Abstract
Rationale: Bimagrumab is a fully human monoclonal antibody that blocks the activin type II receptors, preventing the activity of myostatin and other negative skeletal muscle regulators.
Objectives: To assess the effects of bimagrumab on skeletal muscle mass and function in patients with chronic obstructive pulmonary disease (COPD) and reduced skeletal muscle mass.
Methods: Sixty-seven patients with COPD (mean FEV1, 1.05 L [41.6% predicted]; aged 40–80 yr; body mass index < 20 kg/m2 or appendicular skeletal muscle mass index ≤ 7.25 [men] and ≤ 5.67 [women] kg/m2), received two doses of either bimagrumab 30 mg/kg intravenously (n = 33) or placebo (n = 34) (Weeks 0 and 8) over 24 weeks.
Measurements and Main Results: We assessed changes in thigh muscle volume (cubic centimeters) as the primary endpoint along with 6-minute-walk distance (meters), safety, and tolerability. Fifty-five (82.1%) patients completed the study. Thigh muscle volume increased by Week 4 and remained increased at Week 24 in bimagrumab-treated patients, whereas no changes were observed with placebo (Week 4: +5.9% [SD, 3.4%] vs. 0.0% [3.3%], P < 0.001; Week 8: +7.0% [3.7%] vs. −0.7% [2.8%], P < 0.001; Week 16: +7.8% [5.1%] vs. −0.9% [4.5%], P < 0.001; Week 24: +5.0% [4.9%] vs. −1.3% [4.3%], P < 0.001). Over 24 weeks, 6-minute-walk distance did not increase significantly in either group. Adverse events in the bimagrumab group included muscle-related symptoms, diarrhea, and acne, most of which were mild in severity.
Conclusions: Blocking the action of negative muscle regulators through the activin type II receptors with bimagrumab treatment safely increased skeletal muscle mass but did not improve functional capacity in patients with COPD and low muscle mass.
Clinical trial registered with www.clinicaltrials.gov (NCT01669174).
Keywords: bimagrumab, thigh muscle volume, 6-minute-walk distance, lean body mass
At a Glance Commentary
Scientific Knowledge on the Subject
In patients with chronic obstructive pulmonary disease (COPD), locomotor muscle weakness is associated with a poor prognosis, worse health-related quality of life, and increased health care use. Although pulmonary rehabilitation can partially reverse skeletal muscle weakness in COPD, there is no drug approved for this purpose or to recover lost muscle mass. Myostatin and activin A are negative regulators of muscle mass that are increased in patients with COPD; myostatin and other regulators of muscle mass signal through the activin type II receptors. Bimagrumab is a fully human monoclonal antibody that blocks the activin type II receptors and is currently under investigation for multiple indications associated with muscle wasting.
What This Study Adds to the Field
We report the first placebo-controlled, double-blind, randomized trial that investigated the effects of activin type II receptors blockade in patients with COPD and evidence of low body weight or muscle mass. Bimagrumab substantially increased thigh muscle volume and total lean body mass, but this did not translate into improved muscle function or functional performance.
Chronic obstructive pulmonary disease (COPD) is a common and often progressive condition, with some patients experiencing disabling symptoms despite the use of approved therapies. Reduced quadriceps muscle mass, and consequent weakness, is present in approximately one-third of patients with COPD (1), and multiple reports have confirmed that this extrapulmonary feature is associated with poor clinical outcomes. Specifically, quadriceps weakness (2) and wasting (3) have been associated with increased mortality, and reduced rectus femoris cross-sectional area predicts a higher risk of readmission after acute exacerbation (4).
Myostatin (growth and differentiation factor 8), activin A, activin B, and growth and differentiation factor 11 are all negative regulators of skeletal muscle mass that act through ActRII (activin type II) receptors. The activin receptor is a heterodimer consisting of a type I subunit (ALK4 [activin receptor-like kinase 4] or ALK5) and a type II subunit (ActRIIA or ActRIIB). Humans (5) and animals (6) with genetic mutations that result in reduced or nonfunctional myostatin, exhibit increased muscularity but are otherwise healthy. Conversely, studies have shown that quadriceps myostatin RNA is increased in patients with COPD with low muscle strength and functional limitation (7, 8).
Pulmonary rehabilitation, an exercise-based therapy that is known to increase both exercise capacity and quadriceps strength (9), also reduces quadriceps myostatin expression in patients with COPD in both acute (10) and chronic settings (11). Completion of pulmonary rehabilitation is associated with reduced frequency of COPD exacerbations, emergency department visits, hospitalization, and mortality (12–14). As such, a pharmacological approach that also increases muscle mass and exercise capacity might have the potential to improve quality of life and reduce costs associated with the care of patients with COPD.
Clinical experience with non–androgen-based pharmacological anabolic agents is sparse. An antibody against myostatin has been previously studied in older men and women with a history of falls. Six months of dosing resulted in a modest increase in lean body mass (LBM), with no clinically relevant improvement in functional endpoints (15). In another study, a molecule with a different mechanism of action, a soluble form of the ActRIIB, increased skeletal muscle mass in healthy volunteers by binding to circulating myostatin and other ligands (16). Bimagrumab, an ActRII blocker, has been shown to cause substantial skeletal muscle hypertrophy in animal models (17) and humans (18) by preventing the activity of myostatin and other negative regulators of muscle mass in a SMAD 2/3 (small mothers against decapentaplegic 2/3; the main signal transducers for ActRII)–dependent manner. A phase II study showed that in men and women with sarcopenia, treatment with bimagrumab resulted in increased skeletal muscle mass and strength and improved mobility in subjects with low baseline function (18). Therefore, we theorized that reducing myostatin activity via blockade of the ActRII in patients with COPD and associated low skeletal muscle mass might result in muscle hypertrophy and improved muscle function and functional performance. Some of these data were previously presented at the American Thoracic Society International Conference, May 13 to 18, 2016, in San Francisco, California.
Methods
Study Design and Participants
This 24-week, randomized, double-blind, placebo-controlled, parallel-arm, phase IIa study was conducted across 11 sites in the United Kingdom, United States, and the Netherlands from June 2013 to December 2014. Patients were randomly assigned (1:1) to receive two doses of either bimagrumab 30 mg/kg or placebo (see Figure E1 in the online supplement) administered intravenously on study Days 1 and 57. The study included men and women with a clinical diagnosis of COPD, Global Initiative for Obstructive Lung Disease (GOLD) spirometric stage 2 or worse (19), with a tobacco exposure of more than 10 pack-years. Eligible patients had evidence of low skeletal muscle mass, assessed as body mass index (BMI) less than 20 kg/m2 or appendicular skeletal muscle mass index (lean mass of upper and lower limbs/height2) of less than 7.25 kg/m2 for men or less than 5.45 kg/m2 for women, measured by dual energy X-ray absorptiometry (DXA) (20). Other key inclusion criteria were clinical stability, no participation in pulmonary rehabilitation in the 3 months before dosing, and an average daily consumption of more than 20 kcal/kg and more than 0.6 g protein/kg, as confirmed by dietician’s evaluation (21). Exclusion criteria focused on conditions that would impact improvement in mobility (i.e., Medical Research Council dyspnea score of grade 5 or hospitalization 2 weeks before screening) or confound changes in muscle mass (i.e., drugs known to affect skeletal muscle size, such as an androgen or anti-androgen). A complete list of inclusion and exclusion criteria is available in the online supplementary (Table E1).
Novartis Drug Supply Management produced a randomization list using a validated, automated system that randomly assigned participants to treatment arms. The Novartis Biostatistics Quality Assurance Group approved the randomization scheme. All participants, investigators, and sponsor representatives associated with the study were masked to treatment allocation.
The study was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guidelines for Good Clinical Practice, with applicable local regulations, and with the ethical principles as laid down in the Declaration of Helsinki. All participants provided written informed consent before enrollment. Participants were free to withdraw from the study at any time.
Measurements
Changes from baseline in thigh muscle volume (TMV), assessed by magnetic resonance imaging (MRI), at 8 weeks was the primary outcome of this study to determine differences in muscle hypertrophy from baseline between the treatments. Patients were imaged using a 1.5T scanner and a Q-body coil at all sites, which allowed for intermuscular and subcutaneous lipid quantification (22).
DXA was used to evaluate body composition and total and appendicular lean and fat mass. A calibration phantom was used to ensure consistency in DXA readings across study sites (23). Body positioning, including that of hands and feet, was standardized across sites. In addition to body composition, data regarding appendicular lean mass and bone density were assessed.
Tests to assess mobility, muscle strength and power, and common daily tasks and a questionnaire to measure patients’ health status were used to quantify functional status. These included the 6-minute-walk test, which was performed to conform with the American Thoracic Society Guidelines (24), bilateral handgrip dynamometry, bilateral one-repetition maximum leg press to assess muscle strength (25), stair climbing time to assess lower limb muscle power (26), and the Timed Up and Go test (27). Maximum inspiratory and expiratory pressures were measured from residual volume and total lung capacity, respectively, according to European Respiratory Society/American Thoracic Society guidelines (28). Health status was measured by using the St. George’s Respiratory Questionnaire (29).
Statistical Methodology
A sample size calculation was conducted for the primary endpoint (TMV) on the basis of 8-week data from a previous first-in-human study (30), and this calculation showed that 25 patients in each arm would have a 77% power to detect a 7% increase in TMV by a one-sided test at significance level 5%. Dropouts were estimated at 17%, generating a recruitment target of 60 patients, which was exceeded. Efficacy data were analyzed at each time point by analysis of covariance with a fixed effect for treatment (bimagrumab or placebo) and a continuous covariate for the baseline measurement. TMV data were log-transformed before analysis, but all results were back-transformed and reported on the original scale. Findings were considered statistically significant if a two-tailed P value was less than 0.05.
Results
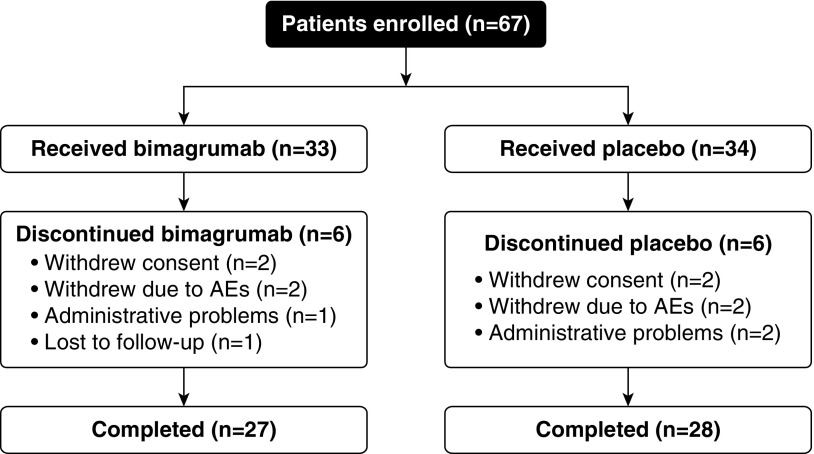
A schematic of the study design is presented in Figure 1. Sixty-seven patients were randomized (bimagrumab group: 33; placebo group: 34), and 55 (82.1%; bimagrumab: 27; placebo: 28) completed the study.
Figure 1.
Study design. All 67 patients (bimagrumab, n = 33; placebo, n = 34) were included in the safety and the efficacy analysis sets. AEs = adverse events.
Baseline characteristics were similar in both treatment arms (summarized in Table 1). The study population had a mean age of 64 years, was predominantly white (90%), had a mean BMI of 19.3 kg/m2, and had a mean FEV1 of 1.05 L (41.6% predicted).
Table 1.
Characteristics of the Participants
| Baseline Data | Bimagrumab (n = 33) | Placebo (n = 34) |
|---|---|---|
| Age, yr | 64.5 (5.9) | 63.1 (7.5) |
| 51–75 | 49–80 | |
| Men/women | 17/16 | 16/18 |
| BMI, kg/m2 | 19.5 (2.0) | 19.1 (1.9) |
| 15.2–23.4 | 15.5–23.6 | |
| FEV1, L | 1.1 (0.5) | 1.0 (0.5) |
| 0.3–2.8 | 0.3–2.4 | |
| FEV1, % predicted | 42.5 (16.4) | 40.6 (15.9) |
| 20–79 | 17–77 | |
| FEV1/FVC, % | 36.1 (9.7) | 38.9 (11.3) |
| 19–56 | 19–65 | |
| TMV, cm3 | 2,533.8 (677.7) | 2,469.6 (748.7) |
| 1,465.7–3,731.6 | 823.2–3,998.1 | |
| Total LBM, kg | 35.5 (7.8) | 33.6 (6.8) |
| 24.4–48.0 | 32.3–53.3 | |
| ALM, kg | 15.3 (3.7) | 14.8 (3.7) |
| 9.9–21.8 | 8.9–25.5 | |
| 6MWD, m | 361 (94) | 372 (92) |
| 138–535 | 144–606 | |
| SGRQ score | 52.4 (14.0) | 54.2 (18.3) |
| 21.6–75.5 | 57.1–91 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ALM = appendicular lean mass; BMI = body mass index; LBM = lean body mass; SGRQ = St. George’s respiratory questionnaire; TMV = thigh muscle volume.
Data are presented as mean (SD) and range, unless specified otherwise.
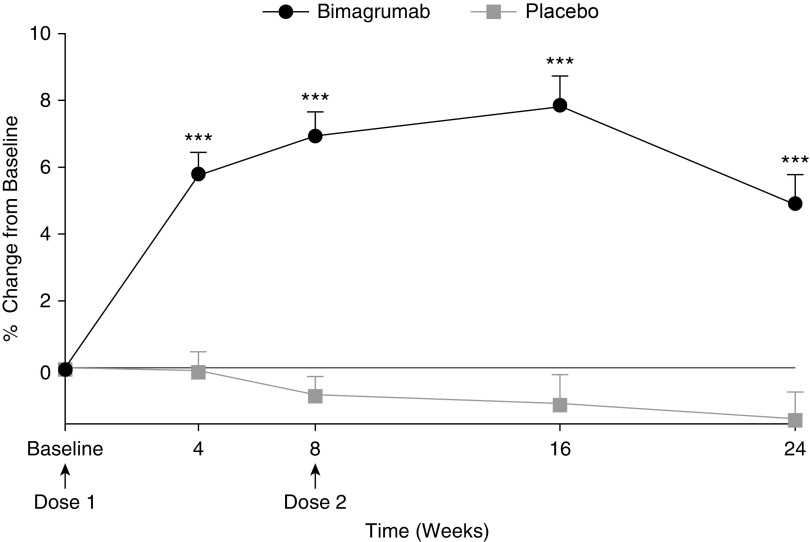
TMV, assessed by MRI, increased significantly 4 weeks after the first dose of bimagrumab and remained above baseline throughout the study (Figure 2), whereas no change was seen in the placebo group. The bimagrumab group had a mean (SD) increase in TMV of +5.9% (3.4%) at Week 4, +7.0% (3.7%) at Week 8, +7.8% (5.1%) at Week 16, and +5.0% (4.5%) at Week 24, whereas those receiving placebo showed a small decline in TMV over the same period (0.0% [3.3%], −0.7% [2.8%], −0.9% [4.5%], and −1.3% [4.1%]). Least squares means differences (90% confidence interval) between the bimagrumab and placebo groups were +6.1% (4.6–7.6%) at Week 4, +7.8% (6.2–9.4%) at Week 8, +8.6% (6.3–11.0%) at Week 16, and +6.6% (4.4–8.9%) at Week 24 (P < 0.001). Differences in LBM, assessed by DXA, from baseline confirmed the systemic effects of bimagrumab. Patients from the group receiving bimagrumab showed mean (SD) improvements in total LBM from baseline of +1.3% (5.7%) at Week 4, +1.6% (5.8%) at Week 8, +2.7% (5.7%) at Week 12, +3.1% (6.4%) at Week 16, and +1.2% (6.2%) at Week 24. These percentage increases translated into mean group gains of 0.4 to 1.1 kg of lean mass. Concurrent with the anabolic effect, the bimagrumab group showed substantial reductions in both intermuscular and subcutaneous adipose tissue on MRI (Figures E2 and E3), with mean (SD) changes at 24 weeks of −13.1% (6.1%) and −15.7% (10.1%), respectively; no changes were observed in the placebo group. Assessment of total and appendicular lean and fat mass by DXA scanning confirmed the MRI findings (Figures E4 and E5). There was no change in bone mineral density in either group.
Figure 2.
Effect of bimagrumab on thigh muscle volume. ***P < 0.001, bimagrumab versus placebo. Data are presented as mean (SE).
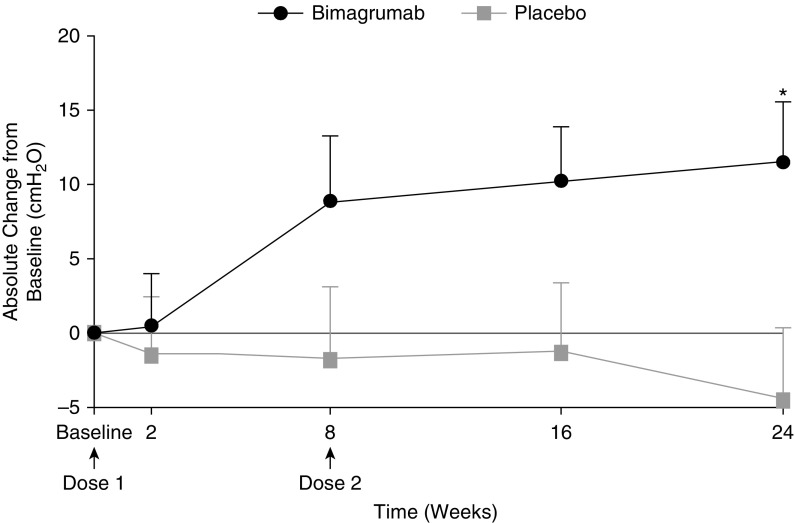
There was no effect of either treatment on any measure of muscle strength or mobility. In the bimagrumab group, there was a progressive increase over placebo in maximum static expiratory pressure (Figure 3); statistical significance was noted at Week 24 (P = 0.017). In addition, no statistically significant differences were recorded in spirometry parameters between both groups throughout the study (Table 2). No statistically significant difference was observed in 6-minute-walk distance (6MWD), stair climbing time, Timed Up and Go test, one-repetition maximum leg press, or hand-grip strength between groups at any time during the study (Table 2). Similarly, no differences in St. George’s Respiratory Questionnaire scores were observed between groups at any time during the study (Table 2).
Figure 3.
Effect of bimagrumab on maximal expiratory pressure. *P = 0.017, bimagrumab versus placebo. Data are presented as mean (SE).
Table 2.
Effect of Bimagrumab on Pulmonary, Physical Performance, and Quality-of-Life Parameters at Weeks 0, 16, and 24
| Bimagrumab |
Placebo |
|||||
|---|---|---|---|---|---|---|
| 0 wk | 16 wk | 24 wk | 0 wk | 16 wk | 24 wk | |
| FEV1, % predicted | 42.5 (16.4) | 39.3 (16.7) | 42.6 (18.4) | 40.6 (15.9) | 38.8 (16.5) | 36.1 (16.3) |
| FEV1/FVC, % | 36.1 (9.7) | 34.5 (9.5) | 36.4 (10.1) | 38.9 (11.3) | 40.0 (13.2) | 37.3 (11.8) |
| Total LBM, kg | 35.5 (7.8) | 36.9 (8.3)* | 36.2 (7.9) | 33.6 (6.8) | 32.1 (6.3) | 32.3 (6.1) |
| ALM, kg | 15.3 (3.7) | 16.3 (3.8)† | 15.9 (3.6)* | 14.8 (3.7) | 13.9 (3.5) | 14.0 (3.4) |
| 6MWD, m | 361 (94) | 380 (84) | 375 (98) | 372 (92) | 353 (104) | 379 (82) |
| Stair climb, s | 5.2 (1.9) | 4.8 (1.7) | 4.9 (1.5) | 4.6 (1.9) | 4.5 (1.2) | 4.4 (1.2) |
| Timed Up and Go, s | 8.8 (2.3) | 8.3 (1.5) | 8.6 (1.6) | 8.2 (1.9) | 8.3 (2.0) | 8.0 (1.9) |
| Hand grip, RH, kg | 28.9 (11.1) | 31.1 (12.8) | 29.6 (11.8) | 27.3 (10.0) | 26.2 (8.4) | 25.2 (9.0) |
| 1-RM leg press, kg | 58.2 (29.4) | 73.5 (38.6) | 72.2 (38.9) | 62.0 (31.0) | 60.8 (30.0) | 63.8 (28.1) |
| MIP, cm H2O | 58.5 (22.3) | 61.7 (26.0) | 61.6 (21.7) | 58.9 (22.6) | 62.0 (23.8) | 57.8 (23.9) |
| MEP, cm H2O | 84.8 (30.9) | 93.9 (39.2) | 96.6 (36.8)‡ | 91.2 (38.8) | 87.3 (33.7) | 86.9 (33.6) |
| SGRQ score | 52.4 (14.0) | 52.0 (17.8) | 50.8 (16.1) | 54.2 (18.3) | 51.9 (20.8) | 55.2 (19.6) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; 1-RM = one-repetition maximum; ALM = appendicular lean mass; LBM = lean body mass; MEP = maximal expiratory pressure; MIP = maximal inspiratory pressure; RH = right hand; SGRQ = St. George’s Respiratory Questionnaire.
Data are presented as mean (SD).
P < 0.01 for difference between treatments.
P < 0.001 for difference between treatments.
P < 0.05 for difference between treatments.
On analysis of safety outcomes, two patients from each treatment group withdrew because of adverse events (AEs), and one patient from each group withdrew after the discovery of neoplasms that were undetected at study entry. Of the 67 participants, 64 (96%) experienced an AE, (33 [100%], in the bimagrumab group and 31 [91.2%] in the placebo group; Table E2). The three most commonly reported AEs with bimagrumab treatment were muscle spasms, tightness, or twitching (∼46–79%); diarrhea (21.2%); and acne (9.1%). Incidences of muscle spasms and muscle tightness were significantly higher in the bimagrumab group than in the placebo group (P = 0.011, muscle spasms; P < 0.001, muscle tightness; Fisher exact test, two-sided); however, no other significant differences in AE incidence were observed between the bimagrumab and placebo groups. In addition, nasopharyngitis (12.1%, 4 of 33), sinusitis (9.1%, 3 of 33), and elevated liver enzymes (alanine aminotransferase, aspartate aminotransferase, or γ-glutamyl transpeptidase) (9.1%, 3 of 33) were reported only in the bimagrumab group. These AEs were mild and self-limited and resolved without treatment. Exacerbations and chest infections were frequent AEs, as would be expected in patients with COPD and low muscle mass, but their prevalence did not differ between study arms.
Discussion
The present study demonstrates that in patients with COPD and associated low muscle mass, ActRII blockade with bimagrumab resulted in a significant increase in TMV. However, the observed hypertrophy of thigh muscles and an increase in total LBM did not result in significant improvement in performance on measures of physical function, except in regard to maximum expiratory pressure.
Critique of the Method
Overall, bimagrumab was safe and well tolerated in this sample of patients with COPD with lower-than-normal muscle mass. The three most commonly reported AEs with bimagrumab treatment—muscle spasms, tightness, and twitching; diarrhea; and acne—were also reported as mostly mild in severity in a recent study of older adults with sarcopenia (18). Physical frailty and muscle wasting, however measured, is a well-established predictor of hospital admission due to acute exacerbation of COPD (4, 31, 32). We expected that our patients, who were selected to benefit from an anabolic agent, would have high rates of acute exacerbation, but it is reassuring that the rates of exacerbation did not differ significantly between treatment arms. In fact, bimagrumab was proven effective in recovery from atrophy in animal models of steroid exposure (17), and a high likelihood of intercurrent exacerbation is not itself a barrier to anabolic drug therapy.
A key rationale for this study was that myostatin levels are increased in the most disabled patients with COPD and tend to decrease with strength training. We acknowledge, however, that our study design would have been stronger if we had ensured that the participants had elevated skeletal muscle myostatin before enrollment. However, we considered that a muscle biopsy might slow recruitment; moreover, because ActRIIs act as a pathway for multiple negative regulators of muscle mass, it is unlikely that the observed increases in TMV and LBM were entirely mediated by blocking the effects of myostatin. In fact, a preclinical study demonstrated that ActRII blockade could induce muscle hypertrophy in a myostatin knockout mouse, proving that other molecules signal through those receptors (17).
The magnitude of the anabolic response with bimagrumab treatment observed in this study was comparable with those reported in a prior study in patients with sarcopenia (18) and with reports on prior anabolic agents used in COPD (33, 34). In individuals with sarcopenia, 16 weeks of treatment with bimagrumab resulted in mean peak increases of +8.0% in TMV and of +6.0% in LBM (18), compared with +7.8% and +3.1%, respectively, in the present study with patients with COPD.
Significance of the Findings
Several prior interventions have attempted to improve functional performance in COPD by increasing skeletal muscle mass. Schols and colleagues performed a large three-way trial of nutrition with and without nandrolone, an anabolic steroid (33). They found that the nutrition plus nandrolone group gained LBM (mean, 1.6 kg vs. placebo), but the available data did not support any benefits on exercise performance (33). Casaburi and coworkers randomized 47 men to a four-way trial of testosterone against placebo with and without exercise training (34). Although the treatment group gained 2.5 kg of LBM over placebo, the use of testosterone without exercise training increased strength but not endurance exercise performance (34). Similar results were obtained by Yeh and colleagues, in an uncontrolled study (35), and by Ferreira and colleagues (36). Similarly, a small (n = 16) randomized study of growth hormone as an adjunct to rehabilitation demonstrated an LBM gain of slightly greater than 1 kg in favor of the treatment group without an improvement in exercise performance (37). Conversely, the appetite-stimulating hormone ghrelin, used as an adjunct to pulmonary rehabilitation, resulted in an increased 6MWD in a small randomized controlled trial, although without a change in overall body weight, compared with placebo (38).
Taken together with prior reports, the current data indicate that anabolic agents in isolation do not, in unselected patients with COPD, translate increased muscle mass into superior exercise performance. Although the anabolic response seen in this study was positive, there are several possibilities why muscle-mass gains did not improve exercise performance in the population, and these may be insightful for the planning of future trials, given that other anabolic agents are emerging (39). First, in a proportion of patients with COPD, the basis of exercise limitation is breathlessness rather than skeletal muscle weakness (40, 41). Given the lack of effect of anabolic agents on lung function and gas exchange, one might not expect the former group to derive as much benefit as the latter. Second, exercise performance might only be improved when it is subnormal. Although the average 6MWD was reduced in the present study, the range of 6MWD baseline values was wide, as some participants with near-normal 6MWD were included; future studies should consider narrower inclusion criteria for reduced exercise performance at baseline. Indeed in a study of sarcopenic seniors who had both slow gait speed and reduced mass, bimagrumab did increase 6MWD and gait speed, suggesting this could be a model for future studies in COPD (18). Third, although the 6MWD is considered a useful stratification tool (31), it has been shown to be poorly responsive to interventions that improve the physiologic ability to exercise (e.g., bronchodilators) (42) and may be less responsive than a constant work rate exercise test (43). Fourth, gain in muscle mass may be a necessary but not sufficient condition for the delivery of improvements in whole-body exercise performance. Studies of isolated high-intensity training in COPD have shown that mitochondrial bioenergetic capacity can improve without mass gains (44), and it may be that concurrent exercise is required to demonstrate improved exercise capacity. Fifth, although we stratified by low muscle mass, we might have obtained greater focus on the population of interest had we made recent weight loss an inclusion criterion; however, unlike (for example) patients with cancer cachexia, most patients with COPD are not managed in secondary or tertiary care, so obtaining accurate sequential weight measures is difficult.
An interesting post hoc analysis showed significant gains in maximal expiratory pressure in the bimagrumab group compared with placebo at Week 24. Unlike maximum inspiratory pressure, where the principal determinant in COPD is the degree of hyperinflation (45), expiratory muscle function is less influenced by lung volume (46). Expiratory muscle strength is necessary to achieve the necessary transient supramaximal flow, which characterizes effective cough (47). Weak expiratory muscles are associated with chest infection in neuromuscular disease, and difficulty in clearing secretions is a major cause of extubation failure in the ICU in such cases (48). Therefore, activin receptor blockade might be effective in these settings, but this, of course, requires further investigation.
Another interesting secondary effect of bimagrumab treatment was a significant reduction in intermuscular and subcutaneous fat. We have previously reported that reduced intermuscular and subcutaneous fat is a marker of anaerobic metabolism in COPD (49), and, thus, ActRII blockade may be of added value in such patients. In general, because muscle weakness is associated with impaired glucose tolerance in COPD (50), this effect could potentially be beneficial.
To conclude, substantial gains in thigh muscle volume were observed after ActRII blockade using bimagrumab treatment, but these did not translate into improved muscle and functional performance in patients with COPD with low muscle mass who were unstratified with respect to exercise capacity. The current findings provide confirmation of efficacy that bimagrumab is anabolic in patients with COPD. Options for demonstrating positive results from trials of anabolic medicines might include translating this anabolic effect into enhanced physical functioning by selecting populations with impaired exercise capacity and considering trials in combination with aerobic exercise programs.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Hardik Ashar (scientific writer, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for providing medical writing support and editorial assistance, coordinating author reviews, and incorporating author comments.
Footnotes
Funded by the Novartis Institutes for BioMedical Research. M.I.P.’s contribution to this work was supported by the National Institute for Health Research Respiratory Biomedical Research Unit at the Royal Brompton and Harefield National Health Service Foundation Trust and Imperial College, London, UK, which partly funds his salary. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, The National Institute for Health Research, or the Department of Health.
Author Contributions: M.I.P. contributed to recruitment of participants, implementation of intervention, and data acquisition and created the initial draft of the manuscript. J.P. was involved in conceptualization of study design, data analysis, and interpretation of results. A.B. contributed to clinical trial management. F.M.E.F., D.S., M.C.S., and R.C. were involved in recruitment of participants, implementation of intervention, and data acquisition. H.-C.T. and D.S.R. provided medical oversight during the study. E.L.-T., R.R., and D.S.R. contributed to conceptualization of study design and with H.-C.T. were involved in interpretation of results. All authors edited the manuscript for intellectual content, provided input for manuscript drafts, and approved the final version submitted for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201802-0286OC on August 10, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40:1115–1122. doi: 10.1183/09031936.00170111. [DOI] [PubMed] [Google Scholar]
- 2.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 4.Greening NJ, Harvey-Dunstan TC, Chaplin EJ, Vincent EE, Morgan MD, Singh SJ, et al. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med. 2015;192:810–816. doi: 10.1164/rccm.201503-0535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 6.Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 7.Man WD, Natanek SA, Riddoch-Contreras J, Lewis A, Marsh GS, Kemp PR, et al. Quadriceps myostatin expression in COPD. Eur Respir J. 2010;36:686–688. doi: 10.1183/09031936.00032510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomiès P, Rodriguez J, Blaquière M, Sedraoui S, Gouzi F, Carnac G, et al. Reduced myotube diameter, atrophic signalling and elevated oxidative stress in cultured satellite cells from COPD patients. J Cell Mol Med. 2015;19:175–186. doi: 10.1111/jcmm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109:207–212. doi: 10.1016/s0002-9343(00)00472-1. [DOI] [PubMed] [Google Scholar]
- 10.Troosters T, Probst VS, Crul T, Pitta F, Gayan-Ramirez G, Decramer M, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1072–1077. doi: 10.1164/rccm.200908-1203OC. [DOI] [PubMed] [Google Scholar]
- 11.Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–310. doi: 10.1183/09031936.00112909. [DOI] [PubMed] [Google Scholar]
- 12.Moore E, Palmer T, Newson R, Majeed A, Quint JK, Soljak MA. Pulmonary rehabilitation as a mechanism to reduce hospitalizations for acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2016;150:837–859. doi: 10.1016/j.chest.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12:CD005305. doi: 10.1002/14651858.CD005305.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner M, McMillan V, Lowe D, Saleem Khan M, Holzhauer-Barrie J, Van Loo V, et al. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: outcomes from the clinical audit of pulmonary rehabilitation services in England 2015. London: Royal College of Physicians; 2017. Pulmonary rehabilitation: beyond breathing better. [Google Scholar]
- 15.Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, et al. STEADY Group. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–957. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]
- 16.Attie KM, Borgstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47:416–423. doi: 10.1002/mus.23539. [DOI] [PubMed] [Google Scholar]
- 17.Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34:606–618. doi: 10.1128/MCB.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65:1988–1995. doi: 10.1111/jgs.14927. [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease; Global strategy for the diagnosis, management and prevention of COPD. 2018 [accessed 2018 Nov 2]. Available from: http://goldcopd.org/
- 20.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8:359. doi: 10.3390/nu8060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Positano V, Christiansen T, Santarelli MF, Ringgaard S, Landini L, Gastaldelli A. Accurate segmentation of subcutaneous and intermuscular adipose tissue from MR images of the thigh. J Magn Reson Imaging. 2009;29:677–684. doi: 10.1002/jmri.21699. [DOI] [PubMed] [Google Scholar]
- 23.Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19:626–631. doi: 10.1183/09031936.02.00279602. [DOI] [PubMed] [Google Scholar]
- 24.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 25.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56:2118–2123. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88:604–609. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 30.Rooks D, Bartlett M, Petricoul O, Laurent D, Roubenoff R. Safety, pharmacokinetics and pharmacodynamics of bimagrumab: a phase I, randomised, double-blind study in elderly and obese healthy human participants. J Frailty Aging. 2017;6(S1):13. [Google Scholar]
- 31.Spruit MA, Polkey MI, Celli B, Edwards LD, Watkins ML, Pinto-Plata V, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13:291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Kon SS, Jones SE, Schofield SJ, Banya W, Dickson MJ, Canavan JL, et al. Gait speed and readmission following hospitalisation for acute exacerbations of COPD: a prospective study. Thorax. 2015;70:1131–1137. doi: 10.1136/thoraxjnl-2015-207046. [DOI] [PubMed] [Google Scholar]
- 33.Schols AMW, Soeters PB, Mostert R, Pluymers RJ, Wouters EFM. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease: a placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–1274. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 34.Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 35.Yeh SS, DeGuzman B, Kramer T M012 Study Group. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest. 2002;122:421–428. doi: 10.1378/chest.122.2.421. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira IM, Verreschi IT, Nery LE, Goldstein RS, Zamel N, Brooks D, et al. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest. 1998;114:19–28. doi: 10.1378/chest.114.1.19. [DOI] [PubMed] [Google Scholar]
- 37.Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease: a prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156:1800–1806. doi: 10.1164/ajrccm.156.6.9704142. [DOI] [PubMed] [Google Scholar]
- 38.Miki K, Maekura R, Nagaya N, Nakazato M, Kimura H, Murakami S, et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: a multicenter, randomized, double-blind, placebo-controlled trial. PLoS One. 2012;7:e35708. doi: 10.1371/journal.pone.0035708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 40.Deschênes D, Pepin V, Saey D, LeBlanc P, Maltais F. Locus of symptom limitation and exercise response to bronchodilation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2008;28:208–214. doi: 10.1097/01.HCR.0000320074.73846.3b. [DOI] [PubMed] [Google Scholar]
- 41.Man WD, Soliman MG, Gearing J, Radford SG, Rafferty GF, Gray BJ, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:562–567. doi: 10.1164/rccm.200302-162OC. [DOI] [PubMed] [Google Scholar]
- 42.Celli B, Tetzlaff K, Criner G, Polkey MI, Sciurba F, Casaburi R, et al. COPD Biomarker Qualification Consortium; Insights from the COPD Biomarker Qualification Consortium. The 6-minute-walk distance test as a chronic obstructive pulmonary disease stratification tool. Am J Respir Crit Care Med. 2016;194:1483–1493. doi: 10.1164/rccm.201508-1653OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borel B, Provencher S, Saey D, Maltais F. Responsiveness of various exercise-testing protocols to therapeutic interventions in COPD. Pulm Med. 2013;2013:410748. doi: 10.1155/2013/410748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brønstad E, Rognmo O, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Håberg AK, et al. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J. 2012;40:1130–1136. doi: 10.1183/09031936.00193411. [DOI] [PubMed] [Google Scholar]
- 45.Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:1310–1317. doi: 10.1164/ajrccm.154.5.8912741. [DOI] [PubMed] [Google Scholar]
- 46.Polkey MI, Luo Y, Guleria R, Hamnegård CH, Green M, Moxham J. Functional magnetic stimulation of the abdominal muscles in humans. Am J Respir Crit Care Med. 1999;160:513–522. doi: 10.1164/ajrccm.160.2.9808067. [DOI] [PubMed] [Google Scholar]
- 47.Polkey MI, Lyall RA, Green M, Nigel Leigh P, Moxham J. Expiratory muscle function in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 1998;158:734–741. doi: 10.1164/ajrccm.158.3.9710072. [DOI] [PubMed] [Google Scholar]
- 48.Epstein SK, Ciubotaru RL. Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med. 1998;158:489–493. doi: 10.1164/ajrccm.158.2.9711045. [DOI] [PubMed] [Google Scholar]
- 49.Shields GS, Coissi GS, Jimenez-Royo P, Gambarota G, Dimber R, Hopkinson NS, et al. Bioenergetics and intermuscular fat in chronic obstructive pulmonary disease-associated quadriceps weakness. Muscle Nerve. 2015;51:214–221. doi: 10.1002/mus.24289. [DOI] [PubMed] [Google Scholar]
- 50.Wells CE, Polkey MI, Baker EH. Insulin resistance is associated with skeletal muscle weakness in COPD. Respirology. 2016;21:689–696. doi: 10.1111/resp.12716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.