Abstract
Background:
Previous simultaneous liver-kidney transplant (SLK) allocation was based on serum creatinine, a metric that disadvantaged women relative to men. A recent SLK policy change utilizes eGFR, which accounts for sex-based differences in creatinine.
Methods:
To understand the impact of this new policy, we analyzed nonstatus 1 adults listed for liver transplantation (LT) from 5/2007–7/2014, excluding those with exceptions. We defined patients who met the new SLK policy as having an eGFR<60 ml/min for 90 days, with a final eGFR<30 ml/min.
Results:
Of 40 979 candidates, 1683 would have met only the new criteria (N-SLK), 2452 would have met only the old criteria (O-SLK), and 1878 would have met both criteria (B-SLK). Compared to those in the B-SLK or O-SLK groups, those in the N-SLK group were significantly more likely to be female (52v.36v.39%, p<0.001). Cox-regression analysis demonstrated that in adjusted analysis those in the N-SLK group were significantly less likely to die post liver transplant (HR 0.03,p<0.001). Further, in cox regression subgroup analyses both in women (HR 0.04,p<0.001) and in men (HR 0.02, p<0.001) those in the N-SLK group who underwent liver transplant were significantly less likely to die post liver transplant, even after adjustment for confounders.
Conclusions:
We anticipate that implementation of the new SLK policy will increase the proportion of women and decrease the proportion of men who are listed for SLK but may not improve posttransplant survival. Our data highlight the need for monitoring of SLK outcomes after implementation of the new policy.
Introduction
The decision to list a patient for a simultaneous liver-kidney transplant (SLK) has important clinical implications: not only does SLK involve greater operative complexity than liver transplantation alone, it takes a scarce resource – a kidney – from the already depleted deceased donor kidney pool. Despite this, the rates of SLK have risen substantially over the last decade, with 4241 patients undergoing SLK since 2005 (1).
Until recently, there were no standardized criteria for listing for SLK, and this decision was made based on subjective criteria, leading to significant regional variations in its use (2). These data prompted the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) on August 10, 2017 to implement the new SLK allocation policy to standardize utilization.
This policy change marked a dramatic transition from utilizing serum creatinine, a metric that disadvantaged women relative to men, to using formulas that estimate glomerular filtration rate (eGFR). It has been well established that women are underserved by the model for end-stage liver disease including serum sodium (MELDNa) score (3–5), a shortcoming driven predominantly by women having on average lower serum creatinine levels (6). We hypothesized that the implementation of the new SLK allocation policy, specifically the transition to formulas that estimate GFR, would disproportionately impact SLK listings among women relative to men. This study aimed to determine what effect this impact may have on post liver transplant outcomes.
Methods
All patients listed for liver transplantation in the UNOS/OPTN registry from May 1st 2007 through July 1st 2014, which represents a time frame of relatively stable liver allocation policy, were evaluated for inclusion in this study. These dates were chosen for use of the Social Security Death Master File for a valid assessment of post liver transplantation survival. Patients who were less than 18 years old or listed as Status 1, including those with fulminant hepatic failure were excluded. Those who received exceptions points or underwent a living donor liver transplantation were also excluded, as these patients have a likelihood of receiving a liver transplant that is independent of their renal function.
Covariates
Data were obtained from the UNOS/OPTN registry as of June 17th, 2016. Data included gender, age at listing, race, height, weight, ABO blood group, UNOS region (1–11), date of listing, etiologies of liver disease, death date, date of removal from the waitlist, reason for removal, transplant date, insurance status, and Karnofsky Performance Status score. The following data were collected at listing and delisting: total bilirubin, international normalized ratio (INR), serum creatinine, presence of hepatic encephalopathy, and presence of ascites. Serum creatinine and estimated glomerular filtration rate (eGFR) were determined longitudinally from the time of listing for liver transplantation to removal from the liver transplant waitlist. Cutoffs deemed to be implausible were as follows: height <120 and >240 cm, weight <30 and >180 kg, total bilirubin ≤ 0 mg/dL, INR ≤ 0, and creatinine ≤ 0 mg/dL (7). The MELDNa score was calculated using the standard formula (8) using a lower limit of 1 for all variables. MELDNa scores were capped at 6 and 40, per current liver allocation policy.
Listing diagnoses were grouped into the following common diagnostic categories hepatitis C virus (HCV), nonalcoholic fatty liver disease (NAFLD, including cryptogenic cirrhosis and nonalcoholic steatohepatitis), alcoholic cirrhosis, autoimmune etiologies (including primary biliary cirrhosis, primary sclerosing cholangitis and autoimmune hepatitis), and other etiologies of cirrhosis (any other listing code that met inclusion criteria).
Renal function, hemodialysis status, and SLK allocation policy
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine based equation (9). We chose this equation, because of the GFR calculators that can be used with the data available in the UNOS/OPTN registry, the CKD-EPI creatinine based equation most closely estimates GFR relative to GFR as measured by iothalamate clearance in patients with cirrhosis (10–13). Those on hemodialysis were treated as having an eGFR <30 ml/min (14) and were not included in the descriptive statistics for serum creatinine and eGFR. Those meeting the new SLK allocation policy were defined based on the following criteria: eGFR <60 mL/min for 90 days with a final eGFR <30 mL/min. Those meeting the old SLK allocation policy were determined as being listed for SLK in the UNOS/OPTN registry.
We defined SLK study groups as follows:
N-SLK: patients who only met the new SLK criteria (ie, those patients who will now qualify for SLK under the new policy but received a liver transplant alone under the old policy)
O-SLK: patients who only met the old SLK criteria (ie, those patients who will not qualify for SLK under the new policy but received a SLK under the old policy)
B-SLK: patients who met both the old and the new SLK criteria (ie, those patients who will qualify for SLK under the new policy and received a SLK under the old policy)
All observations given in parentheses are given in the following order: (N-SLK v. B-SLK v. O-SLK).
Outcomes and censoring
We sought to examine the impact that the new SLK allocation policy will have on post liver transplant outcomes. The primary outcome was death after liver transplant. Patient follow-up began on the date of listing for liver transplantation and ended at the time of death, regardless of the outcome on the liver transplantation waitlist. Those who underwent liver transplantation were followed to last data update with special attention when death occurred by referencing the Social Security Death Master File.
Statistical analysis
Continuous variables were compared between groups by rank-sum or Kruskall-Wallis. Categorical variables were compared between groups by chi-square test. Unadjusted models were used to assess the association of all listed covariates. All covariates with a p<0.2 in univariate analysis were considered for inclusion in multivariate models. Sequential backward selection was used to eliminate those not reaching significance of p<0.05. Cox-regression analysis was used to assess the association between SLK Group and other covariates with death after liver transplant in all patients who met either or both SLK criteria. We hypothesized that under the new SLK policy women will represent a larger proportion of patients listed for SLK than before. This prompted a subgroup analysis looking at post liver transplant mortality in women and men.
Two-sided p values <0.05 were considered statistically significant. Analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco.
Results
Demographics of those meeting SLK criteria
From May 1st 2007 through July 1st 2014, 83 397 patients were listed for liver transplantation in the United States. Of the 40 980 patients who were included in this study, 2452 (6%) would have met only the old SLK criteria (O-SLK), 1638 (4%) would have met only the new SLK criteria (N-SLK), and 1878 (5%) would have met both SLK criteria (B-SLK) (Figure 1). To demonstrate the changes expected by the new policy, we compared the baseline characteristics by SLK Group (Table 1). First, compared to the B-SLK and O-SLK groups, the N-SLK group was significantly more likely to be female (52 v. 39 v. 36%, p<0.001). Additionally, compared to the B-SLK and O-SLK groups, the N-SLK group was more likely to be older (59 v. 59 v. 57 years, p<0.001), to have a listing diagnosis of NASH (30 v. 25 v. 25%, p<0.001), to be non-Hispanic white (74 v. 58 v. 66%, p<0.001), and were less likely to be on hemodialysis (48 v. 74 v. 58%, p<0.001).
Figure 1. Flow diagram of patients included in this study.
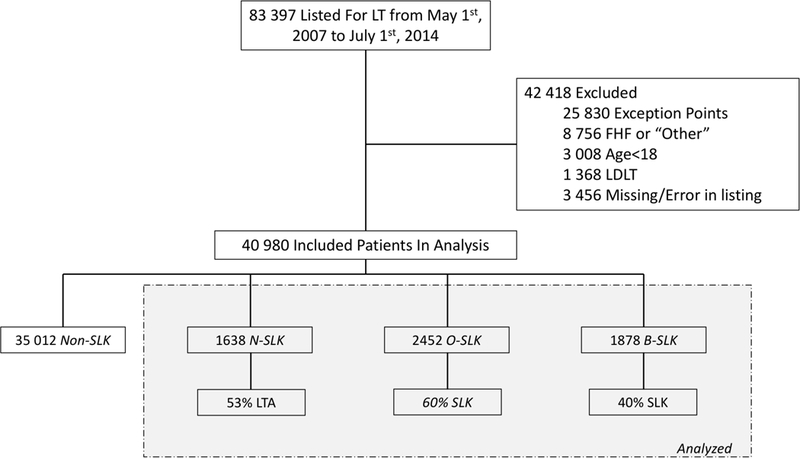
Definitions = Non-SLK, patients met neither SLK criteria; N-SLK, patients met only the new SLK criteria; O-SLK, patients met only the old SLK criteria; B-SLK, patients met both SLK criteria; LTA, % patients who received a transplant, all of which were liver transplant alone; SLK, % patients who received a transplant, all of which were SLK; Abbreviations = HCC, hepatocellular carcinoma; FHF = fulminant hepatic failure; AHN = acute hepatic necrosis; LDLT = living donor liver transplant
Table 1.
Characteristics of patients listed for SLK in the United States from May 1st 2007 to July 1st 2014 under the old policy alone, the new policy alone, both policies, or neither policy.
| Non-SLK (n=35 012) |
O-SLK (n=2452) |
N-SLK (n=1638) |
B-SLK (N=1878) |
P value |
|
|---|---|---|---|---|---|
| Days on waitlist, m(IQR) | 197 (28 – 799) | 33 (12 – 83) | 288 (160 – 627) | 377 (182 – 803) | <0.001 |
| Age at delisting, m(IQR) | 56 (50 – 62) | 57 (51 – 62) | 59 (54 – 65) | 59 (53 – 64) | <0.001 |
| Female sex, no (%) | 13 050 (37) | 881 (36) | 843 (52) | 730 (39) | <0.001 |
| Listing diagnosis, no. (%) | |||||
| Alcohol | 7630 (22) | 613 (25) | 262 (16) | 356 (19) | <0.001 |
| HCV | 13 077 (37) | 852 (35) | 615 (38) | 765 (41) | |
| NAFLD/NASH | 7060 (20) | 613 (25) | 483 (30) | 476 (25) | |
| Autoimmune1 | 4815 (14) | 181 (7) | 220 (13) | 118 (6) | |
| Other | 2429 (7) | 193 (8) | 58 (4) | 163 (9) | |
|
Non-Hispanic white, no. (%) |
25708 (73) | 1622 (66) | 1216 (74) | 1087 (58) | <0.001 |
| Ascites, no. (%) | 13214 (38) | 1441(59) | 920 (56) | 940 (50) | <0.001 |
| Diabetes, no. (%) | 7516 (22) | 814 (33) | 509 (31) | 846 (45) | <0.001 |
|
Hepatic encephalopathy, no. (%) |
5740 (16) | 609 (25) | 469 (29) | 328 (18) | <0.001 |
|
MELDNa at listing, m(IQR) |
19 (14 – 26) | 29 (22 – 36) | 18 (15 – 23) | 21 (19 – 24) | <0.001 |
|
MELDNa at delisting, m(IQR) |
23 (15 – 32) | 32 (25 – 39) | 34 (28 – 40) | 26 (22 – 33) | <0.001 |
|
Final CKD-EPI eGFR, ml/min, m(IQR)* |
69 (42 – 97) | 32 (20 – 45) | 22 (17 – 26) | 18 (12 – 23) | <0.001 |
|
Final MDRD4 eGFR, ml/min, m(IQR)* |
71 (46 – 98) | 33 (21 – 45) | 23 (18 – 27) | 19 (13 – 24) | <0.001 |
|
Final serum creatinine, mg/dL, m(IQR)* |
1.1 (0.8 – 1.5) | 2.1 (1.6 – 3.1) | 2.7 (2.2 – 3.3) | 3.3 (2.7 – 4.6) | <0.001 |
|
Hemodialysis at Delisting, no. (%) |
3235 (9) | 1425 (58) | 792 (48) | 1385 (74) | <0.001 |
| Waitlist Outcome, no. (%) | <0.001 | ||||
| Still Waiting | 9915 (28) | 220 (9) | 143 (8) | 419 (22) | |
| ”Too Sick” or Death | 9140 (26) | 751 (31) | 631 (39) | 717 (38) | |
| DDLT | 15 956 (46) | 1481 (60) | 864 (53) | 742 (40) |
Table Legend: Hepatitis C (HCV); Nonalcoholic Steatohepatitis (NASH); CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration); Modification of Diet in Renal Disease (MDRD); Estimated Glomerular Filtration Rate (eGFR); International Normalized Ratio (INR); Model for End-Stage Liver Disease including Sodium (MELDNa); median (m); interquartile range (IQR)
autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis
excludes those on hemodialysis
Post liver transplant survival by SLK criteria
Of the 5968 patients who met any of the SLK criteria, 3087 (52%) ultimately underwent liver transplantation, with 864 (28%) undergoing liver transplant alone and 2223 (72%) undergoing SLK. The median post liver transplant follow-up time was 5.0 (3.1 – 6.9) years. There were 325 (11%) post liver transplant deaths, with a median time to post liver transplant death of 3.9 (2.3 – 6.0) years.
There were significant differences in the proportion of patients with post liver transplant mortality by SLK group – those in the N-SLK group were significantly less likely to die post liver transplant than both those in the B-SLK and O-SLK group (0.4 v. 14.9 v. 13.5%, p<0.001).
In univariable Cox regression, the factors significantly associated with post liver transplant mortality were etiology of NASH [hazard ratio (HR) 0.76, p=0.04], body mass index (BMI) (HR 0.97, p=0.007), donor risk index (HR 1.40, p=0.03), hepatic encephalopathy (HR 1.45, p=0.004), final pretransplant albumin (HR 0.83 per 1 g/dL, p=0.01), and being in the N-SLK group (HR 0.03 compared to the B-SLK group, p<0.001). In the final multivariable, being in the N-SLK group (ie, the group that met the new SLK criteria but underwent liver transplant alone in the past) was significantly associated with decreased post liver transplant mortality (HR 0.03 compared to the B-SLK group, p<0.001) even after adjusting for confounders (Figure 2).
Figure 2. Kaplan Meier Plot of Survival by SLK Group.
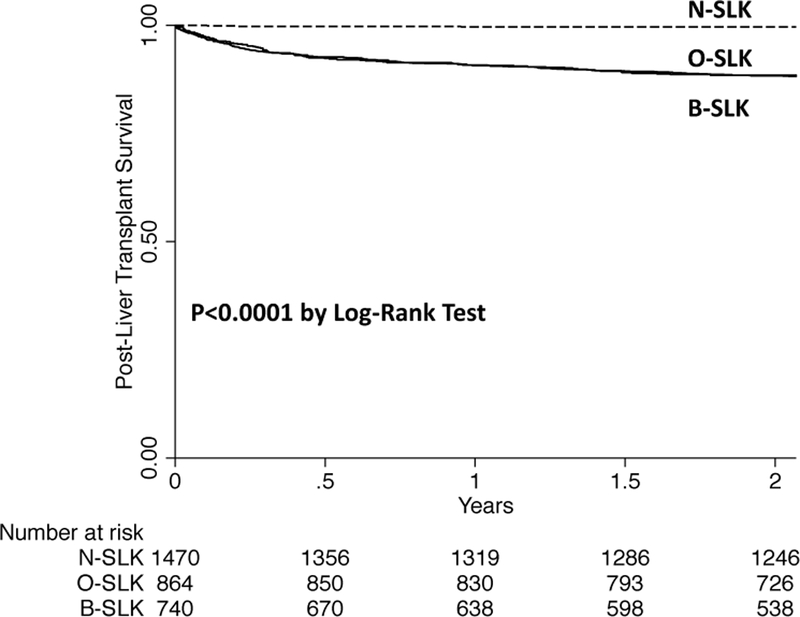
N-SLK: patients who only met the new SLK criteria (ie, those patients who will now qualify for SLK under the new policy but received a liver transplant alone under the old policy); O-SLK: patients who only met the old SLK criteria (ie, those patients who will not qualify for SLK under the new policy but received a SLK under the old policy); B-SLK: patients who met both the old and the new SLK criteria (ie, those patients who will qualify for SLK under the new policy and received a SLK under the old policy)
Post liver transplant survival by SLK criteria in Women
Next, we completed a subgroup analysis of post liver transplant mortality in women by SLK group (n=2454). Compared to women in the B-SLK and O-SLK groups, significant differences existed in the proportion of women in the N-SLK group who were non-Hispanic white (73 v. 64 v. 58%, p<0.001) or had autoimmune-related liver disease (19 v. 14 v. 11%, p<0.001). Compared to women in the B-SLK and O-SLK groups, those in N-SLK group had significantly different MELDNa scores at listing (18 v. 21 v. 29, p<0.001), MELDNa scores at delisting (33 v. 27 v. 33, p<0.001), and final eGFR (24 v. 13 v. 21 ml/min, p<0.001).
Of the women who would have met either or both SLK criteria and underwent single- or dual-organ liver transplant (n=1175), those in the N-SLK group were significantly less likely to die post liver transplant than those in the B-SLK or O-SLK groups (0.5 v. 11 v. 12%, p<0.001). A cox regression analysis demonstrated that the factors that were significantly associated with post liver transplant mortality in univariable analysis were the presence of hepatic encephalopathy (HR 1.76, p=0.02), final eGFR (1.08 per 10 ml/min, p=0.001), final serum albumin (HR 0.75 per 1 g/dL, p=0.04), and being in the N-SLK group (HR 0.04 compared to the B-SLK group, p<0.001). The final multivariable model for post liver transplant death among women included the presence of hepatic encephalopathy (HR 1.94, p=0.005), and being in the N-SLK group (HR 0.04 compared to the B-SLK group, p<0.001) (Table 2).
Table 2.
Cox Regression Analysis for post liver transplantation survival in Women
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age per Year | 0.99 | 0.98 – 1.01 | 0.41 | |||
| NASH | 0.76 | 0.52 – 0.99 | 0.04 | |||
| Non-Hispanic White | 0.84 | 0.67 – 1.06 | 0.15 | |||
| BMI per 1 kg/m2 | 0.97 | 0.96 – 0.99 | 0.007 | |||
| Ascites | 1.02 | 0.82 – 1.28 | 0.83 | |||
| HE | 1.45 | 1.13 – 1.87 | 0.004 | 1.94 | 1.22 – 3.08 | 0.005 |
| SLK Criteria | ||||||
| B-SLK | - | - | - | - | - | - |
| N-SLK | 0.03 | 0.01 – 0.09 | <0.001 | 0.04 | 0.01 – 0.16 | <0.001 |
| O-SLK | 1.02 | 0.81 – 1.31 | 0.83 | 0.84 | 0.54 – 1.31 | 0.44 |
|
Final CKD-EPI eGFR per 10 ml/min |
1.01 | 1.00 – 1.02 | 0.21 | |||
|
Hemodialysis Pre-LT Week Prior |
0.92 | 0.73 – 1.16 | 0.48 | |||
|
Donor Risk Index per point |
1.16 | 0.65 – 2.34 | 0.62 | |||
|
Final MELDNa per point |
1 | 0.99 – 1.01 | 0.51 | |||
|
Final Albumin per 1 g/dL |
0.83 | 0.71 – 0.96 | 0.01 | |||
Table Legend: Nonalcoholic Steatohepatitis (NASH); Body Mass Index (BMI); HE (Hepatic Encephalopathy); DM (Diabetes Mellitus); LT (Liver transplantation); CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration); Modification of Diet in Renal Disease (MDRD); Estimated Glomerular Filtration Rate (eGFR); Hazard Ratio (HR); International Normalized Ratio (INR)
Post liver transplant survival by SLK criteria in Men
We completed the same subgroup analysis of post liver transplant survival by SLK group in men (n=3514). Of the men who underwent either single- or dual-organ transplant (n=1912), those in the N-SLK group (n=456) were significantly less likely to die post liver transplant (0.4 v. 14 v. 17%, p<0.001) than those in the B-SLK or O-SLK group (n=1456).
A cox regression analysis in men who underwent any type of liver transplantation demonstrated that the factors that were significantly associated with post liver mortality in univariable analysis were: BMI (HR 0.97 per 1 kg/m2, p=0.007), Donor Risk Index (HR 1.64 per 1 point, p=0.007), final MELDNa (HR 0.98 per 1 point, p=0.04), and being in the N-SLK group (HR 0.03 comparted to B-SLK group, p<0.001). The final multivariable model for post liver transplant death among men included the presence of hepatic encephalopathy (HR 1.49, p=0.01), Donor Risk Index (2.22 per 1 point, p<0.001), and being in the N-SLK (HR 0.02 compared to the B-SLK group, p<0.001) (Table 3).
Table 3.
Cox Regression Analysis for post liver transplantation survival in Men
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age per Year | 1.00 | 0.98 – 1.01 | 0.75 | |||
| NASH | 0.72 | 0.47 – 1.11 | 0.13 | |||
| Non-Hispanic White | 0.86 | 0.66 – 1.13 | 0.28 | |||
| BMI per 1 kg/m2 | 0.97 | 0.94 – 0.99 | 0.007 | |||
| Ascites | 0.95 | 0.73 – 1.24 | 0.70 | |||
| HE | 1.35 | 1.00 – 1.82 | 0.05 | 1.49 | 1.10 – 2.03 | 0.01 |
| SLK Criteria | ||||||
| B-SLK | - | - | - | - | - | - |
| N-SLK | 0.03 | 0.01 – 0.11 | <0.001 | 0.02 | 0.01 – 0.10 | <0.001 |
| O-SLK | 0.93 | 0.70 – 1.24 | 0.60 | 1.10 | 0.82 – 1.48 | 0.51 |
|
Final CKD-EPI eGFR per 10 ml/min |
1.00 | 0.99 – 1.01 | 0.75 | |||
|
Hemodialysis Pre-LT Week Prior |
0.78 | 0.60 – 1.02 | 0.07 | |||
|
Donor Risk Index per point |
1.64 | 1.15 – 2.34 | 0.007 | 2.22 | 1.55 – 3.18 | <0.001 |
|
Final MELDNa per point |
0.98 | 0.96 – 1.00 | 0.04 | |||
|
Final Albumin per 1 g/dL |
0.88 | 0.74 – 1.05 | 0.17 | |||
Table Legend: Nonalcoholic Steatohepatitis (NASH); Body Mass Index (BMI); HE (Hepatic Encephalopathy); DM (Diabetes Mellitus); LT (Liver transplantation); CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration); Modification of Diet in Renal Disease (MDRD); Estimated Glomerular Filtration Rate (eGFR); Hazard Ratio (HR); International Normalized Ratio (INR)
Discussion
In this study, we aimed to evaluate the potential impact of the new SLK policy – which establishes strict medical criteria for SLK based on eGFR – on rates and outcomes of SLK. Here, we demonstrated that there will be changes in the demographics of patients undergoing SLK; particularly, women will represent a substantially larger proportion of patients listed for SLK than prior to implementation of this new SLK policy (45 v. 37%, p<0.001). Furthermore, based on our analyses, we anticipate that there will be substantial heterogeneity in post liver transplant outcomes among those who meet the new SLK criteria. Specifically, those in the N-SLK group (ie, those who met the new SLK criteria but underwent a liver transplant alone under the old policy) had a significantly higher post liver transplant survival compared to those who only met the old (O-SLK) or both (B-SLK) SLK criteria, even in adjusted analysis. In fact, nearly all the post liver transplant deaths occurred in those in the B-SLK and O-SLK groups (ie, those patients who received a SLK under the old policy).
These findings highlight that not all patients who now qualify for SLK under the new SLK policy may equally benefit from SLK versus liver transplantation alone, a finding that has been raised by a number of previous studies (15–17). We acknowledge the following limitations of our study. As with any analysis of UNOS registry data, our results are limited by the accuracy and content of the registry. However, by focusing on hard outcomes, such as post liver transplant survival, we attempted to minimize any impact input errors may have had in our results. That being said, changes in public policy regarding the Social Security Death Master File may have led to an underreporting of post liver transplant mortality. Next because post liver transplant renal function is not routinely captured in the UNOS registry, we could not accurately comment on post liver transplant renal outcomes by SLK group. Moreover, there are likely to be substantial differences in patients who would have met the new SLK criteria who underwent SLK or liver transplant alone in the past. These differences, which cannot be accounted for in the UNOS registry – such as cause and duration of renal dysfunction, time on dialysis and dialysis practices, and unaccounted for comorbidities (18) – may be contributing to the lower survival that we observed in those who underwent SLK. Despite these limitations, in this specific population our data supports previous work demonstrating that SLK may be associated with a survival decrement (15), and highlights the need for more granular studies to address these potential confounders and expand on the descriptive statistics presented here.
Our study raises important concerns about the downstream effects of the newly-implemented SLK allocation policy. Despite the intentions of the new SLK allocation policy to reduce regional/center variation and standardize the allocation of kidneys, this new SLK allocation policy will likely result in a substantially larger proportion of patients listed for SLK than before, who may, based on historical data (from before the new SLK policy was implemented), not need dual-organ transplantation to achieve favorable posttransplant outcomes. These findings prompt the need for close monitoring of SLK utilization and outcomes, after implementation of the new policy.
Acknowledgments
Grants and Financial Support
This study was funded by K23AG048337 (Paul B. Beeson Career Development Award in Aging Research; Lai) and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (UCSF Liver Center P30 DK026743, T32 DK060414; Cullaro), both of which played no role in the analysis of the data or the preparation of this manuscript
Abbreviations:
- (HCV)
Hepatitis C Virus
- (KALT)
Kidney Transplantation after Liver Transplantation
- (MELDNa)
Model for End-Stage Liver Disease including serum sodium
- (NAFLD)
Nonalcoholic Fatty Liver Disease
- (OPTN)
Organ Procurement and Transplantation Network
- (UNOS)
United Network for Organ Sharing
- (SLK)
simultaneous liver-kidney transplant
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
References
- 1.Boyle G. Simultaneous Liver Kidney (SLK) Allocation Policy. https://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf. Accessed December 18, 2017. [Google Scholar]
- 2.Nadim MK, Davis CL, Sung R, Kellum JA, Genyk YS. Simultaneous Liver-Kidney Transplantation: A Survey of US Transplant Centers. Am J Transplant 2012;12:3119–27. [DOI] [PubMed] [Google Scholar]
- 3.Cholongitas E, Thomas M, Senzolo M, Burroughs AK. Gender disparity and MELD in liver transplantation. J Hepatol 2011;55:500–1. [DOI] [PubMed] [Google Scholar]
- 4.Myers RP, Shaheen AAM, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: Assessment of revised MELD including estimated glomerular filtration rate. J Hepatol 2011;54:462–70. [DOI] [PubMed] [Google Scholar]
- 5.Cholongitas E, Marelli L, Kerry A, et al. Female Liver Transplant Recipients with the Same GFR as Male Recipients Have Lower MELD Scores?A Systematic Bias. Am J Transplant 2007;7:685–92. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok ASF. Re-weighting the model for end-stage liver disease score components. Gastroenterology 2008;135:1575–81. [DOI] [PubMed] [Google Scholar]
- 7.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height Contributes to the Gender Difference in Wait-List Mortality Under the MELD-Based Liver Allocation System. Am J Transplant 2010;10:2658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N Engl J Med 2008;359:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krones E, Fickert P, Zitta S, et al. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol 2015;16:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Souza V, Hadj-Aissa A, Dolomanova O, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology 2014;59:1522–31. [DOI] [PubMed] [Google Scholar]
- 12.Francoz C, Nadim MK, Baron A, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology 2014;59:1514–21. [DOI] [PubMed] [Google Scholar]
- 13.Francoz C, Prié D, AbdelRazek W, et al. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: Impact on the model for end-stage liver disease score. Liver Transplant 2010;16:1169–77. [DOI] [PubMed] [Google Scholar]
- 14.Boyle G. Simultaneous Liver Kidney (SLK) Allocation Policy. https://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf. Accessed November 3, 2017. [Google Scholar]
- 15.Fong T- L, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined Liver-Kidney Transplantation Is Preferable to Liver Transplant Alone for Cirrhotic Patients With Renal Failure. Transplant J 2012;94:411–6. [DOI] [PubMed] [Google Scholar]
- 16.Choudhury RA, Reese PP, Goldberg DS, Bloom RD, Sawinski DL, Abt PL. A Paired Kidney Analysis of Multiorgan Transplantation. Transplantation 2017;101:368–76. [DOI] [PubMed] [Google Scholar]
- 17.Locke JE, Warren DS, Singer AL, et al. Declining Outcomes in Simultaneous Liver-Kidney Transplantation in the MELD Era: Ineffective Usage of Renal Allografts. Transplantation 2008;85:935–42. [DOI] [PubMed] [Google Scholar]
- 18.Nadim MK, Sung RS, Davis CL, et al. Simultaneous Liver-Kidney Transplantation Summit: Current State and Future Directions. Am J Transplant 2012;12:2901–8. [DOI] [PubMed] [Google Scholar]


