Abstract
Rationale: Pulmonary dead space fraction (Vd/Vt) is an independent predictor of mortality in acute respiratory distress syndrome (ARDS). Yet, it is seldom used in practice. The ventilatory ratio is a simple bedside index that can be calculated using routinely measured respiratory variables and is a measure of impaired ventilation. Ventilatory ratio is defined as [minute ventilation (ml/min) × PaCO2 (mm Hg)]/(predicted body weight × 100 × 37.5).
Objectives: To determine the relation of ventilatory ratio with Vd/Vt in ARDS.
Methods: First, in a single-center, prospective observational study of ARDS, we tested the association of Vd/Vt with ventilatory ratio. With in-hospital mortality as the primary outcome and ventilator-free days as the secondary outcome, we tested the role of ventilatory ratio as an outcome predictor. The findings from this study were further verified in secondary analyses of two NHLBI ARDS Network randomized controlled trials.
Measurements and Main Results: Ventilatory ratio positively correlated with Vd/Vt. Ordinal groups of ventilatory ratio had significantly higher Vd/Vt. Ventilatory ratio was independently associated with increased risk of mortality after adjusting for PaO2/FiO2, and positive end-expiratory pressure (odds ratio, 1.51; P = 0.024) and after adjusting for Acute Physiologic Assessment and Chronic Health Evaluation II score (odds ratio, 1.59; P = 0.04). These findings were further replicated in secondary analyses of two separate NHLBI randomized controlled trials.
Conclusions: Ventilatory ratio correlates well with Vd/Vt in ARDS, and higher values at baseline are associated with increased risk of adverse outcomes. These results are promising for the use of ventilatory ratio as a simple bedside index of impaired ventilation in ARDS.
Keywords: ARDS, ventilatory ratio, dead space
At a Glance Commentary
Scientific Knowledge on the Subject
Numerous studies have found that impaired ventilation, as measured by pulmonary dead space, is an important predictor of outcome in acute respiratory distress syndrome (ARDS). Because of complexities in measuring it, however, dead space is seldom used in the critical care setting. Consequently, PaO2/FiO2 remains the only measured physiologic variable used to define severity in ARDS.
What This Study Adds to the Field
The study describes a new index of impaired ventilation called ventilatory ratio (VR). VR can be calculated easily using measured PaCO2 and minute ventilation. In two cohorts of ARDS, VR was found to have significant association with pulmonary dead space fraction. When measured at baseline, VR was found to be independently associated with increased risk of mortality in observational and randomized controlled trial cohorts of ARDS. VR provides a simple and pragmatic solution of monitoring impaired ventilation in mechanically ventilated patients and may be used to stratify patients with ARDS.
Gas exchange consists of two essential functions, oxygenation and ventilation. The former, as quantified by the PaO2/FiO2 ratio, is the primary method of diagnosing and stratifying patients in acute respiratory distress syndrome (ARDS). The latter is best monitored by measuring pulmonary dead space fraction (Vd/Vt). Considerable evidence supports pulmonary dead space fraction as an independent predictor of mortality in ARDS (1, 2). Yet, it is seldom measured or used in clinical practice. Primarily, this is because of the lack of a simple bedside index for monitoring dead space and the additional expense associated with measuring it in the critical care setting (3). Consequently, failure of ventilation, despite its importance, is completely excluded as a stratifying variable in ARDS.
To address this issue, we described ventilatory ratio (VR), a simple bedside index of impaired efficiency of ventilation (4). VR is described as:
| (1) |
where emeasured is the measured minute ventilation (ml/min), PaCO2measured is the measured arterial pressure of carbon dioxide (mm Hg), epredicted is the predicted minute ventilation calculated as predicted body weight × 100 (ml/min) (5), and PaCO2ideal is the expected arterial pressure of carbon dioxide in normal lungs if ventilated with the predicted minute ventilation. PaCO2ideal is set as 37.5 mm Hg (5 kPa) for all patients (4).
VR is a unitless ratio, and a value approximating 1 would represent normal ventilating lungs. Physiologic analysis shows that VR is influenced both by pulmonary dead space fraction and the co2 (ml/min) (Supplementary Equations 2–7). An elevated value of VR would represent either increased pulmonary dead space, increased co2 or both. Previously, we reported that VR is an independent predictor of mortality in two distinct ARDS randomized controlled trial populations, but pulmonary dead space fraction was not measured in either of those studies (6, 7). Thus, the relationship of VR with pulmonary dead space fraction in ARDS remains unknown. Hypothetically, a significant association between the two variables would suggest that VR could be used as a practical bedside marker of impaired ventilation.
There were two main objectives for this study. First, we tested the relationship of VR with measured pulmonary dead space in ARDS. Second, in the same population, we tested whether VR predicts outcome independent of other known prognostic factors in a large observational cohort. Finally, in two separate NHLBI-supported randomized clinical trials, we sought to replicate the findings of the primary dataset.
Methods
Study Population for Primary Analysis
Data were extracted from an ongoing ARDS quality assurance database that has been accrued prospectively at the Zuckerberg San Francisco General Hospital. The database was initiated in September 2000 following publication of the landmark NHLBI ARDS Clinical Trials Network study (8), and the decision to implement standardized ventilator protocols for patients with ARDS. Volume assist-control ventilation was the primary ventilator mode used when implementing these protocols.
The Zuckerberg San Francisco General Hospital is a county hospital and Level-1 trauma center affiliated with the University of California, San Francisco and has multiple ICUs covering medical, surgical, and trauma patients. Patients were diagnosed with ARDS using the American-European Consensus Conference criteria for ARDS (see detailed protocol in the online supplement) (9). Local institutional review board granted a consent-waiver for the review of deidentified data for research purposes.
Measurements
An arterial blood gas was drawn with simultaneous recording of mean expired CO2 (PCO2) as measured by volumetric capnography (NICO monitor, Philips/Respironics) and other ventilatory variables, as described in our previous studies (10). Pulmonary dead space fraction was measured using the Enghoff-Bohr equation (Vd/Vt = [PaCO2 −PCO2]/PaCO2). Only the first measurements from each patient were used for analysis in this study because repeated dead space measurements were inconsistently recorded. Details of the study protocol are outlined in the online supplement.
Additionally, at the time of PCO2 measurement, CO2 elimination (co2) was also measured. co2 as measured by volumetric capnography is the volume of expired CO2 per minute. In steady state, this is considered to represent the volume of CO2 production per minute. Both arterial blood gases and volumetric capnography measurements were obtained after a period of stabilization on a prescribed ventilator setting and after a period of rest where patients were free from nursing and/or physiotherapy interventions.
Predicted body weight was calculated using the NHLBI ARDS network formula (see online supplement). Values for minute ventilation (e) and PaCO2 at the time of PCO2 measurements were used to calculate VR using Equation 1. Respiratory variables recorded simultaneously were used to calculate PaO2/FiO2, driving pressure (plateau pressure−positive end-expiratory pressure [PEEP]), and oxygenation index [OI = (FiO2 × mean airway pressure × 100)/PaO2]. In addition to Vd/Vt and VR, a third index of ventilatory insufficiency used by the Berlin consensus group called corrected minute ventilation (VE-corr = e ×PaCO2/40) was also calculated (11).
Verification Analyses
To verify the findings in the above dataset, we performed secondary analyses of two multicenter NHLBI Network ARDS trials. First, to test the correlation of Vd/Vt with VR, we analyzed a substudy of the clinical trial of aerosolized albuterol for ARDS (ALTA) (12). In that substudy, serial Vd/Vt and co2 were measured over 6 days in 126 patients. To replicate the measurements in the primary dataset, only those patients that had these measurements taken within 12 hours of recruitment into the trial were included for analysis. As with primary dataset, arterial blood gas measurements and ventilatory variables were used to calculate VR. In addition, co2 measurements were used to test its correlation with VR.
In a second randomized controlled trial (FACTT), we tested the prognostic ability of VR to predict outcomes in ARDS. The FACTT trial enrolled 1,000 patients with ARDS between 2000 and 2005. Patients were randomized to receive either liberal or conservative fluid therapy. No dead space measurements were available from this trial. Measurements labeled “baseline ventilator setting” in the FACTT dataset were used to calculate VR because these data were most complete for E and PaCO2, and they were temporally closest to the primary dataset. Details of both these trials can be found in the original published reports (13, 14).
Finally, to test prognostic utility of VR in ARDS, we undertook a post hoc analysis such that the primary and FACTT datasets were stratified using a simple cutoff for VR. The cohorts were stratified into “low VR” if VR was less than two and “high VR” if VR was greater than or equal to two. A value of two was selected for practical purposes and approximated to with median values in this and our previous studies (7). Additionally, the value of two also approximated closely to the most accurate cutoff for VR (1.96) derived from the univariate logistic regression model using the Youden index (analysis not shown) in the primary dataset. Differences in clinical outcomes were compared between the high- and low-VR groups. All patients were included in the main stratified analyses; however, for secondary analyses in ARDS severity subgroups, mild ARDS patients were excluded for two reasons. First, in the primary dataset, the incidence of high VR in mild ARDS was low (n = 18). Second, previous analyses of part of this dataset showed that there was no significant difference in Vd/Vt between survivors and nonsurvivors with mild ARDS (10).
Statistical Analyses
Parametric data are presented as mean ± SD and nonparametric data are presented as median (interquartile range) depending on the distribution of variables. For intergroup comparisons, Student’s t test was used for parametric data and Mann-Whitney test was used to compare nonparametric data. One-way ANOVA was used to compare means across multiple groups. Categorical data were compared using chi-square test. For ordinal data, chi-square test for trends was used to test for association of dependent variable across ordinal groups.
Previous in silico analysis of the relationship between VR and Vd/Vt showed a curvilinear relationship between the variables (6). To test the correlation between these variables, a linear transformation of Vd/Vt was performed by using the following formula: 1/[1 − (Vd/Vt)] (see Supplementary Equation 7) (6). A modified Pearson correlation coefficient was calculated to test the linear relationship between 1/[1 − (Vd/Vt)] and VR (15). The modification was necessary to correct for mathematical coupling between the two variables, given PaCO2was used to calculate both. Spearman correlation coefficient was used to examine the association between CO2 production (co2) and VR. To test for differences in Vd/Vt with increased values of VR, we split the population into quartiles of VR and plotted the distribution of Vd/Vt within each quartile.
For prognostic analysis, we used hospital mortality and ventilator-free days as the main outcome measures. For the primary dataset, mortality was defined as in-hospital mortality, whereas in the FACTT trial, it was defined as 60-day in-hospital mortality. In FACTT, patients discharged from hospital before Day 60 were assumed to be alive at end of the study (14). Ventilator-free days was defined as days free of invasive ventilatory support up to Day 28. Death before this time point was considered as zero ventilator-free days.
Aside from the Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) score, only respiratory variables associated with increased mortality in prior studies were chosen as predictors variables for logistic regression modeling (16–18). Odds ratios (OR) were calculated per unit change of variables, except for Vd/Vt, for which OR were calculated per increase of 0.05 (Equation 1). For multivariate analysis, VR and VE-corr were the primary predictor variables and served as the base models. A combination of backward stepwise procedure and omission of highly colinear variables were used to select variables for adjustment to the base model (see online supplement). All analyses were performed using RStudio version 1.0.143 software (The R project for statistical computing).
Results
From the primary dataset, of the 1,017 patients screened, 685 were managed using ARDS network protocol and considered for the study. Of these, 520 study patients had dead-space, co2, and height measured on the day of ARDS diagnosis and were included for analysis. This sample represented 76% of the patients who had dead-space measurements made between March 2010 and April 2016 (see Figure E1 in the online supplement). There were no differences of note in demographics, clinical characteristics, or mortality between the studied population and the missing data (see Table E1). Measurements of Vd/Vt were made within 12 hours of protocol initiation. Ninety-seven percent of the measurements occurred on the day of ARDS onset. An additional 2% of measurements were done within 48 hours of ARDS onset, and the remainder within 96 hours. Table 1 summarizes demographics and baseline characteristics of the population stratified by vital status at discharge.
Table 1.
Baseline Population Characteristics with Stratification by ICU Mortality
| Population (n = 520) | Survivors (n = 317) | Nonsurvivors (n = 203) | P Value | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 135 (26) | 87 (27) | 48 (24) | 0.755* |
| Male | 376 (72) | 225 (71) | 152 (75) | |
| Transgender | 8 (2) | 5 (2) | 3 (1) | |
| Ethnicity, n (%) | ||||
| White | 204 (39) | 126 (40) | 78 (38) | 0.845* |
| Hispanic | 116 (22) | 73 (23) | 43 (21) | |
| Black | 97 (19) | 61 (19) | 36 (18) | |
| Asian | 94 (18) | 52 (16) | 42 (21) | |
| Other | 9 (2) | 5 (2) | 4 (2) | |
| Age, yr | 52 ± 16 | 49 ± 16 | 56 ± 16 | <0.001 |
| Weight, kg | 82 ± 24 | 84 (25) | 79 (23) | 0.042 |
| Height, cm | 169 ± 10 | 170 ± 10 | 169 ± 10 | 0.203 |
| BMI, kg/m2 | 29 ± 8 | 29 ± 8 | 28 ± 8 | 0.165 |
| APACHE II score | 23 (18–31) | 21 (16.25–26.75) | 29 (23–35) | <0.001† |
| ARDS risk factors, n (%) | ||||
| Pneumonia | 142 (27) | 95 (30) | 47 (23) | 0.007* |
| Sepsis | 110 (21) | 62 (20) | 48 (24) | |
| Trauma | 108 (21) | 77 (24) | 31 (15) | |
| Aspiration | 98 (19) | 52 (16) | 46 (23) | |
| Other | 62 (12) | 31 (10) | 31 (15) | |
| ARDS category, n (%) | ||||
| Mild | 79 (15) | 63 (20) | 16 (8) | <0.001* |
| Moderate | 243 (47) | 164 (52) | 79 (39) | |
| Severe | 198 (38) | 90 (28) | 108 (53) | |
| Systolic blood pressure, mm Hg | 83 ± 27 | 84 ± 24 | 81 ± 31 | 0.177 |
| Bicarbonate, mEq/L | 21 ± 6 | 22 ± 6 | 20 ± 7 | <0.001 |
| Creatinine, mg/dl | 1.6 ± 1.6 | 1.6 ± 1.7 | 1.8 ± 1.5 | 0.163 |
| Bilirubin, mg/dl | 1.8 ± 1.9 | 1.2 ± 1.5 | 2.7 ± 4.2 | <0.001 |
| White cell count, cells/L | 14 ± 11 | 14 ± 10 | 13 ± 11 | 0.341 |
| Platelets | 180 ± 124 | 202 ± 125 | 146 ± 113 | <0.001 |
| pH | 7.26 ± 0.14 | 7.29 ± 0.13 | 7.22 ± 0.14 | <0.001 |
| Urine output, L/24 h | 2.2 ± 1.9 | 2.4 ± 1.7 | 1.9 ± 2.2 | 0.006 |
| Outcomes | ||||
| Hospital mortality | 203 (39%) | |||
| Ventilator-free days | 5 (0–21) | 19 (10.25–23) | 0 (0–0) | <0.001* |
Definition of abbreviations: APACHE = Acute Physiologic Assessment and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; BMI = body mass index.
P values were determined by Student’s t test unless stated otherwise stated.
Chi-square test.
Mann-Whitney test.
The mean APACHE II score for the primary dataset was 23 (18–31). A total of 203 (39%) patients died before hospital discharge. The mean PaO2/FiO2 was 133 ± 60 mm Hg, and mean tidal volume was 6.4 ± 1.5 ml/kg. Mean Vd/Vt was 0.57 ± 0.17, and mean VR was 1.9 ± 0.6. Table E2 summarizes the distribution of respiratory variables at the time of volumetric capnography measurements with stratification by in-hospital mortality.
VR Correlates with Dead Space but Not with CO2 Production co2
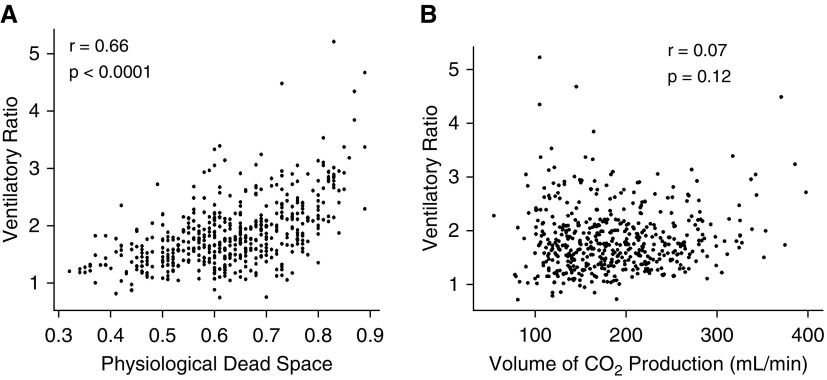
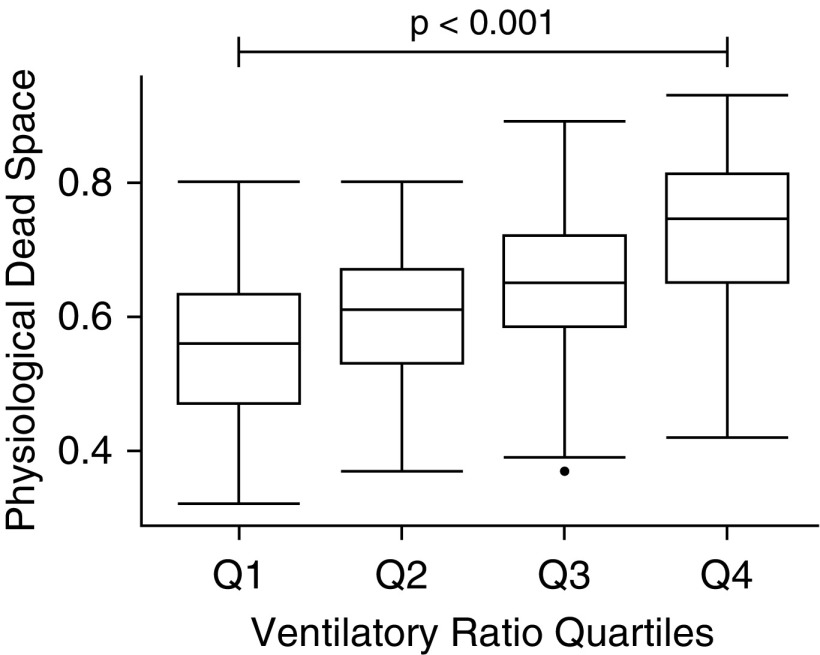
There was moderate positive linear correlation between VR and 1/[1 − (Vd/Vt)] (modified r = 0.66; P < 0.0001) (Figure 1A). The uncoupled slope, corrected for the mathematically coupled variable (PaCO2) was highly significant suggesting a true relationship between the two variables (P < 0.0001). There was no correlation observed between VR and co2 (r = 0.07; P = 0.12) (Figure 1B). When split into ordinal quartiles of VR, Vd/Vt was significantly higher in each sequential VR quartile (P < 0.001) (Figure 2).
Figure 1.
(A) Correlation between physiologic dead space and ventilatory ratio in the primary dataset. (B) Correlation between CO2 production and ventilatory ratio in the primary dataset.
Figure 2.
Physiologic dead space in ordinal groups of ventilatory ratio in the primary dataset. Q1, <1.44; Q2, ≥1.44–<1.76; Q3, ≥1.76–< 2.13; Q4, >2.13. P value represents ANOVA test.
VR Was a Predictor of Hospital Mortality in Multivariate Analysis
VR was higher in nonsurvivors compared with survivors (2.02 ± 0.8 vs. 1.75 ± 0.5; P < 0.001) (see Table E2). In univariate logistic regression analysis, increasing VR was associated with increased odds for hospital mortality (OR, 2.07; confidence interval [CI], 1.53–2.83). PaO2/FiO2, OI, driving pressure, PEEP, Vd/Vt, and VE-corr were all also associated with increased risk of mortality in univariate analysis (see Table E3). In multivariate analysis, VR was independently associated with increased odds of mortality after adjusting for PaO2/FiO2 ratio and driving pressure (OR, 1.51; 95% CI, 1.09–2.12), OI and driving pressure (OR, 1.44; 95% CI, 1.01–2.07), and after adjusting for APACHE II score (OR, 1.59; 95% CI, 1.15–2.32) (Table 2). In contrast, VE-corr was no longer associated with increased risk of mortality after adjusting for PaO2/FiO2 ratio and driving pressure (see Table E3). Likewise, VE-corr was not associated with increased risk of mortality after adjusting for either OI or APACHE II score (data not shown). Interestingly, in multivariate analysis neither PaCO2 (OR, 1.01; 95% CI, 0.98–1.03) nor minute ventilation (OR, 1.04; 95% CI, 0.98–1.12) were associated with increased risk of mortality after adjusting for PaO2/FiO2.
Table 2.
Odds Ratio for ICU Mortality Using Multivariate Logistic Regression with Ventilatory Ratio as the Base Model in the Primary Dataset
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Univariate analysis | |||
| Ventilatory ratio (base model) | 2.07 | 1.53–2.85 | <0.001 |
| Multivariate analysis | |||
| Base model + PF-ratio | 1.60 | 1.16–2.24 | 0.005 |
| Base model + PF-ratio + PEEP | 1.48 | 1.06–2.08 | 0.024 |
| Base model + PF-ratio + DP | 1.51 | 1.09–2.12 | 0.015 |
| Base model + OI | 1.42 | 1.01–2.03 | 0.045 |
| Base model + OI + PEEP | 1.42 | 1.00–2.02 | 0.049 |
| Base model + OI + DP | 1.44 | 1.01–2.07 | 0.047 |
| Base model + APACHE II score | 1.59 | 1.15–2.32 | 0.004 |
Definition of abbreviations: APACHE = Acute Physiologic Assessment and Chronic Health Evaluation; DP = driving pressure; OI = oxygenation index; PEEP = peak end-expiratory pressure; PF-ratio = PaO2/FiO2 ratio.
Odds ratios are per unit change of ventilatory ratio.
VR Increases with Disease Severity in ARDS
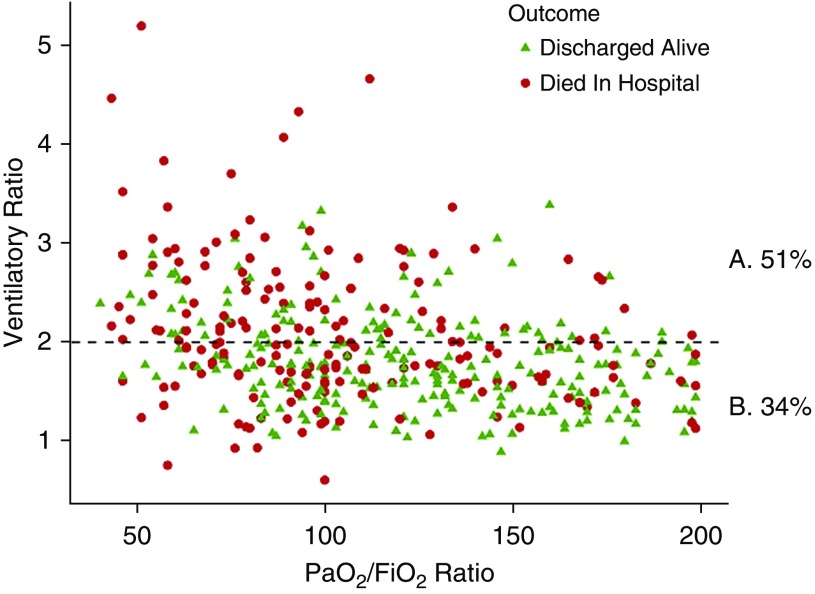
Mean values of VR were significantly higher in more severe groups of ARDS: mild 1.5 ± 0.5, moderate 1.8 ± 0.5, and severe 2.1 ± 0.7 (ANOVA, P < 0.001) (see Figure E2). Worsening hypoxemia (PaO2/FiO2) was associated with worsening impaired ventilation as measured by VR (Figure 3). There was a weak but significant negative correlation between VR and PaO2/FiO2 (Pearson, r = −0.37; CI, −0.29 to −0.44).
Figure 3.
Ventilatory ratio (VR) plotted against PaO2/FiO2 ratio in moderate and severe acute respiratory distress syndrome in the primary dataset. Percentages represent mortality rates in the population split into high-VR (VR ≥2) and low-VR (VR <2) groups. (A) Percentage mortality in high-VR group. (B) Percentage mortality rate in the low-VR group (horizontal dashed line = 2; P < 0.001).
Verification Studies Validate Findings of the Primary Study
Requisite data from the NHLBI ALTA trial were available for 94 patients at baseline. As in the primary dataset, a moderate positive correlation was observed between VR and 1/[1 − (Vd/Vt)] (r = 0.64; P < 0.0001). No correlation was observed between VR and co2 (r = 0.14; P = 0.19) (see Figure E3). Vd/Vt was significantly higher in sequential quartiles of VR (P < 0.0001) (see Figure E4).
For the second verification cohort, complete data were available for 946 patients in the FACTT trial. VR was independently associated with increased risk of mortality in both unadjusted analysis and in models adjusted for a combination of PaO2/FiO2, OI, PEEP, and driving pressure (see Table E5). VR was also associated with increased odds of mortality after adjusting for APACHE III score. Interestingly, in this population, within the described model, PaO2/FiO2 ratio was no longer independently associated with increased odds of mortality (data not shown).
In both ALTA and FACTT, VR was significantly higher in more severe forms of ARDS (P = 0.001), and mortality was significantly higher in ordinal quartiles of VR (P < 0.001). As with the primary dataset, in FACTT, there was modest negative correlation between VR and PaO2/FiO2 ratio (r = −0.32; P < 0.001).
Stratifying Outcomes by VR: High VR versus Low VR
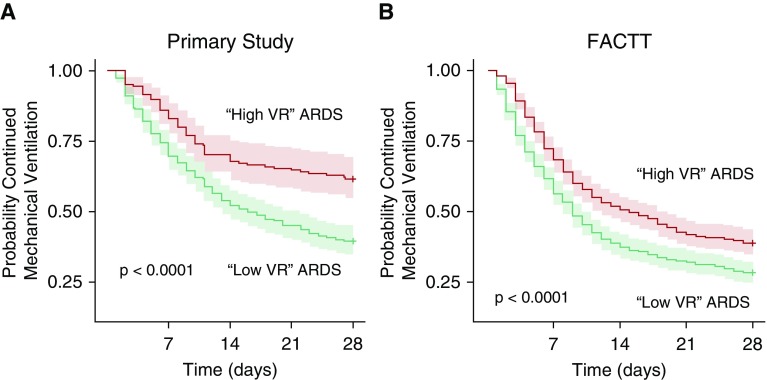
Using this simple stratification strategy, we found mortality was higher in the high-VR compared with the low-VR group (46% vs. 33%; P = 0.003). This difference in mortality was more pronounced in patients with moderate and severe ARDS (51% vs. 34%; P < 0.001) (Table 3) and in severe ARDS (61% vs. 46%) (Table 3). The risk of prolonged mechanical ventilation was also significantly higher in high-VR ARDS (Figure 4A).
Table 3.
Moderate and Severe ARDS Split into Binary Groups according to VR: Data from the Primary Dataset
| ARDS Groups | N (Total) | ARDS with Low VR (<2) [n (%)] |
ARDS with High VR (≥2) [n (%)] |
P Value | ||
|---|---|---|---|---|---|---|
| n | Hospital Mortality | n | Hospital Mortality | |||
| All patients | 520 | 255 | 83 (33) | 255 | 117 (46) | 0.003 |
| Moderate + severe ARDS | 433 | 217 (50) | 74 (34) | 216 (50) | 110 (51) | <0.001 |
| Moderate ARDS | 238 | 136 (57) | 37 (27) | 102 (43) | 41 (40) | <0.001 |
| Severe ARDS | 195 | 81 (42) | 37 (46) | 114 (58) | 69 (61) | <0.001 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; N = number of observations; VR = ventilatory ratio.
P values represent chi-square test.
“ARDS with Low VR” refers to patients with VR <2. “ARDS with High VR” refers to VR ≥2.
Figure 4.
Probability of ventilator-free breathing to 28 days in acute respiratory distress syndrome (ARDS) population according to ventilatory ratio (VR) classification; patients with ARDS with low VR had a VR <2, and patients with ARDS with high VR had a VR ≥2. (A) Data from the primary dataset. (B) Data from the FACTT dataset. P values represent Cox proportional hazards test comparing low-VR ARDS and high-VR ARDS. FACTT = Fluid and Catheter Treatment Trial.
These findings were also replicated in the FACTT dataset, albeit with lower overall mortality and lower mechanical ventilation duration. Mortality was higher in ARDS with high VR compared with low VR (30% vs. 22%; P = 0.007) (Table 4). Similarly, the risk of prolonged mechanical ventilation was also significantly higher in high-VR patients in the FACTT dataset (Figure 4B).
Table 4.
Moderate and Severe ARDS Split into Binary Groups according to VR: Data from the FACTT Dataset
| ARDS Groups | N (Total) | ARDS with Low VR (<2) [n (%)] |
ARDS with High VR (≥2) [n (%)] |
P Value | ||
|---|---|---|---|---|---|---|
| n | 60-d Mortality | n | 60-d Mortality | |||
| All patients | 946 | 477 | 107 (22) | 469 | 140 (30) | 0.011 |
| Moderate + severe ARDS | 731 | 326 (45) | 70 (21) | 405 (55) | 123 (30) | 0.007 |
| Moderate ARDS | 491 | 276 (56) | 60 (22) | 225 (44) | 52 (24) | 0.521 |
| Severe ARDS | 240 | 76 (32) | 20 (26) | 164 (68) | 61 (37) | 0.097 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; FACTT = Fluid and Catheter Treatment Trial; N = number of observations; VR = ventilatory ratio.
P values represent chi-square test.
“ARDS with Low VR” refers to patients with VR <2. “ARDS with High VR” refers to VR ≥2.
Discussion
The main results of this study can be summarized as follows. VR positively correlated with pulmonary dead space in patients with ARDS. Higher values of VR were associated with higher pulmonary dead space. In multivariate analysis, VR was independently associated with increased risk of hospital mortality after adjusting for a combination of markers of mechanical support and hypoxemia (Table 2; see Table E5). VR also remained associated with increased risk of mortality after adjusting for APACHE scores (Table 2; see Table E5). Furthermore, we demonstrated the prognostic utility of VR at baseline in an observational and a randomized controlled trial cohort. The findings of this study confirm the hypothesis that VR could be used as a simple bedside index of impaired ventilation and at baseline it is a useful prognostic physiologic marker in ARDS.
Sixteen years have passed since the study from Nuckton and colleagues (1) showed pulmonary dead space fraction to be an independent predictor of mortality in ARDS. Several studies since that time have reinforced these findings (12, 19, 20). However, measurement of pulmonary dead space and impaired ventilation remain largely unused in either clinical practice or as a research variable. Our current approach to stratification in ARDS neglects this major physiologic abnormality. In the presented study, analysis of the FACTT dataset showed that the PaO2/FiO2 was no longer an independent predictor of mortality when adjusted for VR. These findings add to concerns, raised by others (21, 22), that the PaO2/FiO2 has limitations as the primary stratifying variable in ARDS.
As the severity of ARDS increases, alveolar units with heterogeneous ventilation/perfusion mismatch lead to hypoxemia and hypercarbia, in part explaining the negative correlation between VR and PaO2/FiO2. Mostly, however, ventilation and oxygenation are physiologically distinct processes and hence the observed correlation between the two variables was weak (Figure 3), indicating that the prognostic and pathophysiologic information provided by VR cannot be extracted from PaO2/FiO2 ratio alone and vice versa.
An advantage of VR is that it can be easily calculated using routine bedside variables. Its value reflects the ability of the lungs to excrete carbon dioxide adequately. As highlighted in Supplementary Equation 7, VR is determined by both Vd/Vt and CO2 production (co2). VR is not a direct measure of Vd/Vt and cannot be used to estimate its value. The relationship between the two variables shown in this study does, however, indicate that VR, like Vd/Vt, may be a useful bedside index to monitor impaired ventilation in critically ill patients. These findings in ARDS are similar to our previous findings in patients with acute hypoxemic respiratory failure (23). Although the correlation coefficient was lower in the current study (r = 0.66 and r = 0.64 versus r = 0.71), the greatly enhanced sample size in the presented study adds to the strength and generalizability of the observed relationship of the variables.
Conversely, the lack of correlation between VR and co2 indicates that compared with Vd/Vt, co2 is a less influential determinant of VR. Carbon dioxide production is an important factor in CO2 homeostasis in critically ill patients and impacts any measure of impaired ventilation, be it VR or Vd/Vt. Factors influencing the basal metabolic rate, such as feeding and sepsis, may alter co2 (24). Additionally, nursing interventions, physiotherapy, and resetting the ventilator are also known to affect co2, albeit transiently (25).
Under normal functioning conditions, in response to increases in co2 the lungs can increase CO2 elimination by increasing minute ventilation. In ARDS, two factors limit these responses. First, the ability to increase minute ventilation may be impaired or intentionally limited to achieve lung protection. Second, CO2 eliminating capacity is impaired by ventilation-perfusion mismatch. The precise influence of co2 on ventilatory demands in critically ill patients is unknown. Ravenscraft and colleagues (26) found that dead space was the primary determinant of excessive minute ventilation in mechanically ventilated ICU patients. Specifically, the authors found that in early ARDS the contribution of co2 to excess minute ventilation was minimal. These findings are similar to the data presented in this study.
Regardless, co2 would undoubtedly account for some of the observed discrepancies between VR and Vd/Vt. Given that VR is adjusted for individual height, some of the natural variance in co2 observed across populations is incorporated in the ratio. Individuals with the same dead space fraction may, however, have differing VR depending on their co2. It is unlikely that the influence of co2 is in unison for both VR and Vd/Vt thereby leading to some of the observed variance between the two variables.
A second potential source of variance between VR and Vd/Vt is that VR assumes a linear relationship between E and PaCO2. The physiologic relationship of tidal volume to PaCO2 is, however, known to be asymptotic. Moreover, for the same minute ventilation, the ratio of tidal volume and respiratory rate influences Vd/Vt. For example, assuming the anatomic portion of dead space is constant, lowering tidal volumes and increasing the respiratory rate to maintain the same minute ventilation, would increase Vd/Vt, yet transiently VR would remain unchanged. Any such increases in Vd/Vt over time would lead to decreased CO2 elimination and an increase in PaCO2, which would be captured by VR. This limitation of VR underlines the importance of achieving a period of stability on the prescribed ventilatory settings before calculating VR for optimal interpretation of its value.
Other sources of variance between VR and Vd/Vt may be attributed to the influence of the cardiovascular system on Enghoff-Bohr Vd/Vt. Both shunt and low cardiac output states are known determinants of Vd/Vt. The approximation of arterial PCO2 to alveolar PCO2 (see Supplementary Equation 6) is an assumption that is common to both Enghoff-Bohr Vd/Vt and VR. Consequently, increasing shunt fraction leads to an increase in venous admixture leading to elevated PaCO2. This results in an increase in Vd/Vt that is not directly a factor of impaired ventilation (3). This phenomenon will also be observed in VR. It is improbable, however, that increases in VR would be proportional to Vd/Vt, leading to some variance between the two variables.
Low cardiac output can also affect Vd/Vt (27). Decreased pulmonary blood flow in low cardiac output states lead to reduced alveolar CO2 delivery and decreased CO2 elimination. This in turn results in lower PCO2 and consequently elevated Vd/Vt that is once again a feature of perfusion rather than ventilation per se. Unlike shunt, however, it is unlikely that this influence of low cardiac states on gas exchange would be captured by VR. The contributions of perfusion on Vd/Vt is more likely to manifest in instances when Vd/Vt is high (>0.75) and may in part explain why the correlation with VR is not as strong in this range (Figure 1A). Interestingly, in low cardiac output states it is conceivable that VR may be a more accurate measure of impaired ventilation than Vd/Vt; however, this theory needs prospective evaluation.
Despite the outlined sources of variance, the presented data indicate that VR and pulmonary dead space fraction are closely associated variables. It is, therefore, not surprising that VR, like pulmonary dead space, is a predictor of outcome in ARDS. The findings in this study are consistent with our previous study, which also showed VR to be associated with increased risk of mortality in NHLBI ARDS Network trials (7). Furthermore, these findings are now also generalizable to observational cohorts of ARDS.
The authors of the Berlin definition of ARDS tried the study importance of impaired ventilation. They used VE-corr as a marker of impaired ventilation in ARDS and found no prognostic validity (11, 28). In the present study, and as alluded to by the Berlin investigators, corrected minute ventilation was no longer a predictor of outcome once adjusted for PaO2/FiO2. These findings suggest VR outperforms corrected minute ventilation. Other models have also been described to estimate pulmonary dead space (29). In comparison with VR, the complexity inherent to these multivariate models makes them unappealing for clinical use and negates any value derived from stronger correlation with measured pulmonary dead space fraction. The scarcity of adoption of these models and measured pulmonary dead space in clinical practice underlines the importance of the pragmatism and simplicity of VR, albeit with known inherent limitations.
When conducting research in heterogeneous syndromes, such as ARDS, prognostic enrichment need not be limited to biologic measurements. Practical physiologic methods, as offered by VR, may be as useful (30). Recent studies suggest stratifying by severity of hypoxemia is a useful strategy in ARDS research (31, 32). The data from this study similarly indicate that incorporating VR in screening for clinical trials may facilitate prognostic enrichment and permit a more physiologically meaningful approach to patient selection (Table 2 and Figure 3).
Furthermore, in contrast to PaO2/FiO2 (21), impaired ventilation, as measured by pulmonary dead space fraction, is known to be a more reliable variable that retains its prognostic value over time (20, 33). Beyond prognostication, measuring pulmonary dead space fraction has also been shown to be a useful variable when optimizing PEEP, predicting failure of extubation, and in monitoring response to prone-positioning (34–38). Assuming that ventilatory strategies remain unchanged, VR could be a more stable prognostic variable than PaO2/FiO2 and its delta values within individuals may be used as a tool to monitor impaired ventilation following interventions. Further studies that test the prognostic utility of VR over differing time-points, in various modes of ventilation, and following therapeutic interventions are required to test these hypotheses. The simplicity of calculating VR may increase the feasibility of studies that examine the role of impaired ventilation in the clinical setting.
To demonstrate the value of VR as a stratifying variable, we created a simple schematic that further subdivides the Berlin definition into ARDS with low VR, where VR was less than two, and ARDS with high VR, where VR was greater than or equal to two (Tables 3 and 4 and Figure 4). In the primary dataset, using this simple stratification, mortality was higher in the high-VR group in the total population and in moderate and severe ARDS. In FACTT, mortality in the high-VR group was also significantly higher. Within ARDS severity groups, however, there was a trend toward higher mortality but these were not statistically significant.
In part, these findings may be explained by the lower overall mortality in FACTT compared with the primary dataset. These findings are consistent with an established trend toward better clinical outcomes in randomized controlled trials compared with observational studies because of more stringent inclusion criteria in the former (39). Another limitation in the FACTT dataset that may explain these findings is that arterial PCO2 measurements used to calculate VR may not be contemporaneous to the minute ventilation recordings in all patients. The original trial instructions stated, “If blood gases are not available within reference period, use values closest to reference period on same calendar date.”
This study has other limitations. Although data were accrued prospectively, all datasets were interrogated retrospectively. In addition, only a single time-point was used to calculate VR, limiting interpretation of its value to baseline prognostication. Furthermore, the correction used for the mathematical coupling when testing for correlation between VR and pulmonary dead space fraction is a major limitation for extrapolating these data to delta values in individuals.
Another limitation of the study is that although measurements of dead space and VR in this study were made with the patients in mandatory modes of ventilation, it was not possible to ascertain whether additional spontaneous respiratory effort was being made at the time of these measurement. Spontaneous breaths may have led to dynamic changes in both VR and pulmonary dead space, which may have led to further variance between the two variables (40). Data on the use of paralytic agents and levels of sedation were also unavailable and may have influenced ventilatory impairment. VR also remains untested in patients ventilated in pressure support modes. Prospective studies are needed to address these limitations.
In summary, VR is a simple and physiologically grounded method to assess impaired ventilation at the bedside. VR correlates with pulmonary dead space and higher values are associated with increased risk of mortality. VR shows promise as a simple method to stratify ARDS patients into clinically meaningful subgroups and may be a useful tool for prognostic enrichment in future clinical trials.
Supplementary Material
Footnotes
Supported by the NHLBI (HL140026, C.S.C.; HL51856, M.A.M.) and NIH (K23HL133489, J.R.B.).
Author Contributions: P.S., M.A.M., and R.H.K. were involved in study conception, design, data analysis, and data interpretation. R.H.K. and K.H. were involved in data collection and cleaning. C.S.C., J.R.B., and N.S. were involved in study conception and data interpretation. P.S., C.S.C., J.R.B., N.S., K.H., M.A.M., and R.H.K. were involved in manuscript preparation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0692OC on September 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 2.Lucangelo U, Bernabè F, Vatua S, Degrassi G, Villagrà A, Fernandez R, et al. Prognostic value of different dead space indices in mechanically ventilated patients with acute lung injury and ARDS. Chest. 2008;133:62–71. doi: 10.1378/chest.07-0935. [DOI] [PubMed] [Google Scholar]
- 3.Sinha P, Flower O, Soni N. Deadspace ventilation: a waste of breath! Intensive Care Med. 2011;37:735–746. doi: 10.1007/s00134-011-2194-4. [DOI] [PubMed] [Google Scholar]
- 4.Sinha P, Fauvel NJ, Singh S, Soni N. Ventilatory ratio: a simple bedside measure of ventilation. Br J Anaesth. 2009;102:692–697. doi: 10.1093/bja/aep054. [DOI] [PubMed] [Google Scholar]
- 5.Radford EP., Jr Ventilation standards for use in artificial respiration. J Appl Physiol. 1955;7:451–460. doi: 10.1152/jappl.1955.7.4.451. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, Singh S, Hardman JG, Bersten AD, Soni N Australia and New Zealand Intensive Care Society Clinical Trials Group. Evaluation of the physiological properties of ventilatory ratio in a computational cardiopulmonary model and its clinical application in an acute respiratory distress syndrome population. Br J Anaesth. 2014;112:96–101. doi: 10.1093/bja/aet283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha P, Sanders RD, Soni N, Vukoja MK, Gajic O. Acute respiratory distress syndrome: the prognostic value of ventilatory ratio--a simple bedside tool to monitor ventilatory efficiency. Am J Respir Crit Care Med. 2013;187:1150–1153. doi: 10.1164/rccm.201211-2037LE. [DOI] [PubMed] [Google Scholar]
- 8.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.Kallet RH, Zhuo H, Ho K, Lipnick MS, Gomez A, Matthay MA. Lung injury etiology and other factors influencing the relationship between dead-space fraction and mortality in ARDS. Respir Care. 2017;62:1241–1248. doi: 10.4187/respcare.05589. [DOI] [PubMed] [Google Scholar]
- 11.Wexler HR, Lok P. A simple formula for adjusting arterial carbon dioxide tension. Can Anaesth Soc J. 1981;28:370–372. doi: 10.1007/BF03007805. [DOI] [PubMed] [Google Scholar]
- 12.Kallet RH, Zhuo H, Liu KD, Calfee CS, Matthay MA National Heart Lung and Blood Institute ARDS Network Investigators. The association between physiologic dead-space fraction and mortality in subjects with ARDS enrolled in a prospective multi-center clinical trial. Respir Care. 2014;59:1611–1618. doi: 10.4187/respcare.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 14.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 15.Stratton HH, Feustel PJ, Newell JC. Regression of calculated variables in the presence of shared measurement error. J Appl Physiol (1985) 1987;62:2083–2093. doi: 10.1152/jappl.1987.62.5.2083. [DOI] [PubMed] [Google Scholar]
- 16.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 17.Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balzer F, Menk M, Ziegler J, Pille C, Wernecke KD, Spies C, et al. Predictors of survival in critically ill patients with acute respiratory distress syndrome (ARDS): an observational study. BMC Anesthesiol. 2016;16:108. doi: 10.1186/s12871-016-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:149–158. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 20.Raurich JM, Vilar M, Colomar A, Ibáñez J, Ayestarán I, Pérez-Bárcena J, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care. 2010;55:282–287. [PubMed] [Google Scholar]
- 21.Villar J, Blanco J, del Campo R, Andaluz-Ojeda D, Diaz-Dominguez FJ, Muriel A, et al. Spanish Initiative for Epidemiology S, Therapies for AN. Assessment of PaO(2)/FiO(2) for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open. 2015;5:e006812. doi: 10.1136/bmjopen-2014-006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allardet-Servent J, Forel JM, Roch A, Guervilly C, Chiche L, Castanier M, et al. FIO2 and acute respiratory distress syndrome definition during lung protective ventilation. Crit Care Med. 2009;37:202–207. doi: 10.1097/CCM.0b013e31819261db. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Fauvel NJ, Singh P, Soni N. Analysis of ventilatory ratio as a novel method to monitor ventilatory adequacy at the bedside. Crit Care. 2013;17:R34. doi: 10.1186/cc12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liposky JM, Nelson LD. Ventilatory response to high caloric loads in critically ill patients. Crit Care Med. 1994;22:796–802. doi: 10.1097/00003246-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Weissman C, Kemper M, Damask MC, Askanazi J, Hyman AI, Kinney JM. Effect of routine intensive care interactions on metabolic rate. Chest. 1984;86:815–818. doi: 10.1378/chest.86.6.815. [DOI] [PubMed] [Google Scholar]
- 26.Ravenscraft SA, McArthur CD, Path MJ, Iber C. Components of excess ventilation in patients initiated on mechanical ventilation. Crit Care Med. 1991;19:916–925. doi: 10.1097/00003246-199107000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Mosing M, Kutter AP, Iff S, Raszplewicz J, Mauch J, Bohm SH, et al. The effects of cardiac output and pulmonary arterial hypertension on volumetric capnography derived-variables during normoxia and hypoxia. J Clin Monit Comput. 2015;29:187–196. doi: 10.1007/s10877-014-9588-0. [DOI] [PubMed] [Google Scholar]
- 28.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.Beitler JR, Thompson BT, Matthay MA, Talmor D, Liu KD, Zhuo H, et al. Estimating dead-space fraction for secondary analyses of acute respiratory distress syndrome clinical trials. Crit Care Med. 2015;43:1026–1035. doi: 10.1097/CCM.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goligher EC, Kavanagh BP, Rubenfeld GD, Ferguson ND. Physiologic responsiveness should guide entry into randomized controlled trials. Am J Respir Crit Care Med. 2015;192:1416–1419. doi: 10.1164/rccm.201410-1832CP. [DOI] [PubMed] [Google Scholar]
- 31.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5:524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 32.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 33.Kallet RH, Alonso JA, Pittet JF, Matthay MA. Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care. 2004;49:1008–1014. [PubMed] [Google Scholar]
- 34.Maisch S, Reissmann H, Fuellekrug B, Weismann D, Rutkowski T, Tusman G, et al. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg. 2008;106:175–181. doi: 10.1213/01.ane.0000287684.74505.49. [DOI] [PubMed] [Google Scholar]
- 35.Fengmei G, Jin C, Songqiao L, Congshan Y, Yi Y. Dead space fraction changes during PEEP titration following lung recruitment in patients with ARDS. Respir Care. 2012;57:1578–1585. doi: 10.4187/respcare.01497. [DOI] [PubMed] [Google Scholar]
- 36.Charron C, Repesse X, Bouferrache K, Bodson L, Castro S, Page B, et al. PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: a physiological study. Crit Care. 2011;15:R175. doi: 10.1186/cc10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González-Castro A, Suárez-Lopez V, Gómez-Marcos V, González-Fernandez C, Iglesias-Posadilla D, Burón-Mediavilla J, et al. [Utility of the dead space fraction (Vd/Vt) as a predictor of extubation success] Med Intensiva. 2011;35:529–538. doi: 10.1016/j.medin.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Tusman G, Suarez-Sipmann F, Böhm SH, Pech T, Reissmann H, Meschino G, et al. Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med. 2006;32:1863–1871. doi: 10.1007/s00134-006-0371-7. [DOI] [PubMed] [Google Scholar]
- 39.Pais FM, Sinha P, Liu KD, Matthay MA. Influence of clinical factors and exclusion criteria on mortality in ARDS observational studies and randomized controlled trials. Respir Care. 2018;63:1060–1069. doi: 10.4187/respcare.06034. [DOI] [PubMed] [Google Scholar]
- 40.Rose DK, Froese AB. Changes in respiratory pattern affect dead space/tidal volume ratio during spontaneous but not during controlled ventilation: a study in pediatric patients. Anesth Analg. 1980;59:341–349. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.