Abstract
Humans with alcohol use disorder typically abstain because of the negative consequences associated with excessive drinking, and exposure to contexts previously associated with alcohol use can trigger relapse. We used a rat model that captures a characteristic of this human condition: namely voluntary abstinence from alcohol use because of contingent punishment. There is substantial variability in the propensity to relapse following extended periods of abstinence, and this is a critical feature preventing the successful treatment of alcohol use disorder. Here we examined relapse following acute or prolonged abstinence. In male alcohol preferring P rats, we found an increased propensity to relapse in Context B, the punishment context after prolonged abstinence. Next, we found that neither alcohol intake history nor the motivational strength of alcohol predicted the propensity to relapse. We next examined the putative circuitry of context-induced relapse to alcohol seeking following prolonged abstinence using Fos as a marker of neuronal activation. The anterior insular cortex (AI) was the only brain region examined where Fos expression correlated with alcohol seeking behavior in Context B after prolonged abstinence. Finally, we used local infusion of GABAA and GABAB receptor agonists (muscimol + baclofen) to show a causal role of the AI in context-induced relapse in Context B, the punishment context after prolonged abstinence. Our results show that there is substantial individual variability in the propensity to relapse in the punishment-associated context after prolonged abstinence, and this is mediated by activity in the AI.
SIGNIFICANCE STATEMENT A key feature of alcohol use disorder is that sufferers show an enduring propensity to relapse throughout their lifetime. Relapse typically occurs despite the knowledge of adverse consequences including health complications or relationship breakdowns. Here we use a recently developed rodent model that recapitulates this behavior. After an extended period of abstinence, relapse propensity is markedly increased in the “adverse consequence” environment, akin to humans with alcohol use disorder relapsing in the face of adversity. From a circuitry perspective, we demonstrate a causal role of the anterior insular cortex in relapse to alcohol seeking after extended abstinence following punishment imposed voluntary cessation of alcohol use.
Keywords: alcohol use disorder, anterior insular cortex, context, iP rat, punishment, relapse
Introduction
Individual variation in the expression of particular traits contributes to the onset of neuropsychiatric disease states, including drug addiction (Piazza et al., 1989). Variation in the propensity to relapse following extended abstinence is a critical feature preventing successful treatment of addiction (Gossop et al., 1989). In abstinent alcoholics, environments previously associated with alcohol use provoke relapse (Wikler, 1973; O'Brien, 1997). In rodents, this is modeled using ABA renewal (Bouton and Bolles, 1979) where self-administration of alcohol occurs in one context (Context A) followed by extinction (experimenter-imposed) of alcohol-reinforced responding in a difference context (Context B). Renewal is observed when the rodent is returned to the original alcohol-associated context (Context A; Crombag and Shaham, 2002; Hamlin et al., 2007). However, abstinence in humans is often voluntary because of the adverse consequences of alcohol use (Klingemann, 1991; Blume et al., 2006) without any treatment intervention.
We modified a variation of the ABA renewal model where abstinence is self-imposed in Context B because of adverse consequences (punishment; Marchant et al., 2013) by examining alcohol seeking following extended abstinence (30 d). First, we determined the propensity to relapse to context-induced alcohol seeking following prolonged abstinence after punishment-imposed suppression of alcohol use. We found an increased propensity to relapse in Context B, the punishment context after prolonged abstinence. Second, we determined the predictability of this propensity to relapse using alcohol intake history and the motivational strength of alcohol. Neither factor predicted relapse. Third, we examined the putative circuitry of context-induced relapse to alcohol seeking following prolonged abstinence using Fos expression (Dragunow et al., 1987). The anterior insular cortex (AI) was the only brain region examined where Fos expression correlated with alcohol seeking in the punishment context after prolonged abstinence. Finally, we demonstrated a causal role of the AI in context-induced relapse using local infusion of muscimol and baclofen to reversibly inactivate the AI which essentially prevented relapse in Context B, the punishment context after prolonged abstinence, but not acute abstinence.
Materials and Methods
Ethics statement
All procedures performed were in accordance with the Prevention of Cruelty to Animals Act (2004), under the guidelines of the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Experimental Purposes (2013) and approved by The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee.
Animals
Inbred male iP rats (∼8 weeks old, total n = 129) were obtained from the breeding colony at the Florey. Parental stock was previously obtained from T. K. Li (while at Indiana University). All rats were pair-housed except during Experiments 4 and 5 where they were single-housed. Food (Barastoc rat and mouse, Ridley) and water were available ad libitum and all rats were maintained on a normal 12 h light/dark cycle (07:00 lights on).
Apparatus
Standard operant chambers (Med Associates) enclosed in a ventilated sound-attenuating cubicle were used for self-administration. Each chamber was equipped with two retractable levers and grid floors were connected to shockers. An active lever press resulted in the delivery of 20% ethanol (0.1 ml/delivery) into the receptacle. An inactive lever press had no consequence. Contexts A and B were manipulated as in our previous study (Campbell et al., 2018): illumination level (white/no house light), background (stripes/none), bedding (saw dust/recycled paper), background noise (fan off/on).
Experiment 1: effect of context-induced relapse to alcohol seeking following acute or prolonged abstinence
The behavioral procedure (Fig. 1A) was the same as previously published (Campbell et al., 2018).
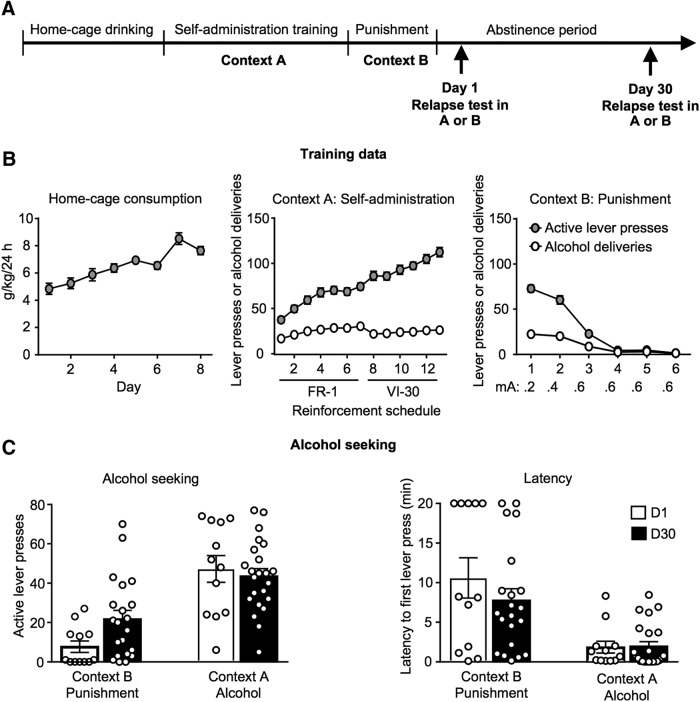
Figure 1.
Home-cage intake, alcohol self-administration in Context A, and punishment of alcohol self-administration in Context B and alcohol seeking behavior after acute and prolonged abstinence. Outline of the behavioral procedure (A). Alcohol intake (grams per kilogram) during the home-cage access phase. Context A active lever presses and alcohol deliveries under the FR-1 and VI-30 schedules of reinforcement. Context B active lever presses and alcohol deliveries with the foot shock punishment range from 0.2 to 0.6 mA (B). Number of active lever presses (left) and latency to first lever press (right) during the relapse test at either Day 1 abstinence or Day 30 abstinence (C). Data are presented as mean ± SEM. Total n = 68, n = 12/group Day 1 abstinence, n = 21 Context B, punishment Day 30 abstinence group, n = 23 Context A, alcohol Day 30 abstinence group. D1, Day 1 abstinence; D30, Day 30 abstinence.
Behavioral procedure (4 phases)
Phase 1: home-cage alcohol intake.
An intermittent access (3–4 times/week) alcohol procedure (Wise, 1973; Simms et al., 2008) was used where rats received 8 × 24 h sessions of access to 20% v/v alcohol. In Experiments 4 and 5, rats received 12 × 24 h home-cage sessions. Alcohol solutions were prepared in tap water from 100% (v/v) ethanol. Daily sessions began at 09:00. After 24 h, the alcohol was replaced with a second water bottle for the subsequent 24–48 h alcohol-free period. The following day, the second water bottle was replaced with 20% alcohol, and the location of the alcohol was alternated from the previous session. Total alcohol consumption was calculated for each session, using the weight difference between the beginning and end of the session, minus 1 g for spillage, multiplied by 0.97 (density of 20% ethanol), and divided by 2 (number of rats per cage).
Phase 2: operant self-administration: Context A.
All rats were given one 16 h overnight training session where only the active lever was presented. An active lever press resulted in the delivery of 0.1 ml of 20% alcohol into a receptacle followed by a 2 s light cue above the active lever. Food and water was provided ad libitum. Rats were then trained for seven 20 min self-administration sessions under a fixed-ratio 1 (FR-1) schedule. Responding on the active lever resulted in the delivery of 0.1 ml of 20% alcohol and the 2 s light cue followed by a 20 s timeout period where lever presses were recorded but not reinforced. Inactive lever presses were recorded but had no consequence. Following FR-1 training, rats progressed to a variable-interval 30 s (VI-30) schedule for six 20 min sessions where alcohol delivery was available after an active lever press at pseudorandom intervals (1–59 s) after the preceding alcohol delivery.
Phase 3: punishment: Context B.
During 20 min sessions, rats self-administered alcohol in an alternate context (Context B) under the same VI-30 schedule. Active lever presses resulted in the delivery of 0.1 ml of alcohol paired with the 2 s light cue. 50% of the reinforced active lever presses randomly resulted in a 0.5 s footshock (0.2–0.7 mA). Punished active lever presses resulted in footshock, 2 s light cue, and alcohol delivery. Inactive lever presses had no consequence. All rats were punished in Context B for up to 6 d, and footshock intensity was increased by 0.2 mA per session up to 0.6 mA, or to 0.7 mA if rats made >25 active lever presses after three punishment sessions.
Phase 4: context-induced relapse test following either acute or prolonged abstinence.
Rats in the acute abstinence protocol were tested for alcohol seeking the day after punishment-imposed abstinence (i.e., Day 1 Context A alcohol group, n = 12; Day 1 Context B punishment group, n = 12). Rats in the prolonged abstinence protocol were moved to a separate holding room for 29 d and tested for alcohol seeking on Day 30 (i.e., Day 30 Context A alcohol group, n = 23; Day 30 Context B punishment group, n = 21). All rats were tested under extinction conditions during 20 min sessions in either Context A or B in a counterbalanced order. During test, an active lever press under a VI-30 schedule resulted in delivery of the 2 s light cue but no alcohol or footshock.
Experiment 2: predicting the propensity to relapse in the punishment context following prolonged abstinence
On the third last day of Context A training, 14 rats were tested on a progressive ratio (PR)3–4 schedule for a single 2 h session. For the PR3–4 schedule, 32 active lever presses were required for the 10th infusion of alcohol (Farid et al., 2012), breakpoint was defined as the final ratio completed within the 2 h session. Subsequently, all rats received two further Context A sessions. Alcohol-reinforced responding was then punished in Context B. All rats were placed in a separate room for the prolonged abstinence phase. On abstinence Day 30, all rats were tested for alcohol seeking behavior in Context B.
Experiment 3: effect of context-induced alcohol seeking following prolonged abstinence on Fos-protein expression
Ninety minutes following the initiation of the relapse test, a subset of rats from Experiment 1 (i.e., Day 30 Context A alcohol group, n = 6; Day 30 Context B punishment low relapsing group, n = 7; Day 30 Context B punishment high relapsing group, n = 8) were anesthetized (sodium pentobarbitone100 mg/kg, i.p.; Virbac). Rats were transcardially perfused with ∼100 ml of 0.1 m PBS followed by ∼400 ml of 4% paraformaldehyde (PFA). Brains were removed and postfixed in PFA (2 h), then transferred to 30% sucrose in PBS (48 h at 4°C). Brains were frozen over dry ice and stored at −80°C. Serial (40 μm) coronal sections were cut using a Leica Microsystems cryostat and stored in 0.1 m PBS containing 0.1% sodium azide at 4°C.
A 1-in-4 series of the whole brain was processed for Fos-immunoreactivity, as published (Campbell et al., 2015, 2017a). Free-floating sections were washed in PBS followed by blocking in 5% normal horse serum (NHS). Sections were incubated (48 h at 4°C) in PBS containing 0.5% Triton-X with 2% NHS and Fos primary antibody (1:3000; rabbit polyclonal, sc-52, Santa Cruz Biotechnology). Following primary incubation, sections were incubated (2 h) in biotinylated horse anti-rabbit secondary (1:300; BA-1100, Vector Laboratories). Subsequently, sections were incubated (1 h) in ABC reagent (Vector Laboratories) followed by incubation (10 min) in 0.1 m sodium acetate with 0.025% diaminobenzidine in 2% nickel sulfate containing 2 mg/ml d-glucose, and 0.4 mg/ml ammonium chloride. Glucose oxidase (0.2 μl/ml) was added to visualize Fos. Sections were mounted onto gelatin-coated slides, air dried, and coverslipped.
Bright-field images of Fos-immunoreactive cells were captured using a MBF Biosciences Color 12-Bit (QImaging) camera attached to an upright Leica DMLB-2 microscope using a 10× objective. Images were quantified using iVision (BioVision) by an observer blinded to experimental conditions. Bilateral cell counts were quantified for each rat across 17 brain regions. These brain regions included the AI, posterior insular cortex (AIp), prelimbic cortex (PrL), infralimbic cortex (IL), lateral orbitofrontal cortex (LOFC), entorhinal cortex (EC), ventral subiculum (vSub), paraventricular thalamus (PVT), lateral hypothalamus (LH), lateral habenula (LHb), basolateral amygdala (BLA), central amygdala (CeA), medial amygdala (MeA) dorsal dentate gyrus (DDG), paraventricular nucleus of the hypothalamus, medial parvicellular part (PVN), nucleus accumbens core (NAcC), and nucleus accumbens shell (NAcSh). Please refer to Table 1 for more detail on bregma levels quantified. All brain coordinates were from Paxinos and Watson (2007).
Table 1.
Bregma levels and references for Fos quantification across several brain regions
| Brain region | Bregma level, mm | References |
|---|---|---|
| AI | +3.08 to +2.76 | Arguello et al., 2017; Venniro et al., 2017; Nasser et al., 2018 |
| AIp | −1.68 to −2.00 | Contreras et al., 2012 |
| PrL | +3.72 to +2.76 | Perry and McNally, 2013; Brown et al., 2016; Burgos- Robles et al., 2017 |
| IL | +3.72 to +2.76 | Perry and McNally, 2013 |
| LOFC | +3.72 to +2.76 | Perry and McNally, 2013 |
| EC | −5.72 to −6.24 | Ge et al., 2017 |
| vSub | −5.72 to −6.24 | Marchant et al., 2016 |
| PVT | −1.68 to −3.12 | Marchant et al., 2014; Campbell et al., 2017b |
| LH | −1.68 to −3.12 | Marchant et al., 2014; Campbell et al., 2017b |
| LHb | −2.96 to −3.44 | Marchant et al., 2014; Zuo et al., 2017 |
| BLA | −2.48 to −2.80 | Campbell et al., 2017a |
| CeA | −2.48 to −2.80 | Campbell et al., 2017a |
| MeA | −2.48 to −2.80 | Campbell et al., 2017a |
| DDG | −3.24 to −3.62 | Ge et al., 2017 |
| PVN | −1.52 to −1.84 | James et al., 2014 |
| NAcC/NAcSh | +2.04 to +1.72 | Marchant et al., 2014; Campbell et al., 2017a |
Bregma levels and references for Experiment 3, which examined the effect of context-induced alcohol seeking following prolonged abstinence on Fos-protein expression.
Experiment 4: effect of AI inactivation on context-induced relapse to alcohol seeking in Context B, the punishment context, following prolonged abstinence
Surgery.
Eighteen rats underwent behavioral training as per Experiment 1. During the abstinence period, rats were anesthetized with isoflurane (5% induction, 2% maintenance) before being placed in a stereotaxic frame (Stoelting Instruments). Bilateral guide cannula (26-gauge; Plastics One) were positioned 1.5 mm above the AI (anteroposterior: +2.8 mm, mediolateral: ±4.4 mm, and dorsoventral: −4.7 mm from bregma; Venniro et al., 2017; Nasser et al., 2018). Cannulae were anchored to the skull with screws and dental cement. After surgery, rats were administered meloxicam (3 mg/kg, i.p.; Troy Laboratories) and Baytril (3 mg/kg, i.p.; Bayer Health Care) for 3 d. All rats were given 7 d recovery from surgery before experimentation.
Intracranial infusions.
Three days preceding the test, rats underwent three daily habituation injections. These occurred in the operant training room and involved the connection of 40 cm polyethylene connectors (Plastics One) to both cannulae and activation of an automated syringe pump (Harvard Apparatus) for 2 min. Following this, connectors were left in place for an additional 2 min. Connectors were then removed and rats were left in their home cages for 10 min before being moved back to their holding room. After 30 d abstinence, rats were tested for alcohol seeking (under extinction conditions) in Context B. Muscimol + baclofen [M+B; Tocris Bioscience; (50 + 50)ng/0.5 μl/hemisphere] was dissolved in sterile saline. The injectors extended 1.5 mm below the guide cannula tips. 0.5 μl/hemisphere of M+B (n = 8) or vehicle (n = 10, 0.9% saline) was infused bilaterally into the AI over 2 min (0.25 μl/min) using a Harvard Apparatus syringe pump connected to two 1 μl microsyringes (SGE Analytical Science) via polyethylene tubing. Injectors were left in place for a further 2 min. Rats were tested in Context B 5–10 min after infusions. Following this, rats were anesthetized (pentobarbitone 100 mg/kg, i.p.; Virbac) and methylene blue (0.5 μl/hemisphere) was infused into the AI. Brains were removed and frozen over super-cooled isopentane, then cut into 40 μm coronal sections using a Leica Microsystems cryostat and counterstained with neutral red to verify cannula placements (see Fig. 4C). One rat was excluded due to misplaced cannula.
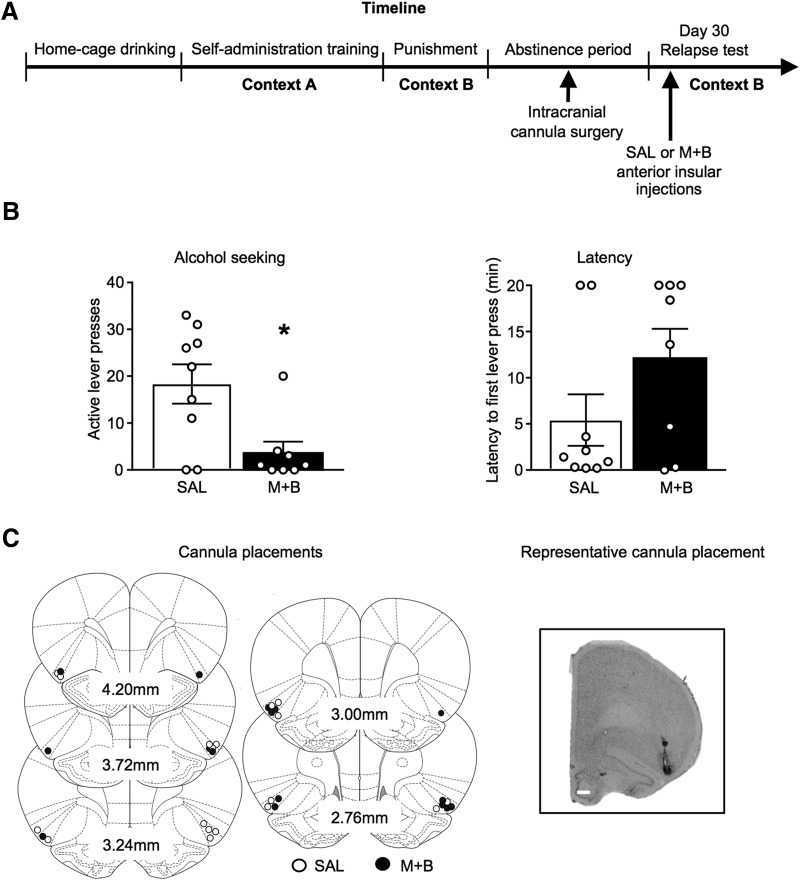
Figure 4.
Anterior insular cortex inactivation prevents context-induced relapse to alcohol seeking in Context B, the punishment context following prolonged abstinence. Outline of the experimental timeline (A). Number of active lever presses (left) and latency to first lever press (right) in the punishment context following prolonged abstinence (B). Approximate cannula placements (mm from bregma) of the injector tips for the AI (C). SAL, saline, M+B, muscimol + baclofen. Data are presented as mean ± SEM. n = 8–9/group. Scale bar, 1000 μm. *p < 0.05.
Experiment 5: effect of AI inactivation on context-induced relapse to alcohol seeking following acute abstinence
Surgery.
Twenty-nine rats underwent behavior training as per Experiment 1. After 11 Context A self-administration sessions, rats received AI intracranial guide cannula surgery as described in Experiment 4. All rats were given at least 7 d to recover from surgery before experimentation. Following recovery, all rats were exposed to six additional Context A sessions followed by punishment in Context B as described in Experiment 1.
Intracranial infusions.
All rats received two habituation injections in Context A and two habituation injections in Context B before the relapse test. This involved the connection of 40 cm polyethylene connectors (Plastics One) to both cannulae and activation of an automated syringe pump (Harvard Apparatus) for 2 min. Following this, connectors were left in place for an additional 2 min. After 1 d abstinence, rats were tested for alcohol seeking (under extinction conditions) in either Context A or B. M+B [Tocris Bioscience (50 + 50)ng/0.5 μl/hemispher] was dissolved in sterile saline. The injectors extended 1.5 mm below the guide cannula tips. 0.5 μl/hemisphere of M+B (Context A: n = 6, Context B: n = 5) or vehicle (Context A: n = 6, Context B: n = 5; 0.9% saline) was infused bilaterally into the AI over 2 min (0.25 μl/min) using a Harvard Apparatus syringe pump connected to two 1 μl microsyringes (SGE Analytical Science) via polyethylene tubing. Injectors were left in place for a further 2 min. Three rats were excluded due to misplaced cannula, three rats were excluded due to ill health following surgery and 1 rat was excluded due to a program operational error.
Locomotor testing.
Twenty-four hours after the relapse test, a subset of rats from Experiment 5 received either M+B (n = 8) or vehicle (n = 8) infusions into the AI and locomotor activity was recorded for 1 h (Med Associates; 43.2 × 43.2 × 30.5 cm). Distance traveled (m) was recorded using photobeam detectors. Following this, rats were anesthetized (pentobarbitone 100 mg/kg, i.p.; Virbac) and methylene blue (0.5 μl/hemisphere) was infused into the AI. Brains were removed and frozen over super-cooled isopentane, then cut into 40 μm coronal sections using a Leica Microsystems cryostat and counterstained with neutral red to verify cannula placements (see Fig. 5C).
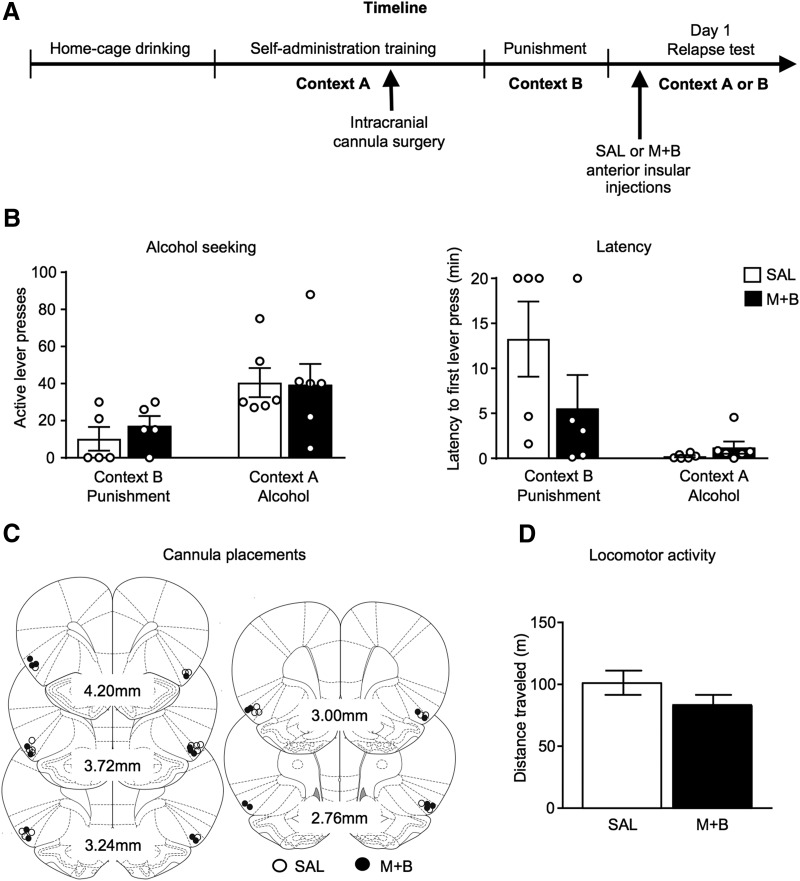
Figure 5.
Anterior insular cortex inactivation has no effect on context-induced relapse to alcohol seeking in following acute abstinence. Outline of the experimental timeline (A). Number of active lever presses (left) and latency to first lever press (right) following acute abstinence (B). Approximate cannula placements (mm from bregma) of the injector tips for the AI (C). SAL, saline, M+B, muscimol + baclofen. There was no effect of AI inactivation on locomotor activity (D). Data are presented as mean ± SEM n = 5–6/group.
Statistical analysis
Data were analyzed separately for the four behavioral phases: home-cage alcohol intake, Context A training, Context B punishment, and context-induced relapse tests. For Experiment 1, training and punishment data were analyzed using repeated-measures ANOVA examining a main effect of session. For the relapse test, between-subjects factors were Context (Context A or Context B) and Abstinence Day (Days 1 or 30). The dependent variables were total number of active lever presses (inactive lever presses as covariate) or number of minutes to first active lever press (latency). The relapse test data were also analyzed using a χ2 test to examine the relationship between Abstinence Day and the relapse status of rats (low vs high relapsing).
Pearson's correlations examined the relationship between alcohol seeking in Context B after 30 d abstinence and average home-cage consumption (g/kg/24 h), average self-administration alcohol deliveries, active lever presses and timeout lever presses for an early time point (first 5 min), late time point (first 10 min), or the total for the 20 min operant session. These data were divided into the first seven sessions when the reinforcement schedule was FR-1 and the last six sessions when the reinforcement schedule was VI-30. Alcohol seeking was also correlated with average punishment alcohol deliveries, active lever presses and timeout lever presses for an early time point (first 5 min), late time point (first 10 min) or the total for the 20 min operant session. For Experiment 2, Pearson's correlations examined the relationship between alcohol seeking and breakpoint as well as alcohol seeking and the number of alcohol deliveries, active lever presses and timeout lever presses for an early time point (first 5 min), late time point (first 10 min), or the total for the 2 h PR session.
In Experiment 3, immunohistochemical data were analyzed as cell counts/mm2 of a given brain region as the dependent variable. To compare Fos expression between rats with a heightened propensity to relapse versus rats with a low propensity to relapse in Context B, a median split was performed on the Day 30 Abstinence Context B relapse data (James et al., 2011). This resulted in a relapse score of 20; rats that had relapse scores <20 were allocated to the “Low relapsing” group and rats that had relapse scores 20 were allocated to the “High relapsing” group. One-way ANOVAs then assessed the effect of abstinence Day 30 treatment condition (Alcohol, Low relapsing, High relapsing) on the number of Fos cells/mm2. Post hoc comparisons were assessed using least significant differences tests. Pearson's correlations examined the relationship between alcohol seeking in Context B and Fos expression.
In Experiment 4, one-way ANOVAs assessed the effect of Treatment (vehicle, M+B) on alcohol seeking (inactive lever presses as covariate) and latency in Context B after 30 d abstinence. In Experiment 5, a two-way between-subjects ANOVA assessed the effect of Drug Treatment (vehicle, M+B) or Context (Contexts A or B) on alcohol seeking (inactive lever presses as covariate) and latency after 1 d abstinence. A one-way ANOVA assessed the effect of M+B or vehicle on distance traveled (m) in the locomotor arena. All analyses were performed using SPSS v25 (α 0.05). Data are presented as mean ± SEM.
Results
Experiment 1: context-induced relapse to alcohol seeking following acute or prolonged abstinence
Rats consumed high quantities of alcohol during the home-cage period and reliably self-administered alcohol in Context A (Fig. 1B). In Context B, rats reduced alcohol self-administration with increasing shock intensity (Fig. 1B).
Alcohol seeking behavior following acute or prolonged abstinence
There was a significant interaction between Test Context × Abstinence Day (F(1,63) = 4.578, p = 0.036; Fig. 1C). There was also a significant main effect of Context (F(1,63) = 40.081, p < 0.0001) with rats tested in Context A having a greater number of active lever presses compared with rats tested in Context B. There was no significant main effect of Abstinence Day (F(1,63) = 0.021, p = 0.884). In Context B there was variability in relapse data on Day 30, therefore rats were divided using a median split (James et al., 2011), which resulted in a criterion Relapse Score of 20 active lever presses. Rats with relapse scores <20 formed the “Punishment low relapsing” group and rats with relapse scores ≥20 formed the “Punishment high relapsing” group. Using this criterion, there was a ∼threefold increase in the number of rats relapsing in Context B after 30 d abstinence, from 16% at Day 1 compared with 52% at Day 30 (Fig. 1C). Chi-square analyses revealed a significant relationship between Abstinence Day and the relapse status of rats in Context B (χ2 = 4.080, p = 0.043). There was no significant relationship between Abstinence Day and relapse status in Context A (χ2 = 0.001, p = 0.971). Analysis of latency to first lever press revealed no interaction between Test Context × Abstinence Day (F(1,64) = 1.082, p = 0.302). There was a significant main effect of Context on latency to first lever press (F(1,64) = 26.715, p < 0.0001) with rats tested in Context B having a greater latency to first lever press compared with Context A. There was no significant main effect of Abstinence Day on latency (F(1,64) = 0.911, p = 0.343). These data indicate that 30 d abstinence following punishment-imposed abstinence increases relapse propensity in the punishment context (Fig. 1C).
Experiment 2: predicting the propensity to relapse in Context B, the punishment context following prolonged abstinence
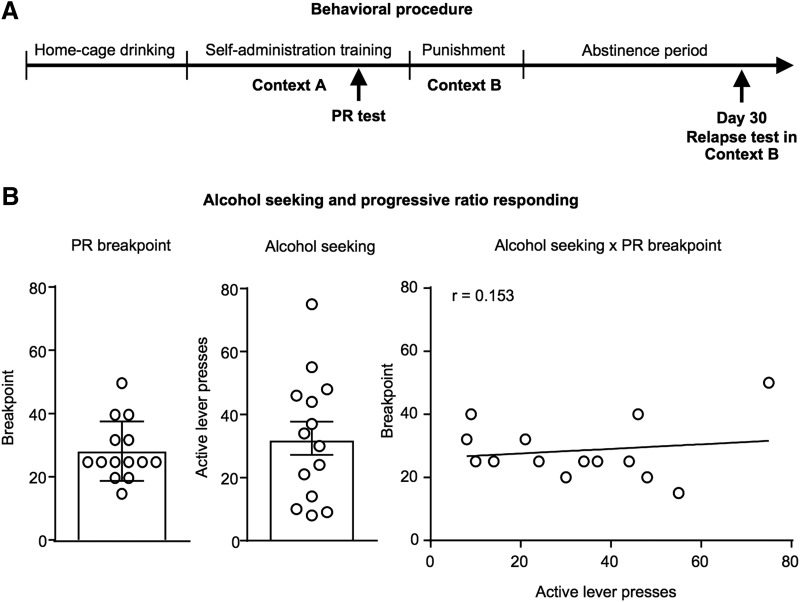
Retrospective Pearson's correlations were performed between home-cage drinking, self-administration, punishment and propensity to relapse after 30 d abstinence. There were no significant correlations between alcohol drinking history and relapse propensity in Context B (p values >0.05; Table 2). Evidence suggests that a history of high alcohol intake does not necessarily predict future alcohol seeking behavior, rather there is a relationship between motivation for alcohol and compulsive alcohol seeking (Giuliano et al., 2015, 2018). Therefore, 14 rats were tested using a PR task (Richardson and Roberts, 1996) during self-administration to assess motivation for alcohol (Fig. 2A). There was no significant correlation between Context B active lever presses after prolonged abstinence and PR breakpoint, r = 0.153, p = 0.602 (Fig. 2B). Additionally, there were no significant correlations between Context B active lever presses after prolonged abstinence and the number of alcohol deliveries (r = −0.034, p = 0.909) or the number of active lever presses r = 0.063, p = 0.830) in the first 20 min of the PR task. Thus motivation for alcohol does not predict propensity for relapse in Context B in this paradigm.
Table 2.
Correlations between alcohol drinking history and the propensity to relapse after 30 d abstinence in the punishment context
| Alcohol drinking history measure | Pearson correlation with propensity to relapse | FR-1 schedule Pearson correlation with propensity to relapse | VI-30 schedule Pearson correlation with propensity to relapse | Punishment Pearson correlation with propensity to relapse |
|---|---|---|---|---|
| Home-cage consumption | 0.000 | |||
| Early alcohol deliveries | 0.236 | 0.389 | 0.361 | |
| Early active lever presses | 0.201 | 0.397 | 0.309 | |
| Early timeout lever presses | 0.182 | 0.275 | 0.282 | |
| Late alcohol deliveries | 0.286 | 0.223 | 0.396 | |
| Late active lever presses | 0.283 | 0.329 | 0.337 | |
| Late timeout lever presses | 0.394 | 0.341 | 0.305 | |
| Total alcohol deliveries | 0.268 | 0.294 | 0.404 | |
| Total active lever presses | 0.242 | 0.374 | 0.309 | |
| Total timeout lever presses | 0.226 | 0.381 | 0.303 |
Retrospective Pearson's correlations were performed between alcohol seeking behavior in Context B after 30 d abstinence and average home-cage consumption (g/kg/24 h), average self-administration alcohol deliveries, active lever presses and timeout lever presses for an early time point (first 10mins), late time point (last 10 min) or the total for the 20 min operant session. These data were divided into the first seven sessions when the reinforcement schedule was FR-1 and the last six sessions when the reinforcement schedule was VI-30. Alcohol seeking was also correlated with average punishment alcohol deliveries, active lever presses and timeout lever presses for an early time point (first 10 min), late time point (last 10 min) or the total for the 20 min operant session. Data presented as the Pearson correlation coefficient (r). n = 21.
Figure 2.
Predicting the propensity to relapse in Context B, the punishment context following prolonged abstinence. Outline of the behavioral procedure (A). PR breakpoint and number of active lever presses during the relapse test in the punishment context (B, left). Correlation between PR breakpoint and alcohol seeking active lever presses in the punishment context (B, right). Data are presented as mean ± SEM. n = 14.
Experiment 3: Fos expression associated with context-induced relapse to alcohol seeking following prolonged abstinence
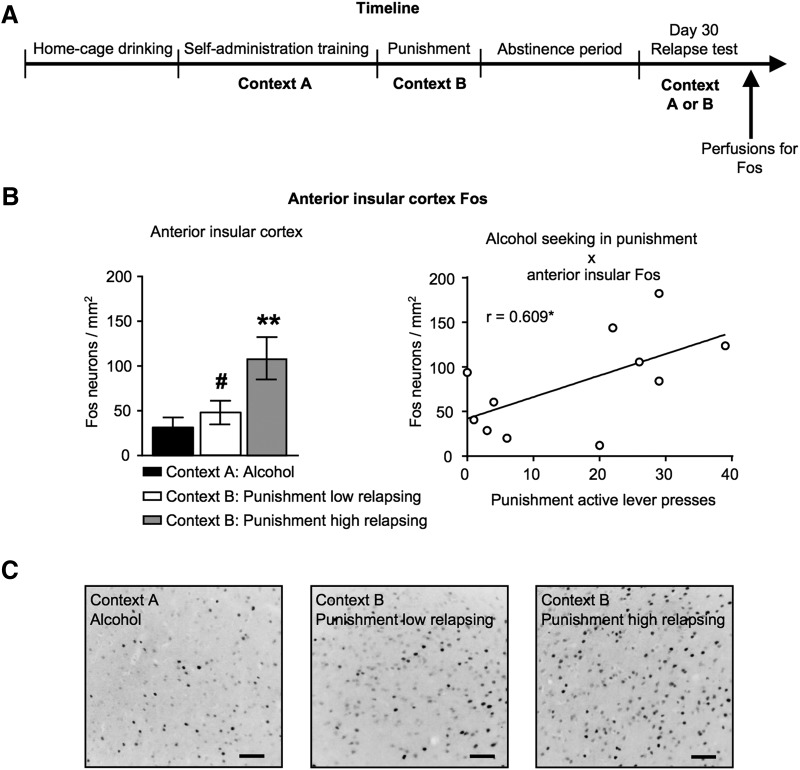
There was a significant effect of Abstinence Group (Day 30 Alcohol n = 6, Day 30 Punishment low relapsing n = 7, Day 30 Punishment high relapsing n = 8) on the number of Fos-positive cells in the LH (F(2,18) = 4.112, p = 0.034), PVT (F(2,18) = 4.494, p = 0.026), LHb (F(2,17) = 4.943, p = 0.020), and AI (F(2,17) = 5.682, p = 0.016). Post hoc analyses showed significant differences between groups Day 30 Alcohol and Day 30 Punishment high relapsing in the LH, PVT, LHb, and AI (p values <0.05; Table 3). In the AI and LHb, there was also a significant difference between groups Day 30 Punishment low relapsing and Day 30 Punishment high relapsing (p values <0.05; Fig. 3; Table 3). Only the AI had a significant correlation between alcohol seeking in the punishment context and Fos counts, r = 0.609, p = 0.047 (Fig. 3B; Table 4).
Table 3.
Fos-protein expression across several brain regions following 30 d abstinence
| Brain region | Context A, alcohol | Context B, punishment low relapsing | Context B, punishment high relapsing |
|---|---|---|---|
| AI | 32.102 ± 10.460 | 48.679 ± 13.150# | 108.517 ± 23.707** |
| AIp | 16.064 ± 5.467 | 24.793 ± 5.639 | 34.041 ± 6.944 |
| PrL | 32.645 ± 7.045 | 30.354 ± 15.082 | 73.565 ± 26.275 |
| IL | 36.773 ± 9.896 | 24.377 ± 12.281 | 61.105 ± 17.219 |
| LOFC | 120.322 ± 32.167 | 110.660 ± 34.262 | 148.329 ± 33.813 |
| EC | 44.752 ± 10.964 | 38.191 ± 9.895 | 73.703 ± 18.003 |
| vSub | 67.540 ± 17.905 | 56.231 ± 8.594 | 71.599 ± 5.483 |
| PVT | 60.854 ± 7.797 | 101.111 ± 21.192 | 131.584 ± 15.626** |
| LH | 19.615 ± 2.517 | 27.611 ± 4.990 | 37.364 ± 4.561* |
| LHb | 12.300 ± 3.617 | 16.474 ± 3.929 | 39.292 ± 9.577* |
| BLA | 12.862 ± 4.768 | 9.913 ± 2.630 | 20.926 ± 4.162 |
| CeA | 26.909 ± 9.721 | 54.359 ± 19.369 | 38.956 ± 9.851 |
| MeA | 40.849 ± 11.937 | 37.668 ± 9.557 | 62.679 ± 14.002 |
| DDG | 12.218 ± 2.844 | 16.454 ± 4.637 | 23.966 ± 3.917 |
| PVN | 43.589 ± 12.772 | 71.525 ± 40.149 | 67.019 ± 19.133 |
| NAcC | 22.970 ± 7.921 | 19.236 ± 8.475 | 29.519 ± 11.549 |
| NAcSh | 15.894 ± 4.935 | 17.451 ± 7.186 | 38.213 ± 11.570 |
Data presented as average number of Fos-positive cells per mm2 ± SEM. n = 6–8/group.
*p < 0.05 versus Alcohol group,
**p < 0.01 versus Alcohol group,
#p < 0.05 versus Punishment high relapsing group.
Figure 3.
Context-induced relapse to alcohol seeking is associated with selective activation of the AI. Outline of the experimental procedure (A). Number of Fos-positive neurons per square millimeter in the AI in rats tested in Context A: alcohol context, or in Context B: punishment context who were either low relapsing or high relapsing. Correlation between alcohol seeking behavior in Context B: punishment context and active lever presses on relapse test (B). Photomicrographs representing Fos-protein expression across each treatment group (C). Data are presented as mean ± SEM. n = 6–8/group. Scale bar, 200 μm. **p < 0.01 versus Alcohol group, #p < 0.05 versus Punishment high relapsing group, *p < 0.05.
Table 4.
Correlations between Fos expression and alcohol seeking behavior in either Context B: Punishment context, or Context A: Alcohol context following prolonged abstinence
| Brain region | Pearson correlation |
|
|---|---|---|
| Context B, punishment | Context A, alcohol | |
| AI | 0.609* | −0.033 |
| AIp | 0.193 | −0.122 |
| PrL | 0.277 | −0.241 |
| IL | 0.393 | −0.362 |
| LOFC | 0.414 | −0.556 |
| EC | 0.190 | −0.150 |
| vSub | 0.175 | 0.480 |
| PVT | 0.193 | −0.088 |
| LH | 0.446 | −0.291 |
| LHb | 0.324 | 0.310 |
| BLA | 0.277 | −0.431 |
| CeA | −0.226 | −0.198 |
| MeA | 0.365 | −0.780 |
| DDG | 0.315 | 0.159 |
| PVN | −0.118 | 0.239 |
| NAcC | −0.031 | −0.707 |
| NAcSh | 0.083 | 0.017 |
Data presented as the Pearson correlation coefficient (r). n = 6–8/group.
*p < 0.05.
Experiment 4: effect of AI inactivation on context-induced relapse to alcohol seeking in the punishment context following prolonged abstinence
Experiment 4 tested a causal role of AI in context-induced relapse to alcohol seeking in Context B, the punishment context after prolonged abstinence (Fig. 4A), using reversible inactivation with intra-AI injections of muscimol + baclofen. AI inactivation reduced context-induced relapse to alcohol seeking in the punishment context following prolonged abstinence [1/8 rats relapsed (12.5%), compared with 5/9 for vehicle (56%); Fig. 4B]. There was a significant main effect of Drug (F(1,14) = 5.700, p = 0.032). Analysis of latency to first lever press revealed no significant effect of Drug (F(1,15) = 2.542, p = 0.132). These results suggest the AI plays a critical role in context-induced relapse to alcohol seeking in Context B, the punishment context after prolonged abstinence.
Experiment 5: effect of AI inactivation on context-induced relapse to alcohol seeking following 1 d abstinence
Experiment 5 tested a causal role of AI in context-induced relapse to alcohol seeking after 1 d abstinence (Fig. 5), using reversible inactivation with intra-AI injections of muscimol + baclofen. AI inactivation did not change alcohol seeking behavior in either context following acute abstinence (Fig. 5B). There was no significant interaction between Drug × Test Context (F(1,17) = 0.010, p = 0.921). There was a significant main effect of Context (F(1,17) = 8.959, p = 0.008) with rats tested in Context A having a greater number of active lever presses compared with rats tested in Context B. There was no significant main effect of Drug (F(1,17) = 0.004, p = 0.952). Analysis of latency to first lever press revealed no significant interaction between Drug × Test Context (F(1,17) = 2.626, p = 0.124). There was a significant main effect of Context (F(1,17) = 10.543, p = 0.005) with rats tested in Context B having a greater latency to first lever press compared with rats tested in Context A. There was no significant main effect of Drug (F(1,17) = 1.589, p = 0.224). There was also no significant effect of AI inactivation on spontaneous locomotor activity (F(1,14) = 2.302, p = 0.151; Fig. 5D). These results suggest the AI does not play a critical role in context-induced relapse to alcohol seeking after acute abstinence.
Discussion
We extended a recently developed model of context-induced relapse to alcohol seeking following punishment-imposed abstinence (Marchant et al., 2013), by examining relapse after prolonged abstinence. We found an increased propensity to relapse in Context B, the punishment context after prolonged abstinence. Neither prior alcohol use history, nor the motivational strength of alcohol, predicted relapse vulnerability after prolonged abstinence. Increased propensity to relapse in the punishment context was correlated with activation of the AI, and reversible inactivation of the AI dramatically reduced the proportion of rats relapsing in the punishment context after prolonged abstinence but not following acute abstinence. Our results demonstrate a critical role of the AI in context-induced relapse to alcohol seeking in Context B, the punishment context, following prolonged abstinence.
Individual variability in the propensity to relapse and predicting relapse vulnerability following prolonged abstinence
In humans with alcohol use disorder, relapse can occur following weeks, months, or years of abstinence, and environments previously associated with alcohol availability are often potent precipitants of relapse (Wikler, 1973; Collins and Brandon, 2002; Ferri et al., 2006). In preclinical models, drug seeking progressively increases during abstinence (Shalev et al., 2001) termed incubation of craving (Grimm et al., 2001). Time-dependent increases in cue-induced reinstatement of alcohol seeking have also been demonstrated (Bienkowski et al., 2004), as well as after punishment-imposed abstinence of methamphetamine and food seeking behavior (Krasnova et al., 2014). Interestingly, in follow-up studies this group found that the incubation effect was predominantly due to enhanced cue-induced methamphetamine seeking after withdrawal (Torres et al., 2017). We did not observe increased alcohol seeking in Context A after 30 d abstinence. This lack of “incubation” has also been demonstrated for context-induced reinstatement of alcohol and methamphetamine seeking following extinction (Zironi et al., 2006; Adhikary et al., 2017). Our findings suggest that alcohol seeking in the alcohol-associated context following voluntary abstinence does not increase over the time span of our procedure, or if it does, the peak of relapse behavior may occur either before or sometime after 30 d. Indeed, a potential criticism of the punishment model used is the relatively brief alcohol-free period before Day 1 relapse testing, because alcohol is still delivered and consumed during punished responding. As previously mentioned, our data do however show a clear effect of context on Day 1 relapse testing, suggesting that even after a short period of abstinence, rats can differentiate between “safe” and “dangerous” environments.
Our most striking finding is increased alcohol seeking, and increased individual variability, in the punishment context, Context B, after 30 d abstinence. This equates to increased alcohol seeking despite the knowledge of likely negative consequences. In contrast to the alcohol context, we repeatedly observed a clear increase in alcohol seeking in the punishment context, Context B, over time. Previous studies have shown that context-induced reinstatement of alcohol seeking following 15 d abstinence does not increase responding for alcohol in the extinction context (Zironi et al., 2006). Thus, our study identifies an important difference between extinction-based and punishment-based models of abstinence and relapse.
In our procedure, the punishment context is associated with alcohol availability, but alcohol seeking is suppressed by increasing shock intensity. Thus, the neural mechanisms responsible for behavioral control in this context likely involve an interaction between those processing reward and those processing the aversive stimulus (Barberini et al., 2012; Marchant et al., 2018b). In contrast, the extinction context involves experimenter-imposed extinction where the reward is no longer delivered. Although there are distinct neural substrates responsible for this, this operational difference results in vastly different learning and neurobiological mechanisms between extinction and punishment. In our model, foot shock presumably causes increased salience of the alcohol-associated cues in the punishment context for relapse vulnerable rats. In this scenario, our data suggest time-dependent increases in alcohol seeking in response to cues with strong valence in susceptible rats. Importantly, the rats that do relapse in the punishment context had higher latency to respond compared with those tested in the alcohol context. This suggests that the extended abstinence period does not completely diminish the contextual association of punishment.
We observed significant variability in the propensity to relapse in Context B, the punishment context, after prolonged abstinence. Our attempt to predict relapse following prolonged abstinence was unsuccessful. Interestingly, these data are in line with human studies which also suggest that history of alcohol intake is not enough to predict relapse (Maisto et al., 2016; DiClemente and Crisafulli, 2017). Additionally, individual interoceptive responses to punishment might impact on alcohol relapse propensity. However, there was no correlation between relapse propensity and punishment self-administration responding in the current dataset, and we have previously shown that variability in the response to shock is not predicted by measures of alcohol self-administration (Marchant et al., 2018a).
Role of the AI in the propensity to relapse following prolonged abstinence
We found a critical role for the AI in context-induced relapse to alcohol seeking in the punishment context following prolonged abstinence, but not acute abstinence. The AI drives motivational behavior through interoception or by modulating approach versus avoidance behavior (Paulus and Stewart, 2014). Reversible inactivation of the AI reduces cue-induced reinstatement of cocaine and nicotine seeking and context-induced reinstatement of cocaine seeking (Cosme et al., 2015; Pushparaj et al., 2015; Arguello et al., 2017). Additionally, chemogenetic inhibition of AI to nucleus accumbens core projections reduces alcohol self-administration (Jaramillo et al., 2018a,b). Recently, Pelloux et al. (2018) showed increased Fos expression in the AI in Context A following context-induced relapse to cocaine seeking after punishment-imposed abstinence. Additionally, Venniro et al. (2017) showed that AI inactivation, and chemogenetic inhibition of AI to central amygdala projections, reduced relapse to methamphetamine seeking in Context A after voluntary abstinence achieved via a model of contingency management in Context B. To our knowledge, the current data are the first to link the AI with the propensity to relapse in the punishment context (B) after prolonged abstinence.
In humans, altered insular cortex volume is associated with alcohol relapse and increased activation of the anterior insular has been associated with compulsive alcohol seeking despite aversive consequences in heavy drinkers (Cardenas et al., 2011; Grodin et al., 2018). Additionally, blood oxygen level-dependent fMRI signals are increased in the insular of cocaine and methamphetamine addicts when exposed to drug-associated cues (Garavan et al., 2000; Yin et al., 2012). Finally, damage to the insular cortex reduces relapse rates in cocaine addicts (Naqvi et al., 2007; Gaznick et al., 2014). These data, combined with our preclinical studies, highlight a pivotal role for the AI in relapse.
Conclusions
In conclusion, we show an increased propensity to relapse in Context B, the punishment context, following prolonged abstinence. Neither alcohol intake history nor the motivational strength of the alcohol predicted relapse. We found increased Fos expression in the AI in rats relapsing in the punishment context, which positively correlated with alcohol seeking. Reversible inactivation confirmed a critical role for the AI in the propensity to relapse in the punishment context after prolonged abstinence. Our findings provide further evidence that alcohol use history cannot be used to accurately predict relapse. Identification of reliable biomarkers that enable precision medicine may ultimately improve relapse prevention.
Footnotes
This work was supported by a National Health and Medical Research Council Project Grant (1105741); A.J.L. is a NHMRC Principal Fellow (1116930) and E.J.C. is supported by the University of Melbourne Early Career Researcher Grant Scheme; and we acknowledge the Victorian State Government Operational Infrastructure Scheme. We thank Lauren McKeogh, Pascale Maynard, Shubham Ranjan, Shubo Jin, and Liubov Lee-Kardashyan for their help with behavioral and Fos experiments and Christina Perry for thoughtful discussions.
The authors declare no competing financial interests.
References
- Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y, Bossert JM (2017) Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine. Addict Biol 22:977–990. 10.1111/adb.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello AA, Wang R, Lyons CM, Higginbotham JA, Hodges MA, Fuchs RA (2017) Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology 234:2431–2441. 10.1007/s00213-017-4632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberini CL, Morrison SE, Saez A, Lau B, Salzman CD (2012) Complexity and competition in appetitive and aversive neural circuits. Front Neurosci 6:170. 10.3389/fnins.2012.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W (2004) Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol 14:355–360. 10.1016/j.euroneuro.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA (2006) Recent drinking consequences, motivation to change, and changes in alcohol consumption over a three month period. Addict Behav 31:331–338. 10.1016/j.addbeh.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC (1979) Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process 5:368–378. 10.1037/0097-7403.5.4.368 [DOI] [PubMed] [Google Scholar]
- Brown RM, Kim AK, Khoo SY, Kim JH, Jupp B, Lawrence AJ (2016) Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol 21:603–612. 10.1111/adb.12251 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, Anandalingam KK, Pagan-Rivera PA, Anahtar M, Beyeler A, Tye KM (2017) Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci 20:824–835. 10.1038/nn.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Watters SM, Zouikr I, Hodgson DM, Dayas CV (2015) Recruitment of hypothalamic orexin neurons after formalin injections in adult male rats exposed to a neonatal immune challenge. Front Neurosci 9:65. 10.3389/fnins.2015.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Barker DJ, Nasser HM, Kaganovsky K, Dayas CV, Marchant NJ (2017a) Cue-induced food seeking after punishment is associated with increased fos expression in the lateral hypothalamus and basolateral and medial amygdala. Behav Neurosci 131:155–167. 10.1037/bne0000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Mitchell CS, Adams CD, Yeoh JW, Hodgson DM, Graham BA, Dayas CV (2017b) Chemogenetic activation of the lateral hypothalamus reverses early life stress-induced deficits in motivational drive. Eur J Neurosci 46:2285–2296. 10.1111/ejn.13674 [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Flanagan JPM, Marchant NJ, Lawrence AJ (2018) Reduced alcohol-seeking in male offspring of sires exposed to alcohol self-administration followed by punishment-imposed abstinence. Pharmacol Res Perspect 6:e00384. 10.1002/prp2.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ (2011) Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry 70:561–567. 10.1016/j.biopsych.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BN, Brandon TH (2002) Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol 70:390–397. 10.1037/0022-006X.70.2.390 [DOI] [PubMed] [Google Scholar]
- Contreras M, Billeke P, Vicencio S, Madrid C, Perdomo G, González M, Torrealba F (2012) Role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology 37:2101–2108. 10.1038/npp.2012.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, LaLumiere RT (2015) The dorsal agranular insular cortex regulates the cued reinstatement of cocaine-seeking, but not food-seeking, behavior in rats. Neuropsychopharmacology 40:2425–2433. 10.1038/npp.2015.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116:169–173. 10.1037/0735-7044.116.1.169 [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Crisafulli MA (2017) Alcohol relapse and change needs a broader view than counting drinks. Alcohol Clin Exp Res 41:266–269. 10.1111/acer.13288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Peterson MR, Robertson HA (1987) Presence of c-fos-like immunoreactivity in the adult rat brain. Eur J Pharmacol 135:113–114. 10.1016/0014-2999(87)90767-9 [DOI] [PubMed] [Google Scholar]
- Farid WO, Lawrence AJ, Krstew EV, Tait RJ, Hulse GK, Dunlop SA (2012) Maternally administered sustained-release naltrexone in rats affects offspring neurochemistry and behaviour in adulthood. PLoS One 7:e52812. 10.1371/journal.pone.0052812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M, Amato L, Davoli M (2006) Alcoholics Anonymous and other 12-step programmes for alcohol dependence. Cochrane Database Syst Rev 3:CD005032. 10.1002/14651858.CD005032.pub2 [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. 10.1176/appi.ajp.157.11.1789 [DOI] [PubMed] [Google Scholar]
- Gaznick N, Tranel D, McNutt A, Bechara A (2014) Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine Tob Res 16:445–453. 10.1093/ntr/ntt172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F, Wang N, Cui C, Li Y, Liu Y, Ma Y, Liu S, Zhang H, Sun X (2017) Glutamatergic projections from the entorhinal cortex to dorsal dentate gyrus mediate context-induced reinstatement of heroin seeking. Neuropsychopharmacology 42:1860–1870. 10.1038/npp.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Goodlett CR, Economidou D, García-Pardo MP, Belin D, Robbins TW, Bullmore ET, Everitt BJ (2015) The novel μ-opioid receptor antagonist GSK1521498 decreases both alcohol seeking and drinking: evidence from a new preclinical model of alcohol seeking. Neuropsychopharmacology 40:2981–2992. 10.1038/npp.2015.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Peña-Oliver Y, Goodlett CR, Cardinal RN, Robbins TW, Bullmore ET, Belin D, Everitt BJ (2018) Evidence for a long-lasting compulsive alcohol seeking phenotype in rats. Neuropsychopharmacology 43:728–738. 10.1038/npp.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B (1989) Lapse, relapse and survival among opiate addicts after treatment: a prospective follow-up study. Br J Psychiatry 154:348–353. 10.1192/bjp.154.3.348 [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. incubation of cocaine craving after withdrawal. Nature 412:141–142. 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, Momenan R (2018) Neural correlates of compulsive alcohol seeking in heavy drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 3:1022–1031. 10.1016/j.bpsc.2018.06.009 [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP (2007) The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146:525–536. 10.1016/j.neuroscience.2007.01.063 [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV (2011) Propensity to “relapse” following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199:235–242. 10.1016/j.neuroscience.2011.09.047 [DOI] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, Dayas CV (2014) Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front Behav Neurosci 8:244. 10.3389/fnbeh.2014.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Van Voorhies K, Randall PA, Besheer J (2018a) Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav Brain Res 348:74–81. 10.1016/j.bbr.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, Besheer J (2018b) Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology 130:42–53. 10.1016/j.neuropharm.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann HK. (1991) The motivation for change from problem alcohol and heroin use. Br J Addict 86:727–744. 10.1111/j.1360-0443.1991.tb03099.x [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, Shaham Y, Cadet JL (2014) Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology 39:2008–2016. 10.1038/npp.2014.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Roos CR, Hallgren KA, Moskal D, Wilson AD, Witkiewitz K (2016) Do alcohol relapse episodes during treatment predict long-term outcomes? Investigating the validity of existing definitions of alcohol use disorder relapse. Alcohol Clin Exp Res 40:2180–2189. 10.1111/acer.13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y (2013) Context-induced relapse to alcohol seeking after punishment in a rat model. Biol Psychiatry 73:256–262. 10.1016/j.biopsych.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Rabei R, Kaganovsky K, Caprioli D, Bossert JM, Bonci A, Shaham Y (2014) A critical role of lateral hypothalamus in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci 34:7447–7457. 10.1523/JNEUROSCI.0256-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, Hope BT, Heins RC, Prisinzano TE, Vardy E, Bonci A, Bossert JM, Shaham Y (2016) Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci 36:3281–3294. 10.1523/JNEUROSCI.4299-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Kaganovsky K (2018a) Punishment of alcohol-reinforced responding in alcohol preferring P rats reveals a bimodal population: implications for models of compulsive drug seeking. Prog Neuropsychopharmacol Biol Psychiatry 87:68–77. 10.1016/j.pnpbp.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM, Shaham Y (2018b) Context-induced relapse after extinction versus punishment: similarities and differences. Psychopharmacology. Advance online publication. Retrieved June 1, 2018. doi: 10.1007/s00213-018-4929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315:531–534. 10.1126/science.1135926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser HM, Lafferty DS, Lesser EN, Bacharach SZ, Calu DJ (2018) Disconnection of basolateral amygdala and insular cortex disrupts conditioned approach in Pavlovian lever autoshaping. Neurobiol Learn Mem 147:35–45. 10.1016/j.nlm.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. (1997) A range of research-based pharmacotherapies for addiction. Science 278:66–70. 10.1126/science.278.5335.66 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL (2014) Interoception and drug addiction. Neuropharmacology 76:342–350. 10.1016/j.neuropharm.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Sydney, NSW: Academic. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Hoots JK, Cifani C, Adhikary S, Martin J, Minier-Toribio A, Bossert JM, Shaham Y (2018) Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions. Addict Biol 23:699–712. 10.1111/adb.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, McNally GP (2013) A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking. Eur J Neurosci 38:2762–2773. 10.1111/ejn.12283 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière J, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245:1511–1513. 10.1126/science.2781295 [DOI] [PubMed] [Google Scholar]
- Pushparaj A, Kim AS, Musiol M, Trigo JM, Le Foll B (2015) Involvement of the rostral agranular insular cortex in nicotine self-administration in rats. Behav Brain Res 290:77–83. 10.1016/j.bbr.2015.04.039 [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y (2001) Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 156:98–107. 10.1007/s002130100748 [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long–Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Cadet JL (2017) Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav Brain Res 326:265–271. 10.1016/j.bbr.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, Chiamulera C, Morales M, Shaham Y (2017) The anterior insular cortex→central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron 96:414–427.e8. 10.1016/j.neuron.2017.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. (1973) Dynamics of drug dependence. implications of a conditioning theory for research and treatment. Arch Gen Psychiatry 28:611–616. 10.1001/archpsyc.1973.01750350005001 [DOI] [PubMed] [Google Scholar]
- Wise RA. (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29:203–210. 10.1007/BF00414034 [DOI] [PubMed] [Google Scholar]
- Yin JJ, Ma SH, Xu K, Wang ZX, Le HB, Huang JZ, Fang KM, Liao LM, Cai ZL (2012) Functional magnetic resonance imaging of methamphetamine craving. Clin Imaging 36:695–701. 10.1016/j.clinimag.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH (2006) Context is a trigger for relapse to alcohol. Behav Brain Res 167:150–155. 10.1016/j.bbr.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Zuo W, Fu R, Hopf FW, Xie G, Krnjević K, Li J, Ye JH (2017) Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol 22:103–116. 10.1111/adb.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]