Abstract
Rationale: Cytomegalovirus (CMV)-related morbidities remain one of the most common complications after lung transplantation and have been linked to allograft dysfunction, but the factors that predict high risk for CMV complications and effective immunity are incompletely understood.
Objectives: To determine if short telomeres in idiopathic pulmonary fibrosis (IPF) lung transplant recipients (LTRs) predict the risk for CMV-specific T-cell immunity and viral control.
Methods: We studied IPF-LTRs (n = 42) and age-matched non-IPF-LTRs (n = 42) and assessed CMV outcomes. We measured lymphocyte telomere length and DNA sequencing, and assessed CMV-specific T-cell immunity in LTRs at high risk for CMV events, using flow cytometry and fluorescence in situ hybridization.
Measurements and Main Results: We identified a high prevalence of relapsing CMV viremia in IPF-LTRs compared with non-IPF-LTRs (69% vs. 31%; odds ratio, 4.98; 95% confidence interval, 1.95–12.50; P < 0.001). Within this subset, IPF-LTRs who had short telomeres had the highest risk of CMV complications (P < 0.01) including relapsing-viremia episodes, end-organ disease, and CMV resistance to therapy, as well as shorter time to viremia versus age-matched non-IPF control subjects (P < 0.001). The short telomere defect in IPF-LTRs was associated with significantly impaired CMV-specific proliferative responses, T-cell effector functions, and induction of the major type-1 transcription factor T-bet (T-box 21;TBX21).
Conclusions: Because the short telomere defect has been linked to the pathogenesis of IPF in some cases, our data indicate that impaired CMV immunity may be a systemic manifestation of telomere-mediated disease in these patients. Identifying this high-risk subset of LTRs has implications for risk assessment, management, and potential strategies for averting post-transplant CMV morbidities.
Keywords: CMV immunity, idiopathic pulmonary fibrosis, lung transplant, telomeres
At a Glance Commentary
Scientific Knowledge on the Subject
Idiopathic pulmonary fibrosis (IPF) is the most common manifestation of short telomere syndromes and the leading indication for lung transplantation. Cytomegalovirus (CMV) remains an important opportunistic pathogen in lung transplant recipients and the factors that regulate CMV immunity and viral control remain incompletely understood. Understanding these mechanisms may lead to new approaches to this common infection that complicates lung transplant.
What This Study Adds to the Field
Our study provides novel insights into CMV-specific T-cell immunity and links important immune responses to telomere length. We show that most IPF lung transplant recipients have short telomeres (71%), whereas 12% carried mutations in telomere-related genes. IPF lung transplant patients had an increased incidence of CMV viremia episodes and other CMV complications compared with age-matched non-IPF transplant recipients and patients with IPF with long telomeres. IPF short telomere patients had impaired in vitro CMV-specific T-cell effector and proliferative responses, and induction of the type-1 immune transcription factor, T-bet (T-box 21; TBX21). Together, our findings show impaired CMV immunity and control in IPF lung transplant recipients with short telomeres.
Cytomegalovirus (CMV), a member of the β-herpesvirus family, remains a significant opportunistic pathogen and cause of morbidity and mortality in solid organ transplant recipients (1–3). Lung-transplant recipients (LTRs) have increased susceptibility to CMV infection, which has been suggested to be related to the lung being a major reservoir for latent virus (4). CMV infection remains a significant cause of morbidity in LTRs, with several studies implicating active CMV pneumonitis as a risk factor for the development of bronchiolitis obliterans syndrome, the major limiting factor for long-term survival in LTRs (5, 6). Recent studies have shown that recurrent episodes of CMV viremia in LTRs are associated with an increased risk of bronchiolitis obliterans syndrome and decreased survival (7–9). Despite the implementation of extended antiviral prophylaxis protocols in the past decade (10), LTRs mismatched for CMV status (donor+/recipient− [D+R−]), which comprise approximately 25% of LTRs, continue to demonstrate increased risk of CMV reactivation, CMV end-organ disease, and overall decreased 5-year mortality (5). The donor+/recipient+ (D+R+) group, which compromises approximately 40% of LTRs, has the second highest burden of CMV disease, with decreased survival compared with donor-negative groups.
We have previously demonstrated immunologic heterogeneity within D+R− LTRs, with respect to CMV-specific T-cell immunity and the capacity for establishing durable immune control, but the determinants of this heterogeneity are not known. Specifically, we have shown important roles for the induction of the major type-1 transcription factor T-bet, and the acquisition of CMV-specific CD8+ and CD4+ T-cell effector function, proliferation, and antigen-presenting cell function (11–13). Another recent study demonstrated polyfunctional T-cell responses in CMV/R+ LTRs that predicted protection from subsequent CMV events (14). Thus, there is an appreciated clinical LTR phenotype with episodes of relapsing viremia (“relapsers”) who differ in CMV-specific T-cell immunity from those who establish immune CMV control (“controllers”) after discontinuation of antiviral therapy. Recently, CMV mismatch (D+R−) status was reported to be a specific risk factor for morbidity and mortality in LTRs 60 years and older (15). Despite these important clinical differences, and the well-appreciated morbidity associated with CMV infection in this setting, the mechanisms that underlie the heterogeneity in CMV immunity are incompletely understood, and the molecular basis that contributes to increased susceptibility in older populations remains unclear.
Idiopathic pulmonary fibrosis (IPF) is now the leading indication for lung transplantation in North America (5). In recent years, IPF pathogenesis has been linked to genetic defects in telomerase and telomere maintenance in one-third of familial pulmonary fibrosis cases and up to 10% of sporadic cases (16, 17). Additionally, abnormally short telomere length (TL) has been reported in at least one-half of patients with IPF (18, 19). These findings have linked IPF, and some severe emphysema cases, to premature aging in the lung that is driven by short telomeres (16, 20). Patients with IPF show an increased risk of systemic short telomere syndrome features that include bone marrow failure, reflecting their limited reserves outside the lung (21, 22). Patients with IPF with telomerase mutations have an increased rate of hematologic complications and invariably require dose reduction of standard myelosuppressive medications after lung transplant (23, 24). Furthermore, CMV viremia was a complication in isolated cases of telomerase mutation LTRs (23, 24) and short telomeres have been associated with defects in adaptive immunity in humans and animal models (21, 25). These observations led us to hypothesize that short telomeres in IPF-LTRs predict the risk for relapsing CMV viremia and other viral-related morbidities. Here we show that IPF-LTRs with short TL demonstrate impaired CMV-specific T-cell immunity and viral control compared with either non-IPF-LTRs or IPF-LTRs with long TL.
Methods
Study Approval and Subjects
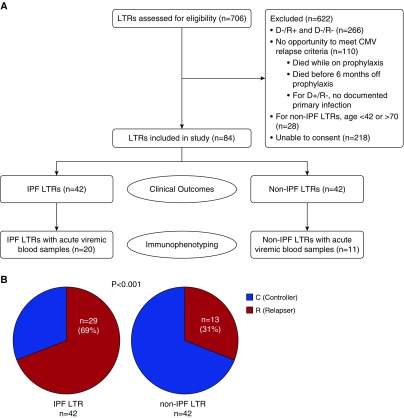
All subjects provided written consent before inclusion in the study for research protocols approved by the University of Pittsburgh and John Hopkins Medicine Institutional Review Boards. Study participants were consecutively identified from among LTRs recruited from Johns Hopkins University (n = 19; 2006–2010) and the University of Pittsburgh (n = 65; 2010–2016) as outlined in Figure 1A. From these two eras, eight of eight (100%) study-eligible IPF-LTRs from Johns Hopkins University and 34 of 66 (52%) study-eligible IPF-LTRs from University of Pittsburgh were consecutively included in our study cohort. Thus, study subjects were either IPF-LTRs (n = 42) or age-matched non-IPF-LTRs (n = 42) who received lung transplants from CMV-positive donors (D+/R+ and D+/R−). CMV serostatus in donors and recipients was determined by standard CMV IgG testing pretransplant. The diagnosis of IPF was based on history and clinicopathologic evidence of usual interstitial pneumonia in the explanted lung tissue. The medical records for each study subject were reviewed by two independent investigators. Baseline characteristics of age, sex, pretransplant diagnosis, type of lung transplant, CMV serostatus, and immunosuppression post-transplant were collected retrospectively. CMV PCR data were reviewed to identify each study subject’s clinical course, and CMV-complications including total number of episodes of CMV end-organ disease (i.e., pneumonitis, gastritis, or colitis), relapsing viremia, the development of CMV antiviral resistance, and/or death associated with CMV infection.
Figure 1.
Lung transplant recipients (LTRs) with idiopathic pulmonary fibrosis (IPF) have an increased incidence of cytomegalovirus (CMV) relapsing viremia compared with age-matched non-IPF control subjects. Study design flow diagram. Of the 84 LTRs included, 31 had acute viremic blood samples available for analysis. (A) All IPF LTRs and those non-IPF LTRs included for immunophenotyping underwent telomere length measurement. (B) Comparison of IPF-LTR cohort (n = 42) and non-IPF-LTR age-matched control subjects (n = 42) for CMV controllers (C) versus relapsers (R) as a percentage of each group. Relapse was defined as at least two consecutive positive CMV PCRs after discontinuation of antiviral treatment for primary infection or standard CMV prophylactic therapy (as per Methods). Analysis was performed using the Fisher exact test with a two-sided P value less than 0.05 considered statistically significant. D/R = donor and recipient.
Immunosuppression, CMV Monitoring, and Prophylaxis/Treatment
All patients in the study were initially treated with a standard three-drug immunosuppression regimen and adjusted as tolerated (Table 1). CMV prophylaxis included either intravenous ganciclovir or oral valganciclovir and administered according to institutional protocols. Plasma CMV viral load was assayed by quantitative PCR in the virology laboratory of the respective institutions. D+R− LTRs who developed primary CMV infection were treated with antiviral therapy until at least two consecutive weekly quantitative PCR measurements revealed undetectable viremia and resolution of symptoms. A similar protocol was followed for D+R+ LTRs with CMV reactivation episodes if clinically indicated. After completion of antiviral therapy, for primary CMV infection in D+R− LTRs or standard CMV antiviral prophylaxis in D+R+ LTRs, patients were prospectively monitored by CMV PCR at least biweekly, and during any symptomatic or clinically indicated time points, for relapsing viremia or end-organ disease. Relapsing viremia was defined as the detection of more than 300 CMV copies/ml on two consecutive samples in the first 6 months after discontinuation of antiviral therapy or prophylaxis (excluding viremia during primary infection in D+R− LTRs). Clearance of CMV viremia was defined as two consecutive undetectable measurements (<300 copies/ml). CMV controllers were defined as patients who did not have evidence of CMV viremia or end-organ disease after treatment of primary infection or discontinuation of CMV prophylaxis. All LTRs with relapsing viremia received antiviral therapy until clearance of viremia (n = 41). Participants were also considered relapsers if they failed to clear acute CMV infection (n = 1).
Table 1.
Baseline Characteristics of Study Cohort
| IPF (n = 42) | non-IPF (n = 42) | P Value | |
|---|---|---|---|
| Age, yr | 61.5 (47–76) | 60.0 (42–69) | NS |
| Male, n (%) | 32 (76.2) | 18 (42.8) | 0.004 |
| CMV serostatus, n (%) | |||
| D+R− (mismatch) | 19 (45.2) | 21 (50.0) | NS |
| D+R+ | 23 (54.8) | 21 (50.0) | |
| Induction, n (%) | |||
| Alemtuzumab | 19 (45.2) | 18 (44.2) | NS |
| Basiliximab | 23 (54.8) | 24 (55.8) | |
| Immunosuppression, n (%) | |||
| Triple-drug* | 36 (85.7) | 33 (78.6) | NS |
| IS reduction† | 6 (14.3) | 9 (21.4) | |
| Follow-up, yr | 3.5 | 3.4 | NS |
| Transplant procedure, n (%) | |||
| Single | 8 (19) | 7 (16.6) | NS |
| Double | 36 (81) | 37 (83.4) |
Definition of abbreviations: CMV = cytomegalovirus; D = donor; IPF = idiopathic pulmonary fibrosis; IS = immunosuppression; NS = not significant; R = recipient.
Triple drug immunosuppression: calcineurin inhibitor, antiproliferative (mycophenolate mofetil or azathioprine), and prednisone.
Reduction in immunosuppression secondary to cytopenias.
Additional detailed methods are provided in an online supplement.
Results
IPF-LTRs Have Increased Risk for Relapsing-Viremia Episodes Compared with Age-matched Non-IPF-LTRs
To investigate whether patients with IPF are at increased risk for CMV reactivation, we first evaluated a retrospective cohort of 84 LTRs from two academic centers, as shown in Figure 1A and described in the Methods section. All of the LTRs within this cohort were either D+R− or D+R+ CMV serostatus, and thus were considered to be high risk for post-transplant CMV complications (26). We compared 42 IPF-LTRs with 42 age-matched non-IPF-LTR (Table 1). Although patients with IPF showed an expected male predominance, there were no significant differences in CMV serology, bilateral versus single lung transplantation, transplant induction therapy, or changes to immunosuppression therapy between groups, with a median follow-up of 3.4 years (Table 1). We assessed the risk of CMV relapsing viremia within our cohort (see Methods) and found that the median antiviral post-transplant prophylaxis duration for D+R− LTRs was 7.5 months and 6 months for D+R+ LTRs. To assess if the underlying lung transplant indication affected the risk of CMV relapsing viremia, we compared patients with and without IPF and found that IPF had a higher rate of relapse (69% vs. 31%; odds ratio, 4.98; 95% confidence interval [CI],1.95–12.50; P < 0.001; Fisher exact test) (Figure 1B). Despite this difference, there was no obvious difference in median viral loads between the groups during relapse (IPF-LTRs = 1,355 copies/ml vs. non-IPF-LTRs = 1,536 copies/ml; P = 0.85), and all relapsers had undergone treatment with antiviral therapy. The episodes of relapsing viremia occurred independent of acute rejection episodes, augmented immunosuppression, or other active infections. Together, these findings show that IPF-LTRs are at increased risk for CMV relapsing viremia compared with age-matched non-IPF-LTRs.
High-Risk IPF-LTRs with Short TL Demonstrate Increased Risk of CMV Relapsing Viremia
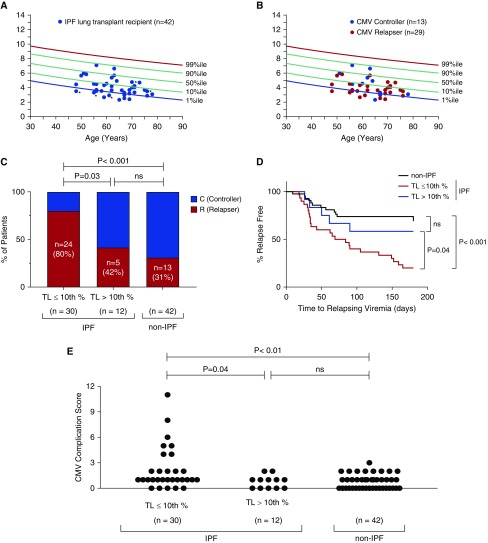
Having determined that IPF-LTRs were at increased risk for CMV relapsing viremia, we asked whether short lymphocyte TL could identify IPF-LTRs at increased risk for CMV relapse. We used the clinically validated flow cytometry and fluorescence in situ hybridization method (flowFISH) (27) and found that 71% of IPF-LTRs had significantly short TL (≤10th) relative to healthy age-matched control subjects (Figure 2A), similar to what has been previously seen (18). To characterize if the short TL was related to inherited mutations, we screened the IPF-LTRs using a next-generation sequencing panel that includes the known causes of familial pulmonary fibrosis including the seven known telomerase and telomere genes (Table 2). We found 10% (4/39 sequenced) had pathogenic mutations in a telomerase or telomere gene: TERT (n = 1), RTEL1 (n = 2), and PARN (n = 1) (Figure 2A and Table 2). Another patient who had classic short TL syndrome features including bone marrow failure and a family history of pulmonary fibrosis, and who had short TL below the age-adjusted first percentile, also carried an ultra-rare RTEL1 variant that was deemed likely pathogenic (Figure 2A and Table 2). These data are consistent with the known prevalence of telomere and telomerase mutations in IPF (16) and confirm what has previously been shown, that short telomeres are a common finding in IPF in the absence of identifiable mutations (18).
Figure 2.
Idiopathic pulmonary fibrosis (IPF) lung transplant recipients (LTRs) with short telomeres have increased risk and more rapid onset for relapsing cytomegalovirus (CMV) viremia and infectious complications. (A) Age-adjusted lymphocyte telomere length (TL) of IPF-LTRs (n = 42) is plotted relative to age-adjusted nomograms of control subjects, as measured by flow cytometry and fluorescence in situ hybridization. Approximately two-thirds of this cohort (n = 29) have TL less than or equal to 10th percentile for age. *The five individuals for whom germline mutations were identified (four pathogenic, one likely pathogenic) as listed in Table 2. (B) TL for CMV controllers (C) (blue circles) and relapsers (R) (red circles). (C) IPF-LTRs with TL less than or equal to 10th percentile (n = 29) demonstrate an increased incidence of CMV relapsing viremia (R) compared with IPF-LTRs with TL greater than 10th percentile (n = 13) or age-matched non-IPF control subjects. Analysis was performed using the Fisher exact test with a two-sided P value of less than 0.05 considered statistically significant. (D) Kaplan-Meier curve analyzed with Mantel-Cox log-rank test showing shortened time to relapsing viremia in IPF-LTRs with TL less than or equal to 10th percentile compared with non-IPF-LTRs (n = 42; P < 0.001) or IPF-LTRs with TL greater than 10th percentile (n = 13; P = 0.02). Time to relapse is defined as time at second consecutive positive CMV PCR after discontinuation of CMV therapy or prophylaxis. (E) Compilation of median CMV Complication Score comparing all groups with 1 point for each 1) relapsing viremia episode, 2) biopsy-proven end-organ disease episode (pneumonitis, gastritis, colitis, or retinitis), or 3) ganciclovir CMV resistance. Results show a higher score for IPF-LTRs with TL less than or equal to 10th percentile versus IPF-LTRs with TL greater than 10th percentile (P = 0.02) and age-matched non-IPF-control subjects (P < 0.01). There is no difference in CMV complications between the latter two groups. Statistical analysis was performed using Mann-Whitney U test with a two-sided P value of less than 0.05 considered statistically significant. ns = not significant.
Table 2.
Rare Variants and Mutations in Telomerase and Telomere Genes Identified in 42 LTRs with IPF
| IPF-LTR No. | Age (yr) | M/F | Family History | Mutant Gene | Protein Position | Prior Disease Association | gnomAD MAF* | Interpretation | CMV Status | R/C | TL Percentile |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 63 | F | No | RTEL1 | S815I | No | 1.72 × 10−3 | VUS | D+/R− | C | 50th–90th |
| 4 | 61 | F | No | RTEL1 | C1244R | Le Guen et al., 2013 (49) | 2.05 × 10−5 | Pathogenic | D+/R− | R | <1st |
| 7 | 48 | M | Yes | TERT | V170M | Parry et al., 2011 (22) | 5.46 × 10−6 | Pathogenic | D+/R− | R | <1st |
| 10 | 67 | M | No | DKC1 | M204R | No, outside of | Absent | Benign | D+/R− | C | 1st–10th |
| pathogenic area | |||||||||||
| 13 | 76 | M | No | RTEL1 | R1264H | Ballew et al., 2013 (50) | 1.31 × 10−4 | Pathogenic | D+/R− | R | 1st–10th |
| 15 | 56 | M | No | TINF2 | A323P | No, outside of | 1.08 × 10−5 | Benign | D+/R+ | R | <1st |
| pathogenic area | |||||||||||
| 21 | 55 | M | Yes | RTEL1 | A687G | No | Absent | Likely pathogenic | D+/R+ | R | <1st |
| 21 | 55 | M | Yes | NAF1 | Q84K | No | Absent | Benign | D+/R+ | R | <1st |
| 25 | 59 | F | No | RTEL1 | R754Q | No | 1.34 × 10−4 | VUS | D+/R+ | R | <1st |
| 26 | 69 | F | Yes | PARN | K609R | No | Absent | VUS | D+/R+ | R | 1st–10th |
| 26 | 69 | F | Yes | TINF2 | G264R | No, outside of pathogenic area | Absent | Benign | D+/R+ | R | 1st–10th |
| 30 | 56 | M | No | PARN | K211R | No | Absent | VUS | D+/R− | R | 1st–10th |
| 33 | 55 | M | No | PARN | F123L | No | Absent | VUS | D+/R- | R | 1st–10th |
| 38 | 71 | F | No | PARN | R128X | Nonsense | 4.06 × 10−6 | Pathogenic | D+/R+ | R | 10th–50th |
| 38 | 71 | F | No | NAF1 | S144P | No | 8.55 × 10−6 | VUS | D+/R+ | R | 10th–50th |
Definition of abbreviations: CMV = cytomegalovirus; D/R = donor and recipient; gnomAD = Genome Aggregation Database; IPF = idiopathic pulmonary fibrosis; LTR = lung transplant recipient; MAF = minor allele frequency; R/C = relapse/controller; TL = telomere length; VUS = variant of unknown significance.
The gnomAD MAF is derived from data from a total of 138,632 individuals (123,136 exomes and 15,496 genomes).
Within this cohort of IPF-LTRs, we tested whether short TL differentiated IPF-LTR CMV relapsers from controllers and found most IPF-LTR relapsers had short TL (Figures 2B and 2C). In fact, in long TL IPF-LTRs (>10th percentile TL), the proportion of relapsers was similar to non-IPF-LTRs (Figure 2C). Furthermore, in those with short TL, the risk of relapse was higher than patients with IPF with long TL (odds ratio, 5.6; 95% CI,1.3–23.3; P = 0.03, Fisher exact test), and non-IPF-LTRs (odds ratio, 8.92; 95% CI, 2.9–28.72; P < 0.001). Importantly, the time to relapsing viremia was significantly shorter in the short TL IPF-LTRs compared with controls by Kaplan-Meier analysis (P = 0.04 for long TL vs. short TL IPF-LTRs, Mantel-Cox log-rank; P < 0.001, for short TL vs. non-IPF-LTRs) (Figure 2D). This difference was not explained by TL differences related to CMV serostatus alone because there was a similar proportion of D+R+ and D+R− LTRs who had short TL (78% [18 of 23] vs. 63% [12 of 19]; P = 0.32; Fisher exact test). Furthermore, using the time on valganciclovir prophylaxis as a covariate, we analyzed the risk of CMV relapse using a Cox proportional hazard model. We found that 10 of 84 patients in our cohort required early discontinuation of valganciclovir because of leukopenia; however, we did not find a difference between the IPF-LTR and non-IPF-LTR groups (4/42 vs. 6/42; P = 0.74 by Fisher exact test), nor between the IPF short TL and IPF long TL (3/30 vs. 1/12; P = 1.00 by Fisher exact test). Thus, valganciclovir exposure had no discernable impact on propensity to CMV relapse (hazard ratio, 1.00; 95% CI, 0.99–1.01; P = 0.88).
We next evaluated whether TL correlates with CMV complications using a cumulative scoring system that included the total number of episodes of relapsing viremia, the number of episodes of biopsy-proven end-organ disease incidence (i.e., pneumonitis, n = 12; gastritis, n = 3; colitis, n = 5; and retinitis, n = 1), and the development of ganciclovir resistance. We observed significantly higher median CMV complication scores in all IPF-LTRs compared with non-IPF-LTRs (see Figure E1A in the online supplement). We found that 13 of 42 (31%) of IPF-LTRs demonstrated end-organ CMV disease compared with 4 of 42 (10%) of non-IPF-LTRs (odds ratio, 4.3; 95% CI, 1.43–12.75; P = 0.02; Fisher exact test). Among the IPF-LTRs with end-organ disease, 11 of 13 (85%) had short TL. Furthermore, of the patients who developed ganciclovir-resistant CMV (6/84), five had IPF and all five patients had short TL (5/6; 83%). Moreover, IPF-LTRs with short TL demonstrated significantly higher median CMV complication scores compared with IPF-LTRs with long TL or non-IPF-LTRs (Figure 2E). This effect was not caused by an imbalance of seropositive and seronegative LTRs, because both D+R− and D+R+ LTRs trended toward higher CMV complication scores compared with their respective non-IPF-LTR control subjects (see Figure E1B). The severity of CMV disease was further documented in the fact that of the patients who died in the follow-up period with CMV-complications, all were IPF-LTRs with short TL. Of these patients (n = 3 D+R−, n = 1 D+R+), all four had CMV viremia immediately before death and two had proven CMV pneumonitis at the time of death (confirmed at autopsy). Together, these clinical observations suggest that high-risk D+R− and D+R+ IPF-LTRs with short TL have an increased risk for CMV relapsing viremia and complications compared with IPF-LTRs with greater than 10th percentile TL or age-matched non-IPF-LTRs.
Impaired CMV-Specific T-Cell Effector Responses in IPF-LTRs with Short Lymphocyte TL
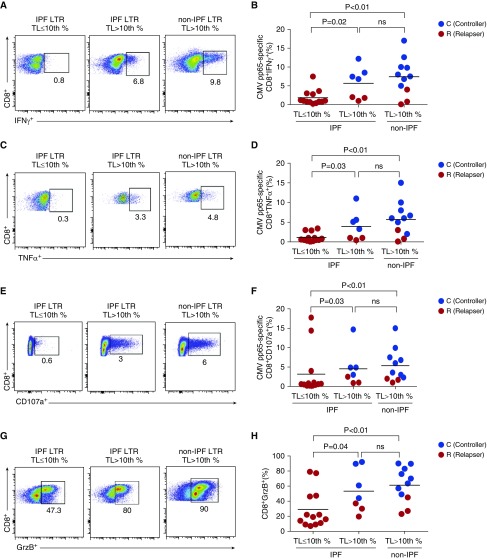
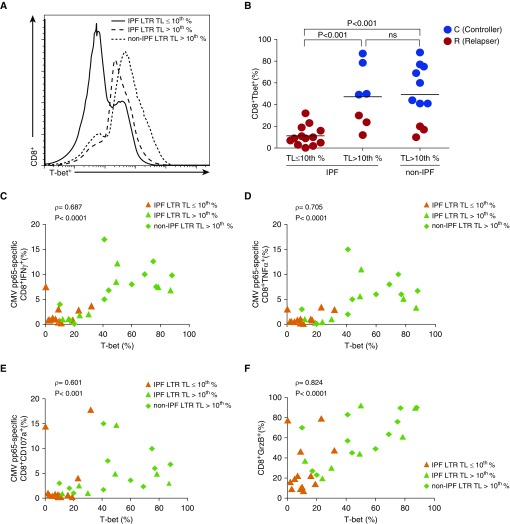
To understand the mechanisms underlying the short telomere-associated CMV morbidity, we studied CMV-specific T-cell immunity. We evaluated peripheral blood mononuclear cell responses to in vitro stimulation with pooled 15-mer overlapping peptides of phosphoprotein-65 (pp65), a major CMV antigen. We measured pp65-specific IFN-γ, TNF-α (tumor necrosis factor-α), the cytotoxic degranulation marker CD107a, and loading of the cytotoxic molecule Granzyme B (GrzB) in CD8+ T cells in a 6-hour assay. We found that pp65-specific CD8+ IFN-γ, TNF-α, CD107a responses, and total CD8+ GrzB loading were significantly reduced in IPF-LTRs with short TL compared with IPF-LTRs with long TL and a subset of age-matched non-IPF-LTRs in which TL was measured and determined to all be greater than 10th percentile (Figure 3). We also found impaired CMV-specific CD4+ effector responses in short TL IPF-LTRs compared with control subjects, including significantly lower frequencies of CMV-specific CD4+IL-2+ cells (see Figure E2).
Figure 3.
Impaired peripheral blood mononuclear cells cytomegalovirus (CMV)-specific CD8+ T-cell effector responses and granzyme B (GrzB) loading capacity in idiopathic pulmonary fibrosis (IPF) lung transplant recipients (LTRs) with short telomere length (TL) (≤10th percentile). Representative frequencies and pooled data of intracellular cytokine staining at 6 hours for CMV pp65-specific CD8+ T-cell function during acute CMV infection are shown. (A–F) Values represent cell frequencies after restimulation with pp65 peptides minus medium alone (unstimulated) of IFNγ+ (A and B), TNF-α+ (C and D), and CD107a+ (E and F), from a subset of 31 LTRs with IPF (TL ≤10th percentile [left panel], IPF TL >10th percentile [middle panel]) or non-IPF-LTR patient with TL greater than 10th percentile (right panel). Also indicated are controllers (C) (blue dots) versus relapsers (R) (red dots) status. (G and H) Representative and pooled frequencies of CD8+GrzB+ (unstimulated peripheral blood mononuclear cells) from the same patients. Bars represent median values, and P values were calculated using the Mann-Whitney-Wilcoxon test. ns = not significant; TNF = tumor necrosis factor.
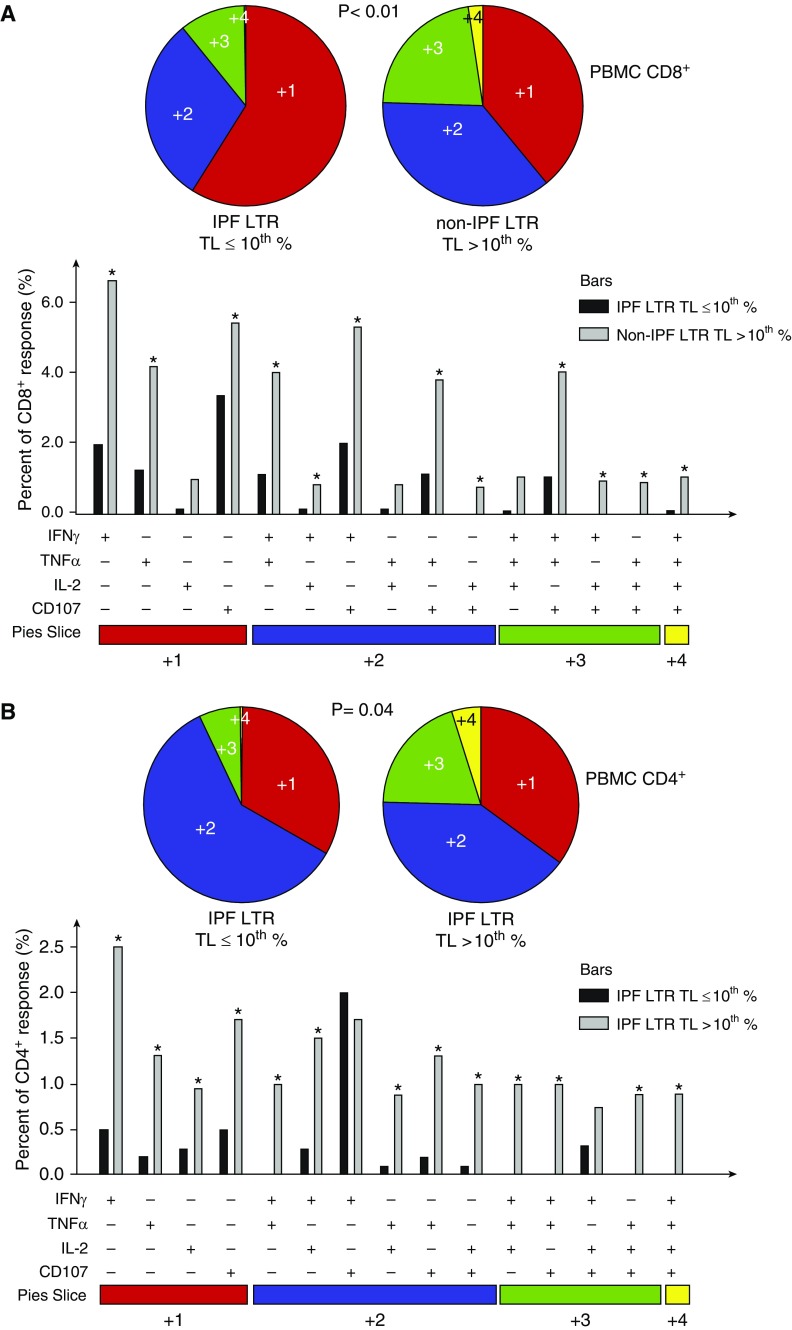
Using Boolean analysis, we next analyzed CMV-pp65-specific multifunctional effector responses (i.e., the capacity of cells to produce more than one cytokine) in a subset of short TL IPF-LTRs and non-IPF-LTRs (long TL) during acute infection. We analyzed pp65-specific IFN-γ, TNF-α, CD107a, and IL-2 production in CD8+ and CD4+ T cells and found multifunctional responses were significantly reduced in short TL IPF-LTRs compared with non-IPF-LTRs (Figure 4). These data demonstrate that short TL is associated with impaired CMV-specific multifunctional effector T-cell responses in IPF-LTRs.
Figure 4.
Idiopathic pulmonary fibrosis (IPF) lung transplant recipients (LTRs) with telomere length (TL) less than or equal to 10th percentile demonstrate impaired quantity and quality of blood CD8+ and CD4+ cytomegalovirus (CMV) pp65-specific effector multifunction during acute CMV infection. Using Boolean analysis from the same subset as Figure 3, the percentage of total and individual CMV pp65-specific effector multifunctional responses for CD8+ (A) or CD4+ (B) T cells from IPF-LTR with TL less than or equal to 10th percentile (black bars) versus non-IPF-LTR with TL greater than 10th percentile (gray bars) is shown in the bar graph for each of the multifunctional subsets. Individual pie charts of peripheral blood mononuclear cells reflecting CMV pp65-specific CD8+ (A) and CD4+ (B) T-cell responses from the IPF-LTR with TL less than or equal to 10th percentile (left pie) and non-IPF-LTR with TL greater than 10th percentile (right pie) cohort. *Significant differences when comparing mean frequencies of single and multifunctional responses, P less than or equal to 0.05. All P values were determined by the Kruskal-Wallis one-way ANOVA or Wilcoxon signed rank test. Data were analyzed using the program SPICE. PBMC = peripheral blood mononuclear cells; TNF = tumor necrosis factor.
IPF-LTRs with Short TL Demonstrate Impaired Induction of T-bet, Which Correlates with T-Cell Effector Function
The transcription factor T-bet plays a fundamental role in coordinating type-1 immune responses (28). We thus evaluated induction of T-bet in the same subset of patients captured during acute CMV infection and found significantly reduced intracellular T-bet levels in total ex vivo CD8+ T cells from IPF-LTRs with short TL, versus control subjects (Figures 5A and 5B). Importantly, most patients with low T-bet levels developed CMV relapsing viremia, consistent with our findings in a previous cohort of D+R− LTRs (11, 12). We performed Spearman rho correlation analysis for CD8+ CMV-specific effector-functions (IFN-γ, TNF-α, and CD107a), and GrzB loading, and found significant correlation between ex vivo CD8+T-bet% levels and each of these effector responses (Figures 5C–5F). These results indicate that short TL is associated with impaired T-bet induction during acute CMV infection and provides a plausible mechanism for reduced CD8+ T-cell effector function.
Figure 5.
Idiopathic pulmonary fibrosis (IPF) lung transplant recipients (LTRs) with short telomere length (TL ≤10th percentile) have impaired upregulation of T-bet in CD8+ T cells, which correlate with impaired effector functions. (A) From the same subset of 31 LTRs, representative flow cytometric histogram showing the intracellular T-bet protein expression of CD8+ T cells overlaid from IPF-LTR (with TL ≤10th percentile [continuous line] and TL >10th percentile [dashed line]) and non-IPF-LTR with TL greater than 10th percentile (dotted line). (B) Pooled data showing the ex vivo CD8+T-bet+ frequencies. Also indicated are controllers (C) (blue dots) versus relapsers (R) (red dots) status. Bars represent median values, and P values were calculated using the Mann-Whitney-Wilcoxon test. (C–E) Cumulative correlation data by scatter plot analysis of CD8+T-bet+ frequencies and cytomegalovirus (CMV)-specific frequencies for IFNγ+ (C), tumor necrosis factor (TNF)α+ (D), and CD107a+ (E), among IPF-LTRs with TL less than or equal to 10th percentile (orange triangles), IPF-LTRs with TL greater than 10th percentile (green triangles), and non-IPF-LTR with TL greater than 10th percentile (green diamonds) during acute CMV infection. (F) From the same patients, cumulative correlation data by scatter plot analysis of CD8+T-bet+ frequencies and total CD8+GrzB+ cells. Correlation coefficient (ρ) and P values were calculated using Spearman rank correlation test. GrzB = granzyme B; ns = not significant; T-bet = T-box21 (TBX21).
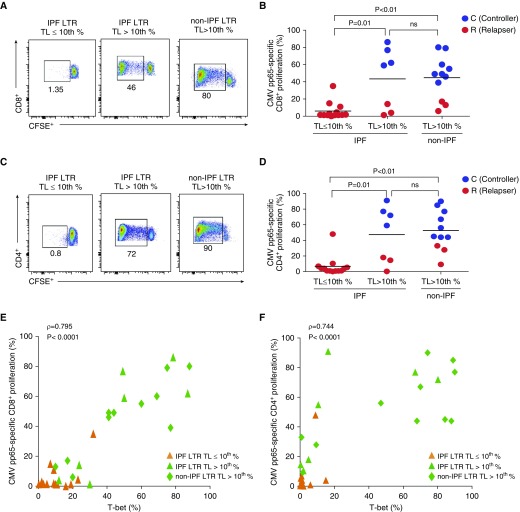
IPF-LTRs with Short Lymphocyte TL Demonstrate a CMV-Specific T-Cell Proliferative Defect That Correlates with Impaired Upregulation of T-bet
Because we found impaired CMV-specific effector T-cell responses in IPF-LTRs with short TL that correlated with T-bet induction, we hypothesized that CMV-specific T-cell proliferation was impaired in IPF-LTRs with short TL and that this defect correlates with low T-bet levels. We measured in vitro pp65-specific T-cell proliferation using the CFSE-dilution assay and observed that short TL IPF-LTRs demonstrated a CMV-specific proliferative defect in both CD8+ and CD4+ T cells and were enriched for CMV-relapsers (Figures 6A–6D) compared with long TL IPF-LTRs and age-matched non-IPF-LTRs. We also assessed concomitant intracellular CD8+ and CD4+ T-bet protein expression and correlated these levels with CMV-specific T-cell proliferative responses (Figures 6E and 6F). We found T-bet levels in the CD8+ and CD4+ pools significantly correlated with respective CMV-specific proliferative responses, with short TL IPF-LTRs exhibiting the lowest T-bet levels and impaired proliferative responses (Figures 6E and 6F). Furthermore, both CD8+T-bet+ levels and CMV-specific proliferative responses did not differ by CMV serostatus with IPF-LTRs (see Figure E3). However, D+R− IPF-LTRs demonstrated significantly reduced CD8+T-bet+ levels and CMV-specific proliferative responses compared with age-matched D+R− non-IPF-LTRs (see Figures E3A and E3B). In addition, there were reduced CD8+ and CD4+ T-cell proliferative responses to the superantigen staphylococcal enterotoxin B, consistent with a global proliferative defect, along with associated decreased staphylococcal enterotoxin B reactive effector cytokine responses (see Figure E4). Together, these data support a CMV-specific T cell and global proliferative defect, along with impaired upregulation of T-bet, in IPF-LTRs with short lymphocyte TL.
Figure 6.
Idiopathic pulmonary fibrosis (IPF) lung transplant recipients (LTRs) with short telomere length (TL) demonstrate a cytomegalovirus (CMV)-specific T-cell proliferative defect, which correlates with impaired upregulation of T-bet. (A–D) From the same subset of 31 LTRs during acute CMV infection, representative flow cytometry plots and cumulative data showing CMV pp65-specific proliferation at 6 days using CFSE dilution for CD8+ (A and B) and CD4+ (C and D) T cells from IPF-LTRs with TL less than or equal to 10th percentile, TL greater than 10th percentile, and non-IPF-LTR patient with TL greater than 10th percentile, where numbers indicate frequencies of live-gated CFSE diluted events (%). Also indicated are controllers (C) (blue dots) versus relapsers (R) (red dots) status in B and D. Bars represent median values, and P values were calculated using the Mann-Whitney-Wilcoxon test. (E and F) Cumulative correlation data of CD8+ (E) and CD4+ (F) proliferative responses and respective Day 6 T-bet+ levels among IPF-LTRs with TL less than or equal to 10th percentile (orange triangles), IPF-LTRs with TL greater than 10th percentile (green triangles), and non-IPF-LTRs with TL greater than 10th percentile (green diamonds) during acute primary CMV infection. Correlation coefficient (ρ) and P values were calculated using Spearman rank correlation test. CFSE = carboxyfluorescein succinimidyl ester; ns = not significant; T-bet = T-box21 (TBX21).
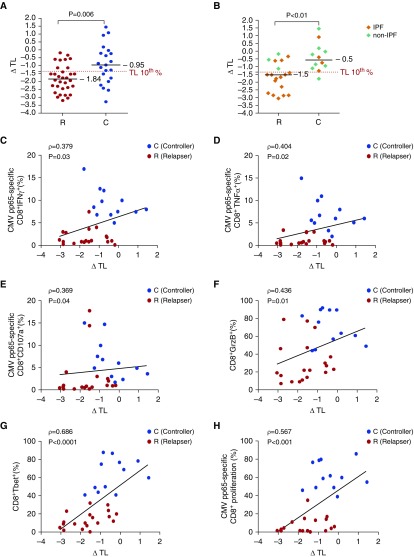
Delta TL Is Increased in CMV Relapsers and Correlates with T-Cell Effector Function
Because we observed impaired CMV-immune responses predominantly in IPF-LTRs with short TL, we hypothesized that TL was an important determinant of viral immunity. To assess this, we evaluated the continuous variable delta TL (ΔTL), which reflects the deviation from the age-adjusted median TL (27). We found that ΔTL was significantly increased (i.e., more negative) in CMV relapsers compared with controllers (Figures 7A and 7B). Next, we determined whether there was a relationship between ΔTL and CD8+ T-cell effector functions. We found significant correlations using Spearmen rho correlation between ΔTL and CMV-specific CD8+IFN-γ+, TNF-α+, and CD107+ frequencies; CD8+T-bet+ and GrzB+ levels; and CMV-specific CD8+ proliferative responses (Figures 7C–7H). Together, these data demonstrate that ΔTL is significantly increased in CMV relapsers compared with controllers and correlates with key immune parameters that differentiate the capacity for CMV immune control (see Figure E5).
Figure 7.
Delta telomere length (ΔTL) differentiates cytomegalovirus (CMV) relapsers versus controllers and correlates with CMV-specific effector and proliferative responses and total CD8+ T-cell T-bet+ and GrzB+ levels. (A and B) Pooled data showing the ΔTL values for CMV relapsers (R) versus controller (C) status for all 53 lung transplant recipients (LTRs) (A) and the ΔTL values for the 31 LTRs assessed for functional immune assays (B). Orange diamonds represent idiopathic pulmonary fibrosis (IPF), and green diamonds represent non-IPF. Shown is the 10% percentile level for ΔTL. Bars represent median values, and P values were determined using the Mann-Whitney-Wilcoxon test. (C–H) Cumulative correlation data of ΔTL and CMV pp65-specific CD8+IFNγ+ frequencies (C), CD8+TNFα+ frequencies (D), CD8+CD107a+ frequencies (E), total CD8+T-bet+ frequencies (F), CD8+GrzB+ frequencies (G), and Day 6 CMV pp65-specific CD8+proliferative responses by CFSE dilution (H) among relapsers (red circles) versus controllers (blue circles) during acute CMV infection. Correlation coefficient (ρ) and P values were calculated using Spearman rank correlation test. GrzB = granzyme B; T-bet = T-box21 (TBX21); TNF = tumor necrosis factor.
Discussion
Here, we show that short lymphocyte TL in IPF-LTRs is associated with increased risk for CMV relapsing viremia after discontinuation of antiviral therapy/prophylaxis, along with increased CMV complications. Additionally, we found that short TL differentiates T-bet expression and CMV-specific T-cell immune responses during active CMV infection in IPF-LTRs, and that ΔTL is reduced in relapsers and correlates with key immune parameters of immune control. In contrast, we determined that CMV serostatus did not differentiate CMV complications in IPF-LTRs. Because TL is genetically determined and short TLs are enriched in patients with IPF (18), our data identify a novel relationship between short telomeres and CMV clinical morbidities and T-cell host defense that may be causal in IPF. Indeed, there are reports of severe T-cell immunodeficiency in telomerase mutation carriers and mice with short telomeres having T-cell quantitative and qualitative defects (21, 29). TL testing by flowFISH is clinically available (27) and our data, especially if replicated in other larger cohorts, may identify patients who are at increased risk for CMV viremia and complications in IPF-LTRs.
Because IPF is now the leading indication for lung transplantation in North America, our study has several implications for this patient population. Our finding that approximately two-thirds of IPF-LTRs in our cohort from two major centers had short TL again confirms enrichment of shortened TL in the IPF population (18, 19). Several recent studies have demonstrated increased transplant complications in IPF-LTRs with short TL and telomerase mutations (23, 30, 31). Our study importantly reveals that the short TL defect in IPF-LTRs has clinical implications even in the absence of mutations as has been previously suggested (18).
Our findings suggest that TL measurement, using the clinically validated flowFISH method (27), may be a useful clinical assessment tool for stratifying IPF lung transplant candidates with respect to CMV risk. Furthermore, several patients in our cohort had difficulty tolerating antiviral therapy, in addition to immunosuppression, likely because of limited bone marrow function or their breaking through of antiviral prophylaxis, suggesting that prolonged prophylaxis strategies alone may not suffice. Adding to this challenge is the prior observation of increased susceptibility to developing ganciclovir resistance, particularly in D+R− LTRs, which could be exacerbated by the pressure of frequent start-stop cycles for antivirals, and is associated with worse outcomes (32–34). Interestingly, most patients in our cohort who developed ganciclovir-resistant CMV were IPF-LTRs with short TL, suggesting these may be the same patients who are at risk for limited bone marrow reserves. An alternative approach to reduce CMV risk by avoidance of CMV-positive donors for IPF candidates who are CMV-negative is theoretically reasonable, but may be practically difficult, because it could exclude up to 70% of the adult donor pool who are CMV seropositive (35). Therefore, a precision medicine approach of identifying high-risk patients by TL and CMV immunity assessment, using validated approaches, and reducing immunosuppression may be the most feasible strategy. Such a strategy needs to be weighed against the potential risk of allograft rejection.
We found that short TL IPF-LTRs had impaired CMV immunity compared with age-matched long TL IPF-LTRs and non-IPF-LTRs. Notably, IPF-LTRs with short TL had significantly reduced CMV-specific T-cell responses during active CMV infection, with reduced CD8+ T-cell T-bet/GrzB levels and acute CMV-specific effector responses (IFN-γ, TNF-α, IL-2, and CD107a) in both CD8+ and CD4+ T cells compared with control subjects. Our data indicate that short telomeres differentiate impaired viral effector responses driven by a CMV-specific T-cell proliferation defect, a strong correlate of T-bet levels, both of which we have previously found to be important for viral control (12, 13). Alternatively, persistent antigenic stimulation could also contribute to impaired T-bet expression (36). Importantly, we also identified impaired proliferative and cytokine responses to staphylococcal enterotoxin B in IPF-LTRs with short TL. Together, our findings demonstrate that short TL in patients with IPF are associated with impaired CMV-specific and global T-cell immunity along with increased CMV morbidities.
Studies from our group and others have demonstrated the importance of CMV-specific T-cell immunity and the heterogeneity within LTRs with respect to this competence (12–14, 36, 37). Our observations in patients with IPF of both viral-specific and global proliferative defects are consistent with telomere shortening contributing to T-cell aging at certain thresholds (29), potentially exacerbated under conditions of chronic immunosuppression and/or active CMV infection. One plausible mechanism contributing to a T-cell proliferative defect is our finding of reduced endogenous CMV-specific CD4+IL-2 production in short TL IPF-LTRs that might be exacerbated by calcineurin inhibitor–based immunosuppression therapy, which directly blocks IL-2 mRNA synthesis (39). However, we and other investigators have reported reduced IL-2 production and/or proliferation from CD4+ T cells during HIV, measles, dengue, and CMV viremia (13, 40–43). Additionally, other T-cell proliferative mechanisms (e.g., IL-15, IL-7) might potentially impact T-cell expansion in patients with short telomeres beyond IL-2 (44, 45). Although we did not find significant differences in immunosuppression dosing or levels between IPF and non-IPF-LTR groups, it is possible those with short telomere lymphocytes are more susceptible to immunosuppression. Indeed, our findings in light of the literature showing impaired T-cell expansion in patients with short TL are consistent with previous reports showing the need for reduced immunosuppression dosing after transplantation caused by reduced bone marrow reserves (23, 29).
There are several caveats to our studies. We recognize there are confounding factors that may impact the capacity for CMV control in our study. Importantly, we did not detect differences in immunosuppression dosing or calcineurin inhibitor levels between our study groups. However, because a functional assessment of the degree of immunosuppression in transplant recipients is lacking, we cannot exclude varying degrees of immunosuppression contributing to CMV susceptibility in our study. With regard to our CMV-immunity studies, we focused on pp65-specific T-cell responses as a dominant response (46), although we acknowledge a broader T-cell response occurs and may play a role defense during chronic infection (47). Finally, although a previous study found TL shortening in the years after primary CMV infection in kidney transplant patients (48), we assessed TL and immunity during acute primary infection, and our results for short TL in IPF-LTRs are highly consistent with short TL in patients with IPF without acute CMV infection (18). Furthermore, although it is possible that acute CMV infection may contribute to incremental telomere shortening, it is unlikely to lead to severe shortening (<10th percentile) that we observed in most IPF-LTRs, in striking contrast to preserved TL in our non-IPF-LTRs control subjects who also had viremia. Thus, despite these caveats, our study provides new evidence that TL impacts CMV-specific T-cell immunity and CMV outcomes in high-risk LTRs.
In summary, we report that short TL IPF-LTRs are at increased risk for CMV relapsing viremia and complications compared with non-IPF-LTRs and have impaired CMV-specific T-cell immunity. Our findings suggest that TL measurement, using the validated method of flowFISH, may be useful for risk-stratification of patients with IPF with the goal of advancing personalized antiviral therapy approaches and optimizing CMV control.
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge the hard work and dedication of all the caregivers to the lung transplant recipients at the University of Pittsburgh Medical Center and The Johns Hopkins Hospital. They thank Christa L. Wagner for technical support.
Footnotes
Supported in part by the NIH grants R01AI079175 and R01HL133184 and the Cystic Fibrosis Foundation (J.F.M.) and by NIH grant R01HL119476, the Commonwealth and Gary Williams Foundations, and a gift from the Harrington family (M.A.).
Author Contributions: I.P. and H.M., acquisition of the data or the analysis and interpretation of such information, design of the study, and writing the article. S.A.W., acquisition of the data or the analysis and interpretation of such information, and writing the article. A.H., F.S., E.M., M.R.P., E.A.L., M.R.M., J.M.P., V.S.H., Y.Z., S.G., P.D.S., C.J.I., and C.R.E., acquisition of the data or the analysis and interpretation of such information. M.A. and J.F.M., acquisition of the data or the analysis and interpretation of such information, writing the article or substantial involvement in its revision before submission, involvement in the conception, hypothesis delineation, and design of the study.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201805-0825OC on August 8, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ljungman P. Beta-herpesvirus challenges in the transplant recipient. J Infect Dis. 2002;186:S99–S109. doi: 10.1086/342962. [DOI] [PubMed] [Google Scholar]
- 2.Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant. 2004;4:1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 4.Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Verleden SE, Todd JL, Sato M, Palmer SM, Martinu T, Pavlisko EN, et al. Impact of clad phenotype on survival after lung retransplantation: A multicenter study. Am J Transplant. 2015;15:2223–2230. doi: 10.1111/ajt.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 8.Kerschner H, Jaksch P, Karigl G, Popow-Kraupp T, Klepetko W, Puchhammer-Stöckl E. Cytomegalovirus DNA load patterns developing after lung transplantation are significantly correlated with long-term patient survival. Transplantation. 2009;87:1720–1726. doi: 10.1097/TP.0b013e3181a60b4e. [DOI] [PubMed] [Google Scholar]
- 9.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010;181:1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlen Copeland CA, Davis WA, Snyder LD, Banks M, Avery R, Davis RD, et al. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J Heart Lung Transplant. 2011;30:990–996. doi: 10.1016/j.healun.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis. 2011;204:1663–1671. doi: 10.1093/infdis/jir624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lendermon EA, Dodd-o JM, Coon TA, Miller HL, Ganguly S, Popescu I, et al. Cd8(+)il-17(+) t cells mediate neutrophilic airway obliteration in t-bet-deficient mouse lung allograft recipients. Am J Respir Cell Mol Biol. 2015;52:622–633. doi: 10.1165/rcmb.2014-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popescu I, Pipeling MR, Mannem H, Shah PD, Orens JB, Connors M, et al. Il-12-dependent cytomegalovirus-specific CD4+ T cell proliferation, T-bet induction, and effector multifunction during primary infection are key determinants for early immune control J Immunol 2016196877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder LD, Chan C, Kwon D, Yi JS, Martissa JA, Copeland CA, et al. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 2016;193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadnerkar A, Toyoda Y, Crespo M, Pilewski J, Mitsani D, Kwak EJ, et al. Age-specific complications among lung transplant recipients 60 years and older. J Heart Lung Transplant. 2011;30:273–281. doi: 10.1016/j.healun.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Stanley SE, Chen JJ, Podlevsky JD, Alder JK, Hansel NN, Mathias RA, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–570. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL, et al. An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:82–93. doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley SE, Gable DL, Wagner CL, Carlile TM, Hanumanthu VS, Podlevsky JD, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8:351ra107. doi: 10.1126/scitranslmed.aaf7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silhan LL, Shah PD, Chambers DC, Snyder LD, Riise GC, Wagner CL, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J. 2014;44:178–187. doi: 10.1183/09031936.00060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borie R, Kannengiesser C, Hirschi S, Le Pavec J, Mal H, Bergot E, et al. et al. Severe hematologic complications after lung transplantation in patients with telomerase complex mutations. J Heart Lung Transplant. 2015;34:538–546. doi: 10.1016/j.healun.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benden C, Edwards LB, Kucheryavaya AY, Christie JD, Dipchand AI, Dobbels F, et al. The registry of the International Society for Heart and Lung Transplantation: sixteenth official pediatric lung and heart-lung transplantation report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:989–997. doi: 10.1016/j.healun.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Alder JK, Hanumanthu VS, Strong MA, DeZern AE, Stanley SE, Takemoto CM, et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc Natl Acad Sci USA. 2018;115:E2358–E2365. doi: 10.1073/pnas.1720427115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner CL, Hanumanthu VS, Talbot CC, Jr, Abraham RS, Hamm D, Gable DL, et al. Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest. 2018;128:5222–5234. doi: 10.1172/JCI120216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokman S, Singer JP, Devine MS, Westall GP, Aubert JD, Tamm M, et al. Clinical outcomes of lung transplant recipients with telomerase mutations. J Heart Lung Transplant. 2015;34:1318–1324. doi: 10.1016/j.healun.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newton CA, Kozlitina J, Lines JR, Kaza V, Torres F, Garcia CK.Telomere length in patients with pulmonary fibrosis associated with chronic lung allograft dysfunction and post-lung transplantation survival J Heart Lung Transplant 201736845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhorade SM, Lurain NS, Jordan A, Leischner J, Villanueva J, Durazo R, et al. Emergence of ganciclovir-resistant cytomegalovirus in lung transplant recipients. J Heart Lung Transplant. 2002;21:1274–1282. doi: 10.1016/s1053-2498(02)00463-1. [DOI] [PubMed] [Google Scholar]
- 33.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant. 2008;22:162–170. doi: 10.1111/j.1399-0012.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 34.Minces LR, Nguyen MH, Mitsani D, Shields RK, Kwak EJ, Silveira FP, et al. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob Agents Chemother. 2014;58:128–135. doi: 10.1128/AAC.00561-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shlobin OA, West EE, Lechtzin N, Miller SM, Borja M, Orens JB, et al. Persistent cytomegalovirus-specific memory responses in the lung allograft and blood following primary infection in lung transplant recipients. J Immunol. 2006;176:2625–2634. doi: 10.4049/jimmunol.176.4.2625. [DOI] [PubMed] [Google Scholar]
- 38.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott JF, Lin Y, Mizel SB, Bleackley RC, Harnish DG, Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984;226:1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- 40.McNeil AC, Shupert WL, Iyasere CA, Hallahan CW, Mican JA, Davey RT, Jr, et al. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc Natl Acad Sci USA. 2001;98:13878–13883. doi: 10.1073/pnas.251539598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilton JC, Luskin MR, Johnson AJ, Manion M, Hallahan CW, Metcalf JA, et al. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J Virol. 2007;81:2713–2725. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittle HC, Dossetor J, Oduloju A, Bryceson AD, Greenwood BM. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978;62:678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew A, Kurane I, Green S, Vaughn DW, Kalayanarooj S, Suntayakorn S, et al. Impaired T cell proliferation in acute dengue infection. J Immunol. 1999;162:5609–5615. [PubMed] [Google Scholar]
- 44.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 45.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pipeling MR, West EE, Osborne CM, Whitlock AB, Dropulic LK, Willett MH, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol. 2010;184:3417–3423. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- 49.Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013;22:3239–3249. doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- 50.Ballew BJ, Joseph V, De S, Sarek G, Vannier JB, Stracker T, et al. A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome. PLoS Genet. 2013;9:e1003695. doi: 10.1371/journal.pgen.1003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.