BBA)-General Subjects 1840(7) (2014) 2351–2360. [DOI] [PubMed] [Google Scholar]
- [99].Chasis JA, Mohandas N, Erythroblastic islands: niches for erythropoiesis, Blood 112(3) (2008) 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, Van Bruggen R, Of macrophages and red blood cells; a complex love story, Frontiers in physiology 5 (2014) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Korolnek T, Hamza I, Macrophages and iron trafficking at the birth and death of red cells, Blood 125(19) (2015) 2893–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Marciano O, Moskovitz Y, Hamza I, Ruthstein S, Histidine residues are important for preserving the structure and heme binding to the C. elegans HRG-3 hemetrafficking protein, JBIC Journal of Biological Inorganic Chemistry 20(8) (2015) 1253–1261. [DOI] [PubMed] [Google Scholar]
- [103].Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, science 306(5704) (2004) 2090–2093. [DOI] [PubMed] [Google Scholar]
- [104].Kim B-E, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ, Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs, Cell metabolism 11(5) (2010) 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sinclair J, Pinter K, Samuel T, Beardsley S, Yuan X, Zhang J, Meng K, Yun S, Krause M, Hamza I, Inter-organ signalling by HRG-7 promotes systemic haem homeostasis, Nature cell biology 19(7) (2017) 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mense SM, Zhang L, Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases, Cell research 16(8) (2006) 681. [DOI] [PubMed] [Google Scholar]
- [107].Igarashi K, Sun J, The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation, Antioxidants & redox signaling 8(1–2) (2006) 107–118. [DOI] [PubMed] [Google Scholar]
- [108].Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F, Identification of heme as the ligand for the orphan nuclear receptors REV- RBα and R V- RBβ, Nature structural & molecular biology 14(12) (2007) 1207–1213 doi 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang L, Guarente L, Heme binds to a short sequence that serves a regulatory function in diverse proteins, The EMBO journal 14(2) (1995) 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pfeifer K, Kim K-S, Kogan S, Guarente L, Functional dissection and sequence of yeast HAP1 activator, Cell 56(2) (1989) 291–301. [DOI] [PubMed] [Google Scholar]
- [111].Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1, The EMBO journal 20(11) (2001) 2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways, Science 318(5857) (2007) 1786–1789. [DOI] [PubMed] [Google Scholar]
- [113].Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy, Nature medicine 19(8) (2013) 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Motterlini R, Foresti R, Biological signaling by carbon monoxide and carbon monoxide-releasing molecules, American Journal of Physiology-Cell Physiology 312(3) (2017) C302–C313. [DOI] [PubMed] [Google Scholar]
- [115].Wegiel B, Gallo D, Csizmadia E, Roger T, Kaczmarek E, Harris C, Zuckerbraun BS, Otterbein LE, Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase, Proceedings of the National Academy of Sciences 108(46) (2011) 18849–18854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wu J, Ma J, Fan S-T, Schlitt HJ, Tsui T-Y, Bilirubin derived from heme degradation suppresses MHC class II expression in endothelial cells, Biochemical and biophysical research communications 338(2) (2005) 890–896. [DOI] [PubMed] [Google Scholar]
- [117].Liu Y, Li P, Lu J, Xiong W, Oger J, Tetzlaff W, Cynader M, Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis, The Journal of Immunology 181(3) (2008) 1887–1897. [DOI] [PubMed] [Google Scholar]
- [118].Hou S, Reynolds MF, Horrigan FT, Heinemann SH, Hoshi T, Reversible binding of heme to proteins in cellular signal transduction, Accounts of chemical research 39(12) (2006) 918–924. [DOI] [PubMed] [Google Scholar]
- [119].Burton MJ, Kapetanaki SM, Chernova T, Jamieson AG, Dorlet P, Santolini J, Moody PC, Mitcheson JS, Davies NW, Schmid R, A heme-binding domain controls regulation of ATP-dependent potassium channels, Proceedings of the National Academy of Sciences 113(14) (2016) 3785–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Barr I, Smith AT, Chen Y, Senturia R, Burstyn JN, Guo F, Ferric, not ferrous, heme activates RNA-binding protein DGCR8 for primary microRNA processing, Proceedings of the National Academy of Sciences 109(6) (2012) 1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graça-Souza AV, Bozza MT, Characterization of heme as activator of Toll-like receptor 4, Journal of Biological Chemistry 282(28) (2007) 20221–20229. [DOI] [PubMed] [Google Scholar]
- [122].Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM, Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease, Blood 123(3) (2014) 377–390 doi 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wegiel B, Hauser CJ, Otterbein LE, Heme as a danger molecule in pathogen recognition, Free Radical Biology and Medicine 89 (2015) 651–661 doi 10.1016/j.freeradbiomed.2015.08.020. [DOI] [PubMed] [Google Scholar]
- [124].Hvidberg V, Maniecki MB, Jacobsen C, Højrup P, Møller HJ, Moestrup SK, Identification of the receptor scavenging hemopexin-heme complexes, Blood 106(7) (2005) 2572–2579 doi 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- [125].Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT, Hemolysis-induced lethality involves inflammasome activation by heme, Proceedings of the National Academy of Sciences 111(39) (2014) E4110–E4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sankar SB, Donegan RK, Shah KJ, Reddi AR, Wood LB, Heme and hemoglobin suppress amyloid β–mediated inflammatory activation of mouse astrocytes, Journal of Biological Chemistry (2018) jbc. RA117. 001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Stec DE, John K, Trabbic CJ, Luniwal A, Hankins MW, Baum J, Hinds TD, Bilirubin Binding to PPARα Inhibits Lipid Accumulation, PLoS ON 11(4) (2016) e0153427 doi 10.1371/journal.pone.0153427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang T, Bu P, Zeng J, Vancura A, Increased heme synthesis in yeast induces a metabolic switch from fermentation to respiration even under conditions of glucose repression, Journal of Biological Chemistry 292(41) (2017) 16942–16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tu BP, McKnight SL, Evidence of carbon monoxide-mediated phase advancement of the yeast metabolic cycle, Proceedings of the National Academy of Sciences 106(34) (2009) 14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL, Cyclic changes in metabolic state during the life of a yeast cell, Proceedings of the National Academy of Sciences 104(43) (2007) 16886–16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hannibal L, Collins D, Brassard J, Chakravarti R, Vempati R, Dorlet P, J.r.m. Santolini, J.H. Dawson, D.J. Stuehr, Heme binding properties of glyceraldehyde-3-phosphate dehydrogenase, Biochemistry 51(43) (2012) 8514–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Stewart JM, Slysz GW, Pritting MA, Muller-Eberhard U, Ferriheme and ferroheme are isosteric inhibitors of fatty acid binding to rat liver fatty acid binding protein, Biochemistry and Cell Biology 74(2) (1996) 249–255 doi 10.1139/o96-026. [DOI] [PubMed] [Google Scholar]
- [133].Reedy CJ, Gibney BR, Heme protein assemblies, Chemical reviews 104(2) (2004) 617–650. [DOI] [PubMed] [Google Scholar]
- [134].Kathiresan M, Martins D, English AM, Respiration triggers heme transfer from cytochrome c peroxidase to catalase in yeast mitochondria, Proceedings of the National Academy of Sciences 111(49) (2014) 17468–17473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Waheed SM, Ghosh A, Chakravarti R, Biswas A, Haque MM, Panda K, Stuehr DJ, Nitric oxide blocks cellular heme insertion into a broad range of heme proteins, Free Radical Biology and Medicine 48(11) (2010) 1548–1558 doi 10.1016/j.freeradbiomed.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yee EL, Pitt BR, Billiar TR, Kim Y-M, Effect of nitric oxide on heme metabolism in pulmonary artery endothelial cells, American Journal of Physiology-Lung Cellular and Molecular Physiology 271(4) (1996) L512–L518. [DOI] [PubMed] [Google Scholar]
- [137].Cooper CE, Nitric oxide and iron proteins, Biochimica et Biophysica Acta (BBA)-Bioenergetics 1411(2–3) (1999) 290–309. [DOI] [PubMed] [Google Scholar]
- [138].Herzik MA, Jonnalagadda R, Kuriyan J, Marletta MA, Structural insights into the role of iron–histidine bond cleavage in nitric oxide-induced activation of H-NOX gas sensor proteins, Proceedings of the National Academy of Sciences 111(40) (2014) E4156–E4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Weichsel A, Maes EM, Andersen JF, Valenzuela JG, Shokhireva TK, Walker FA, Montfort WR, Heme-assisted S-nitrosation of a proximal thiolate in a nitric oxide transport protein, Proceedings of the National Academy of Sciences of the United States of America 102(3) (2005) 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Pietri R, Román-Morales E, López-Garriga J, Hydrogen Sulfide and Hemeproteins: Knowledge and Mysteries, Antioxidants & Redox Signaling 15(2) (2011) 393–404 doi 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kakar S, Hoffman FG, Storz JF, Fabian M, Hargrove MS, Structure and reactivity of hexacoordinate hemoglobins, Biophysical chemistry 152(1–3) (2010) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Koder RL, Anderson JR, Solomon LA, Reddy KS, Moser CC, Dutton PL, Design and engineering of an O 2 transport protein, Nature 458(7236) (2009) 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sher EA, Shaklai M, Shaklai N, Carbon monoxide promotes respiratory hemoproteins iron reduction using peroxides as electron donors, PLoS One 7(3) (2012) e33039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chen AJ, Yuan X, Li J, Dong P, Hamza I, Cheng J-X, Label-Free Imaging of Heme Dynamics in Living Organisms by Transient Absorption Microscopy, Analytical chemistry 90(5) (2018) 3395–3401 doi 10.1021/acs.analchem.7b05046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Rohde KH, Gillaspy AF, Hatfield MD, Lewis LA, Dyer DW, Interactions of haemoglobin with the Neisseria meningitidis receptor HpuAB: the role of TonB and an intact proton motive force, Molecular Microbiology 43(2) (2002) 335–354 doidoi: 10.1046/j.1365-2958.2002.02745.x. [DOI] [PubMed] [Google Scholar]
- [146].Nobles CL, Maresso AW, The theft of host heme by Gram-positive pathogenic bacteria, Metallomics 3(8) (2011) 788–796. [DOI] [PubMed] [Google Scholar]
- [147].Runyen-Janecky LJ, Role and regulation of heme iron acquisition in gramnegative pathogens, Frontiers in cellular and infection microbiology 3 (2013) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Grigg JC, Vermeiren CL, Heinrichs DE, Murphy MEP, Haem recognition by a Staphylococcus aureus NEAT domain, Molecular Microbiology 63(1) (2007) 139–149 doidoi: 10.1111/j.1365-2958.2006.05502.x. [DOI] [PubMed] [Google Scholar]
- [149].Maresso AW, Chapa TJ, Schneewind O, Surface protein IsdC and Sortase B are required for heme-iron scavenging of Bacillus anthracis, Journal of bacteriology 188(23) (2006) 8145–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Moriwaki Y, Terada T, Caaveiro JMM, Takaoka Y, Hamachi I, Tsumoto K, Shimizu K, Heme Binding Mechanism of Structurally Similar Iron-Regulated Surface Determinant Near Transporter Domains of Staphylococcus aureus Exhibiting Different Affinities for Heme, Biochemistry 52(49) (2013) 8866–8877 doi 10.1021/bi4008325. [DOI] [PubMed] [Google Scholar]
- [151].Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O, Passage of Heme-Iron Across the Envelope of Staphylococcus aureus, Science 299(5608) (2003) 906–909 doi 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- [152].Allen CE, Schmitt MP, Novel hemin binding domains in the Corynebacterium diphtheriae HtaA protein interact with hemoglobin and are critical for heme iron utilization by HtaA, Journal of bacteriology 193(19) (2011) 5374–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Allen CE, Schmitt MP, HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae, Journal of bacteriology 191(8) (2009) 2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, McMath LM, Iniguez A, Kimmey JM, Sawaya MR, Whitelegge JP, Discovery and characterization of a unique mycobacterial heme acquisition system, Proceedings of the National Academy of Sciences 108(12) (2011) 5051–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Mitra A, Speer A, Lin K, Ehrt S, Niederweis M, PPE surface proteins are required for heme utilization by Mycobacterium tuberculosis. mBio 8: e01720–16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Burkhard KA, Wilks A, Characterization of the Outer Membrane Receptor ShuA from the Heme Uptake System of Shigella dysenteriae Substrate specificity and identification of the heme protein ligands, Journal of Biological Chemistry 282(20) (2007) 15126–15136. [DOI] [PubMed] [Google Scholar]
- [157].Wei Y, Murphy ER, Shigella iron acquisition systems and their regulation, Frontiers in cellular and infection microbiology 6 (2016) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Tong Y, Guo M, Bacterial heme-transport proteins and their heme-coordination modes, Archives of Biochemistry and Biophysics 481(1) (2009) 1–15 doi 10.1016/j.abb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Ochsner UA, Johnson Z, Vasil ML, Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa, Microbiology 146(1) (2000) 185–198. [DOI] [PubMed] [Google Scholar]
- [160].Weissman Z, Kornitzer D, A family of Candida cell surface haem‐binding proteins involved in haemin and haemoglobin‐iron utilization, Molecular Microbiology 53(4) (2004) 1209–1220 doidoi: 10.1111/j.1365-2958.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- [161].Kuznets G, Vigonsky E, Weissman Z, Lalli D, Gildor T, Kauffman SJ, Turano P, Becker J, Lewinson O, Kornitzer D, A Relay Network of Extracellular Heme-Binding Proteins Drives C. albicans Iron Acquisition from Hemoglobin, PLOS Pathogens 10(10) (2014) e1004407 doi 10.1371/journal.ppat.1004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Weissman Z, Shemer R, Conibear E, Kornitzer D, An endocytic mechanism for haemoglobin‐iron acquisition in Candida albicans, Molecular microbiology 69(1) (2008) 201–217. [DOI] [PubMed] [Google Scholar]
- [163].Hu G, Caza M, Cadieux B, Chan V, Liu V, Kronstad J, Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, for capsule formation, and for virulence, Infection and immunity 81(1) (2013) 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lansky IB, Lukat-Rodgers GS, Block D, Rodgers KR, Ratliff M, Wilks A, The cytoplasmic heme-binding protein (PhuS) from the heme uptake system of Pseudomonas aeruginosa is an intracellular heme-trafficking protein to the δ-regioselective heme oxygenase, Journal of Biological Chemistry 281(19) (2006) 1365213662. [DOI] [PubMed] [Google Scholar]
- [165].Wyckoff EE, Lopreato GF, Tipton KA, Payne SM, Shigella dysenteriae ShuS promotes utilization of heme as an iron source and protects against heme toxicity, Journal of bacteriology 187(16) (2005) 5658–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson Kelsi L., Dunman PM, Joyce S, Skaar EP, A Staphylococcus aureus Regulatory System that Responds to Host Heme and Modulates Virulence, Cell Host & Microbe 1(2) (2007) 109–119 doi 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Pendrak ML, Chao MP, Yan SS, Roberts DD, Heme oxygenase in Candida albicans is regulated by hemoglobin and is necessary for metabolism of exogenous heme and hemoglobin to α-biliverdin, Journal of Biological Chemistry 279(5) (2004) 3426–3433. [DOI] [PubMed] [Google Scholar]
- [168].Kranz RG, Richard-Fogal C, Taylor J-S, Frawley ER, Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control, Microbiology and molecular biology reviews 73(3) (2009) 510–528. [DOI] [PMC free article] [PubMed] [Google Scholar]