Despite decades-long secular improvements in pulmonary care and morbidity in patients with cystic fibrosis (CF), few advancements have been made in preventing diabetes in this highly susceptible population. CF-related diabetes (CFRD) is a major comorbidity of CF, affecting over half of patients with CF by middle age and accelerating their clinical decline. CFRD occurs primarily due to impaired insulin secretion, especially loss of rapid “first-phase” insulin secretion. The recent advent of small-molecule therapies aimed at restoring CFTR (CF transmembrane conductance regulator) function has raised the question as to whether these therapies will treat or even prevent CFRD. These medications have come to market based on their efficacy in improving pulmonary function and weight. To date, only anecdotal and very small studies of their glycemic effects have been reported, providing teasing results suggesting that CFTR restoration might treat CFRD (1–6). The current uncertainty as to whether restoration of CFTR function might improve glucose metabolism in patients with CF stems not only from a lack of clinical data but also from an incomplete understanding of the basic pathophysiology of CFRD. The mechanistic dilemma can be reduced to a simple question: are the causes of poor insulin secretion in patients with CF due to reversible or irreversible defects? In this issue of the Journal, Kelly and colleagues (pp. 342–351) provide the largest and most detailed clinical study to date on this topic (7). Their data show that insulin secretion improves after 16 weeks of ivacaftor therapy. This work adds weight to the optimistic outlook that restoration of CFTR function may help treat and/or prevent CFRD.
In their study, Kelly and colleagues focused on patients with CF and at least one allele encoding a CFTR mutation amenable to ivacaftor potentiation. Twelve subjects, all initially ivacaftor naive, completed the study. The subjects were generally young—all but one were under 18 years of age. Because insulin secretion is impaired starting in early childhood (8), the study was especially well suited to assess the impact of ivacaftor on insulin secretion. Subjects underwent detailed glycemic phenotyping just before starting and upon completing 16 weeks of ivacaftor therapy. During the study, FEV1 and body mass index improved as expected. Crucially, multiple measures of insulin secretion also improved with ivacaftor treatment, including first-phase insulin responsiveness. Furthermore, the improvement of insulin secretion was significant even when evaluated in the context of the prevailing sensitivity to insulin. That so many measures of insulin secretion improved speaks to the robustness of the results and shows that there are reversible aspects to the insulin secretion defects that occur in CF.
Ultimately, to treat CFRD, insulin secretion must be increased sufficiently so as to normalize glucose levels. However, the study by Kelly and colleagues was not well suited to examine the impact of ivacaftor on glycemia because the patients studied had only minor glucose tolerance abnormalities at baseline. Hence, it is not surprising that overall glucose and mixed-meal tolerance did not change with ivacaftor. It thus remains to be determined whether the improvements in insulin secretion induced by ivacaftor will be sufficient to treat CFRD. One limitation of this work is that it did not include an untreated control arm. To help mitigate this limitation, the investigators established safeguards against baseline confounders that might have temporarily altered insulin secretion, such as ensuring that subjects were at their baseline health at the start of the study.
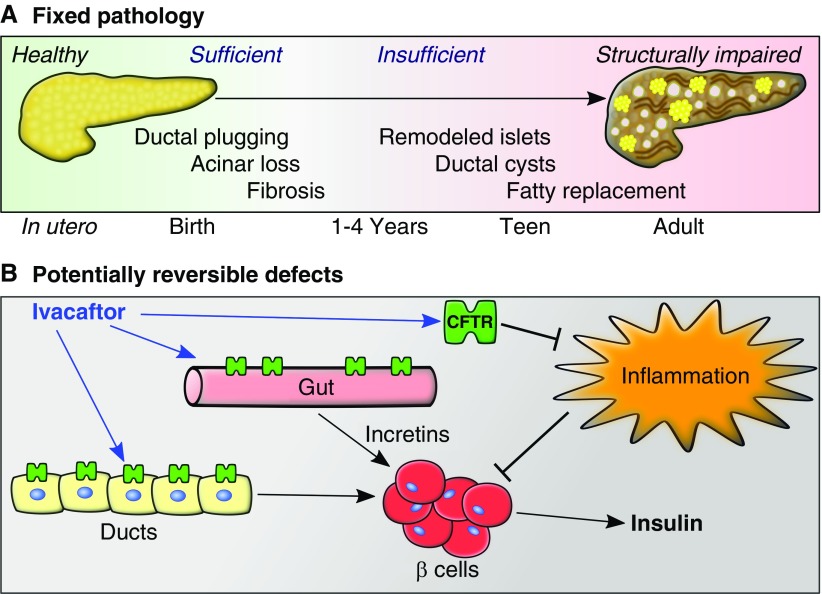
Exocrine pancreatic damage is already extensive and presumably irreversible (Figure 1A) by 4 years of age or earlier in most patients with CF (9, 10). Despite this, considerable numbers of insulin-containing β-cells (roughly 50% of normal) remain (11). These surviving β-cells exist in an irreversibly abnormal environment and thus might be subject to failure over time even in the face of CFTR functional restoration. Only longer-term studies will be able to address whether CFTR functional restoration can prevent CFRD over a span of years to decades.
Figure 1.
Fixed versus CFTR (cystic fibrosis transmembrane conductance regulator)-reversible pathology contributing to CF-related diabetes. (A) Timeline of irreversible pathology contributing to CF-related diabetes. Exocrine pancreatic damage begins in utero, such that by 1–4 years of age most individuals with CF have developed pancreatic insufficiency. The endocrine pancreas survives, albeit in a remodeled state surrounded by an abnormal environment. (B) Restoration of CFTR function might reduce pancreatic inflammation and thus improve β-cell function. Likewise, restoration of CFTR action in the gut might improve incretin secretion and thus enhance β-cell function. Alternatively, enhancement of CFTR function in pancreas ductal epithelium might restore a paracrine environment conducive to β-cell function. Not shown are various potential mechanisms by which ivacaftor might improve glycemia by improving insulin sensitivity in CF.
One of the paradoxes of CFRD is that the quantity of surviving β-cells is generally sufficient to otherwise prevent diabetes. Thus, the surviving β-cells in CFRD are believed to have functional deficiencies (12, 13). Kelly and colleagues’s results are consistent with this, showing that β-cell insulin secretion in CF is improved by short-term CFTR functional restoration. One resulting key question is, what physiological mechanism underlies this improvement in insulin secretion? The simplest explanation involves direct expression of CFTR in β-cells, where its chloride transport actions can augment insulin secretion (14). However, recent reports that CFTR is not expressed in the endocrine pancreas in vivo (12, 15) cast significant doubt on this possibility. Thus, it is likely that CFTR function impacts insulin secretion indirectly (Figure 1B). One possible mechanism for this effect would be relief of islet inflammation by CFTR restoration. CF islets have recently been shown to contain inflammatory ILs (12, 13), and perhaps this could be resolved with CFTR restoration. Another possibility is that restored CFTR might improve secretion of incretins (hormones that enhance β-cell function) from the gut. Kelly and colleagues tested this possibility by examining two incretins, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide, and found no differences induced by ivacaftor. Nonetheless, both of these incretins showed trends in the right direction despite high variability. Thus, it is possible that a larger study might find statistically robust incretin improvements. A final possibility is that CFTR restoration might improve the local environment of islets by enhancing pancreatic ductal function (12).
Kelly and colleagues’s result provides hope that restoration of CFTR function might treat and prevent CFRD. However, given the current limited understanding of CFRD pathogenesis, there remains considerable uncertainty. It is possible that the fixed defects in the pancreas might prove too great a burden for the endocrine pancreas to maintain sufficient long-term function. Because pancreatic disease begins in utero, it could be envisioned that CFTR restoration may need to begin before birth to minimize an individual’s lifetime CFRD risk. Longer-term clinical studies, including in patients with CFRD, will help clarify whether such an intensive approach is needed. Likewise, mechanistic studies of the early-life determinants of CFRD in relevant CF animal models will help unravel the fixed versus reversible underpinnings of diabetes in CF.
Supplementary Material
Acknowledgments
Acknowledgment
The author thanks Cecilia Redmond, M.D., for a critical review of the manuscript.
Footnotes
A.W.N. is supported by NIH grants R01 DK115791 and R24 DK96518, and a Faculty Scholar Award from the Fraternal Order of Eagle Diabetes Research Center at the University of Iowa.
Originally Published in Press as DOI: 10.1164/rccm.201808-1501ED on September 7, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, et al. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14:417–421. doi: 10.1111/pedi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes D, Jr, McCoy KS, Sheikh SI. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190:590–591. doi: 10.1164/rccm.201405-0882LE. [DOI] [PubMed] [Google Scholar]
- 3.Tsabari R, Elyashar HI, Cymberknowh MC, Breuer O, Armoni S, Livnat G, et al. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J Cyst Fibros. 2016;15:e25–e27. doi: 10.1016/j.jcf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Mutyam V, Libby EF, Peng N, Hadjiliadis D, Bonk M, Solomon GM, et al. Therapeutic benefit observed with the CFTR potentiator, ivacaftor, in a CF patient homozygous for the W1282X CFTR nonsense mutation. J Cyst Fibros. 2017;16:24–29. doi: 10.1016/j.jcf.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan A, Cohen-Cymberknoh M, Shteinberg M, Levine H, Vilozni D, Bezalel Y, et al. Ivacaftor for the p.Ser549Arg (S549R) gating mutation–the Israeli experience. Respir Med. 2017;131:225–228. doi: 10.1016/j.rmed.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Thomassen JC, Mueller MI, Alejandre Alcazar MA, Rietschel E, van Koningsbruggen-Rietschel S. Effect of lumacaftor/ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients. J Cyst Fibros. 2018;17:271–275. doi: 10.1016/j.jcf.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Kelly A, De Leon DD, Sheikh S, Camburn D, Kubrak C, Peleckis AJ, et al. Islet hormone and incretin secretion in cystic fibrosis after four months of ivacaftor therapy. Am J Respir Crit Care Med. 2019;199:342–351. doi: 10.1164/rccm.201806-1018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, et al. Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2016;194:974–980. doi: 10.1164/rccm.201512-2518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdani M, Blackman SM, Ridaura C, Bellocq J-P, Powers AC, Aguilar-Bryan L. Structural abnormalities in islets from very young children with cystic fibrosis may contribute to cystic fibrosis-related diabetes. Sci Rep. 2017;7:17231. doi: 10.1038/s41598-017-17404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344–399. [Google Scholar]
- 11.Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab. 1996;81:1267–1272. doi: 10.1210/jcem.81.3.8772610. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Yi Y, Xie W, Liang B, Winter MC, He N, et al. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology. 2017;158:3325–3338. doi: 10.1210/en.2017-00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hull RL, Gibson RL, McNamara S, Deutsch GH, Fligner CL, Frevert CW, et al. Islet interleukin-1β immunoreactivity is an early feature of cystic fibrosis that may contribute to β-cell failure. Diabetes Care. 2018;41:823–830. doi: 10.2337/dc17-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edlund A, Esguerra JLS, Wendt A, Flodström-Tullberg M, Eliasson L. CFTR and anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12:87. doi: 10.1186/1741-7015-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart NJ, Aramandla R, Poffenberger G, Fayolle C, Thames AH, Bautista A, et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation JCI Insight 20183pii: 98240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.