Abstract
Hepatic encephalopathy describes the array of neurological complications that arise due to liver insufficiency and/or portal-systemic shunt. The pathogenesis of hepatic encephalopathy shares a longstanding association with hyperammonemia and inflammation. Recently, aberrant bile acid signaling has been implicated in the development of key features of hepatic encephalopathy due to acute liver failure including neuronal dysfunction, neuroinflammation and blood–brain barrier permeability. This review summarizes the findings of recent studies demonstrating a role for bile acids in hepatic encephalopathy and speculates on the possible downstream consequences of bile acid signaling.
Abbreviations: ASBT, Apical Sodium-Dependent Bile Acid Transporter; FXR, Farnesoid X Receptor; NTCP, Sodium Taurocholate Cotransporting Polypeptide; PXR, Pregnane X Receptor; VDR, Vitamin D Receptor; GR, Glucocorticoid Receptor; TGR5, Takeda G-Protein Receptor 5; S1P2R, Sphingosine 1 Phosphate Receptor 2; Cyp46A1, Cytochrome p450 46A1; CCL2, Chemokine Ligand 2; CCR2, Chemokine Receptor 2
Keywords: blood–brain barrier, farnesoid X receptor, neuroinflammation, sphingosine-1-phosphate receptor 2, Takeda G-protein coupled receptor 5 (TGR5)
Drug-induced liver toxicity is a common cause of liver injury, accounting for approximately one-half of the cases of acute liver failure in the US, with the majority of these due to acetaminophen overdose.1 In patients that do not recover after cessation of exposure, treatment options are limited and transplantation is often required.1 The predominant extrahepatic complication of acute liver failure is the development of Type A hepatic encephalopathy, manifesting as a wide spectrum of neurological or psychiatric abnormalities ranging from subclinical alterations to coma.2 During acute liver failure, mortality is relatively high due to unpredictable systemic complications with 20–25% of its mortality resulting from increased intracranial pressure and the development of hepatic encephalopathy.1
Liver cirrhosis is a consequence of various chronic liver diseases including Hepatitis B and C viral infections, alcoholic liver disease and non-alcoholic fatty liver disease. In the setting of cirrhosis, Type C hepatic encephalopathy develops slowly with many patients having altered sleep patterns and cognitive issues that can progress to more severe symptomology if there is no therapeutic intervention is given.3 Given the poor treatment options for the management of liver cirrhosis and the advances in the clinical diagnosis of cognitive symptoms, the prevalence of hepatic encephalopathy is increasing with an urgent need to develop effective treatment options for these patients.
Associated with the neurobehavioral changes that occur during hepatic encephalopathy is an increase in circulating and neural ammonia concentrations, thought to promote cerebral edema, astrocyte swelling and dysregulation of numerous neurotransmitter systems.4 Currently, the understanding of the events leading to hepatic encephalopathy is limited. Strategies aimed at preventing or reversing the neurological impact of liver damage will improve quality of life and increase the narrow therapeutic window in more severe cases of hepatic encephalopathy, thereby improving the opportunities for liver transplantation.
Our current understanding of the pathogenesis of hepatic encephalopathy has largely centered around the buildup of serum and cortical ammonia during acute liver failure, which can act synergistically with peripheral and central inflammation to precipitate the neurological difficulties observed during hepatic encephalopathy.5, 6 However, the increase of circulating bile acids due to liver failure has been identified as another possible culprit contributing to the complex etiology of hepatic encephalopathy. This review will summarize these recent studies and speculate on possible downstream effects of aberrant bile acid signaling in the brain.
Bile Acid Signaling
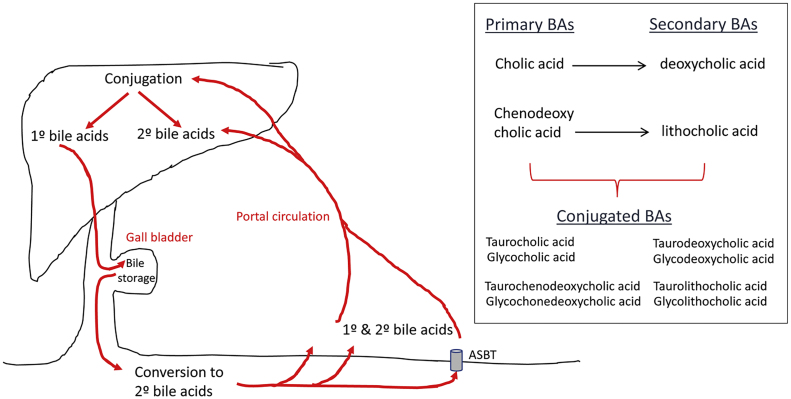
Bile acids are pleiotropic molecules produced predominantly in the liver using cholesterol as their chemical backbone. They are secreted into the intestine where they aide in the digestion and absorption of lipids. When in the intestinal lumen, the primary bile acids cholic acid and chenodeoxycholic acid can be chemically altered by the gut microbiota to form secondary bile acids, deoxycholic acid and lithocholic acid. These bile acids can then be further modified in the liver or the intestine by sulfation or glucuronidation and conjugation to glycine or taurine to make them less toxic7 (Figure 1). These various enzymatic reactions confer a wide variety of characteristics to bile acids, not only in regards to their lipophilicity and hydrophilicity, but also their ability to bind and activate receptors.
Figure 1.
Enterohepatic circulation of bile acids. Primary bile acids are synthesized in the liver and stored in the gall bladder as a component of bile until needed. Once inside the small intestine, primary bile acids can be converted to secondary bile acids via the actions of gut bacteria. Bile acids are taken up into the portal circulation either by passive diffusion throughout the small intestine (minor) or by active transport through the bile acid transporter ASBT. Once returned to the liver both primary and secondary bile acids can be conjugated to glycine or taurine and stored until needed. See Dawson and Karpen55 for a more comprehensive overview of bile acid metabolism.
Various membrane-bound and nuclear receptors are responsive to different bile acids and are summarized in Figure 2. The quintessential bile acid receptor is the nuclear receptor Farnesoid X Receptor (FXR). Bile acid ligands gain entry to the nuclear receptor either by passive diffusion into the cell or via active transport through one of the many bile acid transporters (e.g. Apical Sodium-Dependent Bile Acid Transporter (ASBT), Organic Anion-Transporting Polypeptide (OATP), or Sodium Taurocholate Cotransporting Polypeptide (NTCP)). More recently, certain bile acids have also been identified as ligands for a number of other nuclear receptors, including Pregnane X Receptor8 (PXR), Vitamin D Receptor9 (VDR), and the Glucocorticoid Receptor (GR).10, 11
Figure 2.
Bile acids can bind the membrane receptors TGR5 or S1P2R with the indicated bile acid species having identified affinity for these receptors. In order for bile acids to bind nuclear receptors, they need to passively diffuse across the cell membrane or are actively transported by ASBT, NTCP, or OATP. Once inside the cell, bile acids can bind nuclear receptors FXR, VDR, PXR, or GR which facilitate DNA binding and subsequent regulation of transcription. Similar to the membrane bile acid receptors, these nuclear receptors have differential affinity for specific bile acids, which are indicated on this figure.
Interestingly, a number of membrane-bound bile acid receptors have also been identified. Most studies have focused on the Takeda G-protein coupled receptor 5 (TGR5; also known as G-protein coupled bile acid receptor 1; GPBAR1) which can bind and be activated by the unconjugated bile acids lithocholic acid, deoxycholic acid, and chenodeoxycholic acid, increasing intracellular 3′,5′-cyclic adenosine monophosphate.12, 13 However, more recent reports suggest that the Sphingosine 1-Phophate Receptor 2 (S1P2R) is also responsive to bile acids, in particular conjugated bile acids, resulting in the activation of ERK1/2 and Akt-dependent pathways.14
Increased Serum Bile Acid Content is an Indication of Liver Damage
The majority of the bile acids secreted into the duodenum from the liver are taken up by the enterocytes of the ileum via active transport, secreted into the blood stream and are recirculated back to the liver. However, in conditions where the liver is damaged, serum bile acids are known to increase,15, 16 possibly due to the release of bile acid content from damaged hepatocytes as well as an impaired reuptake of bile acids from the blood stream. The increase in total bile acid content in the serum, as a result of liver damage, occurs in many acute and chronic liver disorders to varying degrees.15 Indeed, this increase has been suggested to have predictive value for the onset of acute decompensation and acute-on-chronic liver failure in patients with cirrhosis,16 both of which are often associated with the development of hepatic encephalopathy. However, the development of hepatic encephalopathy is clearly a multifactorial process, and an increase in serum bile acids in the absence of other factors such as systemic inflammation and hyperammonemia is likely not enough to cause hepatic encephalopathy by itself. Indeed, reports of cognitive deficits in patients with chronic cholestasis that are associated with increased serum bile acid levels17 are sparse. Furthermore, even though a commonly used model of Type C hepatic encephalopathy is bile duct ligation in rats which manifests with chronic cholestatic liver injury, it is not until these rats show evidence of liver cirrhosis that signs of cognitive impairment are evident,18, 19 suggesting that other factors are also necessary for the development of hepatic encephalopathy.
The total bile acid concentration in the serum is the summation of the concentrations of each individual bile acids and as indicated above, the different bile acid species have different properties and affinities for various receptors. Therefore, determining the link between individual bile acid species and the development of hepatic encephalopathy is important. Indeed, in an analysis of serum bile acids in patients with early and advanced cirrhosis there was increased total bile acid content, as well as increased levels of conjugated bile acids that correlated with the severity of the disease.20 There was also increased prevalence of primary bile acids over secondary bile acids in the stool of these patients, which correlated with alterations in the gut microbiota away from those bacteria with the ability to convert primary to secondary bile acids.20 Indeed dysbiosis, or an unfavorable change in the composition of the gut microbiome, is thought to be central to the pathophysiology of chronic liver disease and the development of complications.21 Bile acids are capable of altering the composition of the gut microbiome,22 which in turn, affects the composition of the bile acid pool23 suggesting a link between gut microbiota, individual bile acid species, and the development of hepatic encephalopathy exists.21 In clinical trials, treatment of cirrhotic patients with probiotics, such as VSL#3, have attenuated the neurological and cognitive deficits,24, 25, 26 demonstrating therapeutic value for targeting the gut microbiome for the management of hepatic encephalopathy. Furthermore, VSL#3 treatment of mice alter the composition of the bile acid pool.27 Whether the link between individual bile acid species, the gut microbiota and the development of hepatic encephalopathy is causative or correlative is still uncertain.
Effect of Bile Acids on Blood–Brain Barrier Permeability
One of the clinical features thought to contribute to the pathogenesis of hepatic encephalopathy is an increased permeability of the blood–brain barrier to a number of solutes that is not attributable to a general breakdown in the integrity of the blood–brain barrier.28, 29 Permeability of the blood–brain barrier, as demonstrated by Evans blue extravasation, occurs as a later event well after the onset of neurological symptoms in various rodent models of acute liver failure.30, 31, 32 Furthermore, the increased permeability of the blood–brain barrier seems to be dependent upon the presence of pro-inflammatory factors.30, 33
Over 40 years ago, increased total bile acid content in the cerebrospinal fluid was observed in patients with fulminant hepatic failure,34 although at the time it was concluded that the concentrations were lower than those found to inhibit brain respiration in vitro.34 However, with the more recent recognition of bile acids as signaling molecules and ligands for specific receptors, a role for aberrant bile acid signaling in the pathogenesis of hepatic encephalopathy warranted further investigation. More recently, increases in certain bile acids, particularly taurocholic acid and glycocholic acid were detected in the cerebrospinal fluid of patients with hepatic encephalopathy due to cirrhosis as part of a larger metabolic screen.35 Furthermore, increased total bile acid content in brain tissue has been demonstrated in a rodent model of hepatic encephalopathy due to acute liver failure36 and specifically, increases in taurocholic acid.37 Similarly, increased total bile acid content in brain tissue has been detected as an early event in a model of chronic liver damage prior to the onset of hepatic encephalopathy,11 suggesting that bile acids may play a role in the pathogenesis of hepatic encephalopathy regardless of the underlying liver pathology.
The mechanism by which bile acids are gaining access to the brain and how they come to be present in the cerebrospinal fluid is unknown. Interestingly, in a recent study, certain bile acids themselves were shown to increase the permeability of the blood–brain barrier in vitro and in vivo via a mechanism involving the Rac1-dependent phosphorylation of the tight junction protein occludin.38 The mechanism by which bile acids activated Rac1 was not elucidated, although a role for FXR and TGR5 was ruled out.38 However, given that S1PR2 has recently been shown to regulate blood–brain barrier permeability by the destabilization of adherens junctions,39 it is conceivable that bile acids might be exerting their effects on blood–brain barrier permeability via the activation of S1PR2, which warrants further investigation.
Effect of Bile Acids on Neuronal Dysfunction
A role for aberrant bile acid signaling in the neurological dysfunction associated with hepatic encephalopathy has been demonstrated in a mouse model of acute liver failure.36 Specifically, mice fed a diet enriched with the bile acid sequestrant cholestyramine had reduced serum and brain bile acid content, which alleviated the neurological impairments associated with hepatic encephalopathy such as reflex deficits and the presence of ataxia.36 Furthermore, altering the relative composition of the bile acid pool by feeding mice a diet enriched in cholic acid or deoxycholic acid worsened the neurological decline associated with acute liver failure.36 Identification of the mechanism(s) of action by which bile acids may alter the neurological function in mice with acute liver failure began with the demonstration of bile acid transporter, ASBT, expression, which co-localized with neuronal markers in various brain regions, including the frontal cortex,11, 36 and has been confirmed in other models of neurological disorders.40 In vitro, the uptake of a fluorescent bile acid derivative, cholyl-lysyl fluorescein, into neurons was inhibited by the specific knockdown of ASBT expression.11
Once inside the neuron, it is hypothesized that bile acids are exerting their effects through the activation of FXR.36 In support of this notion, FXR expression has been demonstrated predominantly in neurons36, 41 throughout the brain and the expression of FXR and its cofactor SHP is increased in the frontal cortex during acute liver failure.36 Furthermore, the direct infusion of an FXR-specific vivo morpholino into the frontal cortex proved protective against the neurological complications of acute liver failure.36 To-date, FXR expression has not been demonstrated in other cell types in the brain, however this does not rule out the possibility of non-FXR mediated actions of bile acids, that are described below.
The sequence of events downstream of FXR signaling in hepatic encephalopathy is unknown at this point. However, a number of related observations may give insight into the possible consequences. Firstly, specific deletion of FXR systemically disrupts a number of neurotransmitter systems and altered neurobehavior.42 Specifically, FXR knockout mice showed depressive-like and anxiety-related behavior with increased motor activity.42 Biochemically, there were alterations in glutamatergic, GABAergic, serotonergic and norepinephrinergic neurotransmission in either the hippocampus or cerebellum in the FXR knockout mice.42 Taken together, it is conceivable that the role that aberrant FXR activation plays in the pathogenesis of hepatic encephalopathy may be via the disruption of a number of neurotransmitter systems known to be altered in hepatic encephalopathy.43
Another indication for the possible identity of the downstream consequences of FXR signaling in the pathogenesis of hepatic encephalopathy may lie in the mechanisms by which the brain maintains cholesterol homeostasis. Approximately 25% of the body's cholesterol is found in the brain and the levels are tightly regulated so that fluctuations in dietary cholesterol have minimal effect on brain function.44 In the brain, cholesterol is either incorporated into the cell membrane, where it regulates signal transduction pathways, or influences synapse formation, action potentials and neurotransmitter release.45 Furthermore, intracellular cholesterol serves as the precursor for the synthesis of many neurosteroids that are synthesized in the brain, such as allopregnanolone.45 One of the major ways in which the brain clears cholesterol is via its conversion to 24-(S)-hydroxycholesterol, a reaction catalyzed by the enzyme Cytochrome p450 46A1(Cyp46A1).46 24-(S)-hydroxycholesterol is then able to exit the brain and enter the blood stream where it is integrated into the de novo bile acid synthesis pathway in the liver. We have recently demonstrated that the expression of Cyp46A1 is suppressed in the frontal cortex of mice with Type A hepatic encephalopathy.47 Furthermore, there was a concomitant accumulation of cholesterol, which was predominantly in the un-esterified form and was distributed both intracellularly as well as in the membrane.47 Strategies to prevent aberrant bile acid signaling in the brain (e.g. cholestyramine feeding and icv infusion of FXR antagonists) or accumulation of brain cholesterol (e.g. icv infusion of a cholesterol sequestrant) during acute liver failure prevented the downregulation of Cyp46A1 in the brain, cholesterol accumulation and subsequent neurological symptoms of hepatic encephalopathy.47 Given the known roles for cholesterol in the brain, it is conceivable that aberrant bile acid signaling in the brain may alter cholesterol clearance pathways to bring about alterations in neurotransmitter release and/or neurosteroid synthesis, both of which are known to be altered in hepatic encephalopathy.4, 48
Similar to FXR, an initial increase in TGR5 expression has been demonstrated as an early event in a mouse model of hepatic encephalopathy due to acute liver failure.49 However, TGR5 activation had opposing effects on neurological function during hepatic encephalopathy than those reported for FXR activation.49 Indeed, central infusion of a TGR5 agonist delayed the neurological decline and attenuated the reflex impairment and presence of ataxia.49 Interestingly, TGR5 expression in the brain has been demonstrated to be responsive to ammonia, in that treatment of astrocytes with ammonia reduces the expression of TGR5.50 Given that the ammonia levels in the model of acute liver failure used above do not reach significant levels until later stages of the disease progression,51, 52 it is feasible that there is an initial increase in TGR5 expression as a compensatory attempt to protect the brain during liver failure, which is followed by a decrease in expression at much later stages when ammonia levels are high.
Effect of Bile Acids on Neuroinflammation
The pathogenesis of hepatic encephalopathy shares a longstanding relationship with the initiation of neuroinflammatory processes and microglia activation in both acute and chronic liver diseases. Activation of microglia is a delicate balance between pro-inflammatory and anti-inflammatory signals, which in physiological conditions favors the dampening of microglia activation.53 These signals may be derived from the microglia themselves, or as a result of cell-to-cell communication derived from neurons or astrocytes. Recently, the proinflammatory chemokine ligand 2 (CCL2) was demonstrated to be increased in neurons in a mouse model of hepatic encephalopathy54 with a concomitant decrease in the anti-inflammatory chemokine, fractalkine,53 thereby dysregulating the balance between opposing pro- and anti-inflammatory signal acting on receptors on microglia, resulting in microglia activation.
The activation of microglia can be attenuated by feeding mice the bile acid sequestrant cholestyramine,37 which reduces the buildup of bile acid content in the serum and brain.36 Furthermore, while specific knockdown of FXR expression in neurons had no effect on the hepatic encephalopathy-induced microglia activation, direct infusion of the S1P2R antagonist attenuated the microglia activation, expression of proinflammatory cytokines and subsequent neurological impairment associated with hepatic encephalopathy.37 Together, this suggests that bile acids may regulate neuroinflammatory processes via the activation of S1P2R, rather than through FXR signaling. However, S1P2R immunoreactivity was found to co-localize predominantly with neurons37 and treatment of primary microglia with the bile acid taurocholic acid did not alter the activation state of these cells, suggesting that the proinflammatory outcome of bile acid signaling was likely not due to a direct action on microglia. Indeed, S1P2R activation of neurons led to an increase in expression and release of the proinflammatory chemokine CCL2, which in turn led to an activation of microglia and further release of proinflammatory cytokines.37
In contrast, associated with the neuroprotective actions of TGR5 activation described above, is an associated dampening of neuroinflammation observed in hepatic encephalopathy.49 Treatment of neurons with a TGR5 agonist decreased the expression of CCL2, and the subsequent treatment of microglia with the conditioned media from TGR5 agonist-treated neurons decreased the phagocytic activity and cytokine production in the microglia.49 The mechanism of the anti-inflammatory actions of TGR5 activation therefore appears to be via a downregulation of CCL2 from neurons, which in turn dampens the activation of microglia, rather than through a direct action on microglia themselves.49
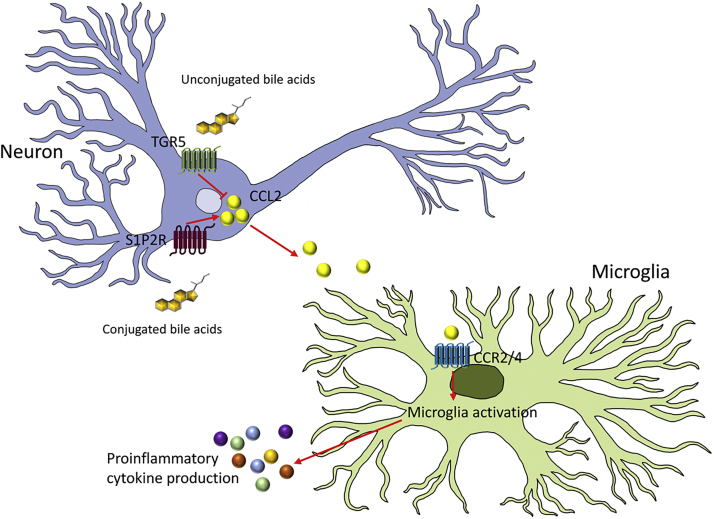
The interplay between S1P2R and TGR5 activation on neuronal CCL2 expression during hepatic encephalopathy is summarized in Figure 3.
Figure 3.
Proinflammatory and anti-inflammatory actions of bile acids during hepatic encephalopathy can be attributed, in part, to the opposing actions of S1P2R and TGR5 activation on the neuronal expression of the chemokine CCL2. Increased CCL2 expression and release from neurons results in the activation of chemokine receptor 2 (CCR2) or chemokine receptor 4 (CCR4) on microglia. This leads to the activation of microglia and the subsequent release of various other proinflammatory cytokines and contributes to neuroinflammation.
Conclusions and Future Directions
While a precise role for bile acid signaling in the brain during hepatic encephalopathy has not yet been completely defined, there is mounting evidence indicating that aberrant bile acid signaling is likely contributing to the pathogenesis of hepatic encephalopathy. The majority of the mechanistic studies have focused on hepatic encephalopathy due to acute liver failure, however there is evidence suggesting that bile acids play a role in the neurological complications of chronic liver diseases as well. Further research is needed to elucidate the role and/or contribution of individual bile acids to the development of hepatic encephalopathy. Furthermore, given that the etiology of hepatic encephalopathy is multifactorial, further research is required to identify potential a synergistic interaction of these bile acids with ammonia and other molecules known to be involved in hepatic encephalopathy pathogenesis. Due to the pleiotropic roles of bile acids and bile acid receptors in normal physiological and metabolic processes, directly targeting bile acid signaling in the brain may not be a feasible target for the development of therapeutic targets. However, further research into the downstream consequences of aberrant bile acid signaling in the brain may identify viable therapeutic options for the management of hepatic encephalopathy.
Conflicts of Interest
The author has none to declare.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas and was funded by a VA merit award (BX002638) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service (BLR&D) and an NIH R01 award (DK082435) to Dr. DeMorrow. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
References
- 1.Bernal W., Auzinger G., Dhawan A., Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.American Association for the Study of Liver Disease, European Association for the Study of the Liver Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth R.F. Complications of cirrhosis III. Hepatic encephalopathy. J Hepatol. 2000;32:171–180. doi: 10.1016/s0168-8278(00)80424-9. [DOI] [PubMed] [Google Scholar]
- 4.Hazell A.S., Butterworth R.F. Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- 5.Bemeur C., Butterworth R.F. Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis. 2013;28:145–150. doi: 10.1007/s11011-012-9361-3. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth R.F. Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab Brain Dis. 2016;31:1211–1215. doi: 10.1007/s11011-015-9746-1. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann A.F. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 8.Staudinger J.L., Goodwin B., Jones S.A. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adachi R., Shulman A.I., Yamamoto K. Structural determinants for vitamin D receptor response to endocrine and xenobiotic signals. Mol Endocrinol. 2004;18:43–52. doi: 10.1210/me.2003-0244. [DOI] [PubMed] [Google Scholar]
- 10.Takigawa T., Miyazaki H., Kinoshita M. Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G427–G438. doi: 10.1152/ajpgi.00205.2012. [DOI] [PubMed] [Google Scholar]
- 11.McMillin M., Frampton G., Quinn M. Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol Endocrinol. 2015;29:1720–1730. doi: 10.1210/me.2015-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruyama T., Miyamoto Y., Nakamura T. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 13.Kawamata Y., Fujii R., Hosoya M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 14.Studer E., Zhou X., Zhao R. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alm R., Carlson J., Eriksson S. Fasting serum bile acids in liver disease. A comparison with histological features. Scand J Gastroenterol. 1982;17:213–218. doi: 10.3109/00365528209182042. [DOI] [PubMed] [Google Scholar]
- 16.Horvatits T., Drolz A., Roedl K. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2017;37:224–231. doi: 10.1111/liv.13201. [DOI] [PubMed] [Google Scholar]
- 17.Bouchier I.A., Pennington C.R. Serum bile acids in hepatobiliary disease. Gut. 1978;19:492–496. doi: 10.1136/gut.19.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan C.Y., Huang S.W., Wang T.F. Lack of detrimental effects of nitric oxide inhibition in bile duct-ligated rats with hepatic encephalopathy. Eur J Clin Invest. 2004;34:122–128. doi: 10.1111/j.1365-2362.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang L.T., Hsieh C.S., Chou M.H. Obstructive jaundice in rats: cause of spatial memory deficits with recovery after biliary decompression. World J Surg. 2004;28:283–287. doi: 10.1007/s00268-003-7209-z. [DOI] [PubMed] [Google Scholar]
- 20.Kakiyama G., Pandak W.M., Gillevet P.M. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acharya C., Bajaj J.S. Gut microbiota and complications of liver disease. Gastroenterol Clin North Am. 2017;46:155–169. doi: 10.1016/j.gtc.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam K.B., Fukiya S., Hagio M. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez-Perez O., Cruz-Ramon V., Chinchilla-Lopez P., Mendez-Sanchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2017;16:s15–s20. doi: 10.5604/01.3001.0010.5494. [DOI] [PubMed] [Google Scholar]
- 24.Dalal R., McGee R.G., Riordan S.M., Webster A.C. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. doi: 10.1002/14651858.CD008716.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhiman R.K., Rana B., Agrawal S. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147 doi: 10.1053/j.gastro.2014.08.031. 1327-1337 e1323. [DOI] [PubMed] [Google Scholar]
- 26.Pratap Mouli V., Benjamin J., Bhushan Singh M. Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: a non-inferiority randomized controlled trial. Hepatol Res. 2015;45:880–889. doi: 10.1111/hepr.12429. [DOI] [PubMed] [Google Scholar]
- 27.Degirolamo C., Rainaldi S., Bovenga F., Murzilli S., Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 28.Scott T.R., Kronsten V.T., Hughes R.D., Shawcross D.L. Pathophysiology of cerebral oedema in acute liver failure. World J Gastroenterol. 2013;19:9240–9255. doi: 10.3748/wjg.v19.i48.9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss N., Rosselli M., Mouri S. Modification in CSF specific gravity in acutely decompensated cirrhosis and acute on chronic liver failure independent of encephalopathy, evidences for an early blood-CSF barrier dysfunction in cirrhosis. Metab Brain Dis. 2017;32:369–376. doi: 10.1007/s11011-016-9916-9. [DOI] [PubMed] [Google Scholar]
- 30.McMillin M.A., Frampton G.A., Seiwell A.P., Patel N.S., Jacobs A.N., DeMorrow S. TGFbeta1 exacerbates blood–brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Invest. 2015;95:903–913. doi: 10.1038/labinvest.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen J.H., Yamamoto S., Steers J. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006;44:1105–1114. doi: 10.1016/j.jhep.2005.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto S., Nguyen J.H. TIMP-1/MMP-9 imbalance in brain edema in rats with fulminant hepatic failure. J Surg Res. 2006;134:307–314. doi: 10.1016/j.jss.2005.11.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chastre A., Belanger M., Nguyen B.N., Butterworth R.F. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood–brain barrier permeability in mice with acute liver failure. Liver Int. 2014;34:353–361. doi: 10.1111/liv.12252. [DOI] [PubMed] [Google Scholar]
- 34.Bron B., Waldram R., Silk D.B., Williams R. Serum, cerebrospinal fluid, and brain levels of bile acids in patients with fulminant hepatic failure. Gut. 1977;18:692–696. doi: 10.1136/gut.18.9.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss N., Barbier Saint Hilaire P., Colsch B. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J Hepatol. 2016;65:1120–1130. doi: 10.1016/j.jhep.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 36.McMillin M., Frampton G., Quinn M. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. Am J Pathol. 2016;186:312–323. doi: 10.1016/j.ajpath.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMillin M., Frampton G., Grant S. Bile acid-mediated sphingosine-1-phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front Cell Neurosci. 2017;11:191. doi: 10.3389/fncel.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn M., McMillin M., Galindo C., Frampton G., Pae H.Y., DeMorrow S. Bile acids permeabilize the blood–brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46:527–534. doi: 10.1016/j.dld.2014.01.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz-Orengo L., Daniels B.P., Dorsey D. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J Clin Invest. 2014;124:2571–2584. doi: 10.1172/JCI73408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nizamutdinov D., DeMorrow S., McMillin M. Hepatic alterations are accompanied by changes to bile acid transporter-expressing neurons in the hypothalamus after traumatic brain injury. Sci Rep. 2017;7:40112. doi: 10.1038/srep40112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C., Wang J., Hu W. Identification of functional farnesoid X receptors in brain neurons. FEBS Lett. 2016;590:3233–3242. doi: 10.1002/1873-3468.12373. [DOI] [PubMed] [Google Scholar]
- 42.Huang F., Wang T., Lan Y. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front Behav Neurosci. 2015;9:70. doi: 10.3389/fnbeh.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liere V., Sandhu G., DeMorrow S. Recent advances in hepatic encephalopathy. F1000Res. 2017;6:1637. doi: 10.12688/f1000research.11938.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orth M., Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartocci V., Servadio M., Trezza V., Pallottini V. Can cholesterol metabolism modulation affect brain function and behavior? J Cell Physiol. 2017;232:281–286. doi: 10.1002/jcp.25488. [DOI] [PubMed] [Google Scholar]
- 46.Lund E.G., Xie C., Kotti T., Turley S.D., Dietschy J.M., Russell D.W. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 47.McMillin M., Grant S., Frampton G. FXR-mediated cortical cholesterol accumulation contributes to the pathogenesis of Type A hepatic encephalopathy. Cell Mol Gastroenterol Hepatol. 2018 doi: 10.1016/j.jcmgh.2018.02.008. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butterworth R.F. Neurosteroids in hepatic encephalopathy: novel insights and new therapeutic opportunities. J Steroid Biochem Mol Biol. 2016;160:94–97. doi: 10.1016/j.jsbmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 49.McMillin M., Frampton G., Tobin R. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. 2015;135:565–576. doi: 10.1111/jnc.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keitel V., Gorg B., Bidmon H.J. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 51.Matkowskyj K.A., Marrero J.A., Carroll R.E., Danilkovich A.V., Green R.M., Benya R.V. Azoxymethane-induced fulminant hepatic failure in C57BL/6J mice: characterization of a new animal model. Am J Physiol. 1999;277:G455–G462. doi: 10.1152/ajpgi.1999.277.2.G455. [DOI] [PubMed] [Google Scholar]
- 52.Belanger M., Cote J., Butterworth R.F. Neurobiological characterization of an azoxymethane mouse model of acute liver failure. Neurochem Int. 2006;48:434–440. doi: 10.1016/j.neuint.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 53.McMillin M., Grant S., Frampton G., Andry S., Brown A., DeMorrow S. Fractalkine suppression during hepatic encephalopathy promotes neuroinflammation in mice. J Neuroinflamm. 2016;13:198. doi: 10.1186/s12974-016-0674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMillin M., Frampton G., Thompson M. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflamm. 2014;11:121. doi: 10.1186/1742-2094-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson P.A., Karpen S.J. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]