To the Editor:
Suppressor cells, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), are important components of the immune response generated against tuberculosis (TB). Tasquinimod (TSQ), a novel antitumor compound currently being developed to treat various cancer forms, is a small molecule that restricts suppressive cell frequency in the tumor microenvironment (1). We sought to determine whether TSQ could be used as host-directed immunotherapy to treat tuberculosis by transiently depleting MDSCs and related cellular populations and thereby serve as a potential adjunctive agent to shorten TB treatment duration.
We evaluated the effect of TSQ in an acute mouse model of TB infection and analyzed the cellular composition and bacterial burden in organs. Briefly, 129S2 (129S2/SvPasCrl) mice (Charles River Laboratory) were infected through aerosol route using Mycobacterium tuberculosis H37Rv as previously described (2). Animals were treated with 10 mg/kg TSQ daily, as reported earlier (3). Mouse organs were homogenized, diluted, and plated for colony-forming units (cfus). For flow cytometry, organs were harvested on Day 21, and single-cell suspensions were stained with CD4, CD8, CD25, CD124, FoxP3, CD11b, and Gr1 fluorescently labeled antibodies. The data were analyzed using BD FACSDiva (BD Biosciences) software. All experiments were in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals (NIH). Lung cfu counts were log10 transformed before analysis. Data, presented as mean ± SEM, were evaluated using Student’s t test.
Results
Tasquinimod monotherapy enhances mycobacterial clearance in the mouse model
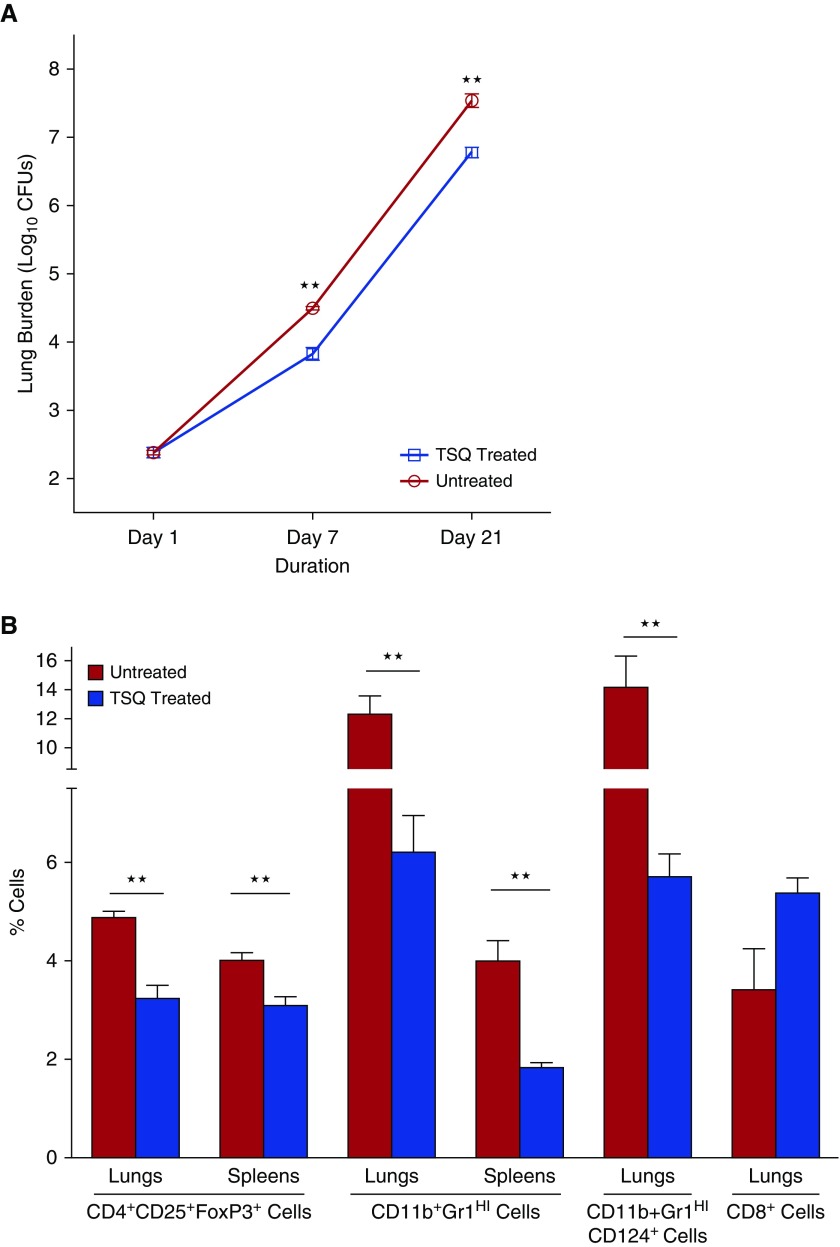
To evaluate the effect of TSQ treatment during tuberculosis, we quantified cfus at 1, 14, and 21 days after infection and TSQ treatment in lungs and spleens. Lung cfu counts in the TSQ-treated group were 0.67 and 0.76 log units lower than the untreated control at Days 7 and 21, respectively (P < 0.001) (Figure 1A). At Day 21, the spleen burden for untreated animals was 4.59 (±0.06) log units, whereas TSQ-treated spleens were 0.55 log units lower than untreated controls. These data demonstrate that TSQ treatment is successful in reducing M. tuberculosis burden in the infected organs.
Figure 1.
Tasquinimod (TSQ) treatment decreases tuberculosis disease progression and depletes regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) in lungs and spleens of Mycobacterium tuberculosis–infected mice. 129S2 mice were infected with M. tuberculosis H37Rv by aerosol route to achieve a lung implantation of 2.4 log10 cfu 1 day after infection. A group of mice was treated with TSQ (10 mg/kg oral) daily for 7 d/wk starting from Day 1. (A) Lungs were homogenized, diluted, and plated for cfu counts at indicated time points. (B) Single-cell suspensions from lungs and spleens of infected mice 21 days after treatment were analyzed by flow cytometry for frequency of CD4+CD25+FoxP3+ Tregs, CD11b+Gr1HI MDSCs, CD124-expressing MDSCs, and CD8+ T cells. ★★P < 0.01.
Adjunctive tasquinimod treatment decreases Treg and MDSC frequencies during TB infection
To better understand the cellular populations involved in the effects of TSQ treatment in TB-infected animals, we analyzed the number of Tregs and MDSCs in treated lungs and spleens. The percentages of CD4+CD25+FoxP3+ cells (Tregs) in lungs at Day 21 were found to be 4.9% for the untreated animals and 3.2% for the TSQ-treated group (P < 0.01), indicating a significant decrease of Tregs after treatment (Figure 1B).
Next, we investigated the numbers of MDSCs in lungs and spleens of untreated or TSQ-treated infected mice. The percentages of CD11b+Gr1Hi cells of total leukocytes in lungs at Day 21 were found to be 12.0% for the untreated animals and 6.2% for the treated group (P = 0.005), indicating a significant decrease in MDSCs in lungs after TSQ treatment (Figure 1B). We observed similar reduction in splenic MDSC frequencies.
It has been recently demonstrated that excessive MDSC accumulation in lungs correlated with elevated surface expression of CD124 (IL-4Rα) on MDSCs during TB infection (4). We determined the frequencies of MDSCs expressing surface CD124 and found that the subset frequencies were reduced from 14.2% in untreated controls to 5.7% in TSQ-treated lungs on Day 21 (Figure 1B). To determine whether the observed inhibition of bacterial growth by TSQ treatment was associated with improved immune responses, we examined CD8+ T cells from lungs of the treated animals. We observed an increase in CD8+ T-cell frequencies from 3.4% in untreated infected animals to 5.4% in TSQ-treated animals on Day 21 (P = 0.07), indicating induction of effector T cells associated with TSQ immunotherapy (Figure 1B).
Tasquinimod enhances antimycobacterial activity of standard TB treatment
To determine the potential of TSQ as an adjunctive therapeutic when given with standard TB treatment, we administered the drugs rifampin (R), isoniazid (H), and pyrazinamide (Z) in the presence and absence of TSQ treatment. An additional group of mice received TSQ monotherapy. Mice were infected with M. tuberculosis H37Rv and treated daily with RHZ with or without TSQ, starting on Day 14 after infection. In mouse lungs (Figure 2A), standard RHZ treatment yielded a 1.91 log10 unit cfu decrease at day 21 compared with the untreated controls, whereas the RHZ-plus-TSQ group gave quantitative cfu counts that were 2.24 log10 units lower (P = 0.003). We observed comparable effects of TSQ addition on spleen burden (Figure 2B, P = 0.002). Taken together, these results demonstrate that TSQ therapy significantly potentiates the bactericidal effectiveness of standard RHZ treatment.
Figure 2.
Tasquinimod (TSQ) treatment potentiates standard tuberculosis chemotherapy. Groups of mice (five mice per time point) were infected with 2.8 log10 units of Mycobacterium tuberculosis H37Rv and treated with rifampin (R; 10 mg/kg), isoniazid (H; 10 mg/kg), and pyrazinamide (Z; 150 mg/kg), with or without TSQ (10 mg/kg oral) from Day 14, daily for 5 d/wk. (A and B) The lungs (A) and spleens (B) were homogenized, diluted, and plated for cfu counts at Day 21. ★★P < 0.01.
Discussion
TSQ is a quinoline-3-carboxamide analog that suppresses the local recruitment and function of MDSCs, Tregs, and their soluble mediators, thereby disrupting the immunosuppressive tumor microenvironment (4–6). Moreover, it was recently shown that granuloma formation was markedly impaired by TSQ treatment in a guinea pig model (7). We recently demonstrated that denileukin diftitox, a recombinant protein toxin targeting CD25+ cells, restricted bacterial replication and depleted suppressor cells in TB-infected organs (8). Our data here reveal that TSQ mono-treatment reduced the bacterial burden in lungs and spleens compared with the untreated animals and was found to be associated with a significant depletion of Tregs and MDSCs in relevant organs. We were unable to observe a direct antimicrobial effect of TSQ against M. tuberculosis H37Rv using in vitro plate assay. Taken together, these observations suggest that the observed effects are likely due to modulation of immune responses during TSQ treatment of TB-infected animals.
Immune system failure during TB dictates the need of immunomodulatory strategies to enhance immune responses. Several strategies targeting different effector mechanisms to modify lung inflammation have shown promise toward shortening TB treatment duration and preventing the development of microbial resistance (9). It has been recently demonstrated that highly susceptible mouse strain 129S2 closely mimics active TB in patients compared with a highly resistant strain, C57BL/6 (10). Using in vivo studies in the TB mouse model, our study demonstrates that TSQ depletes immunosuppressive cells during therapy, leading to reduced bacterial proliferation, and that it potentiates standard antimicrobial therapy for TB treatment. Our results indicate that TSQ offers considerable promise as a novel adjunctive, host-directed therapy for tuberculosis.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. S. Parveen for critical reading of the manuscript and Active Biotech (Lund, Sweden) for providing tasquinimod.
Footnotes
Supported by National Institute of Allergy and Infectious Diseases grants AI 079590, 037856, 036973, and 135280 and the Howard Hughes Medical Institute.
Author Contributions: S.G. conceived and designed the experiments. S.G., S.K., and S.P. performed experiments and analyzed data. S.G. and S.P. designed the flow cytometry experiments. S.G., G.S., and W.R.B. interpreted the data. S.G. and W.R.B. drafted the manuscript. T.L. and J.T.I. provided reagents for the study. All authors have given final approval of the submitted and published versions.
Originally Published in Press as DOI: 10.1164/rccm.201805-0820LE on November 5, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Shen L, Sundstedt A, Ciesielski M, Miles KM, Celander M, Adelaiye R, et al. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res. 2015;3:136–148. doi: 10.1158/2326-6066.CIR-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S, Winglee K, Gallo R, Bishai WR. Bacterial subversion of cAMP signalling inhibits cathelicidin expression, which is required for innate resistance to Mycobacterium tuberculosis. J Pathol. 2017;242:52–61. doi: 10.1002/path.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs JT, Pili R, Qian DZ, Dalrymple SL, Garrison JB, Kyprianou N, et al. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate. 2006;66:1768–1778. doi: 10.1002/pros.20509. [DOI] [PubMed] [Google Scholar]
- 4.Knaul JK, Jörg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med. 2014;190:1053–1066. doi: 10.1164/rccm.201405-0828OC. [DOI] [PubMed] [Google Scholar]
- 5.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 6.Raymond E, Dalgleish A, Damber JE, Smith M, Pili R. Mechanisms of action of tasquinimod on the tumour microenvironment. Cancer Chemother Pharmacol. 2014;73:1–8. doi: 10.1007/s00280-013-2321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka Y, Mizutani T, Mizuta S, Miyamoto A, Murata S, Ano T, et al. Neutrophils and the S100A9 protein critically regulate granuloma formation. Blood Adv. 2016;1:184–192. doi: 10.1182/bloodadvances.2016000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Cheung L, Pokkali S, Winglee K, Guo H, Murphy JR, et al. Suppressor cell-depleting immunotherapy with denileukin diftitox is an effective host-directed therapy for tuberculosis. J Infect Dis. 2017;215:1883–1887. doi: 10.1093/infdis/jix208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol. 2015;15:255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
- 10.Domaszewska T, Scheuermann L, Hahnke K, Mollenkopf H, Dorhoi A, Kaufmann SHE, et al. Concordant and discordant gene expression patterns in mouse strains identify best-fit animal model for human tuberculosis. Sci Rep. 2017;7:12094. doi: 10.1038/s41598-017-11812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.