Despite widespread vaccination, Marek’s disease (MD) continues to pose major challenges for the poultry industry worldwide. MDV causes immunosuppression and deadly lymphomas in chickens, suggesting that this virus has developed a successful immune evasion strategy. However, little is known regarding the initiation and modulation of the host innate immune response during MDV infection. This study demonstrates that the cGAS-STING DNA-sensing pathway is critical for the induction of the IFN-β response against MDV infection in chicken fibroblasts and macrophages. An MDV protein, VP23, was found to efficiently inhibit the cGAS-STING pathway. VP23 selectively inhibits IRF7 but not NF-κB activation. VP23 interacts with IRF7 and blocks its binding to TBK1, thereby suppressing IRF7 activation and resulting in inhibition of the DNA-sensing pathway. These findings expand our knowledge of DNA sensing in chickens and reveal a mechanism through which MDV antagonizes the host IFN response.
KEYWORDS: DNA sensing, IRF7, Marek's disease virus, VP23
ABSTRACT
The type I interferon (IFN) response is the first line of host innate immune defense against viral infection; however, viruses have developed multiple strategies to antagonize host IFN responses for efficient infection and replication. Here, we report that Marek’s disease virus (MDV), an oncogenic herpesvirus, encodes VP23 protein as a novel immune modulator to block the beta interferon (IFN-β) activation induced by cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING) in chicken fibroblasts and macrophages. VP23 overexpression markedly reduces viral DNA-triggered IFN-β production and promotes viral replication, while knockdown of VP23 during MDV infection enhances the IFN-β response and suppresses viral replication. VP23 selectively inhibits IFN regulatory factor 7 (IRF7) but not nuclear factor κB (NF-κB) activation. Furthermore, we found that VP23 interacts with IRF7 and blocks its binding to TANK-binding kinase 1 (TBK1), thereby inhibiting IRF7 phosphorylation and nuclear translocation, resulting in reduced IFN-β production. These findings expand our knowledge of DNA sensing in chickens and reveal a mechanism through which MDV antagonizes the host IFN response.
IMPORTANCE Despite widespread vaccination, Marek’s disease (MD) continues to pose major challenges for the poultry industry worldwide. MDV causes immunosuppression and deadly lymphomas in chickens, suggesting that this virus has developed a successful immune evasion strategy. However, little is known regarding the initiation and modulation of the host innate immune response during MDV infection. This study demonstrates that the cGAS-STING DNA-sensing pathway is critical for the induction of the IFN-β response against MDV infection in chicken fibroblasts and macrophages. An MDV protein, VP23, was found to efficiently inhibit the cGAS-STING pathway. VP23 selectively inhibits IRF7 but not NF-κB activation. VP23 interacts with IRF7 and blocks its binding to TBK1, thereby suppressing IRF7 activation and resulting in inhibition of the DNA-sensing pathway. These findings expand our knowledge of DNA sensing in chickens and reveal a mechanism through which MDV antagonizes the host IFN response.
INTRODUCTION
Innate immunity serves as the first line of host defense against invading pathogens (1). Host cells recognize pathogen-associated molecular patterns present on microbes via a series of pattern recognition receptors (PRRs) to trigger the production of type I interferon (IFN) and other antiviral proteins (1–3). The most well-known PRRs that recognize viral infection are the Toll-like receptors (TLRs), which recognize endosomal nucleic acids; the RIG-I-like receptors (RLRs), which recognize viral RNA; and the cytosolic DNA receptors, which are capable of recognizing viral DNA (3–5). Among these sensors, cyclic GMP-AMP (cGAMP) synthase (cGAS) has been demonstrated to be the principal sensor of cytosolic DNA in various mammalian cells (4, 6, 7). Recently, cGAS was reported to play an important role in the type I IFN responses against DNA viruses, including herpes simplex virus 1 (HSV-1) and Kaposi’s sarcoma-associated herpesvirus (KSHV) (8, 9). After binding DNA, cGAS synthesizes cGAMP from ATP and GTP. As a second messenger, cGAMP binds to and activates the stimulator of interferon genes (STING). Active STING then activates TANK-binding kinase 1 (TBK1) to phosphorylate and activate interferon regulatory factor 3 (IRF3). The phosphorylated IRF3 dimerizes and then translocates to the nucleus, ultimately leading to expression of type I IFNs (5, 10, 11). STING also activates nuclear factor κB (NF-κB), which functions together with IRF3 to initiate transcription of IFNs and inflammatory cytokines (5, 10, 11).
During interaction with hosts, viruses have evolved various strategies to evade host innate immunity, which are essential for viral replication, latency, and persistent infection (12). The cGAS-STING axis plays a crucial role in host antiviral defense; therefore, in order to successfully establish infection, viruses must possess mechanisms to antagonize this signaling pathway (13, 14). HSV-1 tegument proteins UL41 and VP22 have recently been identified to be inhibitors of cGAS (15, 16). The KSHV IRF1 protein and human cytomegalovirus protein UL82 inhibit STING activation either by preventing its binding to TBK1 or by impairing its subcellular trafficking (9, 17). The activation of IRF3 and NF-κB can be inhibited by the VP24, ICP27, and UL24 proteins of HSV-1 (18–20).
Birds are an important reservoir of viruses causing human infections. Similar to PRRs in mammals, several PRRs have been identified in birds, including TLRs and RLRs (21). Chicken PRRs differ from their mammalian counterparts in terms of the absence of TLR9 and RIG-I (22, 23). Chickens are IRF3 deficient; however, the presence of functional IRF7 is considered to compensate for the IRF3 deficiency (22). The NF-κB transcription factor is expressed in chickens and may be functionally similar to that in mammals (22). Furthermore, cytosolic DNA sensors have not yet been reported in chickens.
Marek’s disease virus (MDV), or Gallid herpesvirus 2 (GaHV-2), which is the prototype species of the Mardivirus genus within the Alphaherpesvirinae subfamily, induces immunosuppression and fatal T cell lymphomas in chickens. MDV is genetically similar to two other nonpathogenic Mardivirus species, namely, Gallid herpesvirus 3 (GaHV-3, previously MDV-2) and Meleagrid herpesvirus 1 (MeHV-1), also commonly named herpesvirus of turkeys (HVT; previously MDV-3). Apart from being an economically important virus that affects poultry health, MDV serves as a valuable model organism for understanding virus-induced lymphoma (24–26). In vivo, MDV infection occurs through the respiratory route via the inhalation of infectious dander. After early cytolytic replication in macrophages and B cells, the virus causes latent infection of T lymphocytes, which subsequently undergo transformation, resulting in the formation of deadly lymphomas in the visceral organs (25, 26). Despite many advances in the understanding of MDV pathogenesis, little is known about the innate immune responses during MDV infection and the virus-host interaction in virus-induced lymphoma. To date, the role of the DNA-sensing pathway in the antiviral immune responses upon MDV infection and the mechanism used by MDV for immune evasion remain to be elucidated.
VP23 is an integral capsid protein of herpesvirus. Together with VP19C, VP23 forms the triplex of the capsid shell, which is indispensable for capsid assembly and viral growth (27). VP23 also plays an essential role in the transition from an open to a closed shell (28). Given the critical role played by VP23 in capsid stabilization, mutations in VP23 can abrogate capsid formation and cleavage of replicated DNA (29), and RNA interference with VP23 greatly affects the replication of HSV-1 (30). Although VP23 is essential for capsid assembly and viral replication, whether VP23 affects the host antiviral immune response is unknown. Here, we demonstrate that the cGAS-STING pathway is important for the induction of the beta interferon (IFN-β) response against MDV infection in chicken cells. Moreover, we found that the MDV VP23 protein inhibits the cGAS-STING DNA-sensing pathway. Mechanistically, VP23 targets IRF7 to prevent its interaction with TBK1, thereby suppressing IRF7 activation, leading to a blockade of IFN production.
RESULTS
VP23 inhibits cGAS-STING-mediated IFN-β activation.
To determine the effect of MDV infection on IFN-β production, chicken embryo fibroblasts (CEFs) were inoculated with MDV, and IFN-β mRNA levels were analyzed by real-time quantitative PCR (qPCR) from 4 h to 72 h postinfection. The CEFs exhibited an IFN-β response upon MDV infection (Fig. 1A), confirming that the DNA-sensing pathway is active in chicken cells. However, IFN-β production began to decline at 24 h postinfection and was even lower than that in the mock-infected controls at 72 h postinfection, suggesting that IFN-β production was inhibited during the late phase of MDV infection (Fig. 1A). This observation was confirmed at the protein level by measuring chicken IFN-β levels in the infected cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) (Fig. 1A).
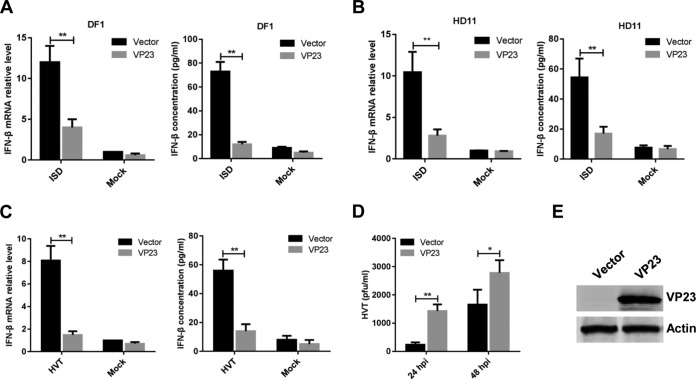
FIG 1.
VP23 inhibits IFN-β activation induced by cGAS-STING. (A) CEFs were infected with MDV at a multiplicity of infection (MOI) of 0.1. IFN-β mRNA and protein levels were measured by real-time qPCR and ELISA, respectively, from 4 h to 72 h after infection. The expression of MDV protein VP23 and gI during viral infection was monitored by immunoblotting. (B) CEFs were transfected with cGAS siRNA (sicGAS), STING siRNA (siSTING), or a nonspecific control (NC) siRNA (siNC) and then infected with MDV (MOI = 0.1). IFN-β mRNA was examined by real-time qPCR at 12 h postinfection, and the IFN-β protein was examined by ELISA at 24 h postinfection. (C) HD11 cells were transfected with cGAS or STING siRNA or a nonspecific control siRNA and then transfected with MDV DNA fragments. IFN-β mRNA was examined by real-time qPCR at 8 h posttransfection, and the IFN-β protein was examined by ELISA at 24 h posttransfection. (D) The knockdown efficiency of cGAS and STING in CEFs was monitored by real-time qPCR and immunoblotting. (E) The knockdown efficiency of cGAS and STING in HD11 cells was monitored by immunoblotting. (F) Various doses of the VP23 expression plasmid or MDV gI expression plasmid were cotransfected with the IFN-β-luc reporter with or without cGAS-STING stimuli into DF-1 cells, and IFN-β promoter luciferase activity was measured at 24 h posttransfection. (G) VP23, the gI expression plasmid, or the empty vector was cotransfected with cGAS and STING plasmids into DF-1 cells. IFN-β mRNA and protein levels were measured by real-time qPCR or ELISA at 24 h posttransfection. (H) Various doses of the VP23 expression plasmid or the MDV gI expression plasmid were cotransfected with the IFN-β-luc reporter with or without cGAS-STING stimuli into HD11 cells, and IFN-β promoter luciferase activity was measured at 24 h posttransfection. (I) VP23, the gI expression plasmid, or the empty vector was cotransfected with the cGAS and STING plasmids into HD11 cells. IFN-β mRNA and protein levels were measured by real-time qPCR or ELISA at 24 h posttransfection. (J) The expression of the transfected plasmids in DF-1 and HD11 cells was detected by Western blotting. The relative amounts of IFN-β, cGAS, and STING mRNA were normalized to the actin mRNA level in each sample, and the fold differences compared with the amounts in the mock-transfected samples were determined. Data are presented as the mean ± SD from three independent experiments. Statistical analysis was performed by using Student's t test (**, P < 0.01; ns, no significant difference).
To determine whether the cGAS-STING pathway responded to MDV infection, CEFs were transfected with cGAS- or STING-specific small interfering RNAs (siRNAs) or a nonspecific control (NC) siRNA and then infected with MDV. The results showed that knockdown of either cGAS or STING markedly decreased IFN-β production in MDV-infected CEFs both at the mRNA level and at the protein level (Fig. 1B). Moreover, a similar decrease in IFN-β mRNA and protein levels was observed in chicken macrophage HD11 cells transfected with siRNAs against cGAS or STING after stimulation by MDV DNA transfection (Fig. 1C). Knockdown of cGAS and STING in CEFs and HD11 cells was confirmed by qPCR and immunoblotting at the mRNA and protein levels (Fig. 1D and E). These results suggest that cGAS and STING play a crucial role in the induction of the IFN-β response upon MDV infection.
Chicken fibroblast (DF-1) and chicken macrophage (HD11) cell lines are widely used in the study of chicken innate immunity (21, 31–33). In DF-1 cells, the IFN-β promoter was highly activated by cotransfecting the same amounts of cGAS and STING expression plasmids (Fig. 1F). With this model, we performed a screen for MDV proteins and found that overexpression of VP23 inhibited cGAS-STING-mediated activation of the IFN-β promoter in a dose-dependent manner; however, the MDV gI protein did not inhibit cGAS-STING-induced IFN-β activation (Fig. 1F). We additionally found that VP23 did not affect IFN-β promoter activity in the absence of exogenous cGAS and STING expression, indicating the specific involvement of VP23 in the cGAS-STING pathway. The results of the dual-luciferase reporter assay were further validated by measuring IFN-β mRNA levels in transfected DF-1 cells by qPCR and IFN-β protein levels in transfected DF-1 cells by ELISA (Fig. 1G). Similarly, in the presence of VP23, IFN-β promoter activity was inhibited in HD11 cells (Fig. 1H); this was confirmed by the decreased induction of IFN-β mRNA and protein in VP23-expressing HD11 cells (Fig. 1I). The successful expression of the transfected plasmids in DF-1 and HD11 cells was confirmed by immunoblotting (Fig. 1J). In addition, we examined the expression of MDV proteins in CEFs at various time points after MDV infection. The viral protein VP23 was detected in the infected cells starting from 24 h postinfection, with continued expression thereafter (Fig. 1A). The VP23 expression course was consistent with the inhibition of the IFN-β response during the late phase of MDV infection, further suggesting that VP23 plays a role in the modulation of the cGAS-STING pathway during viral infection. In one word, these data identified VP23, which had a previously unknown function, to be a viral immune modulator that may inhibit IFN-β production in both fibroblasts and macrophages during viral infection.
VP23 suppresses viral DNA-triggered IFN-β induction.
To determine whether VP23 inhibits the IFN-β production induced by transfected cytosolic DNA, DF-1 and HD11 cells were transfected with the VP23 expression plasmid, and 24 h later, they were transfected with interferon stimulatory DNA (ISD) fragments, which are proven to have a high capability of inducing IFN-β expression in various cells. The mRNA and protein levels of IFN-β in the cells transfected with ISD fragments were measured by qPCR and ELISA. As shown in Fig. 2, IFN-β mRNA and protein levels were greatly increased in DF-1 and HD11 cells in response to ISD stimuli but significantly reduced by VP23 overexpression. These results suggest that VP23 inhibited cytosolic DNA-induced IFN-β production in both fibroblasts and macrophages.
FIG 2.
VP23 suppresses viral DNA-triggered IFN-β induction and promotes viral replication. (A and B) DF-1 (A) or HD11 (B) cells were transfected with the empty vector or the VP23 expression plasmid, and 24 h later they were transfected with ISD fragments. IFN-β mRNA was measured by real-time qPCR at 8 h after ISD transfection, and IFN-β protein levels were measured by ELISA at 24 h after ISD transfection. (C) DF-1 cells transduced with the empty vector or VP23-expressing lentivirus were left uninfected or infected with HVT (MOI = 0.1). IFN-β mRNA in these cells was measured by real-time qPCR at 12 h postinfection, and the IFN-β protein was measured by ELISA at 24 h postinfection. (D) Transduced DF-1 cells were infected with HVT (MOI = 0.01). At 24 or 48 h postinfection, the HVT viral titer was tested by a plaque assay. hpi, hours postinfection. (E) The expression of VP23 in DF-1 cells transduced with the empty vector or VP23-expressing lentivirus was monitored by Western blotting. The relative amount of IFN-β mRNA was normalized to the actin mRNA level in each sample, and the fold differences between the treated samples and the mock-treated samples were calculated. Data are presented as the mean ± SD from three independent experiments. Statistical analysis was performed by using Student's t test (*, P < 0.05; **, P < 0.01).
We generated stable DF-1 cells ectopically expressing VP23 by lentivirus-mediated transduction. The VP23-expressing cells were infected with HVT, and IFN-β production was evaluated. We found that VP23 expression led to a reduced IFN-β response against HVT compared with that of the empty vector-transduced control cells at both the mRNA and the protein levels (Fig. 2C). Concordantly, HVT showed greater replication in VP23-expressing cells than in the vector control-transduced cells (Fig. 2D). The expression of VP23 was confirmed by Western blotting (Fig. 2E). Taken together, these results indicate that VP23 inhibits viral DNA-triggered IFN-β activation and promotes viral replication.
VP23 deficiency enhances IFN-β production in MDV-infected CEFs.
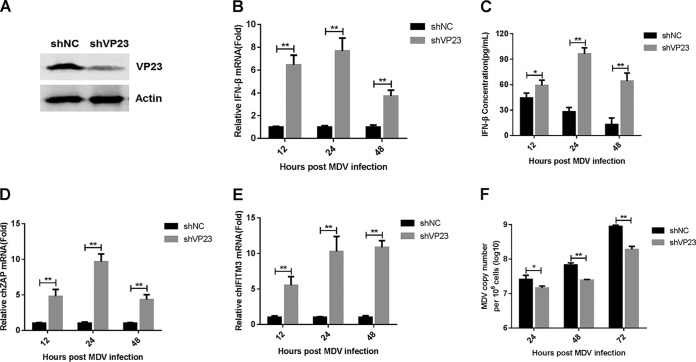
Ectopic expression of VP23 inhibited virus-triggered IFN-β induction; therefore, we next examined the role of endogenous VP23 in the antiviral response to MDV. We generated CEFs stably expressing small hairpin RNA (shRNA) specific for VP23 (shVP23) or control shRNA (shNC). Endogenous VP23 knockdown by VP23-specific shRNA was confirmed by Western blotting during MDV infection (Fig. 3A). Compared with the levels in cells transduced with control shRNA, the IFN-β mRNA and protein levels induced by MDV infection were markedly increased in VP23-knockdown cells from 12 to 48 h postinfection (Fig. 3B and C). Moreover, the knockdown of VP23 promoted the MDV-induced transcription of the IFN-stimulated genes for ZAP and IFN-inducible transmembrane protein 3 (IFITM3) (Fig. 3D and E). Consistently, MDV underwent reduced replication in the VP23-knockdown cells compared with that in the control cells (Fig. 3F). These data suggest that the knockdown of VP23 increases IFN-β production during MDV infection and suppresses viral replication.
FIG 3.
Knockdown of VP23 enhances IFN-β production and attenuates MDV replication. (A) Western blot analysis of CEFs lentivirally transduced with VP23-specific shRNA (shVP23) or a control shRNA (shNC) after 24 h of MDV infection. (B and C) CEFs transduced with shNC or shVP23 were infected with MDV (MOI = 0.1). IFN-β mRNA levels were measured by real-time qPCR (B), and IFN-β protein levels were measured by ELISA (C). (D and E) CEFs transduced with shNC or shVP23 were infected with MDV (MOI = 0.1). The mRNA levels of chZAP (D) and chIFITM3 (E) were measured by real-time qPCR. (F) CEFs transduced with shNC or shVP23 were infected with MDV (MOI = 0.01). The MDV viral titers were tested by real-time qPCR. The amounts of IFN-β, chZAP, or chIFITM3 mRNA were normalized to the actin mRNA level in each sample, and the fold difference relative to the value for the empty vector-transduced controls was determined at each time point. Data are presented as the mean ± SD from three independent experiments. Statistical analysis was performed using Student's t test (*, P < 0.05; **, P < 0.01).
VP23 inhibits IFN-β activation by targeting IRF7.
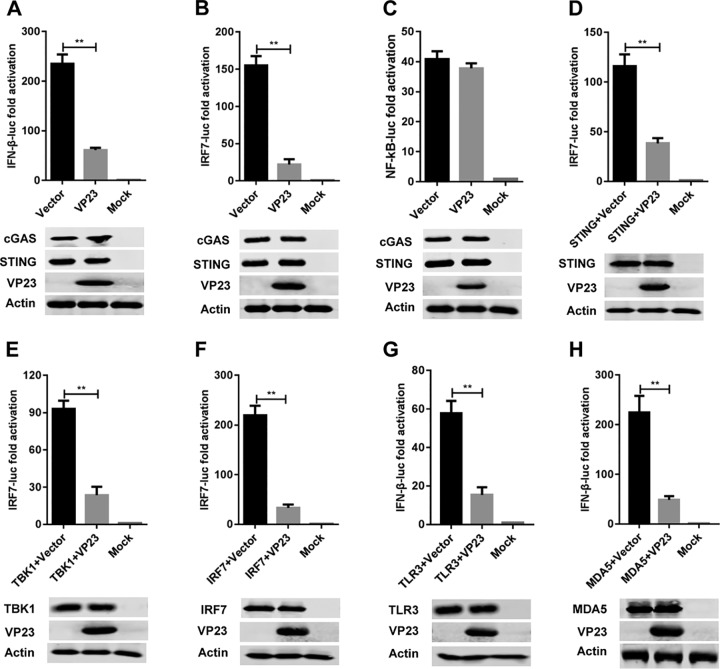
The transcription of IFN-β in chicken cells is dependent on the binding of IRF7 and NF-κB to distinct regulatory domains in the promoter (22). To clarify the mechanism of IFN-β suppression by MDV VP23, we analyzed the activity of IRF7 and NF-κB using a dual-luciferase reporter assay as described previously (32). The results showed that VP23 reduced cGAS-STING-mediated expression of the IFN-β- and IRF7-dependent reporter genes but did not alter NF-κB-dependent luciferase activity (Fig. 4A to C), suggesting that VP23 inhibits the activation of IRF7 but not that of NF-κB.
FIG 4.
VP23 inhibits IFN-β activation by targeting IRF7. (A to C) The IFN-β-luc (A), IRF7-luc (B), or NF-κB-luc (C) reporter was cotransfected with the cGAS and STING constructs as well as the VP23-Flag plasmid or the empty vector into DF-1 cells. Twenty-four hours later, cells were harvested and analyzed by the dual-luciferase reporter assay. (D to H) DF-1 cells were transfected with plasmids expressing chicken STING (D), TBK1 (E), IRF7 (F), TLR3 (G), or MDA5 (H), together with the IRF7-luc or IFN-β-luc reporter and the VP23-Flag or an empty vector plasmid. The dual-luciferase reporter assay was performed at 24 h posttransfection. All cells were transfected with pRL-TK as an internal control to normalize the transfection efficiency, and the fold change relative to the value for the mock-transfected controls was determined. Data are presented as the mean ± SD from three independent experiments. Statistical analysis was performed by using Student's t test (**, P < 0.01).
To determine at what level in the pathway that VP23 blocks IFN-β activation, DF-1 cells were cotransfected with empty vector or a VP23-expressing plasmid along with the luciferase (luc)-labeled IRF7 (IRF7-luc) reporter and plasmids expressing adaptor proteins downstream of cGAS, including STING, TBK1 kinase, and IRF7. All expression constructs elicited a 90- to 200-fold induction of IRF7-luc reporter activity. VP23 inhibited the IRF7-luc activity triggered by all aforementioned constructs (Fig. 4D to F). The results further show that VP23 also reduced the IFN-β promoter activity stimulated by chicken TLR3 and MDA5 (Fig. 4G and H). Although TLR3 and MDA5 also activated the IFN-β promoter in a TBK1-independent manner, these stimuli are known to commonly activate TBK1-IRF7 for type I interferon production (1–3). Therefore, our results suggest that VP23 targets IRF7 activation to evade the host type I interferon response.
VP23 blocks the nuclear translocation of IRF7.
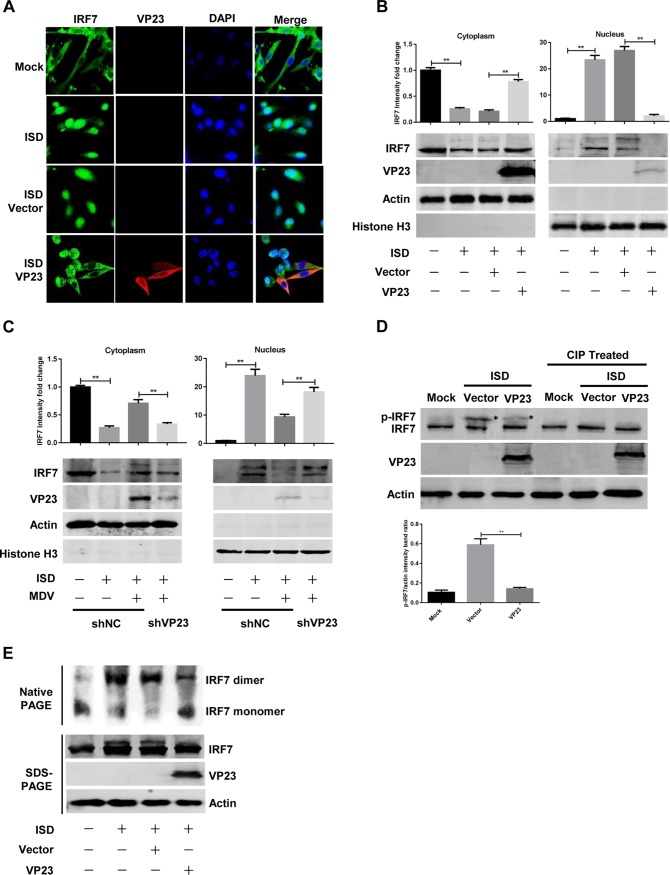
Viral infection triggers IRF7 activation and translocation into the nucleus, where it binds to the promoter regions to activate IFN-β transcription (34). Therefore, we next examined the possibility that VP23 alters the ability of IRF7 to enter the nucleus. DF-1 cells were transfected with a VP23 expression plasmid or an empty vector. At 24 h posttransfection, cells were either transfected with ISD or left untreated. The nuclear accumulation of IRF7 was evaluated by confocal microscopy. Similarly to mammalian IRF7, chicken IRF7 localized exclusively to the cytoplasm in the mock-treated DF-1 cells (Fig. 5A). ISD stimulation induced the nuclear translocation of IRF7 in the majority of cells. However, ectopic expression of VP23 prevented the IRF7 nuclear translocation induced by ISD transfection (Fig. 5A). We further analyzed the levels of IRF7 in cytoplasmic and nuclear extracts by Western blotting, which showed that VP23 obviously increased the level of IRF7 in the cytoplasm and reduced its level in the nuclei of cells treated with ISD (Fig. 5B).
FIG 5.
VP23 blocks the nuclear translocation of endogenous IRF7 by suppressing IRF7 phosphorylation and dimerization. (A) DF-1 cells were transfected with an empty vector or the VP23-Flag expression plasmid, and 24 h later, cells were either transfected with ISD for 12 h or left untreated. Cells were stained with rabbit anti-IRF7 and mouse anti-Flag antibodies. Alexa Fluor 488–anti-rabbit immunoglobulin (green) and Alexa Fluor 546–anti-mouse immunoglobulin (red) were used as the secondary antibodies. Cell nuclei (blue) were stained with DAPI. (B) DF-1 cells were transfected and treated with ISD as described in the legend to panel A. Cell lysates were separated into cytoplasmic and nuclear extracts, and the protein levels of IRF7 and VP23 were analyzed by Western blotting. IRF7 protein levels in the cytoplasm and nucleus were normalized to the amount of actin or histone H3 expression, respectively. (C) shNC- or shVP23-transfected CEFs were infected with MDV at an MOI of 0.1 for 24 h before transfection with ISD. Western blotting was performed at 12 h posttransfection to analyze the level of IRF7 in the cytoplasmic and nuclear extracts. (D) DF-1 cells were transfected and treated with ISD as described in the legend to panel A. Cell lysates were left untreated or treated with calf intestine alkaline phosphatase (CIP) for 1 h, and Western blotting was performed to detect IRF7 phosphorylation with anti-IRF7 antibodies. The protein level of phosphorylated IRF7 (p-IRF7) was normalized to that of actin; the p-IRF7 protein is indicated by an asterisk. (E) DF-1 cells were transfected as described in the legend to panel A and treated with ISD for another 8 h. Native PAGE assays were performed to detect IRF7 dimerization. The data represent the results from one of the triplicate experiments. Statistical analysis was performed by using Student's t test (**, P < 0.01).
To investigate the effects of endogenous VP23 on the nuclear translocation of IRF7, CEFs expressing VP23-shRNA or a control shRNA were infected with MDV before ISD transfection. Then, Western blotting was performed to analyze the level of IRF7 in cytoplasmic and nuclear extracts. Following ISD stimulation, IRF7 nuclear accumulation was observed in CEFs transfected with shNC, and MDV infection suppressed the nuclear translocation of IRF7. However, VP23 knockdown increased the level of IRF7 in the nuclear fraction of MDV-infected cells (Fig. 5C). These observations reveal the capacity of VP23 to inhibit the nuclear localization of IRF7.
Phosphorylation is central to IRF7 activation in response to viral infection, which leads to its dimerization and nuclear accumulation (34). To study the cause of nuclear translocation blocking of IRF7, we determined whether VP23 had any effect on IRF7 phosphorylation. DF-1 cells transfected with the empty vector or a VP23-encoding plasmid were analyzed by Western blotting after ISD treatment. In the absence of VP23, stimulation of transfected cells with ISD led to IRF7 phosphorylation, as determined by the mobility-shifted band in the Western blot, which disappeared following treatment with calf intestine alkaline phosphatase (Fig. 5D). The phosphorylated form of IRF7 was reduced in cells in which VP23 was expressed. Similarly, ISD-triggered IRF7 dimerization was markedly decreased in the presence of VP23 (Fig. 5E). Taken together, these results indicate that VP23 blocks the nuclear translocation of IRF7 by suppressing its phosphorylation and dimerization.
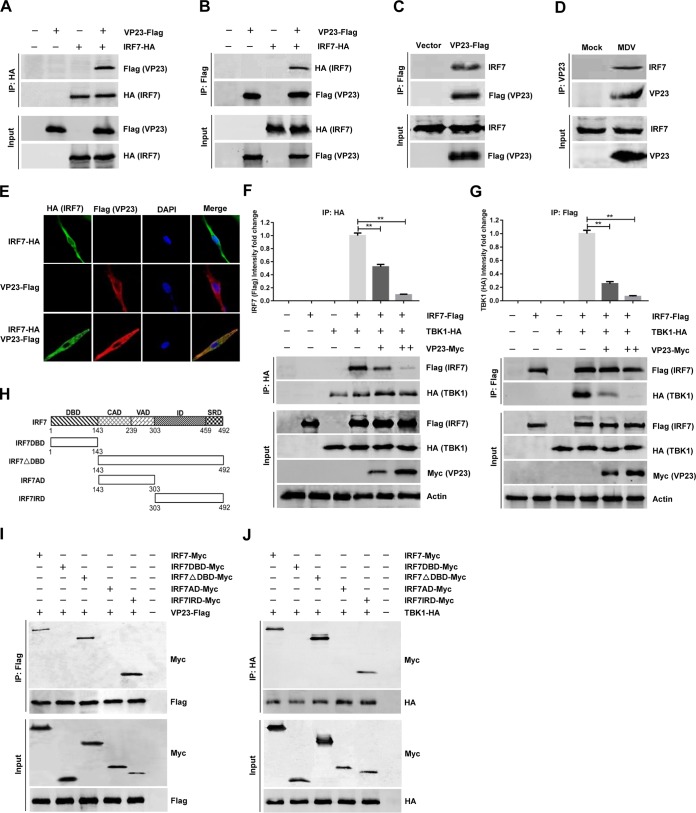
VP23 disrupts the TBK1-IRF7 association by interacting with IRF7.
The specific inhibition of IRF7 by VP23 prompted us to investigate the possibility of an interaction between the two proteins. HEK293T cells were transfected with VP23-Flag along with IRF7-hemagglutinin (HA), and a coimmunoprecipitation assay was performed with antihemagglutinin (anti-HA) and anti-Flag antibodies. We found that the VP23 protein was immunoprecipitated by IRF7, and reciprocally, IRF7 could also be immunoprecipitated by VP23 (Fig. 6A and B). We confirmed the interaction between endogenous IRF7 and ectopically expressed VP23-Flag in DF-1 cells, which was consistent with our finding in HEK293T cells (Fig. 6C). Furthermore, endogenous coimmunoprecipitation experiments indicated that VP23 was associated with IRF7 in CEFs following MDV infection (Fig. 6D). The interaction between VP23 and IRF7 was additionally confirmed by their subcellular colocalization (Fig. 6E).
FIG 6.
VP23 disrupts the association between TBK1 and IRF7 by interacting with IRF7. (A and B) HEK293T cells were cotransfected with the VP23-Flag and IRF7-HA expression plasmids for 36 h, followed by a coimmunoprecipitation (co-IP) assay for VP23-Flag and IRF7-HA using anti-HA (IP: HA) (A) or anti-Flag (IP: Flag) (B) antibody. (C) DF-1 cells were transfected with the VP23-Flag expression plasmid or an empty vector, and at 36 h posttransfection, a coimmunoprecipitation assay was performed with anti-Flag antibody. (D) CEFs were left uninfected or infected with MDV at an MOI of 0.1, and at 48 h postinfection, coimmunoprecipitation was performed with the indicated antibodies. (E) DF-1 cells were transfected with VP23-Flag and/or IRF7-HA expression plasmids for 24 h and then fixed and processed for dual labeling. IRF7 (green) and VP23 (red) proteins were visualized by immunostaining with rabbit anti-HA and mouse anti-Flag antibodies. Cell nuclei were counterstained with DAPI (blue). The areas of colocalization in merged images are shown in yellow. (F and G) DF-1 cells were cotransfected with TBK1-HA and IRF7-Flag expression plasmids with or without different amounts of VP23-Myc. After 36 h of transfection, cell extracts were analyzed by immunoprecipitation (IP) using anti-HA antibody (IP: HA) (F) or anti-Flag antibody (IP: Flag) (G). (H) Schematic representation of the full-length IRF7 (aa 1 to 492) and different truncated IRF7 proteins, including IRF7DBD (aa 1 to 143), IRF7△DBD (aa 143 to 492), IRF7AD (aa 143 to 303), and IRF7IRD (aa 303 to 492). The various domains of IRF7 include the DNA-binding domain (DBD), constitutive activation domain (CAD), virus-activated domain (VAD), inhibitory domain (ID), and signal response domain (SRD). (I) Full-length IRF7-Myc or the truncated IRF7-Myc was transfected with VP23-Flag into DF-1 cells. After 36 h, the cells lysates were immunoprecipitated with anti-Flag and analyzed by Western blotting. (J) Full-length IRF7-Myc or the truncated IRF7-Myc was transfected with TBK1-HA into DF-1 cells. After 36 h, the cell lysates were immunoprecipitated with anti-HA and analyzed by Western blotting. The data represent the results from one of the triplicate experiments. Statistical analysis was performed using Student's t test (**, P < 0.01).
TBK1 has been shown to associate with and phosphorylate IRF7 (34); accordingly, we next aimed to determine whether VP23, as an IRF7-associated protein, could disrupt the association of TBK1 and IRF7. DF-1 cells were cotransfected with TBK1 and IRF7 expression plasmids with or without different amounts of the VP23 expression plasmid. In coimmunoprecipitation assays, we found that TBK1 successfully pulled down IRF7. However, the amount of IRF7 that bound to TBK1 gradually decreased as the amount of VP23 increased (Fig. 6F). Conversely, when VP23 was present, the TBK1 amount immunoprecipitated by IRF7 also decreased in a dose-dependent manner (Fig. 6G).
In order to further verify the domains of IRF7 that are involved in its interaction with VP23 and TBK1, we constructed a series of truncation mutants of IRF7 (Fig. 6H). As shown in Fig. 6I, IRF7 (amino acids [aa] 1 to 492), IRF7△DBD (aa 143 to 492), and IRF7IRD (aa 303 to 492) coimmunoprecipitated with VP23, whereas IRF7DBD (aa 1 to 143) and IRF7AD (aa 143 to 303) did not, suggesting that IRF7 aa 303 to 492 are essential for the association of IRF7 with VP23. Interestingly, we found that the IRF7IRD (aa 303 to 492) domain also mediated the interaction between IRF7 and TBK1 (Fig. 6J). Overall, these results suggest that VP23 disrupts the TBK1-IRF7 association by interacting with the same region of IRF7 as TBK1, thereby preventing IRF7 activation and inhibiting IFN-β production in VP23-expressing cells.
DISCUSSION
In recent years, various DNA sensors that recognize viral nucleic acids during viral infection have been identified (4, 5). Among these, cGAS has been demonstrated to serve as a major cytosolic DNA sensor in response to viral infection in various mammalian cells (4, 6, 7). However, research on DNA sensing in chickens has been minimal, and DNA sensors have not been identified in this species (22). Interestingly, it has been shown that chicken STING can actively sense DNA; in cooperation with MDA5, this protein activates IRF7 and the NF-κB pathway independently of RIG-I (32). The chicken cGAS sequence has recently been deposited in GenBank (accession no. XM_419881); however, its function in DNA sensing has not been identified. In the present study, we found that knockdown of chicken cGAS and STING reduces IFN-β activation in chicken fibroblasts and macrophages, suggesting that the cGAS-STING pathway plays an important role in triggering the innate antiviral immune responses in chickens.
To successfully infect and persist in the host, viruses must possess multiple strategies to subvert host immune responses (12–14). A number of viral proteins that inhibit IFN-I production through modulation of the cGAS-STING DNA-sensing pathway have been identified, including HSV-1 UL41 (15), VP22 (16), VP24 (18), ICP27 (19), UL24 (20), and VP11/12 (35), as well as viral proteins encoded by KSHV (9), human cytomegalovirus (17, 36), and murine gammaherpesvirus 68 (37). Nevertheless, to date, the strategies used by chicken DNA viruses to hinder the DNA-sensing pathway in host cells remain unclear. Here, we identified the VP23 protein from MDV, an alphaherpesvirus that causes lymphomas in chickens, to be an efficient inhibitor of the cGAS-STING pathway.
IRF7 is a crucial transcription factor in the IFN-β signaling pathway (38); in response to viral infection, IRF7 is phosphorylated by TBK1, leading to its dimerization and migration to the nucleus, where it binds to the IFN-β promoter (39–41). IRF7 plays an essential role in host immunity; as a result, viruses have developed various strategies to counteract its activation. It has been demonstrated that the HSV-1 ICP0 protein inhibits IRF7 phosphorylation by TBK1 and IκB kinase ε (IKKε) (42). A KSHV immediate early protein, ORF45, blocks the phosphorylation and nuclear accumulation of IRF7 during viral infection (43). In addition, the Epstein-Barr virus LF2 tegument protein specifically interacts with the central inhibitory association domain of IRF7, leading to the inhibition of IRF7 dimerization (44). The present study adds MDV VP23 to the expanding family of viral proteins that inhibit IRF7 activation; by interacting with IRF7, VP23 disrupts the association between TBK1 and IRF7, which prevents IRF7 phosphorylation and eventually inhibits IFN-β production during MDV infection (Fig. 7). We found that VP23 specifically binds to aa 303 to 492 of IRF7. This region contains two functional domains, the inhibitory domain (aa 303 to 459) and the signal response domain (aa 459 to 492), which was previously shown to be critical for IRF7 dimerization and phosphorylation (44, 45). Importantly, we found that the inhibitory region (aa 303 to 459) is also necessary for the binding of IRF7 to TBK1, which may contribute to the disruption of the TBK1-IRF7 association by VP23.
FIG 7.

Model summarizing the inhibition of the cGAS-STING pathway by MDV VP23. MDV DNA activates the cGAS-STING DNA-sensing pathway during infection, leading to the production of IFN-β. However, the VP23 protein encoded by MDV interacts with chicken IRF7, which disrupts the association between TBK1 and IRF7. This results in the suppression of IRF7 activation and IFN-β production, facilitating viral replication in the host cells.
In the study, we attempted to generate MDV VP23 mutant viruses with the deletion of the whole VP23 protein, the amino terminus only, or the central region of VP23; however, none of these viruses could be rescued (data not shown), thus confirming the indispensable role of VP23 in viral growth. Consistent with the present findings, a previous study also showed that the insertion mutations in various regions, especially the amino terminus of VP23, abolish the function of VP23 and fail to complement the growth of the VP23-null mutant virus (46). We also found that the function of VP23 in IRF7 binding and IFN-β inhibition is conferred by the region spanning aa 1 to 200 on VP23, while the C terminus (aa 201 to 319) is dispensable for its function as an IFN-β inhibitor (data not shown). Interestingly, a region (aa 238 to 255) on the C terminus of HSV-1 VP23 is essential for the closure of capsid shells into icosahedral structures (28), indicating that the multifunctional role of VP23 in viral replication involves different domains.
Notably, we have shown that the knockdown of VP23 during MDV infection induces a stronger IFN-β response and enhances the production of other antiviral effector genes, which results in attenuated viral replication. In contrast, VP23 overexpression inhibited IFN-β production triggered by cytosolic DNA in chicken fibroblasts and macrophages and enhanced viral growth. The reduction in the IFN response to these DNA stimuli by VP23 made the cells expressing VP23 more susceptible to other viruses. Additionally, we found that VP23 inhibits the IFN-β activation stimulated by chicken TLR3 and MDA5. This is not surprising, as VP23 targets IRF7 and the IRF7 activation step is shared by the TLR and RLR pathways (1–3). Thus, it is reasonable to suppose that the VP23 protein affects other pathways, in addition to the DNA-sensing pathway, in response to infection by RNA viruses. These data indicate that MDV has the potential to modulate the host antiviral innate immune response to secondary infection.
In summary, we demonstrate that the cGAS-STING DNA-sensing pathway plays important roles in the IFN-β response upon MDV infection. Moreover, MDV protein VP23 is an efficient IFN-β inhibitor. VP23 interacts with chicken IRF7 and blocks the binding of TBK1 to IRF7, leading to the suppression of IRF7 activation and an impaired IFN-β response. These findings expand our understanding of the pathogenesis of MDV and accelerate the development of more effective control strategies.
MATERIALS AND METHODS
Viruses, cells, and antibodies.
The MDV GA strain (GenBank accession no. AF147806) and HVT FC126 strain (GenBank accession no. AF291866) were propagated in CEFs or DF-1 cells and used in this study. The CEFs and DF-1 and HEK293T cells were prepared and maintained in Dulbecco’s modified Eagle medium as previously described (47). Chicken macrophage HD11 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Commercially available antibodies were used, including mouse anti-Flag, rabbit anti-HA, mouse anti-c-Myc, and rabbit anti-c-Myc (Sigma-Aldrich, St. Louis, MO, USA) and mouse antiactin and rabbit anti-histone H3 (Abcam, Cambridge, UK). The mouse anti-gI, rabbit anti-VP23, rabbit anti-cGAS, rabbit anti-STING, and rabbit anti-IRF7 antibodies were prepared in our laboratory. ISD was purchased from InvivoGen (San Diego, CA, USA).
Plasmid construction.
To construct the VP23 expression plasmid, the VP23 gene was amplified from the MDV genome and cloned into the pCAGGS vector with the Flag tag or c-Myc tag fused to its 3′ end to yield VP23-Flag or VP23-Myc. Plasmids harboring chicken cGAS (GenBank accession no. XM_419881), STING (GenBank accession no. KP893157), TBK1 (GenBank accession no. NM_001199558), or IRF7 (GenBank accession no. KP096419) were constructed by cloning the synthesized sequence into pCAGGS with the Flag, HA, or c-Myc tag fused to the 3′ ends. Truncated IRF7 expression plasmids were constructed by amplifying the indicated fragments of IRF7 into pCAGGS with the c-Myc tag fused to the 3′ ends. The chicken IFN-β promoter luciferase reporter pchIFN-β-luc was constructed by inserting the fragment of the chicken IFN-β promoter from positions −158 to +14 into the pGL3-basic vector, as described previously (32, 45). The pIRF7-luc reporter contained four copies of the IRF7-binding positive regulatory domain (GCA AAT AGA AAG C), and the pNF-κB-luc reporter contained four copies of the NF-κB-binding positive regulatory domain (GGG AAT TCT C).
Real-time qPCR.
Total RNA was extracted from cells by using the RNAiso Plus reagent (TaKaRa, Otsu, Japan). Reverse transcription was performed using a ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan). The quantity of each cDNA was determined by qPCR using the Thunderbird SYBR qPCR mix (Lucigen, Madison, WI, USA) and analyzed with a LightCycler 480 system (Roche, Basel, Switzerland). Primers specific for the genes for IFN-β, chicken ZAP (chZAP), chicken IFITM3 (chIFITM3), cGAS, and STING were synthesized by Invitrogen (Shanghai, China), and the relative mRNA levels of the genes for these proteins were normalized to the actin mRNA level in each sample. The fold differences between the treated samples and the mock-infected samples were calculated. For analyses of the MDV viral titers in the infected cells, total DNA was extracted using an AxyPrep BodyFluid viral DNA/RNA miniprep kit (Corning Life Sciences, Shanghai, China) and tested by real-time qPCR by measuring the copy numbers of the MDV meq gene as an MDV genome target and the chicken ovotransferrin gene as a reference, as described previously (48, 49). All controls and treated samples were examined in triplicate in the same plate.
ELISA.
The levels of IFN-β in cell cultures were analyzed using an ELISA kit for chicken IFN-β (USCN Life Science, Wuhan, China) according to the manufacturer’s instructions.
Transfection and dual-luciferase reporter assays.
DF-1 cells were cotransfected with a firefly luciferase reporter plasmid (IFN-β-luc, IRF7-lun, or NF-κB-luc) and the Renilla luciferase reporter pRL-TK, which served as an internal control, with or without expression plasmids, as indicated above, using a TransIT-X2 dynamic delivery system (Mirus, Madison, WI, USA) according to the manufacturer’s instructions. At 24 h posttransfection, cells were lysed, and samples were assayed for firefly and Renilla luciferase activity with a dual-luciferase reporter assay system (Promega, Madison, WI, USA). Relative luciferase activity was normalized to Renilla luciferase activity. The reporter assays were repeated at least three times.
RNA interference.
siRNAs specifically targeting chicken cGAS (5′-GCA GAA AUA UCA GUG GAC ATT-3′) and STING (5′-AGG UGC UGU GUU CCU GCU UTT-3′) as well as a scramble negative-control siRNA (5′-UUC UCC GAA CGU GUC ACG UTT-3′) were designed and synthesized by GenePharma (Shanghai, China). The siRNA transfections were performed in CEFs using the TransIT-X2 dynamic delivery system (Mirus) according to the manufacturer’s instructions. At 24 h after transfection, cells were harvested or infected with MDV for further analysis. The knockdown efficiency of cGAS or STING was verified by real-time qPCR and Western blotting.
Construction of VP23-expressing cells.
The VP23-encoding sequence was cloned into the pLVX-IRES-ZsGreen1 lentiviral vector (Clontech, Mountain View, CA, USA) with a Flag tag at the C terminus. The recombinant plasmid pLVX-VP23 was sequenced and packaged in HEK293T cells with the helper plasmids psPAX2 and pMD2.G. The resulting lentiviral expression plasmid was transduced into DF-1 cells, and stably transduced cells were selected by flow cytometry. The expression level of VP23 was detected by Western blotting.
Knockdown of VP23 by shRNA lentiviral interference.
A lentiviral vector-based siRNA plasmid (piLenti-siRNA-GFP) expressing shRNA that targets VP23 was designed and constructed by Applied Biological Materials (Richmond, BC, Canada). The recombinant piLenti-shVP23-GFP plasmid was transduced into CEFs according to the manufacturer’s instructions to generate VP23-knockdown cells. Cells transduced with the same vector plasmid expressing a scrambled shRNA served as a negative control. The stably transduced cells were monitored by detection of the green fluorescent protein (GFP) and selected by flow cytometry. The knockdown efficiency of VP23 was detected by Western blotting.
Coimmunoprecipitation assay and Western blotting.
HEK293T or DF-1 cells were transfected with the plasmids using the TransIT-X2 dynamic delivery system (Mirus). At 36 h posttransfection, cells were lysed in Pierce IP buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing protease inhibitor cocktail (Roche). The supernatants were obtained by centrifugation and incubated with the antibodies indicated above at 4°C overnight. Protein G Sepharose beads (Roche) were added, and the mixture was incubated for another 6 h. The beads were washed five times with phosphate-buffered saline (PBS) containing protease inhibitor cocktail and boiled with SDS-loading buffer for 10 min before analysis by Western blotting with the antibodies indicated above.
For Western blotting, whole-cell lysates were obtained by lysing cells in NP-40 lysis buffer (Beyotime, Beijing, China) containing protease inhibitor cocktail (Roche). The cytoplasmic and nuclear proteins were extracted using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific). Protein concentrations were determined with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific). The proteins were separated by electrophoresis on 12% SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, incubated with the primary and secondary antibodies indicated above, and scanned using an Odyssey infrared imaging system (LI-COR Biosciences, USA).
Confocal imaging.
DF-1 cells cultured in 35-mm culture dishes were transfected with the empty vector, the VP23-Flag plasmid, or the IRF7-HA plasmid, as indicated in Fig. 5A and 6E. Twenty-four hours later, cells were harvested or transfected with ISD for another 12 h. For confocal imaging, cells were first fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100 in PBS for 15 min, followed by blocking with 5% bovine serum albumin in PBS for 1 h. Then, the cells were incubated with rabbit anti-IRF7 and mouse anti-Flag or rabbit anti-HA and mouse anti-Flag antibodies diluted in PBS for 1 h. The cells were washed five times with PBS and incubated with the Alexa Fluor 488–anti-rabbit immunoglobulin and Alexa Fluor 546–anti-mouse immunoglobulin secondary antibodies (Abcam). Finally, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). After washing five times with PBS, the cells were examined using a confocal microscope system (model LSM880; Zeiss, Oberkochen, Germany).
Native PAGE.
Native PAGE was performed in order to study IRF7 dimerization as described previously (18), with slight modification. Briefly, the cells were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime) containing protease inhibitor cocktail (Roche). Native acrylamide gels (7.5%) were prerun with 25 mM Tris (pH 8.4) and 192 mM glycine with and without 0.1% deoxycholate (Sigma-Aldrich) in the cathode and anode chambers, respectively, for 30 min at 70 V. The lysates were then applied to the gel and electrophoresed for 150 min at 70 V. The IRF7 monomers and dimers were detected by Western blotting with anti-IRF7 antibodies.
Statistical analysis.
The data are presented as the means ± standard deviations (SD). Statistical significance between groups was determined by Student's t test with GraphPad Prism (version 7.0) software. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This research was supported by grants from the National Key Research and Development Program of China (2017YFD0500101, 2016YFE0203200) and the National Natural Science Foundation of China (31600127).
REFERENCES
- 1.Wu J, Chen ZJ. 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol 32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Akira S. 2015. Toll-like receptors. Curr Protoc Immunol 109:14.12.1–14.12.10. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- 3.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. 2015. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia P, Wang S, Gao P, Gao G, Fan Z. 2016. DNA sensor cGAS-mediated immune recognition. Protein Cell 7:777–791. doi: 10.1007/s13238-016-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Sun L, Chen ZJ. 2016. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinert LS, Lopušná K, Winther H, Sun C, Thomsen MK, Nandakumar R, Mogensen TH, Meyer M, Vægter C, Nyengaard JR, Fitzgerald KA, Paludan SR. 2016. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat Commun 7:13348. doi: 10.1038/ncomms13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Jacobs SR, West JA, Stopford C, Zhang Z, Davis Z, Barber GN, Glaunsinger BA, Dittmer DP, Damania B. 2015. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A 112:E4306–E4315. doi: 10.1073/pnas.1503831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber GN. 2015. STING: infection, inflammation and cancer. Nat Rev Immunol 15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu C, Li X, Li P. 2014. The mechanism of double-stranded DNA sensing through the cGAS-STING pathway. Cytokine Growth Factor Rev 25:641–648. doi: 10.1016/j.cytogfr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beachboard DC, Horner SM. 2016. Innate immune evasion strategies of DNA and RNA viruses. Curr Opin Microbiol 32:113–119. doi: 10.1016/j.mib.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Z, Damania B. 2016. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe 19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng C. 2018. Evasion of cytosolic DNA-stimulated innate immune responses by herpes simplex virus 1. J Virol 92:e00099-17. doi: 10.1128/JVI.00099-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su C, Zheng C. 2017. Herpes simplex virus 1 abrogates the cGAS/STING-mediated cytosolic DNA-sensing pathway via its virion host shutoff protein, UL41. J Virol 91:e02414-16. doi: 10.1128/JVI.02414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, You H, Su C, Li Y, Chen S, Zheng C. 2018. Herpes simplex virus 1 tegument protein VP22 abrogates cGAS/STING-mediated antiviral innate immunity. J Virol 92:e00841-18. doi: 10.1128/JVI.00841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu YZ, Su S, Gao YQ, Wang PP, Huang ZF, Hu MM, Luo WW, Li S, Luo MH, Wang YY, Shu HB. 2017. Human cytomegalovirus tegument protein UL82 inhibits STING-mediated signaling to evade antiviral immunity. Cell Host Microbe 21:231–243. doi: 10.1016/j.chom.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Su C, Zheng C. 2016. Herpes simplex virus 1 serine protease VP24 blocks the DNA-sensing signal pathway by abrogating activation of interferon regulatory factor 3. J Virol 90:5824–5829. doi: 10.1128/JVI.00186-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen MH, Jensen SB, Miettinen JJ, Luecke S, Prabakaran T, Reinert LS, Mettenleiter T, Chen ZJ, Knipe DM, Sandri-Goldin RM, Enquist LW, Hartmann R, Mogensen TH, Rice SA, Nyman TA, Matikainen S, Paludan SR. 2016. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J 35:1385–1399. doi: 10.15252/embj.201593458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Su C, Pearson A, Mody CH, Zheng C. 2017. Herpes simplex virus 1 UL24 abrogates the DNA sensing signal pathway by inhibiting NF-κB activation. J Virol 91:e00025-17. doi: 10.1128/JVI.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Cheng A, Wang M. 2013. Innate sensing of viruses by pattern recognition receptors in birds. Vet Res 44:82. doi: 10.1186/1297-9716-44-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santhakumar D, Rubbenstroth D, Martinez-Sobrido L, Munir M. 2017. Avian interferons and their antiviral effectors. Front Immunol 8:49. doi: 10.3389/fimmu.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karpala AJ, Stewart C, McKay J, Lowenthal JW, Bean AG. 2011. Characterization of chicken Mda5 activity: regulation of IFN-beta in the absence of RIG-I functionality. J Immunol 186:5397–5405. doi: 10.4049/jimmunol.1003712. [DOI] [PubMed] [Google Scholar]
- 24.Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. 2006. Marek's disease virus: from miasma to model. Nat Rev Microbiol 4:283–294. doi: 10.1038/nrmicro1382. [DOI] [PubMed] [Google Scholar]
- 25.Biggs PM, Nair V. 2012. The long view: 40 years of Marek's disease research and avian pathology. Avian Pathol 41:3–9. doi: 10.1080/03079457.2011.646238. [DOI] [PubMed] [Google Scholar]
- 26.Nair V. 2013. Latency and tumorigenesis in Marek's disease. Avian Dis 57:360–365. doi: 10.1637/10470-121712-Reg.1. [DOI] [PubMed] [Google Scholar]
- 27.Yuan S, Wang J, Zhu D, Wang N, Gao Q, Chen W, Tang H, Wang J, Zhang X, Liu H, Rao Z, Wang X. 2018. Cryo-EM structure of a herpesvirus capsid at 3.1 Å. Science 360:eaao7283. doi: 10.1126/science.aao7283. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Huang E, Desai J, Sole M, Pryce EN, Okoye ME, Person S, Desai PJ. 2011. A domain in the herpes simplex virus 1 triplex protein VP23 is essential for closure of capsid shells into icosahedral structures. J Virol 85:12698–12707. doi: 10.1128/JVI.05791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai P, DeLuca NA, Glorioso JC, Person S. 1993. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol 67:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin F, Li S, Zheng K, Zhuo C, Ma K, Chen M, Wang Q, Zhang P, Fan J, Ren Z, Wang Y. 2014. Silencing herpes simplex virus type 1 capsid protein encoding genes by siRNA: a promising antiviral therapeutic approach. PLoS One 9:e96623. doi: 10.1371/journal.pone.0096623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer-Klein J, Givol I, Barsov EV, Whitcomb JM, VanBrocklin M, Foster DN, Federspiel MJ, Hughes SH. 1998. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology 248:305–311. doi: 10.1006/viro.1998.9291. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y, Sun Y, Wang H, Yan Y, Ding C, Sun J. 2015. Chicken STING mediates activation of the IFN gene independently of the RIG-I gene. J Immunol 195:3922–3936. doi: 10.4049/jimmunol.1500638. [DOI] [PubMed] [Google Scholar]
- 33.Crippen TL. 2006. The selective inhibition of nitric oxide production in the avian macrophage cell line HD11. Vet Immunol Immunopathol 109:127–137. doi: 10.1016/j.vetimm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Ning S, Pagano JS, Barber GN. 2011. IRF7: activation, regulation, modification and function. Genes Immun 12:399–414. doi: 10.1038/gene.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deschamps T, Kalamvoki M. 2017. Evasion of the STING DNA-sensing pathway by VP11/12 of herpes simplex virus type 1. J Virol 91:e00535-17. doi: 10.1128/JVI.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang ZF, Zou HM, Liao BW, Zhang HY, Yang Y, Fu YZ, Wang SY, Luo MH, Wang YY. 2018. Human cytomegalovirus protein UL31 inhibits DNA sensing of cGAS to mediate immune evasion. Cell Host Microbe 24:69–80.e4. doi: 10.1016/j.chom.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Kang HR, Cheong WC, Park JE, Ryu S, Cho HJ, Youn H, Ahn JH, Song MJ. 2014. Murine gammaherpesvirus 68 encoding open reading frame 11 targets TANK binding kinase 1 to negatively regulate the host type I interferon response. J Virol 88:6832–6846. doi: 10.1128/JVI.03460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda K, Taniguchi T. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 39.Lin R, Mamane Y, Hiscott J. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J Biol Chem 275:34320–34327. doi: 10.1074/jbc.M002814200. [DOI] [PubMed] [Google Scholar]
- 40.Hwang SW, Kim D, Jung JU, Lee HR. 2017. KSHV-encoded viral interferon regulatory factor 4 (vIRF4) interacts with IRF7 and inhibits interferon alpha production. Biochem Biophys Res Commun 486:700–705. doi: 10.1016/j.bbrc.2017.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Zhao J, Ren J, Hall KH, Moorman JP, Yao ZQ, Ning S. 2016. Protein phosphatase 1 abrogates IRF7-mediated type I IFN response in antiviral immunity. Eur J Immunol 46:2409–2419. doi: 10.1002/eji.201646491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J Virol 78:1675–1684. doi: 10.1128/JVI.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc Natl Acad Sci U S A 99:5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu L, Fossum E, Joo CH, Inn KS, Shin YC, Johannsen E, Hutt-Fletcher LM, Hass J, Jung JU. 2009. Epstein-Barr virus LF2: an antagonist to type I interferon. J Virol 83:1140–1146. doi: 10.1128/JVI.00602-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sick C, Schultz U, Münster U, Meier J, Kaspers B, Staeheli P. 1998. Promoter structures and differential responses to viral and nonviral inducers of chicken type I interferon genes. J Biol Chem 273:9749–9754. doi: 10.1074/jbc.273.16.9749. [DOI] [PubMed] [Google Scholar]
- 46.Okoye ME, Sexton GL, Huang E, McCaffery JM, Desai P. 2006. Functional analysis of the triplex proteins (VP19C and VP23) of herpes simplex virus type 1. J Virol 80:929–940. doi: 10.1128/JVI.80.2.929-940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han C, Zeng X, Yao S, Gao L, Zhang L, Qi X, Duan Y, Yang B, Gao Y, Liu C, Zhang Y, Wang Y, Wang X. 2017. Voltage-dependent anion channel 1 interacts with ribonucleoprotein complexes to enhance infectious bursal disease virus polymerase activity. J Virol 91:e00584-17. doi: 10.1128/JVI.00584-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Liu CJ, Qin YA, Zhang YP, Zhang XW, Hao YQ. 2007. Application of duplex fluorescent quantitative polymerase-chain-reaction for detecting Marek’s disease virus serotype 1. Chin J Prev Vet Med 29:46–51. [Google Scholar]
- 49.Sun GR, Zhang YP, Zhou LY, Lv HC, Zhang F, Li K, Gao YL, Qi XL, Cui HY, Wang YQ, Gao L, Pan Q, Wang XM, Liu CJ. 2017. Co-infection with Marek’s disease virus and reticuloendotheliosis virus increases illness severity and reduces Marek’s disease vaccine efficacy. Viruses 9:158. doi: 10.3390/v9060158. [DOI] [PMC free article] [PubMed] [Google Scholar]