Porcine circovirus type 3 (PCV3), an emerging porcine circovirus, is considered the cause of porcine dermatitis and nephropathy syndrome (PDNS)-like clinical signs and other systemic diseases in piglets and sows. To evaluate the pathogenesis of PCV3 infection in vivo, we used a PCV3 virus stock from the rescue of an infectious PCV3 DNA clone to intranasally inoculate 4- and 8-week-old specific-pathogen-free piglets and demonstrated successful reproduction of PDNS-like disease in animals that were inoculated with PCV3 alone or PCV3 combined with immunostimulation by keyhole limpet hemocyanin. Both 4- and 8-week-old PCV3-inoculated piglets showed similar increases in viral loads in the sera and had seroconverted to PCV3 capsid antibody. Pathological lesions and PCV3-specific antigen were detected in various tissues and organs, while numerous proinflammatory cytokines and chemokines in the sera were significantly upregulated after PCV3 inoculation. These results will provide significant information regarding the pathogenesis of PCV3 in piglets.
KEYWORDS: PCV3 antigen distribution, lesions, pathogenesis, porcine circovirus type 3, PCV3, porcine dermatitis and nephropathy syndrome-like disease
ABSTRACT
Porcine circovirus type 3 (PCV3) is an emerging porcine circovirus that has been associated with porcine dermatitis and nephropathy syndrome (PDNS)-like clinical signs, reproductive failure, cardiac pathologies, and multisystemic inflammation in piglets and sows. Many aspects of PCV3 infection biology and pathogenesis, however, remain unknown. Here, we used a PCV3 virus stock from the rescue of an infectious PCV3 DNA clone to intranasally inoculate 4- and 8-week-old specific-pathogen-free piglets for evaluation of PCV3 pathogenesis. For 4-week-old piglets, typical clinical signs resembling those of PDNS-like disease were observed when piglets were inoculated with PCV3 alone or PCV3 combined with immunostimulation by keyhole limpet hemocyanin, with a mortality of 40% (2/5) for both types of inoculated piglets during a 28-day observation period postinoculation. Both types of inoculated piglets showed similar progressive increases in viral loads in the sera and had seroconverted to PCV3 capsid antibody after inoculation. Pathological lesions and PCV3-specific antigen were detected in various tissues and organs, including the lung, heart, kidney, lymph nodes, spleen, liver, and small intestine, in both types of inoculated piglets. The levels of proinflammatory cytokines and chemokines, including interleukin 1 beta (IL-1β), IL-6, IL-23α, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and chemokine ligand 5 (CCL5), were significantly upregulated in both groups of inoculated piglets. Eight-week-old piglets also exhibited a similar PDNS-like disease but without death after PCV3 inoculation, as evidenced by pathological lesions and PCV3 antigen in various tissues and organs. These results show for the first time successful reproduction of PDNS-like disease by PCV3 infection and further provide significant information regarding the pathogenesis of PCV3 in piglets.
IMPORTANCE Porcine circovirus type 3 (PCV3), an emerging porcine circovirus, is considered the cause of porcine dermatitis and nephropathy syndrome (PDNS)-like clinical signs and other systemic diseases in piglets and sows. To evaluate the pathogenesis of PCV3 infection in vivo, we used a PCV3 virus stock from the rescue of an infectious PCV3 DNA clone to intranasally inoculate 4- and 8-week-old specific-pathogen-free piglets and demonstrated successful reproduction of PDNS-like disease in animals that were inoculated with PCV3 alone or PCV3 combined with immunostimulation by keyhole limpet hemocyanin. Both 4- and 8-week-old PCV3-inoculated piglets showed similar increases in viral loads in the sera and had seroconverted to PCV3 capsid antibody. Pathological lesions and PCV3-specific antigen were detected in various tissues and organs, while numerous proinflammatory cytokines and chemokines in the sera were significantly upregulated after PCV3 inoculation. These results will provide significant information regarding the pathogenesis of PCV3 in piglets.
INTRODUCTION
Porcine circoviruses (PCVs) belong to the genus Circovirus within the family Circoviridae, and currently, two porcine circovirus genotypes, PCV1 and PCV2, have been extensively studied (1, 2). PCV1, which was first identified as a contaminating agent in a pig kidney cell line during the 1970s, is not pathogenic to pigs (3). PCV2 was originally recognized in Canada during the early 1990s and has since been identified as a major pathogen for PCV-associated disease (PCVAD), which is characterized by several clinical conditions, including postweaning multisystemic wasting syndrome (PMWS), reproductive failure, enteritis, and respiratory disorders, and has caused significant economic losses in major swine-producing countries (4–6). PCV2 preferentially targets lymphoid tissues, leading to lymphoid depletion followed by immunosuppression in pigs (7–9). Infection with PCV2 is typically subclinical; clinical PMWS was not reproduced in pigs when infected with PCV2 alone. However, PCVAD is exacerbated by immunostimulation or coinfections with other pathogens (10–14). For example, coinfection of PCV2 with pathogens such as porcine parvovirus (PPV), porcine reproductive and respiratory syndrome virus (PRRSV), or Mycoplasma hyopneumoniae can lead to reproduction of PMWS (10–12).
In 2015, a novel PCV, designated PCV3, was first identified as a pathogenic agent in sows that died and displayed acute porcine dermatitis and nephropathy syndrome (PDNS)-like clinical signs, reproductive failure, cardiac pathology, and multisystemic inflammation in the United States (15, 16) and then in China, Poland, South Korea, Brazil, Thailand, Germany, Denmark, Spain, and Italy (17–24). An epidemic survey suggested that PCV3 was a major pathogen in a total of 356 sows (three farms) that suffered from reproductive failure and acute loss of neonatal piglets in China in 2016 (25). Recently, Fu et al. found that PCV3 is widely distributed in southern China and suggested that PCV3 has been circulating in swine herds for nearly half a century and may have originated from a bat-associated circovirus (26). This might implicate that PCV3, which served as an etiological agent, exhibited severe pathogenicity to pigs as observed for PCVAD. The PCV3 genome contains a single-stranded circular DNA of 2.0 kb (15), and three major open reading frames (ORFs) have been predicated, including ORF1, called the rep gene, ORF2, called the cap gene, and ORF3, which encodes a protein of unknown function. As a major structural protein, the cap protein determines the antigenic characteristics of circoviruses (27), but the cap protein of PCV3 only shares approximately 36% to 37% amino acid identity with that of PCV2 (15). Experimental infection of PCV2 cannot reproduce PDNS, but successful reproduction of PDNS was obtained when coinoculated with PRRSV and a torque teno virus (TTV)-affected tissue homogenate (28), indicating that PCV2 might not be a major causative agent for induction of PDNS-like clinical disease. Although PCV3 was recently proposed to associate with PDNS-like clinical disease in pigs, many aspects of its infection biology and pathogenesis remain unclear.
We report here the pathogenesis of a PCV3 strain from the rescue of an infectious PCV3 DNA clone and the successful reproduction of PDNS-like clinical disease following PCV3 experimental inoculation of 4- and 8-week-old piglets. Pathological lesions and PCV3-specific antigen were detected in various tissues and organs, including the lung, heart, kidney, lymph nodes, spleen, liver, and small intestine, while numerous proinflammatory cytokines and chemokines in the sera were significantly upregulated after PCV3 inoculation. The results presented here provide important insights into the pathogenesis of PCV3-induced PDNS-like clinical disease in piglets.
RESULTS
Generation of PCV3 infectious stock.
The full-length PCV3 genome was synthesized according to the published sequence of the PCV3/CN/Hebei-LY/2015 (MF318451). The synthesized PCV3 genome was ligated into the pSK vector to produce the PCV3 molecular DNA clone (Fig. 1A). The rescued PCV3 from transfection of PCV3 DNA was passaged fifteen times in PK15 cells to increase viral titers. For the detection of PCV3 (designated strain LY) replication, PK15 cells were infected with the recovered viruses and analyzed by immunofluorescence assay using an anti-PCV3-specific antibody. Different passages (5, 10, and 15 passages) of the rescued PCV3 were collected for PCV3 genomic sequencing, and no nucleotide mutation was found in these PCV3 genomes (data not shown), indicating the genetic stability of the rescued PCV3 during continued passage in cultured PK15 cells. Figure 1B shows the results of immunofluorescence staining of PCV3-infected cells. 2,4-Diamidino-2-phenylindole (DAPI) was used for nuclear staining (blue), and cells infected with PCV3 showed a green immunofluorescence signal in the cytoplasm and to some degree in the nucleus. No green fluorescence signal was detected with the anti-PCV3 polyclonal antibody in the mock-infected cells (Fig. 1B). The titer of the PCV3 virus stock after 15 passages was determined to be 106.53 50% tissue culture infective dose (TCID50)/ml at 72 h postinfection.
FIG 1.
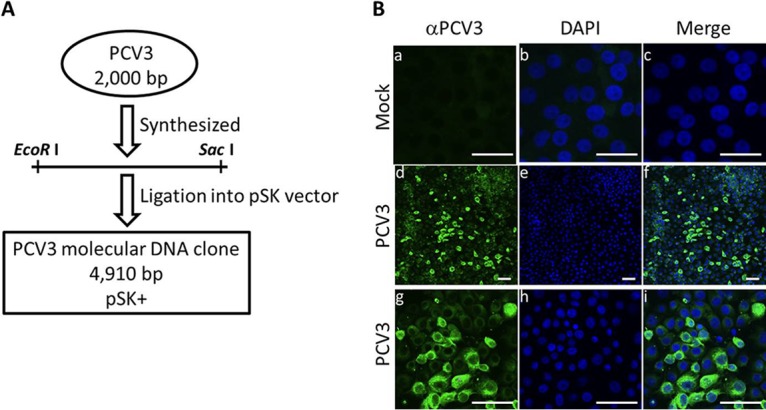
(A) Construction of an infectious PCV3 molecular DNA clone. The full-length PCV3 genome was synthesized according to the published sequence of PCV3/CN/Hebei-LY/2015 (MF318451). A molecular DNA clone containing the PCV3 genome with EcoRI and SacI restriction enzyme sites at 5′ and 3′ ends was cloned into the pBluescript SK (pSK) vector, which was predigested with the EcoRI and SacI restriction enzymes to produce a recombinant plasmid, pSK-PCV3. (B) Immunofluorescence analysis of PCV3-infected cells. PK15 cells infected with the rescued PCV3 virus stock (passage 15) at a multiplicity of infection (MOI) of 0.1 for 72 h. The PCV3-infected cells were immunostained with porcine serum IgG against PCV3 (green) followed by DAPI staining for nuclei (blue). g, h, and i are partial enlargements of d, e, and f, respectively. Mock-infected PK15 cells showed no positivity for PCV3 antigen (a, b, and c). Overlaid images are shown in c, f, and i. Bars, 10 μm.
PCV3 viral loads were present in the sera of PCV3-inoculated piglets.
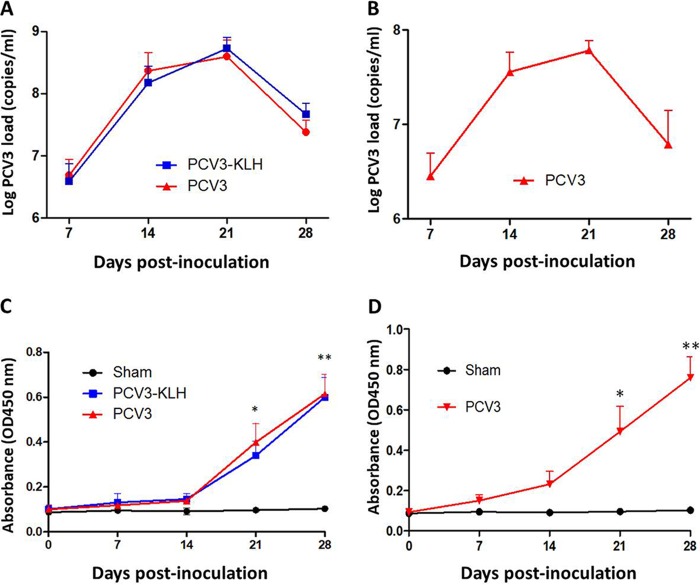
The rescued PCV3 was used to intranasally inoculate 4- and 8-week-old specific-pathogen-free piglets for the evaluation of PCV3 pathogenesis. Serum samples were collected from all piglets at 0, 7, 14, 21, and 28 days postinoculation (dpi) and were assayed for PCV3 viremia by absolute quantitative real-time PCR (qPCR). Sham-inoculated piglets were negative for PCV3 viremia throughout the study. For 4-week-old piglets, both the PCV3- and PCV3-keyhole limpet hemocyanin (KLH)-inoculated groups exhibited PCV3 viremia at the above-indicated time points after inoculation (Fig. 2A). The PCV3 genomic copy numbers in the serum samples ranged from 2.23 × 105 to 7.72 × 108 copies/ml for the PCV3-inoculated piglets and from 3.52 × 105 to 8.31 × 108 copies/ml for the PCV3-KLH-inoculated piglets. The viral loads of the PCV3-inoculated group piglets were slightly lower than those of the PCV3-KLH-inoculated group piglets at 21 and 28 dpi; however, no significant differences between these two groups were observed at any of the time points after inoculation. Eight-week-old piglets also exhibited PCV3 viremia at the above-indicated time points after inoculation, with the maximum level of the mean PCV3 genomic copy number (7.92 × 107 copies/ml) at 21 dpi as observed for 4-week-old piglets in the PCV3- and PCV3-KLH-inoculated groups (Fig. 2B).
FIG 2.
PCV3 loads in sera from 4-week-old (A) and 8-week-old (B) PCV3-inoculated piglets were quantitatively assayed by real-time PCR. PCV3 ORF2-specific antibody dynamics in sera of the 4-week-old (C) and 8-week-old (D) PCV3-inoculated piglets were determined by using an indirect ELISA. The values presented are the means of the results from the five piglets of the 8-week-old PCV3-inoculated experiment at all the indicated time points postinoculation or of the 4-week-old PCV3-inoculated experiment at 7 and 14 dpi in all groups, except for the four piglets at 21 dpi and the remaining three piglets at 28 dpi in groups 2 and 3, due to death of the piglets; error bars show the standard deviations. *, P < 0.05; **, P < 0.01.
The anti-PCV3 cap antibodies in serum samples were examined by recombinant PCV3-cap protein-based enzyme-linked immunosorbent assay (ELISA). Capsid-specific antibody dynamics in the 4- and 8-week-old piglets during the 28-day experimental period are shown in Fig. 2C and D. All the sham-inoculated 4- and 8-week-old piglets were negative for anti-PCV3 antibody during the 28-day experimental period. All the 4- and 8-week-old inoculated piglets, regardless of PCV3 alone or PCV3-KLH inoculation, had seroconverted to PCV3 from 21 dpi onwards. The results indicate that PCV3 resulted in a humoral immune response in the PCV3-inoculated piglets during the late stage of PCV3 infection when the viral burdens of the sera were decreased.
Infection of PCV3 reduced the proliferation of PBMCs.
Conventional 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assays were used to determine the proliferation of peripheral blood mononuclear cells (PBMCs) from the 4-week-old PCV3-inoculated piglets. As shown in Fig. 3, the stimulation index (SI) levels in the presence of phytohemagglutinin (PHA) in the PBMCs of the PCV3- and PCV3-KLH-inoculated groups and the proliferation of PBMCs in both the PCV3- and PCV3-KLH-inoculated groups were significantly lower (P < 0.05) than those in the sham-inoculated groups at all time points after inoculation. The results indicated that PCV3 reduced lymphocyte proliferation in peripheral blood in piglets.
FIG 3.
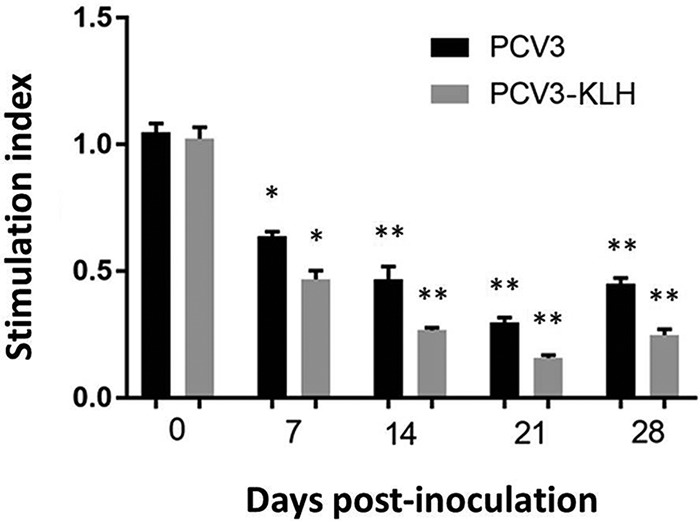
Cell proliferation assay. Culturing of PBMCs from the 4-week-old PCV3-inoculated, PCV3-KLH-inoculated, and sham-inoculated piglets at the indicated time points after PCV3 inoculation was performed three times in the presence of PHA (1 μg/ml). After 72 h, MTT reduction was applied to evaluate the proliferation. Data are presented as the means ± SDs. *, P < 0.05; **, P < 0.01.
Clinical signs induced by PCV3 infection.
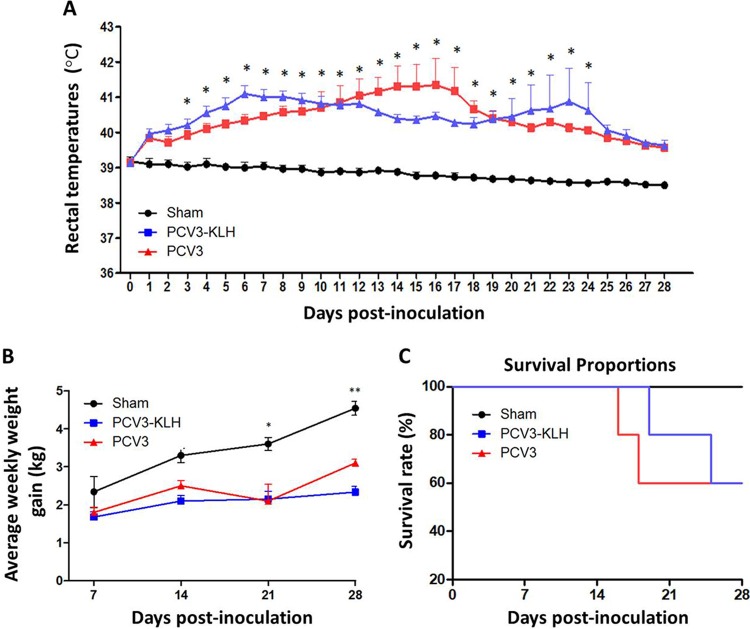
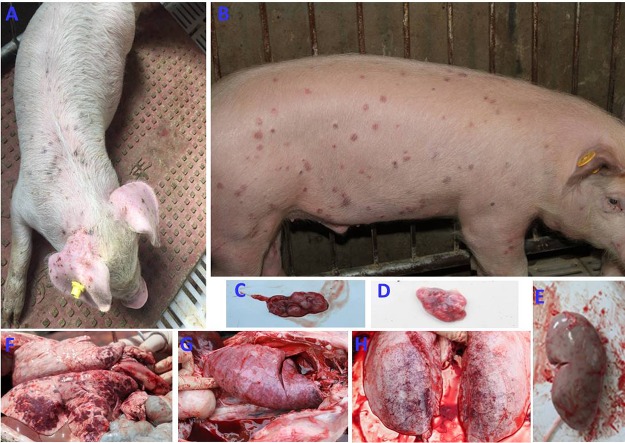
As expected, no obvious clinical signs were observed in the sham-inoculated piglets at any time throughout the animal experiments. For 4-week-old piglets, in group 2 piglets inoculated with PCV3, clinical signs were first observed in two of five piglets at 8 dpi, followed by in three of five piglets at 10 dpi and four of five piglets at 13 dpi; all the piglets exhibited clinical signs at 16 dpi until the end of the experiment. In group 3 piglets that were inoculated with PCV3-KLH, clinical signs were first observed in two of five piglets at 8 dpi, followed by in two of five piglets at 10 dpi and four of five piglets at 13 dpi; all the piglets exhibited clinical signs at 19 dpi until the end of the experiment. All the piglets in groups 2 and 3 exhibited rectal temperatures ≥40.0°C (ranging from 40.0°C to 42.4°C) for approximately 20 consecutive days after inoculation (Fig. 4A). The rectal temperatures in group 2 piglets peaked (42.4°C) at 16 dpi and then decreased, whereas the rectal temperatures of group 3 piglets peaked twice (41.5°C at 6 dpi and 42.3°C at 23 dpi). No obvious rectal temperature changes were observed in the sham-inoculated group piglets during the experimental period. Following PCV3 inoculation, all the piglets in groups 2 and 3 exhibited reductions in body weight gain throughout the experiment (Fig. 4B). The average weekly weight gains of all of the inoculated piglets regardless of PCV3 or PCV3-KLH inoculation were significantly lower (P < 0.05) than those of the sham-inoculated piglets at 21 and 28 dpi. In addition, the PCV3-KLH-inoculated piglets exhibited the maximum body weight loss, with an average weekly weight loss of approximately 1.9 kg at 28 dpi. Two piglets in group 2 died at 16 and 18 dpi (Fig. 4C), while two piglets in group 3 died at 19 and 25 dpi (Fig. 4C). The temperature changes and body weight gains were roughly consistent with the clinical signs after PCV3 inoculation. Clinical signs in piglets from groups 2 and 3 were similar. Initial observations consisted of anorexia, coughing, sneezing, and diarrhea within 8 dpi followed by severer symptoms, such as increased respiratory rates, lethargy, rubefaction on the skin and the ears, multifocal papules, shivering, and/or hyperspasmia, within 15 dpi, and these signs persisted until the end of the experiment. Figure 5A shows multifocal papules, macules, and/or superficial dermatitis. For 8-week-old piglets, typical clinical signs of PDNS-like disease were also observed in piglets inoculated with PCV3 but without death during a 28-day observation period (Fig. 5B and data not shown). These results indicate that inoculation of PCV3 alone was capable of inducing severe clinical PDNS-like diseases in piglets.
FIG 4.
(A) Rectal temperature kinetics of the 4-week-old PCV3-inoculated piglets. *, P < 0.05 for the comparison of the PCV3-inoculated or PCV3-KLH-inoculated and sham-inoculated piglets at the indicated times after inoculation. Data are shown as the means ± SDs. (B) Average weekly weight gains of the 4-week-old PCV3-inoculated piglets following inoculation. *, P < 0.05; **, P < 0.01 for the comparison of the PCV3-inoculated or PCV3-KLH-inoculated and sham-inoculated piglets at the indicated times after inoculation. Average weekly weight gain values are shown as the means ± SDs. (C) Survival rates of the 4-week-old PCV3-inoculated and PCV3-KLH-inoculated piglets.
FIG 5.
Clinical signs and gross lesions of the 4- or 8-week-old PCV3-inoculated piglets. Clinical signs of a 4-week-old PCV3-inoculated piglet at 28 dpi (A) or an 8-week-old PCV3-inoculated piglet at 14 dpi (B) were mainly exudative dermatitis, which was characterized by red or purple papules and macules. Inguinal lymph node (C) of a 4-week-old PCV3-inoculated piglet and tracheobronchial lymph node (D) of a PCV3-KLH-inoculated piglet were markedly enlarged, hemorrhaging, and tan. (E) Kidney from a 4-week-old PCV3-inoculated piglet was edematous with many needle-like bleeding spots. Lungs from a 4-week-old PCV3-inoculated piglet (F), a PCV3-KLH-inoculated piglet (G), and an 8-week-old PCV3-inocluated piglet (H) were rubbery, failed to collapse, and had a diffuse mottled tan-red appearance and severe multifocal dark purple-to-red consolidation.
Gross lesions.
From 4-week-old piglets, lungs, livers, kidneys, spleens, hearts, small intestines, and lymph nodes (mandibular, tracheobronchial, mesenteric, and inguinal) were collected from all the sham-, PCV3-, and PCV3-KLH-inoculated piglets at 28 dpi, and from piglets that died during the experiment, for gross observations. None of the sham-inoculated piglets exhibited notable macroscopic lesions compatible with PNDS-like disease. Moderate-to-severe gross lesions were similar among piglets in the PCV3- and PCV3-KLH-inoculated groups. Lungs exhibited collapse or failure, lobular pneumonia, bronchopneumonia, multifocal hemorrhage, or mottled tan-to-purple consolidation (Fig. 5F and G). Lymph nodes were obviously enlarged two to three times the normal size and/or were firm and showed hyperplasia (Fig. 5C and D). Livers were hyperemic with gray-white nodules and necrosis. Kidneys were swollen, with many needle-like hemorrhages or scattered hemorrhagic foci (Fig. 5E). Spleens were swollen and/or showed necrosis at the edge zone. Eight-week-old PCV3-inoculated piglets had similar gross lesions in various tissues and organs (Fig. 5H and data not shown) as observed for the 4-week-old PCV3-inoculated piglets.
Microscopic lesions.
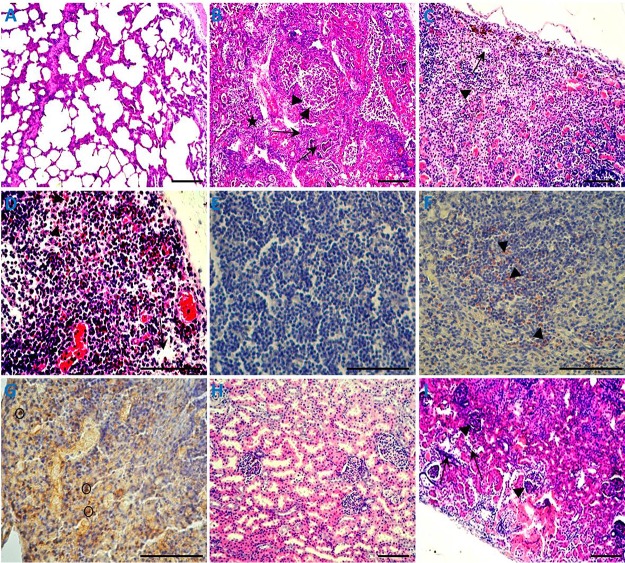
For 4-week-old piglets, microscopic lesions were similar among piglets in the PCV3- and PCV3-KLH-inoculated groups. Lung lesions were characterized by lymphoplasmacytic and histiocytic bronchointerstitial pneumonia (Fig. 6B). Inflammatory cells, including neutrophils/histiocytes, epithelial cells, macrophages, lymphocytes, and plasmacytes, infiltrated near the small blood vessels and bronchioles. Bronchial submucosa and alveolar walls were thickened, and the bronchial lumen and alveolar cavity were filled with purulent inflammatory exudates, including mainly foamy macrophages, neutrophils, epithelial cells, and their degenerative and necrotic debris. Alveolar septa were dilated and congested and infiltrated by neutrophils, lymphocytes, and plasma cells. Cortex and medullary blood vessels in the tracheobronchial lymph nodes were significantly dilated and congested and showed lymphocytic necrosis, with large areas and patches of hemorrhages. Inguinal lymph nodes showed cortex lymphocytic reduction and hyperplasia of epithelial-like cells (Fig. 6C). Mesenteric lymph nodes showed lymphocytic necrosis, lymphoid depletion, deposition of abundant hemosiderin, and natural killer cells (Fig. 6D). Notably, large abundant eosinophil infiltrations were also observed in the tracheobronchial lymph nodes, mesenteric lymph nodes, and inguinal lymph nodes (Fig. 6C, D, and F and data not shown). In addition, natural killer cells were detected in these lymphoid tissues after PCV3 inoculation (Fig. 6D and G and data not shown). For spleens, the numbers of lymphoid follicles in the white pulp were significantly reduced, the follicular area was significantly decreased, hemorrhages and necrosis were observed at the edge of the lymphoid follicles, red blood sinuses were dilated, and the reticular cells were necrotic. The kidneys displayed dilated cortical tubules and attenuation and regeneration of the tubular lining epithelium, and large clusters of lymphocytes and macrophages diffusely infiltrated the cortical interstitium and glomeruli (Fig. 6I). The hepatic lobular interstitial vessels, central veins, and hepatic sinusoids were significantly dilated and congested. The hepatocyte cells became significantly narrowed due to extrusion of congested blood. In the hearts, cardiac muscle fibers were swollen, myolysis, pyknotic nuclear debris, interstitial edema, and focal hemorrhages were observed, the epicardium was thickened and inferior epicardial fibers were coagulated and necrotic, and a large number of eosinophils and a small number of lymphocytes infiltrated necrotic areas. The small intestines showed mucosal epithelium degeneration, local mucosal epithelial cell pyknosis, necrosis, an intrinsic layer of lymphocytes, and eosinophil infiltration. Various organs and tissues from the 8-week-old PCV3-inoculated piglets showed similar microscopic lesions (data not shown) as observed for the 4-week-old PCV3-inoculated piglets.
FIG 6.
Histopathological lesions of organs in the 4-week-old PCV3-inoculated piglets. (A) Normal morphology of a lung section from a sham-inoculated piglet. (B) Lung lesions from a PCV3-inoculated piglet showed peribronchiolar lymphohistiocytic inflammation (arrows), severe necrotizing bronchiolitis (arrowheads), and interstitial pneumonia (star). Lymph node lesions, such as an inguinal lymph node (C) and mesenteric lymph node (D), from the PCV3-inoculated piglets mainly consisted of lymphocytic necrosis and depletion (arrows), a large number of eosinophil infiltrations (arrowheads), and/or hyperplasia of epithelial-like cells (box) and natural killer cells (circle). (E) No staining for eosinophils was observed in the mesenteric lymph node section from a sham-inoculated piglet by immunohistochemical assay. (F) Mesenteric lymph node sections from a PCV3-inoculated piglet showed many eosinophils (arrowheads) when reacted with an antibody raised against eosinophil major basic protein, a pan eosinophil marker. (G) Tracheobronchial lymph node section from a PCV3-inoculated piglet showed some natural killer cells (circles) when reacted with an antibody raised against CD335, a marker of NK cells. (H) Normal morphology of a kidney section from a sham-inoculated piglet. (I) Kidney lesions from a PCV3-inoculated piglet showed dilated cortical tubules and attenuation and regeneration of the tubular lining epithelium (arrows) and large clusters of diffusely infiltrated lymphocytes and macrophages in the cortical interstitium and glomeruli (arrowheads). Bars, 80 μm.
Microscopic lesions in the lung, liver, spleen, heart, kidney, lymph nodes, and small intestine were scored according to previously published scoring systems (29, 30). The average lesion scores of these tissues from 4- or 8-week-old piglets in the PCV3- and PCV3-KHL-inoculated groups were significantly different (P < 0.05) from those in the sham-inoculated piglets (Table 1). The average lesion scores of these tissues from the PCV3-KLH-inoculated group were higher than those in the PCV3-inoculated group for 4-week-old piglets, whereas the 8-week-old PCV3-inoculated piglets displayed lower average lesion scores in these tissues than those in the 4-week-old PCV3-inoculated piglets. However, there was no significant difference between the 4-week-old PCV3- and PCV3-KLH-inoculated groups or the 4- and 8-week-old PCV3-inoculated groups.
TABLE 1.
Distribution of histopathological lesions in sham- and PCV3-inocuated piglets
| Group | Inoculum | No. positive/no. tested (avg score)a for: |
||||||
|---|---|---|---|---|---|---|---|---|
| Lung | Liver | Lymph nodes | Spleen | Kidney | Heart | Small intestines | ||
| Experiment 1 | ||||||||
| 1 | Sham | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) |
| 2 | PCV3b | 5/5 (2.8) | 5/5 (1.8) | 5/5 (2.8) | 5/5 (2.6) | 5/5 (2.6) | 4/5 (1.6) | 5/5 (2.2) |
| 3 | PCV3-KLHb | 5/5 (3.0) | 5/5 (2.4) | 5/5 (3.0) | 5/5 (2.8) | 5/5 (2.8) | 5/5 (2.2) | 5/5 (2.6) |
| Experiment 2 | ||||||||
| 1 | Sham | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) |
| 2 | PCV3 | 5/5 (2.6) | 5/5 (1.8) | 5/5 (2.6) | 5/5 (2.2) | 5/5 (2.2) | 4/5 (1.6) | 5/5 (2.2) |
Average histological scores for organs and tissues: 0, normal; 1, mild; 2, moderate; 3, severe.
All the remaining piglets from each group were necropsied at 28 dpi, and a total of four dead piglets from groups 1 and 2 were also included in histopathological evaluation.
Tissue distribution of PCV3 antigen.
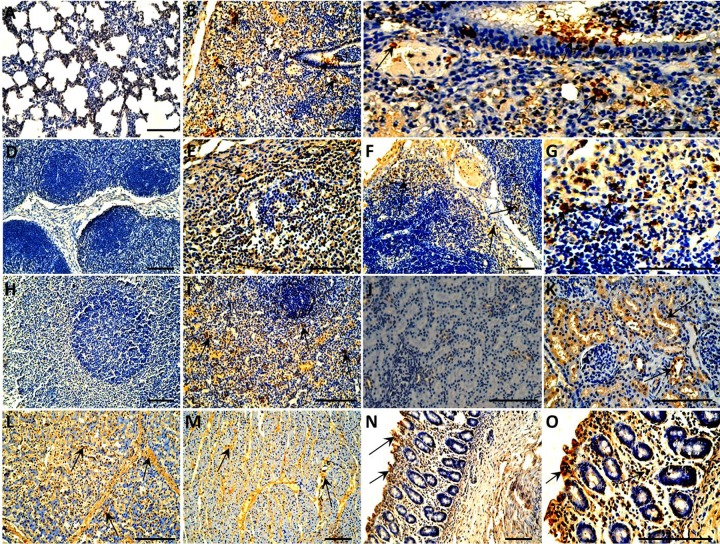
From 4-week-old piglets, lung, liver, kidney, spleen, heart, small intestine, and lymph node samples of all the piglets were further subjected to immunohistochemical staining of the PCV3 antigen. As shown in Fig. 7, all the tissues from the sham-inoculated piglets were negative for PCV3 antigen. Distributions of PCV3 antigen in various tissues and organs were similar regardless of whether they were from the PCV3- or PCV3-KLH-inoculated group piglets. In the lungs, the bronchial epithelial cell surface, interstitial vascular contents, alveolar exudate, dust cells, and septal cells all showed strong positive reactions (Fig. 7B). Cortical and medulla reticular cells, macrophages, and eosinophils showed positive reactions in the tracheobronchial lymph nodes, mesenteric lymph nodes, and inguinal lymph nodes (Fig. 7E and F and data not shown). The necrotic cells showed a positive reaction in the spleen (Fig. 7I). For kidneys, positive reactions were observed in renal tubular epithelial cells, and the necrotic epithelial cells showed strong positive signals for PCV3 antigen (Fig. 7K). Liver lobular stroma, the hepatic sinus antral wall, and the cytoplasm in the sinus were positive (Fig. 7L). Myocardial fibers showed varied positive reactions, and necrotic tissues and vascular contents showed strong positive reactions (Fig. 7M). Intestinal epithelial cells and inflammatory cells in the lamina propria were positive for PCV3 antigen (Fig. 7N). The 8-week-old PCV3-inoculated piglets exhibited similar distributions of PCV3 antigen in theses tissues (data not shown) after PCV3 inoculation as observed in the 4-week-old PCV3-inoculated piglets. For PCV3 antigen scoring, the average scores for the estimated levels of PCV3 antigens in these tissues in the 4-week-old PCV3- and PCV3-KLH-inoculated or 8-week-old PCV3-inoculated animals were significantly different (P < 0.05) from those for the sham-inoculated piglets (Table 2). However, there was no significant difference in the average scores for the estimated levels of PCV3 antigen between the 4-week-old PCV3- and PCV3-KLH-inoculated groups or the 4- and 8-week-old PCV3-inoculated groups.
FIG 7.
Immunohistochemical staining of organs and tissues of the 4-week-old piglets inoculated with PCV3. PCV3 antigen-positive cells are brown. (A) No staining was observed in the lung section from a sham-inoculated piglet. (B) Lung tissues from a PCV3-inoculated piglet showed many cells positive for PCV3 antigen (arrows). (C) Partial enlargement of panel B. (D) No staining was observed in the lymphoid tissue section from a sham-inoculated piglet. Tracheobronchial lymph node (E) and mesenteric lymph node (F) from the PCV3-inoculated piglets show many cells positive for PCV3 antigen (arrows). (G) Partial enlargement of panel F. (H) No staining was observed in the spleen section from a sham-inoculated piglet. (I) Spleen from a PCV3-inoculated piglet showed many cells positive for PCV3 antigen (arrows). (J) No staining was observed in the kidney section from a sham-inoculated piglet. (K) Kidney from a PCV3-inoculated piglet showed many cells positive for PCV3 antigen (arrows). Many cells positive for PCV3 antigen (arrows) were also observed in liver (L), heart (M), and small intestine (N) of a PCV3-inoculated piglet. (O) Partial enlargement of panel N. Bars, 80 μm.
TABLE 2.
Expression and distribution of PCV3 antigen in sham- and PCV3-inoculated piglets
| Group | Inoculum | No. positive/no. tested (avg score)a for: |
||||||
|---|---|---|---|---|---|---|---|---|
| Lung | Liver | Lymph nodes | Spleen | Kidney | Heart | Small intestines | ||
| Experiment 1 | ||||||||
| 1 | Sham | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) |
| 2 | PCV3b | 5/5 (2.8) | 5/5 (2.0) | 5/5 (2.8) | 5/5 (2.4) | 5/5 (2.4) | 4/5 (1.8) | 5/5 (2.4) |
| 3 | PCV3-KLHb | 5/5 (3.0) | 5/5 (2.4) | 5/5 (3.0) | 5/5 (2.8) | 5/5 (2.8) | 5/5 (2.4) | 5/5 (2.6) |
| Experiment 2 | ||||||||
| 1 | Sham | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) | 0/5 (0.0) |
| 2 | PCV3 | 5/5 (2.2) | 5/5 (1.6) | 5/5 (2.6) | 5/5 (2.0) | 5/5 (2.4) | 4/5 (1.6) | 5/5 (2.2) |
Average PCV3-positive antigen scores range from 0 for no signal to 3 for a strong positive signal.
All the remaining piglets from each group were necropsied at 28 dpi, and a total of four dead piglets from groups 1 and 2 were also included in evaluation of PCV3 antigen distribution.
Cytokine expression in the sera of the PCV3-inoculated piglets.
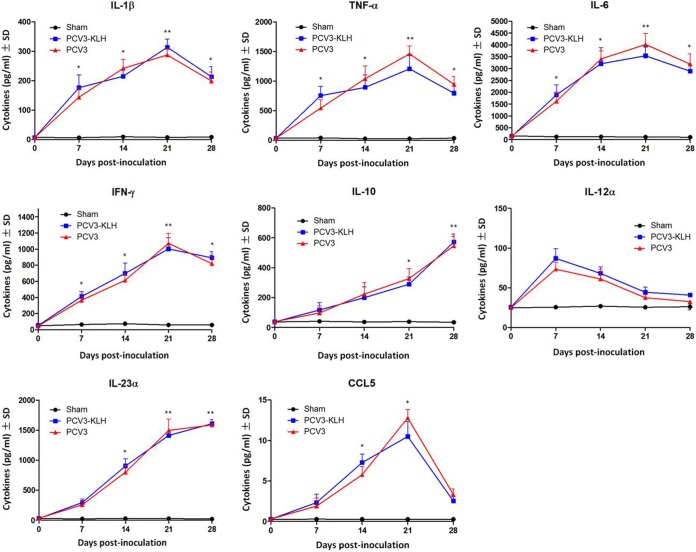
To further characterize the factors that regulate PCV3 pathogenesis in piglets, we analyzed the production of cytokines/chemokines in the sera of 4-week-old piglets after PCV3 inoculation. As shown in Fig. 8, the levels of gamma interferon (IFN-γ) and interleukin 12 alpha (IL-12α) peaked on day 7, of IL-1β peaked on day 14, and of tumor necrosis factor alpha (TNF-α), IL-6, and chemokine ligand 5 (CCL5) peaked on day 21 and thereafter declined in the PCV3-inoculated group; these levels were significantly (P < 0.05) increased compared to those in the sham-inoculated piglets. Levels of IL-23α and IL-10 continued to increase during PCV3 inoculation. The PCV3-KLH-inoculated group exhibited similar production curves of cytokines/chemokines in the sera as those observed for the PCV3-inoculated piglets.
FIG 8.
Cytokine/chemokine levels in the sera of the 4-week-old PCV3-inoculated piglets. *, P < 0.05; **, P < 0.01 for comparisons of PCV3-inoculated or PCV3-KLH-inoculated and sham-inoculated piglets at the indicated time points after inoculation. Data are shown as the means ± SDs.
DISCUSSION
PDNS has been reported in many pig-rearing countries since it was first described in Europe in 1993 (31–40). This disease is a complex syndrome in swine, and multiple factors may be involved in the clinical presentation of PDNS. PCV2 is a major pathogen associated with PMWS, and its nucleic acid has been also detected in PDNS-affected pigs (41, 42). These findings indicate that PCV2 may have a role in PDNS (43). However, experimental infection with PCV2 does not reproduce PDNS successfully. Recently, Palinski et al. (15) used metagenomics sequencing to identify a novel PCV (PCV3) as a major pathogen that might be associated with mummified fetuses aborted from sows with PDNS-like lesions and sows that died acutely with clinical signs consistent with PDNS. Subsequently, increasing amounts of epidemiological data (26) demonstrate that PCV3 is already widely distributed in various pig farms worldwide, suggesting that it serves as a potential causative agent, threatening the swine industry. However, there are no reports on whether experimental infection with PCV3 is solely responsible for the occurrence of PDNS-like disease.
In this study, we found that a homogeneous PCV3 infectious virus stock, which was derived from an infectious PCV3 molecular DNA clone, was capable of reproducing PDNS-like disease when administered to 4- and 8-week-old piglets via the intranasal route. It is advantageous to construct an infectious clone of PCV3 so that a biologically pure and homogeneous infectious virus stock can be generated for definitive characterization of the disease and pathological lesions associated with PCV3 infection in piglets. Oronasal exposure is likely the natural route of PCV3 transmission. Furthermore, we found that the reproduction of PNDS-like clinical disease in the 4- or 8-week-old PCV3-inoculated piglets was similar to that observed when immunostimulating PCV3-inoculated piglets with injections of KLH. This is different from experimental infection of PCV2 in which clinical PMWS was reproduced only in PCV2-infected pigs coinfected with PPV or PRRSV (10, 11, 13) or in PCV2-infected pigs dependent upon immune stimulation by injection of KLH (12–14). Treatment with an immunostimulator is believed to promote the maximal or optimal PCV2 replication to mediate PMWS in piglets when infected with PCV2 (12–14). Piglets that were inoculated with PCV3 alone presented characteristics of PDNS-like clinical signs and lesions similar to those that have been reported in cases of natural infection with the virus (15). All the 4-week-old PCV3-inoculated piglets exhibited fever, anorexia, coughing, sneezing, diarrhea, lethargy, rubefaction on the skin and the ears, multifocal papules, shivering, and/or hyperspasmia after PCV3 inoculation. Two of five piglets inoculated with PCV3 developed severe clinical signs, cardiac pathologies, and multisystemic inflammation and died prior to the termination of the experiment at 16 and 18 dpi. Typical clinical signs of PDNS-like disease were also observed in 8-week-old piglets inoculated with PCV3, although without death during a 28-day observation period. The varieties of clinical and pathological changes in the PCV3-inoculated piglets were different from those obtained for PCV2-infected piglets. For example, none of the PCV2-infected piglets showed obvious signs of disease resembling those of clinical PDNS, a condition where PCV2 is considered the major infectious agent involved (44). The results here corroborate findings from a recent study (15), confirming the role of PCV3 as the etiological agent of PDNS-like disease and further suggesting that PCV3 is more pathogenic for piglets than PCV2 due to the PDNS-like clinical disease reproduced by infection of PCV3 alone.
PCV3 pathogenesis was assessed by viremia, pathological changes, viral distribution in tissues, and the expression of cytokines/chemokines in sera in our study. Viremia, which was detected at all the time points postinoculation, coincided with the clinical phase of the disease in the 4-week-old PCV3-inoculated piglets, with peak viral DNA (approximately 7.72 × 108 PCV3 genome copies/ml) detected in serum at 21 dpi. In the 8-week-old PCV3-inoculated piglets, the highest level of PCV3 (7.92 × 107 copies/ml) in serum was also detected at 21 dpi. In addition, seroconversion to PCV3 antibodies appeared in both the 4- and 8-week-old PCV3-inoculated piglets at 14 dpi, although the largest amount of viral DNA correlated with a low titer of antibody against PCV3 in sera. The coexistence of PCV3 virus with its antibodies suggested that the virus may have evolved a strategy for its maintenance in nature, as observed for encephalomyocarditis virus (45) and foot-and-mouth disease virus (46). MTT assays showed that PBMCs of PCV3-inoculated piglets had a low proliferative response to mitogen (PHA) (Fig. 3), which may be associated with T-cell anergy. Gross and histological lesions in the PCV3-inoculated piglets were similar to those previously reported in clinical naturally PCV3-induced diseases (15). Histopathological lesions were characterized by extensive lymphocyte necrosis and depletion, necrotic epithelial cells, and infiltration of inflammatory cells, including neutrophils/histiocytes, macrophages, and eosinophils, as well as secondary suppurative bronchopneumonia with inflammatory exudates (Fig. 6). Immunohistochemical (IHC) staining showed abundant PCV3-positive cells in various tissues and organs in the PCV3-inoculated piglets, with neutrophils, macrophages, eosinophils, and epithelial cells positive for PCV3 antigen (Fig. 7). Research data suggested that lymphoid depletion and histiocytic replacement in lymphoid tissues are the hallmark lesions of PCV2 infection (29, 47). However, infection with PCV3 triggers inflammatory lesions in various tissues and organs followed by lymphocytic dysplasia and necrosis, leading to disruption of the immune system in piglets. These changes might result in increased susceptibility to infections by primary and secondary pathogens that can affect growth performance as well as increase morbidity and mortality.
An interesting finding of our study is that abundant eosinophil infiltrations were present in the lymphoid tissues of the PCV3-inoculated piglets (Fig. 6). In general, eosinophils are a type of disease-fighting white blood cell that plays an important role in the inflammatory response to allergic reactions, asthma, and infection. Eosinophilic pustular folliculitis (EPF), a type of inflammatory dermatosis, is characterized by recurrent episodes of pruritic follicular papules and pustules and is associated with folliculotropic infiltration of eosinophils (48). An immunocompromised status is believed to be causally related to the development of EPF, as its immunosuppression-associated type is mediated by infection with human immunodeficiency virus (49). The results of the PDNS-like disease led us to hypothesize that PCV3 infection in piglets may cause the immunosuppression-associated type of EPF, mediating allergic reactions and inflammation and eventually leading to clinical symptoms such as skin rashes and asthma. However, whether the PCV3-mediated inflammatory response is predominantly associated with eosinophil infiltrations and the related mechanisms require further investigation. In addition, eosinophils may contribute to antiviral immunity, as observed for various viruses, such as rhinovirus (50), respiratory syncytial virus (51–53), parainfluenza virus (54), and pneumonia virus of mice (55), with reductions in viral burden observed in the presence of eosinophils. However, whether eosinophils activated by PCV3 contribute to antiviral immune responses to PCV3 infection requires further study.
Excessive and sustained upregulation of inflammatory cytokines/chemokines is believed to lead to severe local inflammatory responses and tissue damage, thus, resulting in severe diseases. In the present study, the levels of various inflammatory cytokines and chemokines, such as TNF-α, IL-1β, IFN-γ, IL-6, and CCL5, in the sera of the PCV3-inoculated piglets peaked at the time of the maximal viremia and persisted throughout the experiment (Fig. 8). IL-1β activates lymphocytes and promotes local tissue destruction. Elevated TNF-α levels in PCV2-infected pigs were associated with progressive weight loss (56, 57). The expression of IL-23α and IL-10 continued to increase in the sera of the PCV3-inoculated piglets (Fig. 8). High levels of inflammatory cytokines and chemokines may be responsible for PCV3-mediated clinical signs of illness and tissue damage caused by the migration of inflammatory cells, including neutrophils, eosinophils, and macrophages, to local tissues and organs, which are similar to the symptoms observed for PCV2 infection (2, 58–60). Therefore, elucidation of the underlying mechanisms of the cytokine/chemokine imbalance may help devise a better control strategy to enhance the host immune response against PCV3 infection.
In conclusion, this study shows for the first time the successful reproduction of PDNS-like clinical disease by experimental infection with PCV3 and further provides significant insights into the pathogenesis of PCV3 in piglets. The results presented here demonstrate the nature of the PDNS-like disease caused by PCV3 in piglets and shed light on the dynamics of viremia, the characteristics of gross and histopathological lesions, the tissue distributions of PCV3 antigen, and the profiles of inflammatory cytokines/chemokines, which are attributable to PCV3 pathogenesis. Further studies are needed, however, to assess the importance of these findings for PCV3 infection biology and immunopathology.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were conducted according to the animal welfare guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences.
Cells.
The permanent PK15 cell line, which was free of PCV, was maintained in minimal essential medium (MEM; Gibco) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 5% l-glutamine, 100 U of penicillin G/ml, and 100 μl of streptomycin/ml at 37°C in a humidified 5% CO2 incubator.
Construction of the PCV3 infectious DNA clone.
The full-length PCV3 genome was synthesized by TaiheGene (Taihe Biotech, Beijing) according to the published sequence of the PCV3/CN/Hebei-LY/2015 (MF318451), which was derived from samples of diseased piglets in Heibei, China, in 2015 that had lesions consistent with a PDNS-like disease. A molecular DNA clone containing the PCV3 genome with EcoRI and SacI sites at 5′ and 3′ ends was synthesized and cloned into the pBluescript SK (pSK) vector (Stratagene, La Jolla, CA) for the construction of a recombinant plasmid, pSK-PCV3 (Fig. 1A). The recombinant plasmid was sequenced to confirm that no errors were introduced as a result of PCR amplification.
Transfection and infection.
Briefly, monolayer PK15 cells grown to approximately 85% confluence were transfected with 250 ng of the recombinant plasmid containing the PCV3 molecular DNA clone by using Lipofectamine LTX reagent with Plus reagent in accordance with the manufacturer’s instructions. For the infection test, the recombinant plasmid-transfected cells were subjected to three successive freeze-thaw cycles. The total lysates were collected and used to infect PK15 cells. The cells were additionally treated with 300 mM d-glucosamine at 24 h after transfection as described previously (30). They were then analyzed by indirect fluorescence assays (IFAs) after infection. The generated PCV3 virus stock was then serially passaged 15 times in PK15 cells, and the titer of the PCV3 virus stock (designated strain LY) was determined as described previously for PCV2 titration (29) and expressed as the 50% tissue culture infective dose (TCID50)/ml.
Indirect immunofluorescence assay and confocal microscopy.
PK15 monolayer cells grown on chamber slides (BD) at 72 h after PCV3 strain LY infection were washed with phosphate-buffered saline (PBS) and then fixed in 4% paraformaldehyde (PFA). The cells were incubated with anti-PCV3 polyclonal antibody diluted in 3% bovine serum albumin (BSA)-PBS at room temperature (RT) for 1 h, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (Sigma-Aldrich) at RT for 1 h. Nuclei were stained with DAPI (2,4-diamidino-2-phenylindole) for 30 min at 37°C. The cells were washed with PBS, rinsed in distilled water, dried, mounted with fluorescence mounting medium, and examined under a Nikon AIR confocal laser microscope system.
Animal experimental design.
We designed two experiments for analysis of the pathogenesis of PCV3 infection in piglets. In experiment 1, fifteen 4-week-old specific-pathogen-free (SPF) Duroc crossed with Large White piglets from the Beijing Center for SPF Swine Breeding & Management, China, were randomly assigned to three groups (5 piglets in each group). Each group of piglets was housed in an individual room and fed sterile food and water ad libitum. Prior to inoculation, all the piglets were shown to be negative for antigens to PCV1, PCV2, PCV3, PPV, TTV, pseudorabies virus (PRV), swine influenza virus (SIV), classical swine fever (CSFV), porcine epidemic diarrhea virus (PEDV), Japanese encephalitis virus (JEV), and PRRSV and antibodies to PCV2, PRRSV, CSFV, PPV, transmissible gastroenteritis virus (TGEV), and PEDV. Piglets in group 1 were sham inoculated and served as negative controls. Piglets in group 2 were inoculated intranasally with approximately 2 ml of 106.53 TCID50/ml of the PCV3 infectious virus stock. Activation of the immune system was shown to be a key component of the pathogenesis of PCV2-associated PMWS in gnotobiotic pigs (12, 14). The effect of immunostimulation on the induction of PCV3 was also investigated by immunization with keyhole limpet hemocyanin (KLH) emulsified in incomplete Freund’s adjuvant (ICFA). Piglets in group 3 were first inoculated intranasally with the same amount of the PCV3 as for group 2, and on day 4 postinoculation, all the piglets in group 3 were injected with a 4.0-ml volume of 2.0 mg KLH emulsified in ICFA at four sites (1 ml administered per site): two axillae and two hips. All piglets were monitored daily for rectal temperature and clinical signs. Body weight was recorded from all the piglets at 0, 7, 14, 21, and 28 dpi. Blood samples were collected from the anterior vena cava at weekly intervals following PCV3 inoculation. At 28 dpi, all remaining piglets were humanely euthanized after intravenous injection of 80 mg/kg body weight sodium pentobarbital, and a complete necropsy was performed. Lungs, spleens, lymph nodes (mandibular, tracheobronchial, mesenteric, and inguinal), livers, hearts, kidneys, and small intestines were collected during necropsy and processed for histological examination and immunohistochemical staining.
In experiment 2, ten 8-week-old SPF piglets from the same source as experiment 1 were randomly assigned to two groups (5 piglets in each group). Piglets in group 1 were sham inoculated and served as negative controls. Piglets in group 2 were inoculated intranasally with the same dose of the PCV3 infectious virus stock as for group 2 piglets in experiment 1. Clinical examinations, detections of virus loads and anti-PCV3 antibodies in serum, humane euthanasia, and necropsy of all the piglets at 28 dpi for pathological observations were conducted as described above for experiment 1.
Clinical evaluation.
All the piglets were weighed at the designated dpi time points, and the relative weekly weight gains were determined. Clinical observations, including evidence of central nervous system disease, liver disease (icterus), musculoskeletal disease, and changes in body condition, were also recorded daily.
Pathological examination.
Complete examinations were performed, and gross pathology was recorded for each piglet at necropsy. Lungs, hearts, lymph nodes (mandibular, tracheobronchial, mesenteric, and subinguinal), livers, gallbladders, spleens, small intestines, and kidneys were collected and fixed by immersion in 2.5% glutaraldehyde-polyoxymethylene solution. Fixed tissues were dehydrated, embedded in paraffin wax, sectioned at 4-μm thickness, and then stained with hematoxylin-eosin (HE) for microscopic examination. Evaluations of microscopic lesions and the amounts of PCV3 antigen were performed in a blind fashion. Lymph node scores ranged from 0 (normal) to 3 (severe) based on the estimated amounts of lymphoid necrosis, histiocytic/eosinophil infiltration, histiocytic hyperplasia, and syncytial giant cell formation. Lung scores ranged from 0 (normal) to 3 (severe lymphohistiocytic interstitial pneumonia). Liver scores ranged from 0 (normal) to 3 (severe lymphohistiocytic hepatitis). Other tissue and organ scores ranged from 0 (normal) to 3 (severe lesions).
Immunohistochemical staining.
The tissue samples for histopathological observation were subjected to IHC staining for the detection of PCV3 viral antigen. Briefly, the deparaffinized sections were incubated with a polyclonal antibody against PCV3, followed by incubation with a biotinylated secondary antibody and horseradish peroxidase-labeled avidin-biotin chain working solution (Beijing Zhong Shan Golden Bridge Biotechnology Co., Ltd., China) before the addition of 3,3′-diaminobenzidine (Zymed Laboratories Inc., San Diego, CA) as a substrate. The tissue sections were then counterstained with hematoxylin, dehydrated, and mounted with neutral gums. IHC staining was also used for the detection of eosinophils and natural killer cells in the lymph node samples when incubated with mouse primary antibody against human eosinophil major basic protein (clone BMK-13, catalog number MCA5751; Bio-Rad) and mouse primary antibody against pig CD355 (clone VIV-KM1, catalog number MCA5972GA; Bio-Rad), respectively. The amount of PCV3 antigen was scored in a blinded fashion by assigning scores of 0 for no signal to 3 for a strong positive signal.
MTT assay.
For proliferation assays, a specific proliferative response of porcine peripheral blood mononuclear cells (PBMCs) to PCV3 inoculation at the designated time points was determined using the CellTiter 96 AQueous One Solution assay (Promega) according to the manufacturer’s instructions. Briefly, whole blood samples were heparinized, and PBMCs were isolated after density gradient centrifugation and plated at 1 × 104 cells/well in RPMI 1640 medium containing phytohemagglutinin (PHA; 25 μg/ml) in 96-well plates for incubation at 37°C with 5% CO2 for 3 days, followed by incubation with 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) for 4 h. The media were subsequently aspirated, and 100 μl of dimethyl sulfoxide (DMSO) was added to each well before the plates were placed on a shaker for 10 min to fully dissolve the formazan crystals. The absorbance at 490 nm was measured using an automated microplate reader, and the stimulation index (SI) was calculated using the following formula: SI = (Atest well − Ablank well)/(Acontrol well − Ablank well) × 100.
Quantification of viral DNA by real-time PCR.
Quantitative real-time PCR (qPCR) was performed to determine the PCV3 virus loads in serum samples collected at 7, 14, 21, and 28 dpi. Total DNAs were isolated from serum samples of inoculated or sham-inoculated piglets using a DNeasy Blood & Tissue Minikit (Qiagen) in accordance with the manufacturer’s instructions. The sense primer (5′-GTGCCAGGGCTTGTTATTCT-3′) and the antisense primer (5′-CTATTCATTAGGAGGCCCACAG-3′) were used to amplify a 102-bp fragment of the PCV3 cap gene. The qPCR assay was performed with the SYBR green PCR kit (Bio-Rad) under the following conditions: 95°C for 15 min and 45 cycles of 94°C for 15 s and 59°C for 60 s. The sensitivity and specificity of the assay were determined with a dilution series of a plasmid (cap gene cloned into pMD-18) containing the entire PCV3 cap gene. The threshold cycle (CT) values determined from the plasmid dilution series were used to create a standard curve to determine the genomic copy number. Each assay was run in triplicates.
Serum cytokine levels.
The serum samples from the 4-week-old PCV3-inoculated piglets at the designated time points postinoculation were assessed for cytokine levels of IL-1β, IL-6, IL-10, IL-12α, IL-23α, TNF-α, IFN-γ, and CCL5 by using commercial ELISA kits (Aviva Systems Biology Corp., USA) according to the manufacturer’s instructions. The serum concentrations of cytokines were calculated according to the recombinant standards supplied in the kits.
Detection of serum antibodies to PCV3.
Blood samples were collected from all piglets at the designated times after inoculation, and serum antibodies to PCV3 were detected by an indirect ELISA based upon the recombinant PCV3 cap protein (15).
Statistical analyses.
Results are shown as the means ± standard deviations (SDs). Statistical comparisons were performed using Student’s t tests or analyses of variance (ANOVAs) by using SPSS 17.0 software, and a P value of less than 0.05 was considered significant.
ACKNOWLEDGMENT
This study was supported by a grant from the National Key Research and Development Program of China (2017YFD0500103).
REFERENCES
- 1.Ellis J. 2014. Porcine circovirus: a historical perspective. Vet Pathol 51:315–327. doi: 10.1177/0300985814521245. [DOI] [PubMed] [Google Scholar]
- 2.Meng XJ. 2013. Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci 1:43–64. doi: 10.1146/annurev-animal-031412-103720. [DOI] [PubMed] [Google Scholar]
- 3.Saha D, Lefebvre DJ, Ducatelle R, Doorsselaere JV, Nauwynck HJ. 2011. Outcome of experimental porcine circovirus type 1 infections in mid-gestational porcine foetuses. BMC Vet Res 7:64. doi: 10.1186/1746-6148-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan G, Meehan B, Todd D, Kennedy S, McNeilly F, Ellis J, Clark EG, Harding J, Espuna E, Botner A, Charreyre C. 1998. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet Rec 142:467–468. [PubMed] [Google Scholar]
- 5.Opriessnig T, Meng XJ, Halbur PG. 2007. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19:571–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 6.Afghah Z, Webb B, Meng XJ, Ramamoorthy S. 2017. Ten years of PCV2 vaccines and vaccination: is eradication a possibility? Vet Microbiol 206:21–28. doi: 10.1016/j.vetmic.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Segalés J, Domingo M, Chianini F, Majó N, Domínguez J, Darwich L, Mateu E. 2004. Immunosuppression in postweaning multisystemic wasting syndrome affected pigs. Vet Microbiol 98:151–158. [DOI] [PubMed] [Google Scholar]
- 8.Lee G, Han D, Song JY, Lee YS, Kang KS, Yoon S. 2010. Genomic expression profiling in lymph nodes with lymphoid depletion from porcine circovirus 2-infected pigs. J Gen Virol 91:2585–2591. doi: 10.1099/vir.0.022608-0. [DOI] [PubMed] [Google Scholar]
- 9.Yue F, Cheng A, Zhu Y, Li P, Zhang Y, Sun G, Wang M, Wang X. 2015. Overexpression of programmed death ligands in naturally occurring postweaning multisystemic wasting syndrome. Viral Immunol 28:101–106. doi: 10.1089/vim.2014.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. 1999. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol 121:1–11. doi: 10.1053/jcpa.1998.0295. [DOI] [PubMed] [Google Scholar]
- 11.Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiate PCV2 replication. Arch Virol 145:2421–2429. doi: 10.1007/s007050070031. [DOI] [PubMed] [Google Scholar]
- 12.Krakowka S, Ellis JA, McNeilly F, Ringler S, Rings DM, Allan G. 2001. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2). Vet Pathol 38:31–42. doi: 10.1354/vp.38-1-31. [DOI] [PubMed] [Google Scholar]
- 13.Ha Y, Lee YH, Ahn KK, Kim B, Chae C. 2008. Reproduction of postweaning multisystemic wasting syndrome in pigs by prenatal porcine circovirus 2 infection and postnatal porcine parvovirus infection or immunostimulation. Vet Pathol 45:842–848. doi: 10.1354/vp.45-6-842. [DOI] [PubMed] [Google Scholar]
- 14.Law J, UCVM Class of 2015, McCorkell R, Muench G, Wynne-Edwards K, Schaetzl HM, Solis C, Nourozieh N, Waeckerlin R, Eschbaumer M, Horsman S, Czub M. 2017. Induction of humoral immune response in piglets after perinatal or post-weaning immunization against porcine circovirus type-2 or keyhole limpet hemocyanin. Can J Vet Res 81:5–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Palinski R, Piñeyro P, Shang P, Yuan F, Guo R, Fang Y, Byers E, Hause BM. 2017. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J Virol 91:e01879-16. doi: 10.1128/JVI.01879-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan TG, Giannitti F, Rossow S, Marthaler D, Knutson TP, Li L, Deng X, Resende T, Vannucci F, Delwart E. 2016. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol J 13:184. doi: 10.1186/s12985-016-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen S, Sun W, Li Z, Zhuang X, Zhao G, Xie C, Zheng M, Jing J, Xiao P, Wang M, Han J, Ren J, Liu H, Lu H, Jin N. 2018. The detection of porcine circovirus 3 in Guangxi, China. Transbound Emerg Dis 65:27–31. doi: 10.1111/tbed.12754. [DOI] [PubMed] [Google Scholar]
- 18.Tochetto C, Lima DA, Varela APM, Loiko MR, Paim WP, Scheffer CM, Herpich JI, Cerva C, Schmitd C, Cibulski SP, Santos AC, Mayer FQ, Roehe PM. 2018. Full-genome sequence of porcine circovirus type 3 recovered from serum of sows with stillbirths in Brazil. Transbound Emerg Dis 65:5–9. doi: 10.1111/tbed.12735. [DOI] [PubMed] [Google Scholar]
- 19.Kedkovid R, Woonwong Y, Arunorat J, Sirisereewan C, Sangpratum N, Lumyai M, Kesdangsakonwut S, Teankum K, Jittimanee S, Thanawongnuwech R. 2018. Porcine circovirus type 3 (PCV3) infection in grower pigs from a Thai farm suffering from porcine respiratory disease complex (PRDC). Vet Microbiol 215:71–76. doi: 10.1016/j.vetmic.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Stadejek T, Woźniak A, Miłek D, Biernacka K. 2017. First detection of porcine circovirus type 3 on commercial pig farms in Poland. Transbound Emerg Dis 64:1350–1353. doi: 10.1111/tbed.12672. [DOI] [PubMed] [Google Scholar]
- 21.Kwon T, Yoo SJ, Park CK, Lyoo YS. 2017. Prevalence of novel porcine circovirus 3 in Korean pig populations. Vet Microbiol 207:178–180. doi: 10.1016/j.vetmic.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Faccini S, Barbieri I, Gilioli A, Sala G, Gibelli LR, Moreno A, Sacchi C, Rosignoli C, Franzini G, Nigrelli A. 2017. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound Emerg Dis 64:1661–1664. doi: 10.1111/tbed.12714. [DOI] [PubMed] [Google Scholar]
- 23.Fux R, Söckler C, Link EK, Renken C, Krejci R, Sutter G, Ritzmann M, Eddicks M. 2018. Full genome characterization of porcine circovirus type 3 isolates reveals the existence of two distinct groups of virus strains. Virol J 15:25. doi: 10.1186/s12985-018-0929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzo G, Legnardi M, Hjulsager CK, Klaumann F, Larsen LE, Segales J, Drigo M. 2018. Full-genome sequencing of porcine circovirus 3 field strains from Denmark, Italy and Spain demonstrates a high within-Europe genetic heterogeneity. Transbound Emerg Dis 65:602–606. doi: 10.1111/tbed.12836. [DOI] [PubMed] [Google Scholar]
- 25.Ku X, Chen F, Li P, Wang Y, Yu X, Fan S, Qian P, Wu M, He Q. 2017. Identification and genetic characterization of porcine circovirus type 3 in China. Transbound Emerg Dis 64:703–708. doi: 10.1111/tbed.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu X, Fang B, Ma J, Liu Y, Bu D, Zhou P, Wang H, Jia K, Zhang G. 2018. Insights into the epidemic characteristics and evolutionary history of the novel porcine circovirus type 3 in southern China. Transbound Emerg Dis 65:e296–e303. doi: 10.1111/tbed.12752. [DOI] [PubMed] [Google Scholar]
- 27.Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol 81:2281–2287. doi: 10.1099/0022-1317-81-9-2281. [DOI] [PubMed] [Google Scholar]
- 28.Krakowka S, Hartunian C, Hamberg A, Shoup D, Rings M, Zhang Y, Allan G, Ellis JA. 2008. Evaluation of induction of porcine dermatitis and nephropathy syndrome in gnotobiotic pigs with negative results for porcine circovirus type 2. Am J Vet Res 69:1615–1622. doi: 10.2460/ajvr.69.12.1615. [DOI] [PubMed] [Google Scholar]
- 29.Fenaux M, Halbur PG, Haqshenas G, Royer R, Thomas P, Nawagitgul P, Gill M, Toth TE, Meng XJ. 2002. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J Virol 76:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tischer I, Peters D, Rasch R, Pociuli S. 1987. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch Virol 96:39–57. [DOI] [PubMed] [Google Scholar]
- 31.Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. 2001. Comparative pathogenesis of infection pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol 39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. 1995. Comparison of the pathogenicity of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol 32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 33.Spillane P. 1998. Dermatitis nephropathy syndrome in finisher pigs. Vet Rec 143:28. [PubMed] [Google Scholar]
- 34.van Halderen A. 1995. Dermatitis/nephropathy syndrome in pigs. J S Afr Vet Assoc 66:108–110. [PubMed] [Google Scholar]
- 35.Kavanagh NT. 1994. Dermatitis/nephropathy syndrome in pigs. Vet Rec 134:311. [DOI] [PubMed] [Google Scholar]
- 36.White M, Higgins RJ. 1993. Dermatitis nephropathy syndrome of pigs. Vet Rec 132:199. [DOI] [PubMed] [Google Scholar]
- 37.Smith WJ, Thomson JR, Done S. 1993. Dermatitis/nephropathy syndrome of pigs. Vet Rec 132:47. [DOI] [PubMed] [Google Scholar]
- 38.Gresham A, Giles N, Weaver J. 2000. PMWS and porcine dermatitis nephropathy syndrome in Great Britain. Vet Rec 147:115. [PubMed] [Google Scholar]
- 39.Thomson J, Smith B, Allan G, McNeilly F, McVicar C. 2000. PDNS, PMWS and porcine circovirus type 2 in Scotland. Porcine dermatitis and nephropathy syndrome. Post-weaning multisystemic wasting syndrome. Vet Rec 146:651–652. [PubMed] [Google Scholar]
- 40.Gresham A, Jackson G, Giles N, Allan G, McNeilly F, Kennedy S. 2000. PMWS and porcine dermatitis nephropathy syndrome in Great Britain. Vet Rec 146:143. [PubMed] [Google Scholar]
- 41.Segalés J, Piella J, Marco E, Mateu-de-Antonio EM, Espuña E, Domingo M. 1998. Porcine dermatitis and nephropathy syndrome in Spain. Vet Rec 142:483–486. [DOI] [PubMed] [Google Scholar]
- 42.Thibault S, Drolet R, Germain MC, D'Allaire S, Larochelle R, Magar R. 1998. Cutaneous and systemic necrotizing vasculitis in swine. Vet Pathol 35:108–116. doi: 10.1177/030098589803500204. [DOI] [PubMed] [Google Scholar]
- 43.Thomson JR, MacIntyre N, Henderson LE, Meikle CS. 2001. Detection of Pasteurella multocida in pigs with porcine dermatitis and nephropathy syndrome. Vet Rec 149:412–417. [DOI] [PubMed] [Google Scholar]
- 44.Vlasakova M, Leskova V, Sliz I, Jackova A, Vilcek S. 2014. The presence of six potentially pathogenic viruses in pigs suffering from post-weaning multisystemic wasting syndrome. BMC Vet Res 10:221. doi: 10.1186/s12917-014-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billinis C, Paschaleri-Papadopoulou E, Psychas V, Vlemmas J, Leontides S, Koumbati M, Kyriakis SC, Papadopoulos O. 1999. Persistence of encephalomyocarditis virus (EMCV) infection in piglets. Vet Microbiol 70:171–177. [DOI] [PubMed] [Google Scholar]
- 46.Alexandersen S, Zhang Z, Donaldson AI. 2002. Aspects of the persistence of foot-and-mouth disease virus in animals-the carrier problem. Microbes Infect 4:1099–1110. [DOI] [PubMed] [Google Scholar]
- 47.Sorden SD. 2000. Update on porcine circovirus and postweaning multisystemic syndrome (PMWS). Swine Health Prod 8:133–136. [Google Scholar]
- 48.Long H, Zhang G, Wang L, Lu Q. 2016. Eosinophilic skin diseases: a comprehensive review. Clin Rev Allergy Immunol 50:189–213. doi: 10.1007/s12016-015-8485-8. [DOI] [PubMed] [Google Scholar]
- 49.Afonso JPJM, Tomimori J, Michalany NS, Nonogaki S, Porro AM. 2012. Pruritic papular eruption and eosinophilic folliculitis associated with human immunodeficiency virus (HIV) infection: a histopathological and immunohistochemical comparative study. J Am Acad Dermatol 67:269–275. doi: 10.1016/j.jaad.2011.11.923. [DOI] [PubMed] [Google Scholar]
- 50.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. 1995. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med 151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 51.Kimpen JL, Garofalo R, Welliver RC, Ogra PL. 1992. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr Res 32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Domachowske JB, Bonville CA, Dyer KD, Rosenberg HF. 1998. Evolution of antiviral activity in the ribonuclease A gene superfamily: evidence for a specific interaction between eosinophil-derived neurotoxin (EDN/RNase 2) and respiratory syncytial virus. Nucleic Acids Res 26:5327–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. 2007. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 54.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. 1999. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med 190:1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. 2014. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123:743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J, Ha Y, Chae C. 2006. Potentiation of porcine circovirus 2-induced postweaning multisystemic wasting syndrome by porcine parvovirus is associated with excessive production of tumor necrosis factor-alpha. Vet Pathol 43:718–725. doi: 10.1354/vp.43-5-718. [DOI] [PubMed] [Google Scholar]
- 57.Kreikemeier CA, Engle TB, Lucot KL, Kachman SD, Burkey TE, Ciobanu DC. 2015. Genome-wide analysis of TNF-alpha response in pigs challenged with porcine circovirus 2b. Anim Genet 46:205–208. doi: 10.1111/age.12262. [DOI] [PubMed] [Google Scholar]
- 58.Darwich L, Balasch M, Plana-Dúran J, Segalés J, Domingo M, Mateu E. 2003. Cytokine profiles of peripheral blood mononuclear cells from pigs with postweaning multisystemic wasting syndrome in response to mitogen, superantigen or recall viral antigens. J Gen Virol 84:3453–3457. doi: 10.1099/vir.0.19364-0. [DOI] [PubMed] [Google Scholar]
- 59.Darwich L, Mateu E. 2012. Immunology of porcine circovirus type 2 (PCV2). Virus Res 164:61–67. doi: 10.1016/j.virusres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Opriessnig T, Gerber PF, Matzinger SR, Meng XJ, Halbur PG. 2017. Markedly different immune responses and virus kinetics in littermates infected with porcine circovirus type 2 or porcine parvovirus type 1. Vet Immunol Immunopathol 191:51–59. doi: 10.1016/j.vetimm.2017.08.003. [DOI] [PubMed] [Google Scholar]