Abstract
Memory reconsolidation is hypothesized to be a mechanism by which memories can be updated with new information. Such updating has previously been shown to weaken memory expression or change the nature of the memory. Here we demonstrate that retrieval-induced memory destabilization also allows that memory to be strengthened by additional learning. We show that for rodent contextual fear memories, this retrieval conditioning effect is observed only when conditioning occurs within a specific temporal window opened by retrieval. Moreover, it necessitates hippocampal protein degradation at the proteasome and engages hippocampal Zif268 protein expression, both of which are established mechanisms of memory destabilization-reconsolidation. We also demonstrate a conceptually analogous pattern of results in human visual paired-associate learning. Retrieval-relearning strengthens memory performance, again only when relearning occurs within the temporal window of memory reconsolidation. These findings link retrieval-mediated learning in humans to the reconsolidation literature, and have potential implications both for the understanding of endogenous memory gains and strategies to boost weakly learned memories.
SIGNIFICANCE STATEMENT Memory reconsolidation allows existing memories to be updated with new information. Previous research has demonstrated that reconsolidation can be manipulated pharmacologically and behaviorally to impair problematic memories. In this article, we show that reconsolidation can also be exploited to strengthen memory. This is shown both in rats, in a fear memory setting, and in a human declarative memory setting. For both, the behavioral conditions necessary to observe the memory strengthening match those that are required to trigger memory reconsolidation. There are several behavioral approaches that have previously been shown convincingly to strengthen memory. The present demonstration that reconsolidation can underpin long-lasting memory improvements may both provide an underlying mechanism for such approaches and provide new strategies to boost memories.
Keywords: destabilization, fear conditioning, memory, reconsolidation, retrieval
Introduction
Once acquired, memories are subject to modification. One mechanism by which this can be achieved involves the phenomenon of memory reconsolidation (Lee, 2009; Nader and Hardt, 2009; Lee et al., 2017). In reconsolidation, a memory is first destabilized (Ben Mamou et al., 2006). Following destabilization, the memory is restabilized, or reconsolidated, during which process the memory can be strengthened pharmacologically (Tronson et al., 2006) and new, updating information may be integrated (Lee, 2008, 2010; Inda et al., 2011; De Oliveira Alvares et al., 2013; Olshavsky et al., 2013).
The capacity of reconsolidation to update memories has been exploited behaviorally to weaken fear memory expression by combining memory retrieval with subsequent extinction training in a retrieval–extinction procedure. This was demonstrated initially in a tone fear setting dependent upon amygdala plasticity (Monfils et al., 2009), and subsequently was shown to apply also to contextual fear memories (Flavell et al., 2011; Rao-Ruiz et al., 2011). These latter studies demonstrated that the retrieval–extinction phenomenon depended upon hippocampal L-type voltage-gated calcium channels (Flavell et al., 2011), which are known to be required for memory destabilization (Suzuki et al., 2008).
We hypothesized, based upon the apparent function of reconsolidation to update memories and the success of exploiting this to weaken memory expression, that reconsolidation might be similarly harnessed also to strengthen hippocampal memory expression. While simple additional learning in isolation certainly does strengthen memories (Lee, 2008), retrieval that induces destabilization can also be an effective method of increasing fear memory expression (Inda et al., 2011; De Oliveira Alvares et al., 2013). However, while both of these contextual fear memory-strengthening effects have been shown previously to involve hippocampal destabilization-reconsolidation (Lee, 2008; De Oliveira Alvares et al., 2013), previous contextual fear memory studies have not attempted to combine destabilization-inducing retrieval with additional relearning. Based upon the hypothesized conceptual similarity between retrieval-extinction and the proposed retrieval-relearning, we would predict that any memory-strengthening effect should be subject to the same temporal “reconsolidation window” of effect, which includes 10–60 min intervals, but not a 6 h interval, between retrieval and extinction (Monfils et al., 2009).
Interestingly, studies of human associative memory have traditionally focused on the beneficial, memory-enhancing effects of retrieval, rather than on the destabilizing or updating effects. It is a well established observation in the cognitive psychology literature that memory testing (i.e. retrieval) is at least as effective in supporting subsequent performance as is additional learning (Roediger and Karpicke, 2006), and is much more effective than additional learning when performance is assessed at long delays, especially when combined with immediate feedback. In fact, it has recently been argued that retrieval can act as a fast consolidating event for newly acquired memories (Antony et al., 2017). While some empirical studies have confirmed that memory retrieval, which likely induces destabilization, can itself strengthen memory (Forcato et al., 2011), it has not previously been shown that retrieval, via destabilization and reconsolidation, opens a temporally limited window of opportunity for a memory to be strengthened by additional experience. We here test explicitly such a hypothesis using contextual fear conditioning in rats, in which the cellular mechanisms of destabilization and reconsolidation are well delineated, and associative learning in humans.
For the present series of experiments, we predicted that the combination of a single destabilization-inducing memory retrieval with a single additional relearning session shortly thereafter would confer the greatest memory enhancement when arranged in a manner to engage reconsolidation (i.e., relearning occurring after, rather than before, retrieval and within the reconsolidation window). Moreover, we predicted that this retrieval-relearning double experience would exceed any memory gains afforded by retrieval practice alone and would both rely upon memory destabilization and recruit cellular mechanisms of reconsolidation. Recent evidence using inhibitory avoidance memories supports the behavioral prediction (Du et al., 2017) but does not show a conclusive dependence upon destabilization and reconsolidation. Therefore, using near-threshold parameters of conditioning (to avoid ceiling effects), we exposed rats to subsequent retrieval and relearning within an uninterrupted session or with varying intertrial intervals. We also used a reverse order condition (i.e., relearning followed by retrieval) as a comparative approach to strengthen memories. Following confirmation that the combination of retrieval and relearning strengthened hippocampal contextual fear memories in a reconsolidation-dependent manner, we applied the same strategy to weakly learned human episodic paired-associate memories, which are similarly dependent upon the hippocampus (Eichenbaum, 2000; Konkel et al., 2008).
Materials and Methods
Experimental design and statistical analysis.
Rodent sample size was determined by power analyses assuming the effect size would be equivalent to that observed in memory disruption studies. Sample size for the human studies was arbitrarily set a level 50% greater than that used in previous human memory reconsolidation studies (Hupbach et al., 2007). Given the aim of showing memory strengthening, rats that showed >50% freezing after learning were excluded; pilot studies showed that the mean freezing after learning was 27.7%, and that one-quarter of rats increased the percentage of freezing levels by >50% from learning to test. The principles for exclusion criteria in the human study were that initial learning performance should not preclude the detection of a population mean strengthening effect; specific details are included in the statistical analysis section. No outliers were excluded from the analyses (all data fell within 2 SDs of the mean). The reported endpoints and statistical analytical approach were determined prospectively.
The original objectives of the research were to demonstrate whether relearning within the reconsolidation window strengthens contextual fear memory (Fig. 1A), and whether this depends upon mechanisms of destabilization and reconsolidation. Following the outcomes of these experiments, the further objective of the research was to show analogous results in human paired-associate memory.
Figure 1.
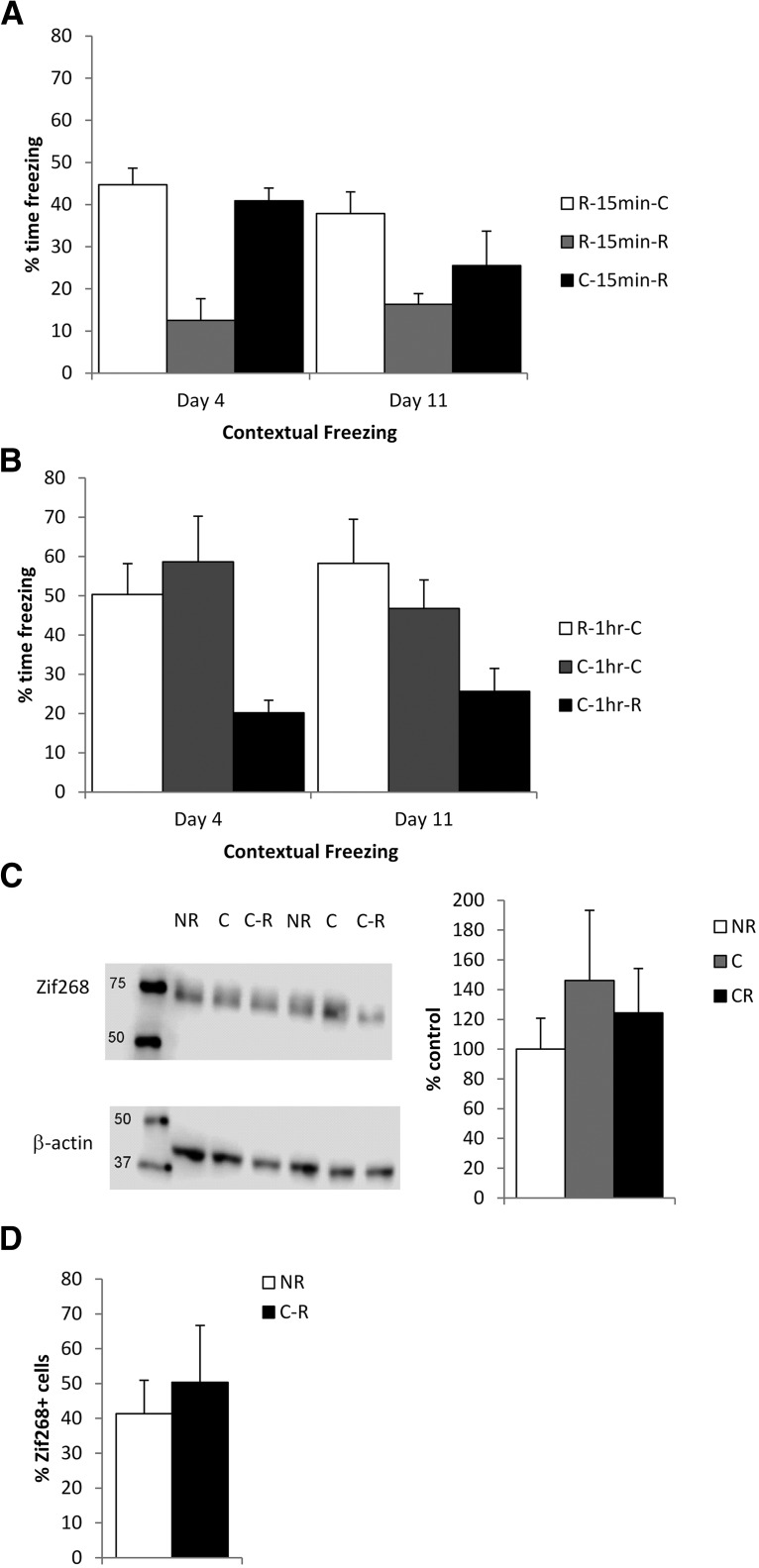
Combination of retrieval and conditioning strengthened contextual fear memory via destabilization and reconsolidation. A, Previously weakly conditioned rats were subjected to retrieval and conditioning on day 2, and were tested again on days 4 and 11. B, With a 15 min interval between retrieval and conditioning on day 2, contextual freezing was increased at the tests compared with when there was no interval. C, There was a similar increase in freezing with a 1 h interval, but not with a 6 h interval. D, Schematic representing the infusion of β-lac into the dorsal hippocampus before retrieval or conditioning within the retrieval-1 h-relearning procedure. E, Infusion of β-lac prevented contextual fear memory strengthening. F, Schematic of the behavioral procedures for the Zif268 expression experiments. G, Retrieval-conditioning, but not retrieval alone, reliably elevated Zif268 levels compared with a nonreactivated control condition, as assessed through Western blots. H, Zif268 expression was also assessed with flow cytometry (image shows representative sample with events plotted according to size [forward scatter (FSC)] and cell granularity [side scatter (SSC)], allowing the isolation of cells from debris and illustrating distinct populations of labeled events [DAPI +ve (blue), NeuN +ve (purple), Zif268 +ve (green), and negative/debris (black)]. I, Flow cytometry also showed an increase in Zif268 expression in retrieval-conditioning. Data are presented as the mean + SEM.
Research subjects and experimental design are described below. Subjects were randomly allocated to experimental group within each cohort of subjects, using a random sequence generator. Experimenters were not strictly blinded to allocation during the conduct of the experiments, but all data processing and analysis was conducted blind to the intervention.
Statistical analyses were conducted in JASP (JASP Team, 2016). Contextual freezing was analyzed using a mixed two-way ANOVA across both test sessions, with separate one-way ANOVA of freezing during retrieval/reconditioning (either the full retrieval session or the preshock period of the reconditioning session). Due to the groupings of cohorts, and a substantial time interval between cohorts, the data are analyzed primarily within cohort, starting with core comparisons, followed by the wider analysis including additional groups. Raw uncorrected p values are presented, but all analyses survive Bonferroni correction for repeated analyses within each cohort. Within the wider analysis, Tukey-corrected post hoc pairwise comparisons were used to explore group differences. We also conducted an exploratory comparison across cohorts, focusing on the effect of delay between retrieval and conditioning. η2p was used as an estimate of effect size, and Bayes Factors (BF10/BFInclusion) are also reported as the outcome of Bayesian analyses for the estimation of posterior probability. Western blot and flow cytometry analyses were conducted using one-way ANOVAs, with Bonferroni-corrected post hoc pairwise comparisons. For the human episodic memory task, a memory improvement score was calculated by the simple numerical difference between the number of correct object associates reported at the final test and the number reported immediately after learning on the first day of training. Data for participants scoring >32 of 40 in the immediate test on the first day of training were excluded to avoid individual ceiling effects, with the criterion determined by the average improvement score of 7.4 in the core experimental group without exclusions. These improvement scores were compared across groups using a series of one-way ANOVAs, each with Tukey-corrected post hoc pairwise comparisons.
Subjects.
One hundred twenty-one experimentally naive adult male Lister Hooded rats (Charles River Laboratories) weighed either 200–225 g (for nonsurgical experiments) or 275–300 g (for cannulated rats) at the start of the experiment. Rats were housed in quads (save for a 24 h recovery period following surgical procedures) under a 12 h light/dark cycle (lights on at 7:00 A.M.) in a specialist animal facility. Individually ventilated cages contained aspen chip bedding and a Plexiglas tunnel for environmental enrichment. Rats had free access to food and water other than during behavioral sessions. Experiments took place between 9:00 A.M. and 4:00 P.M. in a behavioral laboratory. At the end of the experiment, animals were humanely killed using a rising concentration of CO2 to render the animal unconscious, followed by dislocation of the neck and extraction of the brain if required. All procedures were approved by the local animal welfare and ethical review board and performed in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act, Amendment Regulations 2012 (PPL P8B15DC34).
One hundred seventy-one undergraduate students from the University of Birmingham participated in the study. All participants were recruited through the Psychology Research Participation Scheme and received course credit for their participation. Participants gave their informed consent, and all procedures were approved by the University of Birmingham Science, Technology, Engineering and Mathematics Ethics Review Committee.
Surgical procedures.
Twenty-nine rats were implanted with chronic indwelling stainless steel cannulae (Coopers Needleworks) according to our established procedures (for full details, see Exton-McGuinness and Lee, 2015). The cannulae targeted the dorsal hippocampus (Lee and Hynds, 2013). At the end of the experiment, extracted brains were drop perfused in 4% paraformaldehyde for 7 d and then processed for histological assessment of cannula placements by Nissl staining.
Rodent behavioral procedures.
All behavioral procedures were performed in conditioning chambers (Med Associates) as previously described (Lee and Hynds, 2013), with freezing behavior automatically recorded by Videotracking software (Viewpoint Life Sciences). Rats were randomly allocated to an experimental group within each experiment.
All rats (whether cannulated or not) received the same behavioral training. Conditioning consisted of a single 3 min session, without any prior exposure to the context, in which rats were exposed to a single 0.35 mA footshock for 2 s after 2 min. This near-threshold footshock intensity generated appreciable conditioning, in the form of later contextual freezing, in only a subset of rats, and so allowed for the observation of memory strengthening. On the next day, the experimental retrieval-relearning groups received a nonreinforced retrieval session (2 min re-exposure to the conditioning context), followed at varying times later by a reconditioning session (Fig. 1A). Memory strengthening, assessed at tests on days 4 and 11, was compared against a group that had no interval between the retrieval and relearning (retrieval-0 min-relearning; operationally, this consisted of a single conditioning session with footshock delivered after 4 min that acted also as a relearning-only control), given that an interval is necessary to engage the behavioral modification of a destabilized memory (Monfils et al., 2009). Additional control groups included a double retrieval (retrieval-retrieval) group that received two retrieval sessions separated by the same 15 min interval, both to control for the double experience and to act as a retrieval-only comparison, and the reversal of the order of presentation of the retrieval and reconditioning sessions (relearning-retrieval). A final control consisted of two spaced reconditioning sessions (relearning-relearning) that were expected to increase freezing maximally. During all intervals, rats were returned to their homecage in the holding room. Contextual freezing was subsequently assessed in 2 min test sessions 2 and 9 d later.
Cannulated rats were habituated to a dummy infusion procedure (with the injectors loaded with PBS, but no infusion taking place) on the day of conditioning. They were then infused (1 μl/side) with clasto-lactacystin-β-lactone (β-lac; 32 ng/μl) or its vehicle (2% DMSO in 1 m HCl diluted in PBS and adjusted to pH 7.0–7.4 with NaOH; Lee, 2010) immediately before either the retrieval session or the relearning session within the retrieval-1 h-relearning condition on day 2.
Biochemical procedures.
Thirty-six rats were conditioned on day 1. On day 2, there were five conditions: (1) no behavioral session (nonreactivated); (2) retrieval only; (3) retrieval-1 h-relearning; (4) relearning only; and (5) relearning-1 h-retrieval. The rats were killed 2 h after the initial behavioral session on day 2, and their brains were rapidly extracted for the assessment of Zif268 protein levels. The dorsal hippocampus was dissected and frozen on dry ice. For flow cytometry, the tissue was subjected to a standard nuclear extraction protocol and the nuclear fraction was resuspended in 10% normal donkey serum. Five of these samples were unable to be processed by flow cytometry. Flow cytometry was conducted largely based upon established procedures (Li et al., 2014). Samples were then incubated with rabbit anti-Zif268 (1:500; catalog #sc-110, Santa Cruz Biotechnology) and mouse anti-NeuN (1:1000; catalog #MAB377, Millipore) primary antibodies, followed by secondary antibodies (donkey anti-mouse IgG PE, 1:100; catalog #sc-3744, Santa Cruz Biotechnology; donkey anti-rabbit IgG A488, 1:1000; catalog #AB150073, Abcam) and DAPI (0.5 μg; Cell Signaling Technology), and then run through a flow cytometer. All gates were set at a fixed position across samples to include the most fluorescent group of cells. The DAPI+ gate was used as the stopping gate (10,000 events), so that a set number of events was counted for each sample, allowing a more standardized comparison. Zif268+ cells were considered to be those that were simultaneously DAPI+, NeuN+, and Zif268+, and the percentage of Zif268+ labeling for each sample was calculated based on a total cell count of 10,000. Western blot procedures were conducted largely as previously described (Lee and Hynds, 2013). Blots were incubated first with rabbit anti-EGR1 (1:1000 in 5% nonfat milk overnight at 4°C; catalog #4154, Cell Signaling Technology), and then with goat anti-rabbit HRP-linked secondary antibody [1:2000 in 5% nonfat milk for 60 min at room temperature (RT); catalog #7074, Cell Signaling Technology]. After enhanced chemiluminescence visualization (C-Digit, LI-COR), the HRP activity of the goat anti-rabbit secondary antibody was irreversibly quenched with 30% H2O2 for 15 min at 37°C (Sennepin et al., 2009). The blot was then incubated with the mouse anti-actin loading control (1:20,000 in TBST overnight at RT; catalog #ab6276, Abcam) and goat anti-mouse HRP-linked secondary antibody (1:10,000 in TBST at RT; catalog #A4416, Sigma-Aldrich), and revisualized with enhanced chemiluminescence. The Zif268 signal (-background) was normalized against actin expression [(raw Zif268 signal) × (mean actin signal)/(sample actin signal)] and then this figure was normalized against the mean of the nonreactivated control group to generate a percentage control value.
Human behavioral procedures.
All behavioral procedures were conducted using a visual paired-association task, run in PsychoPy (Peirce, 2007) on a desktop computer in a testing cubicle. The visual images were 40 object and 40 scene images that were randomly selected from object and scene stimulus banks (Brady et al., 2008; Konkle et al., 2010). Each object stimulus was randomly associated with a scene image (with the associations determined uniquely for each participant). The object image was presented directly above the scene image for 4 s. During learning, the 40 paired associates were sequentially presented on a single occasion each. Immediate retention of the single-trial learning was tested by presentation of the scene image alone for 6 s, with the participant prompted to recall verbally the associated object image. The experimenter manually recorded the response, which was subsequently coded as correct/incorrect. No feedback was given.
Forty-eight hours after learning, the participants returned to the same testing cubicle, with the same experimenter. In the experimental retrieval-10 min-relearning group, participants were first presented with the scene images alone (as in the immediate test after learning) and were requested to remember, but not verbalize, the associated object image. After a 10 min mathematical distraction task, they were then given a second learning session, which was identical in nature to initial learning (but with a randomized order of paired-associate presentation). Control groups (seven in total) were conducted in three sequential experimental cohorts, with random allocation of participants to the groups within these cohorts, as follows: (1) reversal of the order of retrieval and relearning (relearning-10 min-retrieval), presentation of retrieval or relearning alone (followed by the distractor task), no memory experience (control group; these participants simply completed the Big 5 personality test; John and Srivastava, 1999), followed by the distractor task; (2) double presentation of either the retrieval (retrieval-10 min retrieval) or relearning (relearning-10 min-relearning) sessions, with the same distractor task between the two presentations; and (3) delayed the interval between relearning and retrieval, such that the second experience occurred outside the putative reconsolidation window (retrieval-6 h-relearning and relearning-6 h-retrieval). The distractor task was completed immediately after the first experience.
Another 48 h later, all participants were tested on their paired-associate recall in an identical manner to the immediate test after learning.
Results
Strengthening of contextual fear conditioning in rats
We studied the impact of various intervals between retrieval and the relearning of rodent contextual fear (Fig. 1A) as previous studies had demonstrated that intervals of 10 min and 1 h between retrieval and extinction, but not 0 min or 6 h, successfully and persistently diminished fear expression (Monfils et al., 2009). These conditions were split across different cohorts, and so each cohort was analyzed independently, followed by an exploratory consolidated analysis of all groups. Memory strengthening was assessed at tests on days 4 and 11. Analysis of contextual freezing at these tests revealed that the retrieval-15 min-relearning group (R-15 min-C) displayed higher freezing compared with the unspaced retrieval-0 min-conditioning (R-0 min-C) control (Fig. 1B). A significant main effect of group was observed (F(1,15) = 17.1, p < 0.001, η2p = 0.53, BFInclusion = 16.4), with no effect of session or group × session interaction (F values <1.5, p values >0.24, BFInclusion <0.64). The pattern of results at test were not due to differences in initial conditioning, as freezing on day 2 before footshock delivery was equivalent across groups (R-0 min-C, 14.8 ± 10.4; R-15 min-C, 13.1 ± 9.7; F(1,15) = 0.13, p = 0.72, η2p = 0.009, BF10 = 0.44). Therefore, spacing of retrieval and conditioning resulted in greater memory strengthening. Moreover, the retrieval-1 h-conditioning (R-1 hr-C) group froze at higher levels than the retrieval-6 h-conditioning (R-6 hr-C) group (Fig. 1C). A significant main effect of group was observed (F(1,14) = 9.5, p = 0.008, η2p = 0.41, BFInclusion = 29.8), with no effect of session or group × session interaction (F values <0.98, p values >0.22, BFInclusion <0.46). The pattern of results at test were again not due to differences in initial conditioning, as freezing on day 2 before footshock delivery was equivalent across groups (R-1 h-C = 18.7 ± 12.5, R-6 h-C = 18.0 ± 13.6; F(1,14) = 0.012, p = 0.92, η2p = 0.001, BF10 = 0.43). The exploratory analysis across all delays confirmed that greater strengthening was observed with delays of 15 min and 1 h (F(3,29) = 9.2, p < 0.001, η2p = 0.49, BFInclusion = 108). Frequentist post hoc comparisons (p < 0.05) confirmed that the 0 min and 6 h delay groups did not differ from each other, and neither did the 15 min and 1 h delay groups. While the 1 h delay froze at higher levels than 0 min and 6 h, the 15 min delay group was not significantly higher than the 6 h group. Bayesian post hoc tests largely supported this pattern, although there was some evidence for a difference between the 15 min and 6 h groups (BF10 = 4.1). So far, this pattern of results confirms that retrieval paired with reconditioning produces more substantial benefits on long-term retention when the reconditioning occurs within a critical time window opened by the preceding retrieval, and that this time window is consistent with a reconsolidation-based process.
Contextual fear strengthening is blocked by disrupting memory destabilization
If the retrieval-relearning enhancement of fear memory is mediated by a destabilization-reconsolidation process, the prevention of memory destabilization should block the increase in freezing. This is a strategy that has previously been used to conclude a role of reconsolidation in memory modification (Lee, 2008, 2010; De Oliveira Alvares et al., 2013). Given that hippocampal protein degradation at the proteasome is essential for the destabilization of contextual fear memories (Lee et al., 2008), we infused the proteasome inhibitor β-lac into the dorsal hippocampus immediately before memory retrieval within the retrieval-1 h-relearning condition that appeared to provide the most robust strengthening (Fig. 1D). As a control for any direct effect of β-lac upon the subsequent conditioning session, β-lac was infused in a separate group after retrieval and immediately before relearning. Analysis of contextual freezing at the tests revealed that the preretrieval β-lac group froze at lower levels than the vehicle and preconditioning β-lac groups (Fig. 1E). A significant main effect of group was observed (F(2,18) = 13.7, p < 0.001, η2p = 0.60, BFInclusion = 173), with a significant effect of session (F(1,18) = 13.7, p = 0.001, η2p = 0.44, BFInclusion = 17.0), but less evidence for a group × session interaction (F(2,18) = 3.11, p = 0.069, η2p = 0.26, BFInclusion = 4.5). Post hoc comparisons of the main effect of group confirmed that the preretrieval β-lac group froze at a lower level than each of the other two groups (p < 0.002, Cohen's d > 0.95, BF10 > 885), which did not differ from each other. Given the trend toward an interaction, an analysis of simple main effects confirmed significant group differences at both tests on day 4 (F(2,18) = 15.9, p < 0.001, η2p = 0.64, BF10 = 215) and day 11 (F(2,18) = 8.2, p = 0.003, η2p = 0.48, BF10 = 14.5), with post hoc comparisons revealing lower freezing levels in the preretrieval β-lac group compared with each of the other two groups (p < 0.03, Cohen's d > 0.63, BF10 > 3.6). Therefore, the persistent increase in freezing following retrieval-conditioning was blocked specifically by preretrieval intrahippocampal infusion of β-lac.
Contextual fear strengthening recruits Zif268 expression
This interpretation that retrieval-conditioning engages destabilization-reconsolidation to strengthen memory expression was further explored by analysis of hippocampal Zif268 protein levels by both Western blots and flow cytometry in separate samples. Rats were initially conditioned and then subjected to the retrieval-1 h-relearning procedure, with brains being taken 1 h later (Fig. 1F). The retrieval-conditioning group was compared with a nonreactivation control (no behavioral session) as well as a group that received only the retrieval session to determine the contribution of the initial behavioral experience to the engagement of Zif268 expression. The Western blot analyses showed evidence that retrieval-conditioning increased Zif268 expression compared with nonreactivation, with the retrieval-only group having intermediate and nonsignificantly different levels of Zif268 (Fig. 1G: F(2,8) = 8.5, p = 0.010, η2p = 0.68, BF10 = 5.3; post hoc p = 0.008, BF10 = 8.8 for the nonreactivation vs retrieval-conditioning comparison). Analysis by flow cytometry revealed further evidence for an upregulation of Zif268 expression by retrieval-conditioning (Fig. 1H,I: F(2,9) = 6.8, p = 0.023, η2p = 0.66, BF10 = 3.5; post hoc p = 0.023, BF10 = 3.7 for the nonreactivation vs retrieval-conditioning comparison). Therefore, the increased memory expression at test in the retrieval-conditioning groups is highly likely due to a reconsolidation-mediated updating process.
Contextual fear strengthening depends upon the nature and order of retrieval and conditioning
The retrieval-conditioning groups were compared against additional groups to investigate whether the nature of the sessions (i.e., retrieval vs conditioning) and the order of presentation (i.e., retrieval before conditioning) is important for the strengthening effect. For the 15 min interval, comparison groups included retrieval-retrieval and conditioning-retrieval groups (Fig. 2A). A significant main effect of group was observed (F(2,21) = 10.23, p < 0.001, η2p = 0.49, BFInclusion = 30.8), with no effect of session or group × session interaction (F values <2.7, p values >0.11, BFInclusion < 1.8). Post hoc comparisons (p < 0.05, Cohen's d > 0.62, BF10 > 25.9) confirmed that the retrieval-retrieval group froze at lower levels than both the retrieval-conditioning and conditioning-retrieval groups. Therefore, the spacing of retrieval and conditioning resulted in greater memory strengthening that could not be attributed simply to the spaced retrieval opportunity. There was no difference, however, between the retrieval-conditioning and conditioning-retrieval groups (BF10 = 0.62), suggesting that the order of presentation of retrieval and conditioning might not be important for memory strengthening, at least for the 15 min interval.
Figure 2.
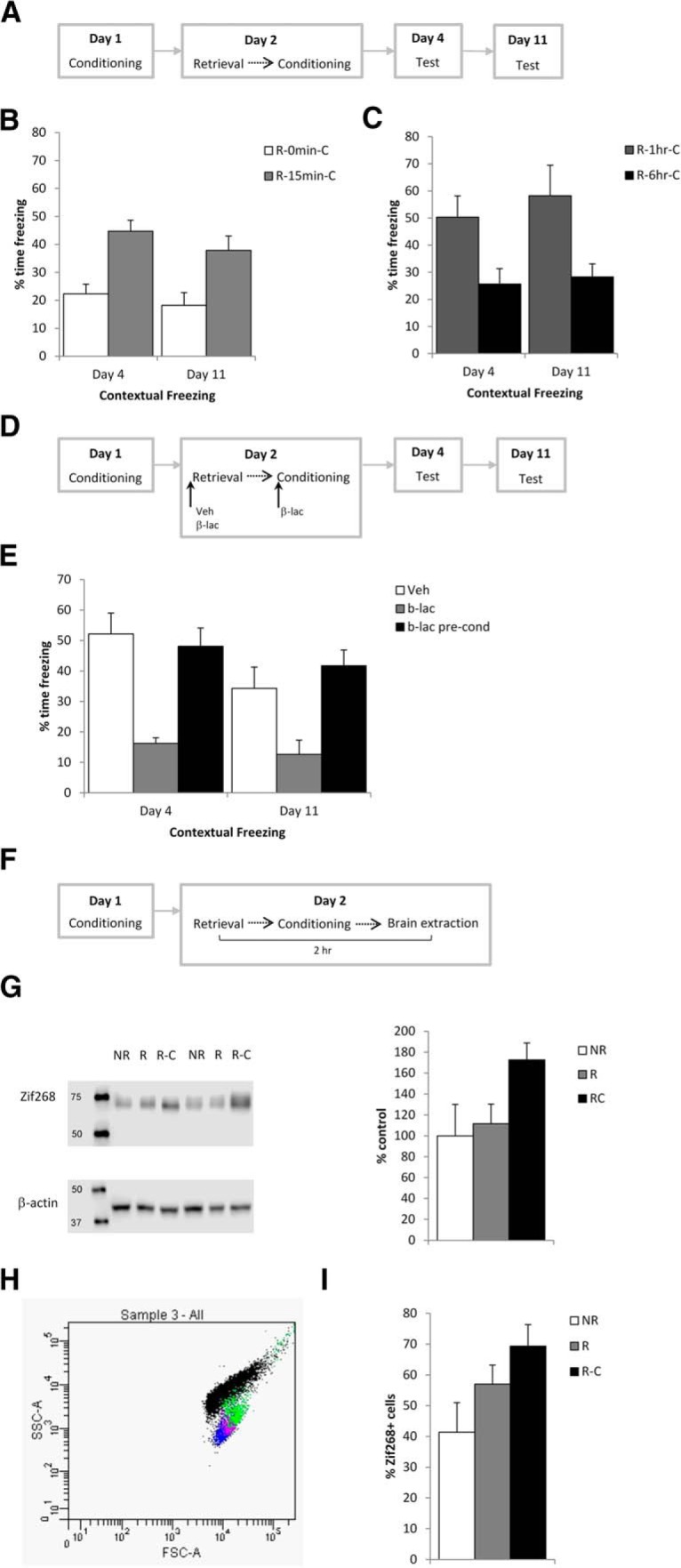
Retrieval-conditioning strengthens contextual fear memory more reliably than other combinations of experiences. A, With a 15 min interval, both retrieval-conditioning and conditioning-retrieval show greater strengthening than retrieval-retrieval. B, With a 1 h interval, retrieval-conditioning strengthened contextual fear to a greater degree than conditioning-retrieval, and to an equivalent degree as double conditioning. C, D, Conditioning-retrieval with a 1 h interval did not upregulate Zif268 expression as assessed with Western blots (C) and flow cytometry (D). Data are presented as the mean + SEM.
For the 1 h interval, we again included a conditioning-retrieval comparison, as well as a conditioning-conditioning group (Fig. 2B). A significant main effect of group was observed (F(2,20) = 7.3, p = 0.004, η2p = 0.42, BFInclusion = 9.4), with no effect of session or group × session interaction (F values <1.9, p values >0.19, BFInclusion <0.64). Post hoc comparisons (p < 0.05, Cohen's d > 0.57, BF10 values >1 54) confirmed that the retrieval-conditioning and conditioning-conditioning groups differed from the conditioning-retrieval group, but did not differ from each other (BF10 = 0.35). Therefore, with the 1 h interval, retrieval-conditioning strengthened contextual fear memory to a similar degree as two spaced conditioning sessions. However, retrieval after conditioning failed to strengthen memory.
Given the apparently qualitatively different effect of conditioning-1 h-retrieval compared with retrieval-1 h-conditioning, we analyzed Zif268 expression following conditioning-1 h-retrieval or conditioning alone, comparing the same nonreactivation control as in our previous cellular analyses. There was little evidence for any difference in Zif268 expression between the groups when assessed through Western blots (Fig. 2C; F(2,9) = 0.60, p = 0.57, η2p = 0.12, BF10 = 0.47). Due to the loss of samples, the conditioning-retrieval group could only be compared by flow cytometry against the nonreactivation group, again demonstrating little evidence for any difference (Fig. 2D; t(4) = 0.58, p = 0.59, d = 0.47, BF10 = 0.62). Therefore, it appears that conditioning-retrieval does not engage cellular mechanisms of reconsolidation, at least with the 1 h interval analyzed here.
Strengthening of paired-associate memory in humans
Given the effect of retrieval-conditioning in strengthening hippocampal contextual fear memories, we conducted a conceptual replication applying an analogous retrieval-relearning procedure to an experimental human episodic memory paradigm. Using single-trial paired-associate learning of background scenes and target images, a relatively poor episodic memory was initially learned (mean, 17.9 of 40 associates recalled immediately after learning across all groups). This allowed for the detection of quantitative memory improvements at a later test (Fig. 3A; strengthening score = test performance − learning performance). In an initial experiment, a retrieval-relearning group (with an interval of 10 min) was compared against groups receiving individual retrieval or relearning experiences, as well as the reverse relearning-retrieval order and a nonmemory control (Fig. 3B). One-way ANOVA revealed a significant effect of group on the memory strengthening (F(4,90) = 51.7, p < 0.001, η2p = 0.70, BF10 = 2.3 × 1019), with planned comparisons (p values <0.05, BF10 values >5.8) confirming that the retrieval-relearning group improved to a greater extent than the relearning-alone, retrieval-alone, and control groups. Exploratory post hoc analyses revealed, surprisingly, that the retrieval-alone group had no performance benefit over the control group (p = 0.55, BF10 = 0.67), and both groups in fact displayed poorer memory performance at test compared with immediately after learning.
Figure 3.
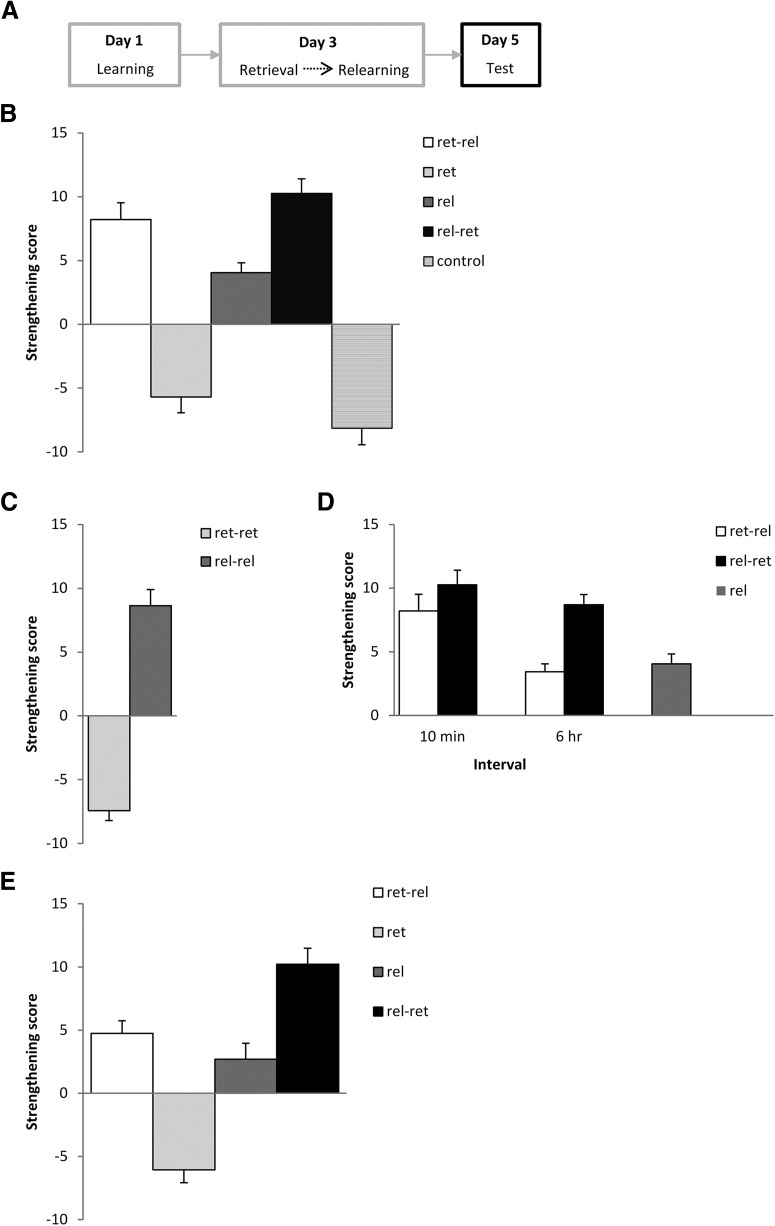
Retrieval-relearning improves human visual paired-associate memory performance. A, Previously weakly learned paired associates were retrieved and/or relearned after 2 d, and tested again 2 d later. B, Test performance was increased by retrieval-relearning, but also by relearning-retrieval. C, When the same experience was repeated, only relearning-relearning improved memory performance. D, When the interval between retrieval and relearning was increased to 6 h, the memory-strengthening effect of retrieval-relearning was decreased, but that of relearning-retrieval was not. E, When participants were instructed to verbalize the answer at the retrieval session, there was no beneficial effect of the retrieval when conducted before relearning. Data are presented as the mean strengthening score (test performance − learning performance) +/− SEM.
The primary conclusion from these initial results is that two experiences are more beneficial to memory improvement than a single or no retrieval or relearning opportunity. It is not clear, however, whether it is the different nature of the two experiences that contributes to the magnitude of memory strengthening. Therefore, we tested two further conditions, in which two identical experiences were repeated − retrieval-retrieval and relearning-relearning. There was a significant difference between the retrieval-retrieval and relearning-relearning groups (Fig. 3C: F(1,36) = 103.9, p < 0.001, η2p = 0.74, BF10 = 1.4 × 109), with the retrieval-retrieval group showing no evidence of memory strengthening, compared with the substantial improvement displayed by the relearning-relearning group. An exploratory analysis of all four double-experience groups confirmed that there were equivalent levels of memory strengthening in all but the retrieval-retrieval group (F(3,72) = 50.4, p < 0.001, η2p = 0.68, BF10 = 4.0 × 1014; post hoc tests: differences with the retrieval-retrieval group, p values <0.001 and BF10 values >1.2 × 108; equivalences, p values >0.61, BF10 values <0.57). Therefore, it is not simply the increased number of experiences that are conducive to memory strengthening, but their nature is also an important factor.
Given that the combination of retrieval and relearning is important for memory strengthening, we again exploited the time-dependent nature of reconsolidation updating to determine whether relearning needs to be presented within the reconsolidation window (Schiller et al., 2010). We also tested whether a similar temporal requirement applied to the memory strengthening observed for relearning-retrieval. Therefore, the retrieval-6 h-relearning and relearning-6 h-retrieval groups were compared against the original relearning-alone, retrieval-relearning, and relearning-retrieval groups (Fig. 3D). ANOVA revealed a significant difference between the groups (F(4,90) = 10.99, p < 0.001, η2p = 0.33, BF10 = 5.8 × 104), with post hoc comparisons demonstrating no difference between the retrieval-6 h-relearning and relearning-alone groups (p = 0.91, BF10 = 0.55), but greater memory strengthening in the relearning-6 h-retrieval group (p values < 0.02, BF10 values > 56). Of particular relevance was the observation that the retrieval-6 h-relearning group performed more poorly than the retrieval-10 min-relearning group (p < 0.002, BF10 = 48), but the relearning-6 h-retrieval and relearning-10 min-retrieval groups performed at similarly high levels (p = 0.56, BF10 = 0.73), These results show that when relearning was delayed until the reconsolidation window had closed, there was no benefit of the prior retrieval experience, strongly indicating that the retrieval-relearning effect is mediated by destabilization-reconsolidation. Moreover, the preserved memory strengthening in the relearning-6 h-retrieval condition suggests that the beneficial effects of relearning-retrieval are mediated by an alternative process. This interpretation is further supported by an additional experiment showing that verbalized recall, which is known to prevent memory destabilization in human paired-associate paradigms (Forcato et al., 2009), prevented the retrieval-relearning memory gain, but not that observed following relearning-retrieval (Fig. 3E). ANOVA revealed a significant effect of group (F(3,70) = 42.2, p < 0.001, η2p = 0.64, BF10 = 4.3 × 1019), with planned comparisons (p values <0.002, BF10 values >25.5) confirming that the retrieval-relearning group improved to a greater extent than the retrieval-alone group, but to a lesser extent than the relearning-retrieval group. However, the retrieval-relearning group did not differ from the relearning-alone group (BF10 = 0.72), whereas an exploratory post hoc comparison showed that relearning-retrieval improved test performance relative to relearning-alone (p < 0.001, BF10 = 708). A further exploratory comparison against the retrieval-relearning group from Figure 3A revealed a weak effect of verbalizing the retrieval at retrieval-relearning (t(36) = 2.16, p = 0.038, d = 0.70, BF10 = 1.85). Therefore, while both retrieval-relearning and relearning-retrieval result in memory gains, they appear not to rely upon the same behavioral conditions.
Discussion
The present results show that relearning within the reconsolidation window opened by retrieval improves subsequent long-term memory expression in both rodent and human hippocampal memory settings. Retrieval followed 10–15 min later by relearning strengthened both contextual fear memory in rats and visual paired-associate memory in humans. The same benefit was present in rodents with an interval of 1 h between retrieval and relearning. Critically, however, when the interval between retrieval and relearning was extended outside the reconsolidation window (Nader et al., 2000; Monfils et al., 2009; Schiller et al., 2010), there was no greater strengthening observed compared with relearning alone. Furthermore, when blocking memory destabilization by preventing protein degradation in the dorsal hippocampus, the retrieval-induced strengthening effect was significantly reduced. Retrieval combined with relearning also reliably elevated the levels of hippocampal Zif268, a cellular correlate of memory destabilization. Together, these core findings strongly suggest that the memory-enhancing effects of retrieval-relearning are mediated by reconsolidation mechanisms.
On a behavioral level, the observed memory improvement is not simply a consequence of retrieval practice, as a single or double retrieval did not have beneficial effects in either setting. While this may, at first, appear to contradict the extensive literature on the retrieval practice effect in humans, it should be noted that retrieval practice is commonly implemented using several retrieval episodes, often interleaved with further learning, and taking place within the same behavioral session as initial learning (Roediger and Butler, 2011; Hulbert and Norman, 2015). The same is true for the related phenomena of test-potentiated learning (Arnold and McDermott, 2013) and the forward effect of testing (Pastötter and Bäuml, 2014), where testing and learning are typically conducted within a single session. This contrasts in a number of ways with the present study, in which retrieval occurred 48 h after learning, and on only one to two occasions, and was not interleaved with relearning or with feedback. Repeated retrieval shortly after learning has been shown to be greatly superior to a single retrieval opportunity (Roediger and Karpicke, 2006). However, a single retrieval 24 h after learning did not improve subsequent performance per se (Potts and Shanks, 2012), although under conditions of increased test difficulty there was evidence for a retrieval practice-like effect. In our study, given the weak learning, the long 48 h interval between study and retrieval practice, and the lack of feedback, the failure of retrieval in itself to produce memory improvement is perhaps not unexpected, as errors in retrieval are likely to strengthen the wrong associate (Roediger and Karpicke, 2006).
In rodent studies, a single or limited number of retrievals can strengthen subsequent aversive memory expression in a manner that is believed to involve memory reconsolidation (Inda et al., 2011; De Oliveira Alvares et al., 2013; Fukushima et al., 2014). However, in contrast, we have previously demonstrated that contextual fear memory retrieval is detrimental to subsequent memory expression regardless of the parameters of initial retrieval (Cassini et al., 2017). It remains unclear whether the capacity for retrieval-relearning to strengthen memory is dependent upon conditions in which retrieval itself does not have memory-improving effects. Perhaps it is more likely that the summative effect of retrieval and relearning is magnified in weak learning settings (Hulbert and Norman, 2015).
A number of lines of evidence point toward the retrieval-relearning effect being mediated by the updating of memory strength via destabilization-reconsolidation. First, it should be noted that the capacity for reconsolidation-mediated memory gains to be observed following postretrieval interventions has been demonstrated both pharmacologically for rodent fear memory (Lee et al., 2006; Tronson et al., 2006) and also for paired-associate memory with postretrieval presentation of negative valence pictures (Finn et al., 2012). Behaviorally, we find that the memory improvement is highly robust with an interval of 15 min or 1 h between retrieval and relearning. When shortening this interval to 0 min, or extending it to 6 h, the improvement was reduced by 20–30%. This temporal window of efficacy matches that shown for retrieval-extinction effects that are dependent upon destabilization-reconsolidation (Monfils et al., 2009; Schiller et al., 2010). With no interval between retrieval and extinction/relearning, it is likely that the absence of an offset signal for the retrieval session results in the failure to trigger reconsolidation, in a similar manner to that of the necessity for conditioned stimulus offset to trigger reconsolidation in crabs (Pedreira and Maldonado, 2003) and humans (Hu et al., 2018). With an extended interval of ≥6 h, the cellular processes of reconsolidation will have proceeded to the extent that pharmacological treatment is without effect (Nader et al., 2000) and behavioral intervention is unable to hijack the reconsolidating memory (Schiller et al., 2010).
For our human memory data, the importance of the nature of the retrieval experience provides further evidence supporting the destabilization-reconsolidation hypothesis. When retrieval preceded relearning, there was a facilitative effect only when the retrieval was incomplete; that is, when the participants were instructed not to verbalize the answer. With a full retrieval, including answer production, there was no benefit for the retrieval. This contrast replicates conceptually the findings of Forcato et al. (2009), who observed that human declarative memory reconsolidation was only triggered when the reminder prevented the production of the answer. Alternative explanations of our human memory strengthening, including retrieval practice (Roediger and Butler, 2011), test-potentiated learning (Arnold and McDermott, 2013), and the forward effect of testing (Pastötter and Bäuml, 2014) are all based upon studies in which an explicit and full retrieval test is used. Therefore, none can account for the dependence of the present memory strengthening upon the specific reminder structure that has previously been demonstrated to be necessary to trigger memory reconsolidation (Forcato et al., 2009).
Within our rodent contextual fear experiments, the mechanistic understanding of destabilization and reconsolidation allows a more direct implication of reconsolidation. First, hippocampal protein degradation at the proteasome has been previously established to be necessary for destabilization (Lee et al., 2008). When blocking this process specifically before retrieval, the memory-enhancing effects of further learning were substantially reduced. A similar dependence on memory destabilization was observed for cued fear memory strengthening with retrieval-relearning in a previous study (Du et al., 2017). The cellular analyses of Zif268 expression further support the interpretation that retrieval-relearning engages reconsolidation processes to update the existing memory. However, it should be noted that our Zif268 expression data relate only to the retrieval-60 min-relearning condition, and so there is somewhat less evidence that retrieval-15 min-relearning similarly engages reconsolidation processes. Nevertheless, there is equally no reason to suggest that the shorter interval fails to engage reconsolidation, especially as the reconsolidation window has been consistently demonstrated to span 10–60 min (Monfils et al., 2009; Schiller et al., 2010; Flavell et al., 2011; Rao-Ruiz et al., 2011), and so it is highly likely that a similar pattern of Zif268 expression would be observed following retrieval-15 min-relearning. Dorsal hippocampal Zif268 has been extensively implicated in contextual fear memory reconsolidation and updating (Lee et al., 2004; Lee, 2008, 2010; Barnes et al., 2012; Cheval et al., 2012; Lee and Hynds, 2013; Besnard et al., 2014; Machado et al., 2015). Here, Zif268 expression was most robustly upregulated following retrieval and conditioning, which strongly supports the engagement of memory reconsolidation processes for the memory-strengthening effect. Somewhat surprisingly, there was less evidence for Zif268 upregulation following retrieval alone, or conditioning alone, given that retrieval alone has been shown previously to upregulate hippocampal Zif268 (Lee et al., 2004; Lee, 2008; Barnes et al., 2012; Lee and Hynds, 2013; Besnard et al., 2014). While we do not have an explanation for this discrepancy, we would note that previous demonstrations of upregulation have used stronger initial fear-conditioning parameters (Lee et al., 2004; Lee and Hynds, 2013; Besnard et al., 2014). The weaker initial conditioning may have contributed to the weaker engagement of Zif268 by retrieval and conditioning alone.
The comparison condition, in which relearning preceded retrieval showed memory strengthening that was quantitatively similar to that observed following retrieval-relearning but differed qualitatively in some important ways. First, in the rodent contextual fear experiments, the strengthening effect of relearning-retrieval was only observed with an interval of 15 min, but not 60 min. The latter time interval is highly suited to reconsolidation effects (Monfils et al., 2009; Flavell et al., 2011), suggesting that the relearning-retrieval memory strengthening is not mediated by reconsolidation. This interpretation is consistent with the human paired-associate memory results, which showed that the memory strengthening following relearning-retrieval occurred regardless of the duration of the interval between relearning and retrieval, and regardless of the nature (verbalized vs nonverbalized) of the retrieval. While the mechanism of the memory strengthening resulting from relearning-retrieval remains unclear, it can be concluded that it is unlikely to involve memory reconsolidation.
The capacity of retrieval-relearning, and indeed relearning-retrieval, to confer substantial memory improvements in hippocampal-dependent memories in both rodents and humans has potential translational application across both educational and clinical settings to maximize learning gains and perhaps to offset memory decline. It remains unclear at present what exactly the nature of the interval/distraction between retrieval and relearning needs to be to enable memory strengthening, and so it is possible even that either or both processes are engaged in everyday memory recall and endogenous relearning.
Footnotes
This study was conducted with funding from the UK BBSRC (Grant BB/J014982/1; https://bbsrc.ukri.org). We thank Madalina Bacalu, Nazma Fathema, Eleanor Keeling, Nathan Rodrigues, Kristina Sourlekova, Chloe Webster, and Courtaney Weekes for assistance with data collection.
The authors declare no competing financial interests.
References
- Antony JW, Ferreira CS, Norman KA, Wimber M (2017) Retrieval as a fast route to memory consolidation. Trends Cogn Sci 21:573–576. 10.1016/j.tics.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold KM, McDermott KB (2013) Test-potentiated learning: distinguishing between direct and indirect effects of tests. J Exp Psychol Learn Mem Cogn 39:940–945. 10.1037/a0029199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P, Kirtley A, Thomas KL (2012) Quantitatively and qualitatively different cellular processes are engaged in CA1 during the consolidation and reconsolidation of contextual fear memory. Hippocampus 22:149–171. 10.1002/hipo.20879 [DOI] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K (2006) NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9:1237–1239. 10.1038/nn1778 [DOI] [PubMed] [Google Scholar]
- Besnard A, Laroche S, Caboche J (2014) Comparative dynamics of MAPK/ERK signalling components and immediate early genes in the hippocampus and amygdala following contextual fear conditioning and retrieval. Brain Struct Funct 219:415–430. 10.1007/s00429-013-0505-y [DOI] [PubMed] [Google Scholar]
- Brady TF, Konkle T, Alvarez GA, Oliva A (2008) Visual long-term memory has a massive storage capacity for object details. Proc Natl Acad Sci U S A 105:14325–14329. 10.1073/pnas.0803390105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassini LF, Flavell CR, Amaral OB, Lee JLC (2017) On the transition from reconsolidation to extinction of contextual fear memories. Learn Mem 24:392–399. 10.1101/lm.045724.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheval H, Chagneau C, Levasseur G, Veyrac A, Faucon-Biguet N, Laroche S, Davis S (2012) Distinctive features of egr transcription factor regulation and DNA binding activity in CA1 of the hippocampus in synaptic plasticity and consolidation and reconsolidation of fear memory. Hippocampus 22:631–642. 10.1002/hipo.20926 [DOI] [PubMed] [Google Scholar]
- De Oliveira Alvares L, Crestani AP, Cassini LF, Haubrich J, Santana F, Quillfeldt JA (2013) Reactivation enables memory updating, precision-keeping and strengthening: exploring the possible biological roles of reconsolidation. Neuroscience 244:42–48. 10.1016/j.neuroscience.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Du J, Price MP, Taugher RJ, Grigsby D, Ash JJ, Stark AC, Hossain Saad MZ, Singh K, Mandal J, Wemmie JA, Welsh MJ (2017) Transient acidosis while retrieving a fear-related memory enhances its lability. eLife 6:e22564. 10.7554/eLife.22564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50. 10.1038/35036213 [DOI] [PubMed] [Google Scholar]
- Exton-McGuinness MTJ, Lee JLC (2015) Reduction in responding for sucrose and cocaine reinforcement by disruption of memory reconsolidation. Eneuro 2:ENEURO.0009–0015.2015. 10.1523/ENEURO.0009-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn B, Roediger HL 3rd, Rosenzweig E (2012) Reconsolidation from negative emotional pictures: is successful retrieval required? Mem Cognit 40:1031–1045. 10.3758/s13421-012-0203-7 [DOI] [PubMed] [Google Scholar]
- Flavell CR, Barber DJ, Lee JL (2011) Behavioural memory reconsolidation of food and fear memories. Nat Commun 2:504. 10.1038/ncomms1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcato C, Argibay PF, Pedreira ME, Maldonado H (2009) Human reconsolidation does not always occur when a memory is retrieved: the relevance of the reminder structure. Neurobiol Learn Mem 91:50–57. 10.1016/j.nlm.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Forcato C, Rodríguez ML, Pedreira ME (2011) Repeated labilization-reconsolidation processes strengthen declarative memory in humans. PLoS One 6:e23305. 10.1371/journal.pone.0023305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Zhang Y, Archbold G, Ishikawa R, Nader K, Kida S (2014) Enhancement of fear memory by retrieval through reconsolidation. eLife 3:e02736. 10.7554/eLife.02736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wang W, Homan P, Wang P, Zheng X, Schiller D (2018) Reminder duration determines threat memory modification in humans. Sci Rep 8:8848. 10.1038/s41598-018-27252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert JC, Norman KA (2015) Neural differentiation tracks improved recall of competing memories following interleaved study and retrieval practice. Cereb Cortex 25:3994–4008. 10.1093/cercor/bhu284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L (2007) Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem 14:47–53. 10.1101/lm.365707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM (2011) Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci 31:1635–1643. 10.1523/JNEUROSCI.4736-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASP Team (2016) JASP version 0.8.1.2. Amsterdam, the Nehterlands: University of Amsterdam. [Google Scholar]
- John OP, Srivastava S (1999) The Big Five trait taxonomy: history, measurement, and theoretical perspectives. In: Handbook of personality: theory and research (Pervin LA, John OP, eds), pp 102–138. New York: Guilford. [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ (2008) Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci 2:15. 10.3389/neuro.09.015.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle T, Brady TF, Alvarez GA, Oliva A (2010) Scene memory is more detailed than you think: the role of categories in visual long-term memory. Psychol Sci 21:1551–1556. 10.1177/0956797610385359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. (2008) Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci 11:1264–1266. 10.1038/nn.2205 [DOI] [PubMed] [Google Scholar]
- Lee JL. (2009) Reconsolidation: maintaining memory relevance. Trends Neurosci 32:413–420. 10.1016/j.tins.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. (2010) Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci 4:168. 10.3389/fnbeh.2010.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Hynds RE (2013) Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus 23:233–244. 10.1002/hipo.22083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL (2004) Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304:839–843. 10.1126/science.1095760 [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ (2006) Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26:10051–10056. 10.1523/JNEUROSCI.2466-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Nader K, Schiller D (2017) An update on memory reconsolidation updating. Trends Cogn Sci 21:531–545. 10.1016/j.tics.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Lee SH, Kim H, Kaang BK (2008) Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319:1253–1256. 10.1126/science.1150541 [DOI] [PubMed] [Google Scholar]
- Li X, Baker-Andresen D, Zhao Q, Marshall V, Bredy TW (2014) Methyl CpG binding domain ultra-sequencing: a novel method for identifying inter-individual and cell-type-specific variation in DNA methylation. Genes Brain Behav 13:721–731. 10.1111/gbb.12150 [DOI] [PubMed] [Google Scholar]
- Machado I, Gonzalez PV, Vilcaes A, Carniglia L, Schiöth HB, Lasaga M, Scimonelli TN (2015) Interleukin-1β-induced memory reconsolidation impairment is mediated by a reduction in glutamate release and zif268 expression and α-melanocyte-stimulating hormone prevented these effects. Brain Behav Immun 46:137–146. 10.1016/j.bbi.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324:951–955. 10.1126/science.1167975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Hardt O (2009) A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10:224–234. 10.1038/nrn2590 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726. 10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- Olshavsky ME, Song BJ, Powell DJ, Jones CE, Monfils MH, Lee HJ (2013) Updating appetitive memory during reconsolidation window: critical role of cue-directed behavior and amygdala central nucleus. Front Behav Neurosci 7:186. 10.3389/fnbeh.2013.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastötter B, Bäuml KH (2014) Retrieval practice enhances new learning: the forward effect of testing. Front Psychol 5:286. 10.3389/fpsyg.2014.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H (2003) Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38:863–869. 10.1016/S0896-6273(03)00352-0 [DOI] [PubMed] [Google Scholar]
- Peirce JW. (2007) PsychoPy—psychophysics software in python. J Neurosci Methods 162:8–13. 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts R, Shanks DR (2012) Can testing immunize memories against interference? J Exp Psychol Learn Mem Cogn 38:1780–1785. 10.1037/a0028218 [DOI] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S (2011) Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci 14:1302–1308. 10.1038/nn.2907 [DOI] [PubMed] [Google Scholar]
- Roediger HL, Karpicke JD (2006) Test-enhanced learning: taking memory tests improves long-term retention. Psychol Sci 17:249–255. 10.1111/j.1467-9280.2006.01693.x [DOI] [PubMed] [Google Scholar]
- Roediger HL 3rd, Butler AC (2011) The critical role of retrieval practice in long-term retention. Trends Cogn Sci 15:20–27. 10.1016/j.tics.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA (2010) Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463:49–53. 10.1038/nature08637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennepin AD, Charpentier S, Normand T, Sarré C, Legrand A, Mollet LM (2009) Multiple reprobing of Western blots after inactivation of peroxidase activity by its substrate, hydrogen peroxide. Anal Biochem 393:129–131. 10.1016/j.ab.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Mukawa T, Tsukagoshi A, Frankland PW, Kida S (2008) Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn Mem 15:426–433. 10.1101/lm.888808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR (2006) Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9:167–169. 10.1038/nn1628 [DOI] [PubMed] [Google Scholar]