Abstract
Background
Blunt traumatic thoracic aortic injury (BTAI) is a life‐threatening surgical emergency associated with mortality up to 8000 per year, most commonly caused by rapid acceleration/deceleration injury sustained through motor vehicle accident and/or blunt thoracic trauma. BTAI has high pre‐hospital mortality following the primary injury, with only 10% to 15% of patients surviving long enough to reach the hospital. Open surgical repair had remained the standard treatment option for BTAI since successfully introduced in 1959. However, with technological advances, thoracic endovascular repair (TEVAR) offers an alternative treatment option for BTAI. TEVAR is a less invasive surgical approach for management of these already critical patients; many reports have described favourable early outcomes.
Thoracic endovascular repair may appear to be superior to open repair for treatment of BTAI. However, its long‐term results and efficacy remain unknown. No randomised controlled trials (RCTs) have provided evidence to support the superiority of the endovascular approach versus open repair in the treatment of BTAI. This review aims to address this matter. This is an update of a review first published in 2015.
Objectives
To determine whether use of thoracic endovascular repair (TEVAR) for treatment of blunt traumatic thoracic aortic injury (BTAI) is associated with reduced mortality and morbidity when compared with conventional open surgery.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 20 August 2018.
Selection criteria
We considered all published and unpublished randomised controlled trials (RCTs) comparing TEVAR and open surgery for BTAI.
Data collection and analysis
Two review authors independently reviewed all RCTs identified by the Cochrane Vascular Information Specialist.
Main results
We found no RCTs that met the inclusion criteria for this review.
Authors' conclusions
We found no RCTs conducted to determine whether use of TEVAR for the treatment of BTAI is associated with reduced mortality and morbidity when compared to conventional open repair. Hence, we are unable to provide any evidence to guide the treatment option for this life‐threatening condition. To perform a randomised controlled trial to clarify the optimal management of BTAI would be highly challenging due to the natural history of the condition. Despite the lack of RCT evidence, clinicians are moving forward with endovascular treatment of BTAI on the basis of meta‐analyses of cohort studies and large clinical series.
Plain language summary
TEVAR versus open surgery for blunt traumatic thoracic aortic injury
Background
Blunt traumatic thoracic aortic injury (BTAI) caused by motor vehicle accident and blunt thoracic trauma is a surgical emergency with high mortality rate. Most patients do not survive long enough to reach the hospital. Two main treatment options for BTAI are open surgery and thoracic endovascular repair (TEVAR).
Study characteristics and key results
We performed a review of the literature (current up to 20 August 2018) to determine whether use of TEVAR is associated with reduced death and illness when compared to open repair. We identified no randomised controlled trials on this topic.
Quality of the evidence
We found no studies undertaken to address our objectives; therefore we were not able to assess the quality of the evidence.
Authors' conclusions
We identified no randomised controlled trials on this topic. To perform a randomised controlled trial to clarify optimal management of BTAI would be very challenging to complete, mainly because of the natural history of the condition, usually seen in combination with other life‐threatening injuries, the requirement for urgent intervention and the potential difficulties surrounding consent. Despite lack of RCT evidence, clinicians are moving forward with endovascular treatment of BTAI on the basis of meta‐analyses of cohort studies and large clinical series.
Background
Description of the condition
Blunt traumatic thoracic aortic injury (BTAI) is a life‐threatening surgical emergency that is most commonly caused by rapid acceleration/deceleration injury sustained through motor vehicle accident and/or blunt thoracic trauma. In the United States, nearly 8000 deaths secondary to BTAI occur each year (Nagy 2000). BTAI has a high pre‐hospital mortality following the primary injury, with only 10% to 15% of patients surviving long enough to reach hospitals (O'Conner 2004). Among these patients, 99% would die without early diagnosis and surgical intervention (O'Conner 2004).
Description of the intervention
Open surgical repair has been the standard treatment option for BTAI since it was successfully introduced in 1959 (Passaro 1959). This procedure generally involves thoracotomy, single‐lung ventilation, systemic anticoagulation, use of cardiopulmonary bypass and aortic cross‐clamping. Although this procedure provides a chance of survival for this highly morbid condition, some aspects of open repair may serve to exacerbate co‐existing injuries sustained, resulting in high postoperative mortality and frequent major postoperative complications (Cowley 1990; von Oppell 1994).
Thoracic endovascular repair (TEVAR) offers an alternative treatment option for BTAI. Since first introduced in 1991 for treatment of abdominal aneurysms (Parodi 1991), TEVAR has been quickly adopted as treatment for BTAI (Dake 1994; Kato 1997). TEVAR offers a less invasive approach of treatment for these already critical patients; therefore, many reports describing favourable early outcomes are available in the literature (Erben 2018; Orford 2003; Tehrani 2006). Thoracic endovascular repair involves meticulous preoperative planning with computed tomo‐angiography (CTA) imaging to size stent grafts, gaining access to the thoracic aorta via an endovascular approach and deployment of the stent grafts in the thoracic aorta. Although TEVAR is a novel treatment compared to open surgery, severe complications may occur and these complications may be classified into two main categories: device‐related complications (endoleak, stent graft migration, stent graft rupture) and ischaemic complications secondary to embolic events (stroke, paraplegia, spinal cord‐related ischaemic injury) (Bavaria 2007; Feezor 2008).
Why it is important to do this review
Endovascular approach appears to be superior to open repair for treatment of BTAI. However, long‐term results and efficacy of the endovascular approach remain unknown. We found no randomised controlled trial evidence to support superiority of the endovascular approach compared with open repair in the treatment of BTAI. This review aims to address this matter. This is an update of a review first published in 2015 (Pang 2015).
Objectives
To determine whether use of thoracic endovascular repair (TEVAR) for treatment of blunt traumatic thoracic aortic injury (BTAI) is associated with reduced mortality and morbidity when compared with conventional open surgery.
Methods
Criteria for considering studies for this review
Types of studies
Trials considered included randomised controlled trials already published and those still being conducted but reporting preliminary results. In addition, we considered randomised trials conducted by stent manufacturers (on file but not published) for inclusion in the review. Studies published in the English language were considered for inclusion in the review. We attempted to obtain translations of non‐English language studies when necessary.
Types of participants
We included in the review all participants with documented BTAI identified on chest computed tomographic scan or aortogram.
Types of interventions
We planned to include randomised controlled trials that compared TEVAR versus conventional open surgery.
Both procedures (TEVAR and conventional open surgery) must have been performed within one week of the diagnosis of BTAI for the study to be eligible for inclusion in this review. We planned to extract the following information for analysis and comparison.
TEVAR
Co‐morbidities and associated lesions.
Time from diagnosis to repair.
Device type, diameter and length.
Type of access utilised.
Use of heparin.
Operating times.
Conventional open surgery
Co‐morbidities and associated lesions.
Time from diagnosis to repair.
Type of repair used (graft interposition, direct suture).
Use of mechanical circulatory support or accessory equipment.
Types of outcome measures
Primary outcomes
Mortality at 30 days, and at one year.
Secondary outcomes
-
Postoperative and follow‐up complications as related to:
device (stent failure, stent fracture, stent migration); or
procedure (endoleak, pseudoaneurysm formation, paraplegia, cerebrovascular accident, recurrent laryngeal nerve injury, acute renal failure, conversion to open repair).
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 22 August 2018);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2018, Issue 7);
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 20 August 2018);
Embase Ovid (searched from 1 January 2017 to 20 August 2018);
CINAHL Ebsco (searched from 1 January 2017 to 22 August 2018);
AMED Ovid (searched from 1 January 2017 to 22 August 2018).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 22 August 2018:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We searched the reference lists of relevant articles retrieved by electronic searches for additional citations.
Data collection and analysis
Selection of studies
Two review authors (DP, DH) independently selected studies eligible for inclusion in the review. The third review author (PB) resolved disagreements if necessary.
Data extraction and management
We planned that two review authors (DP and DH) would independently extract the required data.
Required data include trial design, participant characteristics, therapeutic modalities (surgery or TEVAR), method of diagnosis, time to treatment and information on mortality and morbidity. We planned to review additional information on side effects as reported by each trial. When necessary, we planned to contact the principal authors of included studies to ask for further information. We intended to consult the third review author (PB) to resolve disagreements.
Assessment of risk of bias in included studies
We planned that two review authors (DP, DH) would independently assess the methodological rigour and clinical significance of each trial in accordance with the Cochrane 'risk of bias' domain‐based assessment (Higgins 2011), which includes assessment of different domains of eligible trials such as selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel, blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other potential sources of bias. We planned to classify the domains as having low risk of bias, unclear risk of bias or high risk of bias according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to consult the third review author (PB) for resolution of any disagreements.
Measures of treatment effect
We planned to pool the data on mortality, morbidity and other available outcomes provided by each trial to obtain an overall estimate of the effectiveness of the thoracic stent graft. We planned to present the data as a weighted mean difference (WMD) with 95% confidence interval (CI).
We planned to perform statistical analysis according to the statistical guidelines as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
We planned to use the participant as the unit of analysis.
Dealing with missing data
We intended to contact authors of respective studies for clarification and extraction of missing data.
Assessment of heterogeneity
We intended to evaluate trial heterogeneity using the I2 statistic, with values > 50% considered to show substantial heterogeneity. If no evidence of substantial statistical heterogeneity was found, we planned to use a fixed‐effect model meta‐analysis. If substantial statistical heterogeneity was detected, we planned to use a random‐effects model meta‐analysis.
Assessment of reporting biases
When the number of studies was sufficient, we intended to use forest plots to assess reporting bias. Otherwise, we planned to base assessment of reporting/publication bias for individual studies on comparison of reported study outcomes versus published study protocols.
Data synthesis
We intended to base all analyses on intention‐to‐treat data derived from individual clinical trials. We intended to perform a fixed‐effect model meta‐analysis unless substantial heterogeneity was detected (see also Assessment of heterogeneity).
'Summary of findings' table
We intended to create a 'Summary of findings' table using the following outcomes: mortality at 30 days and one year, post‐operative and follow‐up complications related to device and procedure. We planned to use the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence (Atkins 2004). We planned to use methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions and GRADEpro software (GRADEpro GDT 2015; Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We did not plan to perform a subgroup analysis because of the natural history of the condition.
Sensitivity analysis
We intended to perform a sensitivity analysis to assess the quality of included studies. However, we included no studies in this review; therefore, we did not perform subgroup analysis.
Results
Description of studies
Results of the search
See Figure 1.
1.
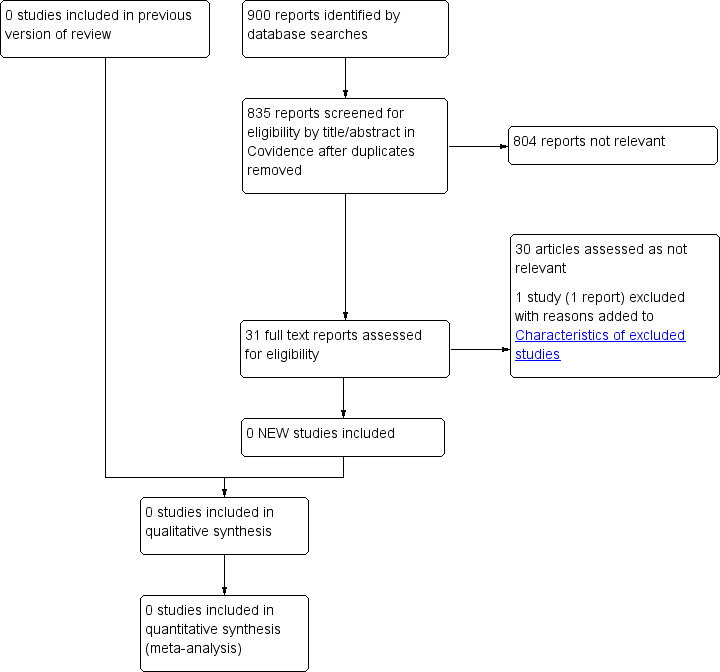
Study flow diagram
Included studies
We found no eligible studies.
Excluded studies
See Characteristics of excluded studies.
One new study was excluded for this update (Shackford 2017). In total four studies were excluded as they did not meet the inclusion criteria (ADSORB Trial; INSTEAD Trial; Liang‐Wan 2011; Shackford 2017). We excluded the ADSORB Trial, as it compares endovascular intervention versus best medical treatment. We excluded the INSTEAD Trial because researchers sought to investigate the benefit of endovascular intervention in type B aortic dissection. We excluded Liang‐Wan 2011 because it provides a clinical comparison of two operational methods (replacing ascending aorta + reconstructing aortic arch with triple‐branched stent graft; replacing ascending aorta + replacing half aortic arch) used to treat aortic dissection. We excluded Shackford 2017 from formal review as, although authors assessed outcomes relevant to this review, it is an observational study.
Risk of bias in included studies
As we identified no eligible studies, it was not possible to assess risk of bias.
Effects of interventions
We found no eligible studies for inclusion.
Discussion
Summary of main results
We found no randomised controlled trials undertaken to determine whether use of thoracic endovascular repair (TEVAR) for treatment of blunt traumatic thoracic aortic injury (BTAI) is associated with reduced mortality and morbidity when compared with conventional surgery.
Overall completeness and applicability of evidence
We found no randomised controlled trials undertaken to assess the benefits of one treatment over the other.
To undertake a randomised controlled trial to clarify the optimal management of BTAI would be very challenging, first because of the natural history of the condition, which is usually seen in combination with other life‐threatening injuries, requirements for urgent intervention and potential difficulties surrounding consent; and second because an adequately powered study of this relatively rare condition would require a multi‐centre study, and potential multi‐national involvement. Well‐conducted observational studies may be useful for guiding the most appropriate management option.
Quality of the evidence
We found no studies conducted to address our objectives; therefore, we were unable to assess the quality of the evidence and create a summary of findings table.
Potential biases in the review process
We found no studies relevant for inclusion in this review. The Cochrane Vascular Information Specialist performed a comprehensive search of the literature, and review authors selected studies in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion.
Agreements and disagreements with other studies or reviews
Use of thoracic endovascular repair (TEVAR) is rapidly expanding, and as experience is gained and devices are improved, trends seem to be pointing towards improved or at least equal outcomes with TEVAR versus open surgical procedures (Buz 2008; Erben 2018; Shackford 2017; Yamane 2008).
Xenos 2008 performed a meta‐analysis of 17 retrospective cohort studies reporting on a total of 589 participants with traumatic thoracic aortic injury. Of these, 369 were treated with conventional open surgical repair and 220 underwent TEVAR. Although authors recognised that the TEVAR cohort had a higher injury severity score than those undergoing open surgical repair, periprocedural mortality, 30‐day mortality and morbidity were significantly lower in the TEVAR group. These findings were also observed in Jonker 2010. In this study, involving 328 patients with thoracic aortic trauma, mortality rates were lower in TEVAR patients, and rates of complications (cardiac, acute renal failure, paraplegia or cerebrovascular events) were similar when compared to open repair. More recently, the non‐randomised study by Shackford 2017 reported significantly lower 30‐day mortality in TEVAR group (5.7% vs 10.7%).
In Azizzadeh 2013, a retrospective single centre cohort study including 106 participants, authors reported that the TEVAR group sustained fewer complications and lower risk of in‐hospital death. Costs were found to be similar between groups (Azizzadeh 2013). This observation was supported by DuBose 2015. In this retrospective multi‐centre study involving nine Level 1 trauma centres, lower aortic‐related mortality among TEVAR patients was reported (odds ratio (OR) 0.21, 95% confidence interval (CI) 0.05 to 0.88). Researchers also identified higher chest Abbreviated Injury Scale (AIS) scores, and grade and injury severity scores as independent predictors of mortality (DuBose 2015).
Non‐RCT evidence would appear to support an endovascular approach to traumatic thoracic aortic injury. The largest meta‐analysis to date, which included 7768 participants, reported lower mortality and spinal ischaemia rates with TEVAR, as well as reduced risk of graft infection and systemic infection when endovascular treatment was compared with open repair (Murad 2011).
However, the safety of TEVAR in emergency settings does remain a concern. Demetriades 2008 reported high (20%) stent graft related complications in TEVAR patients in his study of 193 patients. Although the TEVAR cohort was associated with lower mortality, Demetriades 2008 concluded that this was not without a significant risk from device‐related complications. Jonker 2010 reported 9% of patients undergoing TEVAR developed endoleak or distal embolisations.
The Society for Vascular Surgery has issued guidelines stating that BTAI should be managed through an endovascular technique (Lee 2011).
Authors' conclusions
Implications for practice.
No randomised controlled trials have sought to determine whether use of TEVAR for treatment of BTAI is associated with reduced mortality and morbidity when compared with conventional open surgery. Despite the lack of RCT evidence, clinicians are moving forward with endovascular treatment of BTAI on the basis of meta‐analyses of cohort studies and large clinical series.
Implications for research.
It is now unlikely that an RCT comparing open and endovascular treatment of BTAI will be undertaken, as most recent evidence points towards clinical benefit derived from endovascular repair. However, as new devices are brought into clinical use, comparative trials would be useful.
What's new
| Date | Event | Description |
|---|---|---|
| 6 March 2019 | Review declared as stable | This Cochrane review has been marked stable and will only be updated when new studies are identified. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 9, 2015
| Date | Event | Description |
|---|---|---|
| 28 August 2018 | New search has been performed | Search updated. No new studies included. One new study excluded. |
| 28 August 2018 | New citation required but conclusions have not changed | Search updated. No new studies included. One new study excluded. Text updated. No change to conclusions. |
| 23 April 2008 | Amended | Converted to new review format |
Acknowledgements
We thank A Asmat, CN Lee and P Robless for their work on the protocol of this review. We thank the Cochane Vascular editorial base and editors for their input.
Appendices
Appendix 1. Database search strategies
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Aorta, Thoracic EXPLODE ALL TREES 158 #2 MESH DESCRIPTOR Aortic Rupture EXPLODE ALL TREES 62 #3 MESH DESCRIPTOR Thoracic Injuries EXPLODE ALL TREES 340 #4 ((aort* near3 (transect* or disrupt* or tear or torn or rupture* or injur*))):TI,AB,KY 331 #5 ((thora* near3 (transect* or disrupt* or tear or torn or rupture* or injur*))):TI,AB,KY 331 #6 ((thora* near5 repair)):TI,AB,KY 82 #7 ((trauma* near5 thoracic)):TI,AB,KY 34 #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 893 #9 MESH DESCRIPTOR Stents EXPLODE ALL TREES 3739 #10 MESH DESCRIPTOR Thoracic Surgery EXPLODE ALL TREES 154 #11 MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES 431 #12 MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES 432 #13 (stent* or graft* or tevar or endograft* or endograft* or endoprosthe*):TI,AB,KY 81243 #14 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancurepowerlink or anaconda or Ancure or Advanta or Intracoil or Zilver or Luminex):TI,AB,KY 797 #15 endovascular:TI,AB,KY 2523 #16 MESH DESCRIPTOR Vascular Surgical Procedures 591 #17 MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES 7457 #18 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 88001 #19 #8 AND #18 189 #20 01/01/2015 TO 20/08/2018:CD 465323 #21 #19 AND #20 64 |
64 |
| Clinicaltrials.gov | Aorta, Thoracic OR Aortic Rupture OR Thoracic Injuries | Stents OR Thoracic Surgery OR Blood Vessel Prosthesis OR Endovascular Procedures | Start date on or after 01/01/2015 | 30 |
| ICTRP Search Portal | Aorta, Thoracic OR Aortic Rupture OR Thoracic Injuries | Stents OR Thoracic Surgery OR Blood Vessel Prosthesis OR Endovascular Procedures | Start date on or after 01/01/2015 | 1 |
| MEDLINE | 1 exp Aorta, Thoracic/ 32629 2 exp Aortic Rupture/ 9152 3 exp Thoracic Injuries/ 25241 4 (aort* adj3 (transect* or disrupt* or tear or torn or rupture* or injur*)).ti,ab. 10045 5 (thora* adj3 (transect* or disrupt* or tear or torn or rupture* or injur*)).ti,ab. 7479 6 (thora* adj5 repair).ti,ab. 5001 7 (trauma* adj5 thoracic).ti,ab. 3923 8 or/1‐7 77045 9 exp STENTS/ 69255 10 exp Thoracic Surgery/ 12143 11 exp Blood Vessel Prosthesis/ 27544 12 exp Blood Vessel Prosthesis Implantation/ 20808 13 (stent* or graft* or tevar or endograft* or endograft* or endoprosthe*).ti,ab. 382983 14 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancurepowerlink or anaconda or Ancure or Advanta or Intracoil or Zilver or Luminex).ti,ab. 119849 15 endovascular.ti,ab. 41664 16 Vascular Surgical Procedures/ 29086 17 exp Endovascular Procedures/ 107508 18 or/9‐17 639441 19 8 and 18 15352 20 randomized controlled trial.pt. 467089 21 controlled clinical trial.pt. 92592 22 randomized.ab. 419648 23 placebo.ab. 191146 24 drug therapy.fs. 2041380 25 randomly.ab. 295821 26 trial.ab. 436951 27 groups.ab. 1825297 28 or/20‐27 4264875 29 19 and 28 1691 30 (2017* or 2018*).ed. 1602207 31 29 and 30 226 |
226 |
| EMBASE | 1 exp thoracic aorta/ 17826 2 exp aortic rupture/ 467 3 exp thorax injury/ 65667 4 (aort* adj3 (transect* or disrupt* or tear or torn or rupture* or injur*)).ti,ab. 11570 5 (thora* adj3 (transect* or disrupt* or tear or torn or rupture* or injur*)).ti,ab. 7853 6 (thora* adj5 repair).ti,ab. 5851 7 (trauma* adj5 thoracic).ti,ab. 4349 8 or/1‐7 101954 9 exp stent/ 145005 10 exp thorax surgery/ 495762 11 exp blood vessel prosthesis/ 11224 12 (stent* or graft* or tevar or endograft* or endograft* or endoprosthe*).ti,ab. 503775 13 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancurepowerlink or anaconda or Ancure or Advanta or Intracoil or Zilver or Luminex).ti,ab. 73801 14 endovascular.ti,ab. 56965 15 vascular surgery/ 30862 16 exp endovascular surgery/ 28079 17 or/9‐16 1067561 18 8 and 17 25820 19 randomized controlled trial/ 485275 20 controlled clinical trial/ 453455 21 random$.ti,ab. 1255604 22 randomization/ 78353 23 intermethod comparison/ 224410 24 placebo.ti,ab. 263205 25 (compare or compared or comparison).ti. 440032 26 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1683959 27 (open adj label).ti,ab. 61772 28 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 201124 29 double blind procedure/ 145019 30 parallel group$1.ti,ab. 20918 31 (crossover or cross over).ti,ab. 89816 32 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 271999 33 (assigned or allocated).ti,ab. 320352 34 (controlled adj7 (study or design or trial)).ti,ab. 281246 35 (volunteer or volunteers).ti,ab. 217697 36 trial.ti. 234877 37 or/19‐36 3867888 38 18 and 37 3861 39 (2017* or 2018*).em. 2804651 40 38 and 39 566 41 from 40 keep 1‐566 566 |
566 |
| CINAHL | S31 S29 AND S30 6 S30 EM 2017 OR EM 2018 412,721 S29 S16 AND S28 91 S28 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 292,248 S27 MH "Random Assignment" 39,196 S26 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 32,877 S25 MH "Crossover Design" 11,264 S24 MH "Factorial Design" 922 S23 MH "Placebos" 8,375 S22 MH "Clinical Trials" 93,035 S21 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,544 S20 TX crossover OR "cross‐over" 14,666 S19 AB placebo* 0 S18 TX random* 221,071 S17 TX "latin square" 143 S16 S8 AND S15 1,324 S15 S9 OR S10 OR S11 OR S12 OR S13 OR S14 67,790 S14 TX endovascular 4,827 S13 TX powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancurepowerlink or anaconda or Ancure or Advanta or Intracoil or Zilver or Luminex 4,795 S12 TX stent* or graft* or tevar or endograft* or endograft* or endoprosthe* 34,865 S11 (MH "Blood Vessel Prosthesis") 1,016 S10 (MH "Thoracic Surgery+") 32,807 S9 (MH "Stents+") 10,048 S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 5,437 S7 aort* n3 (transect* or disrupt* or tear or torn or rupture* or injur*) 1,314 S6 TX trauma* n5 thoracic 462 S5 TX thora* n5 repair 0 S4 TX (thora* n3 (transect* or disrupt* or tear or torn or rupture* or injur*)) 2,395 S3 (MH "Thoracic Injuries+") 1,852 S2 (MH "Aortic Rupture") 505 S1 (MH "Aorta, Thoracic") 1,268 |
6 |
| AMED | 1 (aort* adj3 (transect* or disrupt* or tear or torn or rupture* or injur*)).ti,ab. 11 2 (thora* adj3 (transect* or disrupt* or tear or torn or rupture* or injur*)).ti,ab. 106 3 (thora* adj5 repair).ti,ab. 4 4 (trauma* adj5 thoracic).ti,ab. 26 5 exp Aorta/ 130 6 exp Thoracic injuries/ 9 7 or/1‐6 278 8 exp Stents/ 189 9 exp Thoracic surgery/ 350 10 (stent* or graft* or tevar or endograft* or endograft* or endoprosthe*).ti,ab. 1612 11 (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancurepowerlink or anaconda or Ancure or Advanta or Intracoil or Zilver or Luminex).ti,ab. 218 12 endovascular.ti,ab. 28 13 or/8‐12 2116 14 7 and 13 7 15 exp CLINICAL TRIALS/ 3788 16 RANDOM ALLOCATION/ 314 17 DOUBLE BLIND METHOD/ 667 18 Clinical trial.pt. 1212 19 (clinic* adj trial*).tw. 5438 20 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2866 21 PLACEBOS/ 591 22 placebo*.tw. 3132 23 random*.tw. 17749 24 PROSPECTIVE STUDIES/ 1119 25 or/15‐24 22789 26 14 and 25 0 |
0 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADSORB Trial | ADSORB trial compares endovascular intervention versus best medical treatment. This is not the aim of the review |
| INSTEAD Trial | INSTEAD trial investigates benefits of endovascular intervention in type B aortic dissection. This is not the aim of the review |
| Liang‐Wan 2011 | A clinical comparison of two operational methods (replacing ascending aorta + reconstructing aortic arch with triple‐branched stent graft and replacing ascending aorta + replacing half aortic arch to treat the aortic dissection). This study does not reflect the aims of this review |
| Shackford 2017 | Not a randomised controlled trial |
Differences between protocol and review
2015 version
In keeping with updated requirements of The Cochrane Collaboration, we will assess the quality of all future included studies using the risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The title of this review has been changed from 'Thoracic stent graft versus surgery for traumatic thoracic transection' to 'Thoracic endovascular repair (TEVAR) versus open surgery for blunt traumatic thoracic aortic injury', so that all patients who have a thoracic aortic injury will be captured, as well as the thoracic intervention rather than the device used. We have amended the objective of the review and, accordingly, the types of participants included and interventions provided.
Contributions of authors
DP selected studies, assessed the methodological rigour of studies, extracted data and wrote the review. DH selected studies, assessed the methodological rigour of studies, extracted data and wrote the review. PB resolved conflicts related to methodological quality, extracted data and commented on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
DP: none known. DH: none known. PB: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
ADSORB Trial {published data only}
- Brunkwall J. ADSORB: one year results of stent grafts for acute uncomplicated type B dissection. Eurointervention 2013.
- Brunkwall J, Kasprzak P, Heijmen R, Verhoeven E, von‐Tengg‐Kobligk H, Taylor P. ADSORB ‐ a prospective randomised controlled trial in acute uncomplicated Type B dissection: stent graft induces false channel thrombosis and reduces its false lumen size ‐ 1 year results. Abstracts of the European Society for Vascular Surgery XXVI Annual Meeting 2012:64‐5.
- Brunkwall J, Lammer J, Verhoeven E, Taylor P. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. European Journal of Vascular and Endovascular Surgery 2012;44(1):31‐6. [DOI] [PubMed] [Google Scholar]
- Lammer J, Brunkwall JS, Taylor PR, Verhoeven EL, Kasprzak P. The ADSORB trial: acute dissection treatment with stent graft or best medical therapy. Cardiovascular and Interventional Radiology 2012; Vol. 35:S175.
INSTEAD Trial {published data only}
- Nienaber C. INSTEAD Trial ‐ 3 month data (INvestigation of STEntgrafts in Aortic Dissection). More Vascular and Endovascular Controversies, Charing Cross 28th International Symposium; 2006 Apr 8‐11; London. (//bibamed.agcl.com/cx_2006/Sun1615Nienaber.pdf).
- Nienaber C. Long‐term (3‐5 year) results of the INSTEAD trial shows the benefit of early TEVAR treatment of uncomplicated type B aortic dissections: game changing results. Veith 39th Annual Symposium. 2012.
- Nienaber C. Type B aortic dissection ‐ Intervention vs. best medical treatment. Vascular News 2006;Educational Supplement (VN32):2‐3. [Google Scholar]
- Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, Kodolitsch Y, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent‐graft placement. New England Journal of Medicine 1999;340(20):1539‐45. [DOI] [PubMed] [Google Scholar]
- Nienaber CA, Kische S, Akin I, Rousseau H, Eggebrecht H, Fattori R, et al. Strategies for subacute/chronic type B aortic dissection: the Investigation of Stent Grafts in Patients with type B Aortic Dissection (INSTEAD) trial 1‐year outcome. Journal of Thoracic and Cardiovascular Surgery 2010;140(6 Suppl):S101‐8. [DOI] [PubMed] [Google Scholar]
- Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection: long‐term results of the randomized investigation of stent grafts in aortic dissection trial. Circulation: Cardiovascular Interventions 2013;6:407‐16. [DOI] [PubMed] [Google Scholar]
- Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120(25):2519‐28. [DOI] [PubMed] [Google Scholar]
- Nienaber CA, Zannetti S, Barbieri B, Kische S, Schareck W, Rehders TC, et al. INvestigation of STEnt grafts in patients with type B Aortic Dissection: design of the INSTEAD trial ‐ a prospective, multicenter, European randomized trial. American Heart Journal 2005;149(4):592‐9. [DOI] [PubMed] [Google Scholar]
- Nienaber NA. INSTEAD Trial: INvestigation of STEnt Grafts in Patients With Type B Aortic Dissection. clinicaltrials.gov/ct2/show/NCT00525356?term=aortic+dissection&rank=2 (first posted 5 September 2007).
Liang‐Wan 2011 {published data only}
- Liang‐Wan C. The contrast of the outcome between replacing ascending aorta + reconstructing aortic arch with triple‐branched stent graft and replacing ascending aorta +replacing half aortic arch to treat the aortic dissection (ChiCTR‐TRC‐11001828). chictr.org.cn/hvshowproject.aspx?id=1952 (first posted 29 December 2011).
Shackford 2017 {published data only}
- Shackford SR, Dunne CE, Karmy‐Jones R, Long W 3rd, Teso D, Schreiber MA, et al. The evolution of care improves outcome in blunt thoracic aortic injury: a Western Trauma Association multicenter study. Journal of Trauma and Acute Care Surgery 2017;83(6):1006‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azizzadeh 2013
- Azizzadeh A, Charlton‐Ouw KM, Chen Z, Rahbar MH, Estrera AL, Amer H, et al. An outcome analysis of endovascular versus open repair of blunt traumatic aortic injuries. Journal of Vascular Surgery 2013;57(1):108‐14; discussion 115. [PUBMED: 23141678] [DOI] [PubMed] [Google Scholar]
Bavaria 2007
- Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low‐risk patients: a multicenter comparative trial. Journal of Thoracic and Cardiovascular Surgery 2007;133(2):369‐77. [PUBMED: 17258566] [DOI] [PubMed] [Google Scholar]
Buz 2008
- Buz S, Zipfel B, Mulahasanovic S, Pasic M, Weng Y, Hetzer R. Conventional surgical repair and endovascular treatment of acute traumatic aortic rupture. European Journal of Cardiothoracic Surgery 2008;33(2):143‐9. [PUBMED: 18065235] [DOI] [PubMed] [Google Scholar]
Cowley 1990
- Cowley RA, Turney SZ, Hankins JR, Rodriguez A, Attar S, Shankar BS. Rupture of thoracic aorta caused by blunt trauma: a fifteen year experience. Journal of Thoracic and Cardiovascular Surgery 1990;100(5):652‐60. [PubMed] [Google Scholar]
Dake 1994
- Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent‐grafts for the treatment of descending thoracic aneurysms. New England Journal of Medicine 1994;331(26):1729‐34. [DOI] [PubMed] [Google Scholar]
Demetriades 2008
- Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy‐Jones R, Teixeira PG, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma Multicenter Study. Journal of Trauma 2008;64(3):561‐71. [PUBMED: 18332794] [DOI] [PubMed] [Google Scholar]
DuBose 2015
- DuBose JJ, Leake SS, Brenner M, Pasley J, O'Callaghan T, Luo‐Owen X, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. Journal of Trauma and Acute Care Surgery 2015;78(2):360‐9. [PUBMED: 25757123] [DOI] [PubMed] [Google Scholar]
Erben 2018
- Erben Y, Trejo G, Brownstein AJ, Jean RA, Ziganshin BA, Carino D, et al. Endovascular thoracic aortic transection repair has equivalent survival to open repair after blunt thoracic aortic injury. International Angiology 2018;37(2):155‐9. [DOI] [PubMed] [Google Scholar]
Feezor 2008
- Feezor RJ, Martin TD, Hess PJ Jr, Daniels MJ, Beaver TM, Klodell CT, et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Annals of Thoracic Surgery 2008;86(6):1809‐14. [PUBMED: 19021982] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc). GRADEpro GDT. Version accessed 7 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc), 2015.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jonker 2010
- Jonker FH, Giacovelli JK, Muhs BE, Sosa JA, Indes JE. Trends and outcomes of endovascular and open treatment for traumatic thoracic aortic injury. Journal of Vascular Surgery 2010;51(3):565‐71. [PUBMED: 20045619] [DOI] [PubMed] [Google Scholar]
Kato 1997
- Kato N, Dake MD, Miller DC, Semba CP, Mitchell RS, Razavi MK, et al. Traumatic thoracic aneurysm: treatment with endovascular stent‐grafts. Radiology 1997;205(3):657‐62. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. Journal of Vascular Surgery 2011;53(1):187‐92. [PUBMED: 20974523] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available from handbook.cochrane.org.
Murad 2011
- Murad MH, Rizvi AZ, Malgor R, Carey J, Alkatib AA, Erwin PJ, et al. Comparative effectiveness of the treatments for thoracic aortic transection [corrected]. Journal of Vascular Surgery 2011;53(1):193‐9.e1‐21. [PUBMED: 21035988] [DOI] [PubMed] [Google Scholar]
Nagy 2000
- Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. Journal of Trauma 2000;48(6):1128‐43. [DOI] [PubMed] [Google Scholar]
O'Conner 2004
- O'Conner CE. Diagnosing traumatic rupture of the thoracic aorta in the emergency department. Emergency Medicine Journal 2004;21:414‐9. [PMC free article] [PubMed] [Google Scholar]
Orford 2003
- Orford VP, Atkinson NR, Thomson K, Milne PY, Campbell WA, Roberts A, et al. Blunt traumatic aortic transection: the endovascular experience. Annals of Thoracic Surgery 2003;75(1):106‐11. [DOI] [PubMed] [Google Scholar]
Parodi 1991
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Annals of Vascular Surgery 1991;5(6):491‐9. [DOI] [PubMed] [Google Scholar]
Passaro 1959
- Passaro E, Pace WG. Traumatic rupture of the aorta. Surgery 1959;46:787‐91. [PubMed] [Google Scholar]
Tehrani 2006
- Tehrani HY, Peterson BG, Katariya K, Morasch MD, Stevens R, DiLuozzo G, et al. Endovascular repair of thoracic aortic tears. Annals of Thoracic Surgery 2006;82(3):873‐8. [DOI] [PubMed] [Google Scholar]
von Oppell 1994
- Oppell UO, Dunne TT, Groot MK, Zilla P. Traumatic aortic rupture: twenty year meta‐analysis of mortality and risk of paraplegia. Annals of Thoracic Surgery 1994;58(2):585‐93. [DOI] [PubMed] [Google Scholar]
Xenos 2008
- Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, et al. Meta‐analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. Journal of Vascular Surgery 2008;48(5):1343‐51. [PUBMED: 18632242] [DOI] [PubMed] [Google Scholar]
Yamane 2008
- Yamane BH, Tefera G, Hoch JR, Turnipseed WD, Acher CW. Blunt thoracic aortic injury: open or stent graft repair?. Surgery 2008;144(4):575‐80; discussion 580‐2. [PUBMED: 18847641] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Asmat 2007
- Asmat A, Lee CN, Robless P. Thoracic stent graft versus surgery for traumatic thoracic transection. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006642] [DOI] [Google Scholar]
Pang 2015
- Pang D, Hildebrand D, Bachoo P. Thoracic endovascular repair (TEVAR) versus open surgery for blunt traumatic thoracic aortic injury. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD006642.pub2] [DOI] [PubMed] [Google Scholar]


