Sexual transmission of HSV-2 results from viral shedding following reactivation from latency. The immune cell populations and mechanisms that control HSV-2 shedding are not well understood. This study examined the role of CD4+ T cells in control of virus shedding using a guinea pig model of genital HSV-2 infection that recapitulates the shedding of virus experienced by humans. We found that the frequency of virus-shedding episodes, but not the incidence of clinical disease, was increased by depletion of CD4+ T cells. The HSV-specific antibody response was not diminished, but frequency of functional HSV-reactive CD8+ T cells was significantly diminished by CD4 depletion. These results confirm the role of cell-mediated immunity and highlight the importance of CD4+ T cells in controlling HSV shedding, suggesting that therapeutic vaccines designed to reduce transmission by controlling HSV shedding should include specific enhancement of HSV-specific CD4+ T cell responses.
KEYWORDS: CD4+ T lymphocyte, HSV-2, HSV-2 shedding, genital mucosa, guinea pig
ABSTRACT
Reactivation of herpes simplex virus 2 (HSV-2) results in infection of epithelial cells at the neuro-epithelial junction and shedding of virus at the epithelial surface. Virus shedding can occur in either the presence or absence of clinical disease and is usually of short duration, although the shedding frequency varies among individuals. The basis for host control of virus shedding is not well understood, although adaptive immune mechanisms are thought to play a central role. To determine the importance of CD4+ T cells in control of HSV-2 shedding, this subset of immune cells was depleted from HSV-2-infected guinea pigs by injection of an anti-CD4 monoclonal antibody (MAb). Guinea pigs were treated with the depleting MAb after establishment of a latent infection, and vaginal swabs were taken daily to monitor shedding by quantitative PCR. The cumulative number of HSV-2 shedding days and the mean number of days virus was shed were significantly increased in CD4-depleted compared to control-treated animals. However, there was no difference in the incidence of recurrent disease between the two treatment groups. Serum antibody levels and the number of HSV-specific antibody-secreting cells in secondary lymphoid tissues were unaffected by depletion of CD4+ T cells; however, the frequency of functional HSV-specific, CD8+ gamma interferon-secreting cells was significantly decreased. Together, these results demonstrate an important role for CD4+ T lymphocytes in control of virus shedding that may be mediated in part by maintenance of HSV-specific CD8+ T cell populations. These results have important implications for development of therapeutic vaccines designed to control HSV-2 shedding.
IMPORTANCE Sexual transmission of HSV-2 results from viral shedding following reactivation from latency. The immune cell populations and mechanisms that control HSV-2 shedding are not well understood. This study examined the role of CD4+ T cells in control of virus shedding using a guinea pig model of genital HSV-2 infection that recapitulates the shedding of virus experienced by humans. We found that the frequency of virus-shedding episodes, but not the incidence of clinical disease, was increased by depletion of CD4+ T cells. The HSV-specific antibody response was not diminished, but frequency of functional HSV-reactive CD8+ T cells was significantly diminished by CD4 depletion. These results confirm the role of cell-mediated immunity and highlight the importance of CD4+ T cells in controlling HSV shedding, suggesting that therapeutic vaccines designed to reduce transmission by controlling HSV shedding should include specific enhancement of HSV-specific CD4+ T cell responses.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is one of the most common sexually transmitted pathogens and is responsible for 20 million new infections each year worldwide (1). Genital herpes infection is also a leading cause of genital ulcer disease (2, 3) and increases the likelihood of acquiring other sexually transmitted diseases, including HIV (4–6). HSV-2 infection may result in the development of self-limiting painful lesions in immunocompetent individuals but is a cause of severe morbidity in immunocompromised populations (7, 8) and in newborns exposed to virus in the birth canal (9, 10).
HSV-2 initially infects epithelial cells and is transported via neurons to sensory and autonomic ganglia. Although the acute infection of the epithelium and ganglia is controlled by the host immune response, a latent infection is established in sensory and autonomic ganglion neurons (11, 12). While the role of virus reactivation from autonomic ganglia in disease is incompletely understood, numerous studies have shown that HSV-2 periodically reactivates from latency in sensory ganglia and is transported back down the sensory nerve axon to the epithelium at or near the site of original infection (13). Virus shedding may occur concomitantly with recurrent lesions or in the absence of clinical symptoms. The virus titer and duration of shedding are generally greater during symptomatic shedding (14); however, more transmissions occur as a result of physical contact during asymptomatic shedding, when individuals may be unaware they are shedding virus (15, 16). Asymptomatic shedding episodes are usually of short duration, occur relatively frequently, and result in various magnitudes of virus shedding (14, 17). Release of newly reactivated virus in a defined time interval can also be widespread in genital tract regions that are innervated by multiple sacral ganglia, suggesting several reactivations occur simultaneously (18). The short duration of the majority of shedding events in immunocompetent individuals suggests active and rapid immune control at the site of virus release from neurons. Consistent with this concept, populations of a novel CD8αα+ T cell subsets have been shown to accumulate in epithelial sites of humans previously infected by HSV-2, and HSV-specific CD8+ cells have been detected at the site of virus release at neuro-epithelial junctions (19–21). HSV-specific CD8+ resident memory T cells (TRM) in cutaneous and genital tissue have been shown to provide rapid effector responses to virus challenge in murine HSV-2 infection models (22–24). While much attention has focused on HSV-specific CD8+ TRM in controlling virus shedding in the genital tract, less is known about protection afforded by CD4+ TRM. In HSV-infected humans, HSV-specific CD4+ T cells were enriched in the cervix compared to the level in peripheral blood and expressed effector memory T cell markers and the CD69 and CD103 markers of tissue residency (25, 26). In mice previously infected with HSV-2, CD4+ TRM were detected in memory lymphocyte clusters in the vaginal mucosa and were required for full protection against a single high-titer HSV-2 challenge (27, 28). However, the role of CD4+ T cells in controlling HSV-2 shedding during the multiple intermittent exposures to relatively low virus titers following reactivation of latent HSV is not understood. Examination of the role of specific T cell subsets in control of HSV-2 shedding has not been performed due to difficulties in the controlled modulation of cellular immune responses in animal models that experience spontaneous reactivation and shedding of HSV-2. Utilizing a novel CD4 depletion approach in guinea pigs, we examined the effect that loss of this T cell subset had on HSV-2 shedding. Overall, the depletion of CD4+ T cells from guinea pigs with a latent HSV-2 infection resulted in more frequent shedding and an increase in the duration of concurrent shedding days but did not alter the frequency or severity of recurrent disease. The diminished immune control of HSV-2 shedding in CD4-depleted animals appeared to reflect loss of important cell-mediated mechanisms rather than alteration of the HSV-specific antibody response, strongly supporting an important role for T cell responses in control of HSV-2 shedding. Further, these results directly support an important role for CD4+ T cells in control of virus shedding in the vagina after HSV reactivation and further support the notion that effective therapeutic vaccines for control of HSV-2 shedding should target strong CD4+ T cell responses.
RESULTS
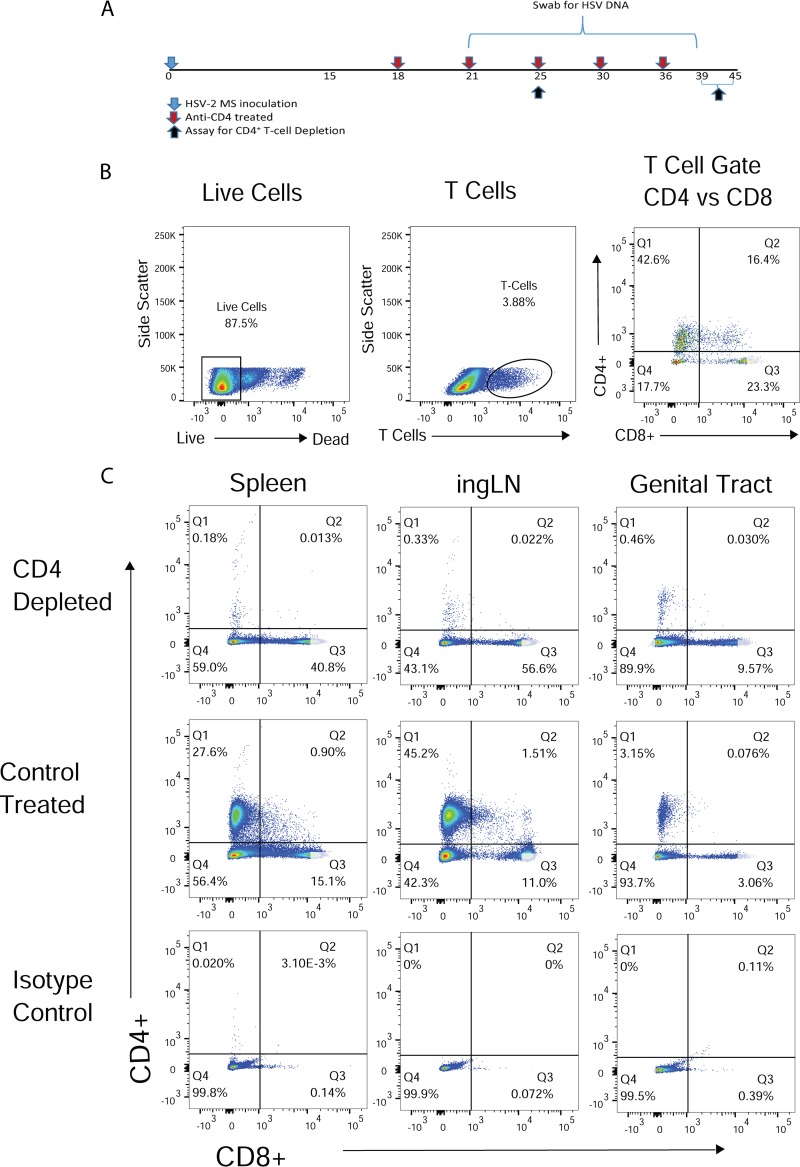
Cell-mediated responses are thought to be responsible for the rapid control and clearance of epithelial infections by newly released virus after HSV-2 reactivation, yet the role of specific T cell subsets is unclear. To determine the role of CD4+ T lymphocytes in control of HSV shedding, we depleted CD4+ cells from guinea pigs with an established latent HSV-2 infection. Guinea pigs were treated over a 3-week period with a modified rat anti-guinea pig CD4 monoclonal antibody (MAb) or control treated with rat serum beginning day 18 postinfection (p.i.) (Fig. 1A). The presence of CD4+ and CD8+ T lymphocytes was determined in a subset of the treated animals on day 25 p.i. and after the termination of the experiment between days 39 and 45 p.i. (7 and 21 to 27 days after initiating MAb treatment, respectively). The gating strategy and T cell subset histograms from T cells obtained from the spleen, inguinal lymph node (ingLN), and genital tract from representative anti-CD4 MAb- and control-treated animals are shown in Fig. 1B and C.
FIG 1.
Depletion of CD4+ T cells from peripheral tissue and secondary lymphoid tissue of HSV-2-infected guinea pigs by treatment with anti-guinea pig CD4 MAb. (A) Illustration of CD4 depletion regimen. Female guinea pigs received anti-CD4 MAb or diluted rat serum as a control treatment beginning on day 21 after HSV-2 inoculation and on days indicated by red arrows. Lymphocytes from representative animals of each treatment group were harvested on day 25 p.i. or at the termination of the experiment, between days 39 and 45 p.i. (B) Representative histograms demonstrating the gating strategy for analysis of CD4+ and CD8+ T lymphocyte populations. (C) Representative histograms of T lymphocyte populations from a CD4-depleted and control-treated animal. Results are from a single experiment, representative of three identical experiments performed.
Table 1 shows that treatment resulted in approximately 99% depletion of CD4+ T lymphocytes in the spleen at day 25 p.i. and 96% on days 39 to 45 p.i. Similarly, approximately 97% of CD4+ T cells were depleted from the ingLN over the course of the experiment. Depletion of CD4+ T cells from the genital tracts of infected animals occurred more slowly but was approximately 85% on day 25 p.i. and increased to ∼99% depletion at the termination of the experiment (days 39 to 45 p.i.). Approximately 87% of CD4+ T cells were depleted from the lumbosacral ganglia and adjacent spinal cord at the termination of the experiment. Although depletion of CD4+ T lymphocytes was nearly complete, CD8+ T lymphocytes were detected at high levels at both time points in spleen, ingLN, and genital tracts of anti-CD4-depleted animals compared to control-treated animals (Fig. 1C).
TABLE 1.
Maintenance of CD4 depletiona
| Tissue | Mean % depletion ± SEM (n)b on day: |
|
|---|---|---|
| 25 | 39–45 | |
| Spleen | 99.2 ± 0.2 (3) | 95.9 ± 2.0 (7) |
| ingLN | 96.9 ± 1.2 (3) | 97.2 ± 1.1 (7) |
| Genital tract | 84.9 ± 3.6 (3) | 98.9 ± 1.0 (2) |
| Spinal cord/ganglia | NDc | 86.5 ± 1.8 (2) |
Depletion was calculated as 1 – (% CD4 cells in anti-CD4-treated tissue/% CD4 cells in control-treated tissue) × 100.
n represents the number of total samples, obtained from three depletion experiments performed.
ND, not determined.
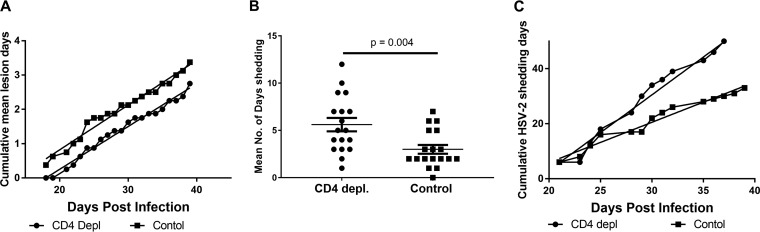
CD4 depletion did not impact recurrent disease. As shown in Fig. 2A, the incidence of recurrent disease, measured as the cumulative mean lesion days, was not different between CD4-depleted and control-treated animals over the course of the study. Although lesions were detected in control-treated animals on day 18 but not detected until day 21 in CD4-treated animals, the slopes of the cumulative mean lesion day curves were not different between the two groups (P = 0.36 by linear regression). To assess effects of CD4 depletion on HSV-2 shedding, vaginal swabs were collected from CD4-depleted and control-treated guinea pigs on days 21 to 39 p.i., and the frequency and magnitude of HSV-2 shedding was determined by quantitative PCR (qPCR) (29, 30). From two separate experiments, all (18/18) of the CD4-depleted and 17/18 control-treated animals shed virus during the observation period (Fig. 2B). However, the mean number of shedding days experienced by individual animals was significantly greater in CD4-depleted animals than in control-treated animals (P < 0.004 by unpaired, 2-tailed Student's t test), resulting in the cumulative number of HSV-2 shedding days over the treatment period being significantly greater in CD4-depleted animals than in control-treated animals (P < 0.0001 by linear regression analysis) (Fig. 2C). Overall, control-treated animals shed virus on 20% of the days compared to 34% in CD4-depleted animals (P < 0.0001 by Fisher’s exact test). In contrast, the mean number of HSV-2 genomes detected during shedding over the 21-day treatment period was comparable between the two groups (data not shown), suggesting that the magnitude of shedding was unaffected by CD4 depletion. Together, these results demonstrate that loss of CD4+ T cells resulted in the diminished ability to control the frequency of HSV-2 shedding but did not significantly alter the magnitude of HSV-2 shedding events or the number of recurrent lesion days.
FIG 2.
Depletion of CD4+ T lymphocytes from HSV-2-infected guinea pigs resulted in diminished control of HSV-2 shedding. (A) Depletion of CD4+ T cells from HSV-2-infected guinea pigs did not result in exacerbation of recurrent disease. HSV-infected guinea pigs (n = 8 animals/group) were scored for incidence of recurrent lesions between days 21 and 39 p.i. Results are expressed as the cumulative mean lesion days for CD4-depleted and control-treated animals and are from a single representative experiment of two experiments performed. The linear regression line for each curve is shown, and the slopes of the cumulative mean lesion day curves are not different between the two groups (P = 0.36 by linear regression analysis). (B) Mean number of days shedding by HSV-2-infected, CD4-depleted, and control-treated animals. Results shown are the number of days of shedding by individual CD4-depleted and control-treated animals between days 21 and 39 p.i. (P < 0.004 by unpaired, 2-tailed Student's t test). Results shown are pooled from 2 experiments performed (n = 18 animals/group). (C) Cumulative days shedding by HSV-2-infected, CD4-depleted, and control-treated guinea pigs. Animals were swabbed on the indicated days for detection of HSV-2 shedding by qPCR from day 21 through day 39 p.i. Results shown are from a single experiment representative of two experiments performed (n = 8). The linear slope of cumulative shedding by CD4-depleted animals is significantly different from the cumulative shedding slope of control-treated animals (P < 0.0001 by linear regression analysis).
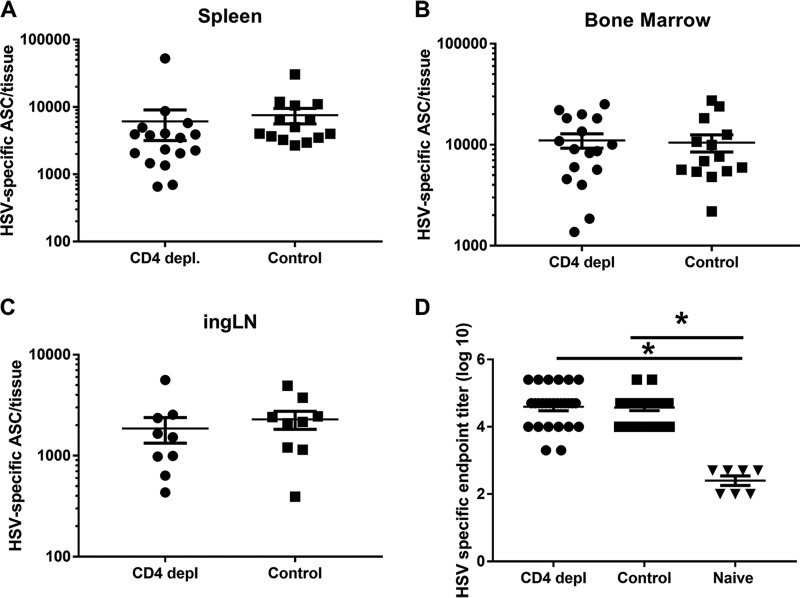
Because CD4+ T cells play a role in a variety of immune functions, we determined if other adaptive immune responses were altered in CD4-depleted animals. To determine if CD4 depletion altered the HSV-specific antibody response, enzyme-linked immunospot (ELISPOT) assays and enzyme-linked immunosorbent assays (ELISAs) were performed to quantify the HSV-specific antibody-secreting cell (ASC) response and HSV-specific serum IgG antibody levels. HSV-specific ASCs were detected at large numbers in the spleen, bone marrow, and ingLN of CD4-depleted and control-treated HSV-2-infected guinea pigs at the termination of the depletion experiment (Fig. 3A to C). The mean number of HSV-specific ASCs was not significantly different between groups (P > 0.05 by unpaired, 2-tailed Student's t test) in any tissue tested. Consistent with these results, the endpoint ELISA IgG titers of HSV-specific serum antibodies from anti-CD4-treated and control-treated HSV-2-infected guinea pigs were significantly higher than those of uninfected controls (P < 0.0001 by ANOVA with Tukey’s multiple-comparison test), but titers in the two treatment groups were not different (P = 0.83 by ANOVA with Tukey’s multiple-comparison test) (Fig. 3D). Together, these results support the notion that depletion of CD4+ T cells did not modify the antibody response to HSV-2 infection and strongly suggest that the diminished control of shedding in CD4-depleted animals reflected a diminished cell-mediated response to reactivated virus.
FIG 3.
HSV-specific ASC responses and serum antibody levels are not diminished by CD4 depletion. Sera and lymphocytes from spleen, bone marrow, and ingLN were obtained from guinea pigs after the termination of the CD4 depletion experiment (between days 39 and 45 p.i.). (A to C) The number of HSV-specific ASC in the indicated tissues of individual animals was quantified by ELISPOT assay, and the results are reported as the mean number of HSV-specific ASC/tissue ± standard errors of the means (SEM). Results represent data pooled from two identical experiments, n = 9 to 17. (D) The titer of serum HSV-gD-specific antibody was determined in individual animals by ELISA and reported as the mean endpoint dilution titer ± SEM (log10). Results represent data pooled from three identical experiments (n = 7 naive, 27 CD4-depleted, and 22 control-treated animals). *, P < 0.0001 (ANOVA with Tukey’s multiple-comparison test).
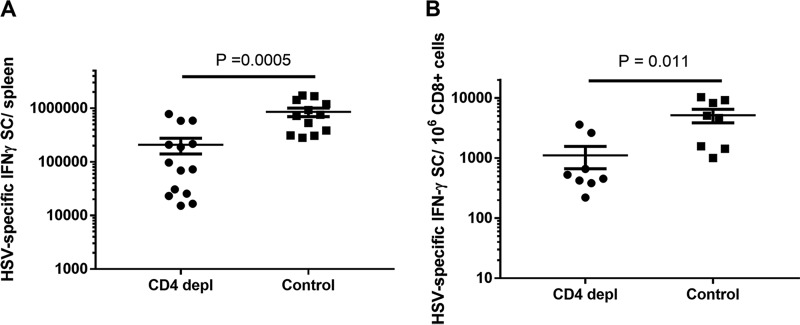
While CD8+ T cells were detected in the secondary lymphoid tissues and genital epithelium of both CD4-depleted and control-treated animals (Fig. 1C), it was possible that the effector function of these cells was altered in the absence of CD4+ T cells (31). To assess the functional activity of the HSV-specific T cell populations in treated animals, we quantified the HSV-specific, gamma interferon (IFN-γ)-secreting cells (SC) from spleens of CD4-depleted and control-treated animals at the termination of the experiment (between days 39 and 45 p.i.). As shown in Fig. 4A, IFN-γ SC were detected in both treatment groups; however, the total number of HSV-specific IFN-γ SC obtained from CD4-depleted, HSV-immune animals was significantly lower than that in control-treated, HSV-immune animals (P= 0.0005 by 2-tailed Student's t test). This difference was anticipated, because the response measured would theoretically include both CD4+ and CD8+ T cells in the control-treated group but only CD8+ T cells in the CD4-depleted group. To test if CD4 depletion altered the frequency of functional HSV-specific, CD8+ IFN-γ SC, we enriched CD8+ T cells from spleens of CD4-depleted and control-treated animals and quantified the number of HSV-specific IFN-γ SC by ELISPOT assay. As shown in Fig. 4B, a significant decrease in the frequency of HSV-specific, CD8+ IFN-γ SC was detected in CD4-depleted animals compared to that of the control-treated group (P= 0.0109 by 2-tailed Student's t test). Together, these results suggest that, in addition to the loss of CD4+ T cell effector activity, depletion of CD4+ T cells significantly decreased the frequency of functional HSV-specific CD8+ IFN-γ SC but did not affect HSV-specific ASC or HSV-specific serum antibody levels.
FIG 4.
Frequency of HSV-specific, CD8+ IFN-γ SC is significantly diminished by depletion of CD4+ T cells. (A) Total number of IFN-γ SC in spleens of CD4-depleted and control-treated animals obtained after the termination of the depletion experiment (between days 39 and 45 p.i.). Results from individual animals were determined and expressed as the mean number of IFN-γ SC per spleen ± SEM. Results represent data pooled from two identical experiments (n = 14 CD4-depleted and 12 control-treated animals). (B) Frequency of CD8+ HSV-specific, IFN-γ SC in spleens of CD4-depleted and control-treated animals. Splenic CD8+ lymphocytes were enriched by antibody-coated beads and the number of HSV-specific IFN-γ SC determined by ELISPOT assay. Results from individual animals (n = 8 CD4-depleted and 8 control-treated animals) were determined and expressed as the mean number of CD8+ IFN-γ SC per 106 enriched CD8+ T lymphocytes ± SEM.
DISCUSSION
Both CD4+ and CD8+ T cells have been shown to be involved in control of genital herpes infections in murine models (27, 32, 33) by effector mechanisms such as IFN-γ production and cytolytic activity (34, 35). In murine models, CD4+ T cells appear to play a central role in protection through direct antiviral activity and mediation of the migration and retention of innate and adaptive immune cells to the site of infection (27, 36). Because murine genital HSV-2 infection results in high lethality and surviving animals do not experience spontaneous recurrent disease or virus shedding into the genital tract, studies examining CD4+ T cell function in genital HSV-2 infection generally utilize viral challenge of HSV immune animals. In the current studies, we used a guinea pig model of genital HSV-2 infection to examine the role of CD4+ T cells in control of HSV-2 shedding, because guinea pigs mimic human HSV-2 infections by experiencing spontaneous recurrences resulting in virus shedding from the genital epithelium (37, 38). The development of a reagent to manipulate the local and systemic cellular immune response to HSV-2 infection in the current study represents an important step forward and significantly increases the utility of the guinea pig model by allowing assessment of the cellular immune components involved in control of HSV-2 shedding, providing new information directly relevant to virus transmission in humans and to the development of therapeutic vaccines.
While much attention has focused on HSV-specific CD8+ TRM in controlling virus shedding, less is known about protection afforded by CD4+ TRM and effector memory cells. Evidence for a contribution of CD4+ T cells in controlling HSV shedding comes from clinical studies showing that HIV+ patients with low CD4+ T cell counts experience more frequent HSV-2 shedding but no difference in the incidence of recurrent disease (39, 40). However, it is unclear if this loss of control of HSV shedding reflects diminished antiviral activity by CD4+ T cells or, alternatively, altered innate and adaptive immune responses in HIV+ individuals. The results of our current study are similar, showing that acute depletion of CD4+ T cells in HSV-infected guinea pigs resulted in more frequent HSV-2 shedding but no difference in recurrent disease. Clinical studies utilizing frequent sampling show that most shedding events in humans are of very short duration, usually lasting only a few hours (17). Additionally, multiple-site sampling of humans experiencing HSV shedding have shown that shedding may occur simultaneously at nonadjacent sites, suggesting the occurrence of multiple individual reactivation events (18). Because sampling in our current study was limited to daily sampling of the entire vaginal vault, we were unable to determine if the increased shedding in CD4-depleted animals reflected extended virus replication following single reactivation/shedding episodes or an increase in the number of independent but contemporaneous shedding episodes.
Interestingly, similar to the studies in HIV+ individuals (39, 40), the absence of CD4+ T cells in depleted guinea pigs altered shedding frequency but not magnitude. Our current study also did not determine if the mechanism of protection manifested by CD4+ T cells was active at the site of latency in the sensory ganglia, at the site of virus shedding in the genital epithelia, or both, because treatment with the anti-CD4 MAb depleted cells at both the genital epithelium and sensory ganglia (Table 1). Populations of CD8+ TRM reside in the sensory ganglia of HSV-infected humans and laboratory animals and have been shown to modulate reactivation from latency (41, 42). However, CD4+ T cells have also been demonstrated in HSV-infected sensory ganglia and have been shown to play a role in clearance of HSV-1 from the sensory ganglia and spinal cords (43). Future studies will be aimed at dissecting the protective role of CD4+ T cells at these two sites of HSV-2 pathogenesis.
Several mechanisms could play a role in CD4+ T cell control of HSV-2 shedding. CD4+ T cells exhibiting IFN-γ secretion and cytolytic activity, suggestive of direct antiviral activity, have been detected in the vaginal mucosa of HSV-infected mice (28, 32, 44, 45). More recently, large numbers of HSV-specific CD4+ T cells were detected in lymphocyte clusters in the vaginal mucosa (27, 28). While there was evidence for direct antiviral activity by these cells, their protective function was also manifested partially through the IFN-γ-dependent induction of CCL5 production by vaginal macrophages, which subsequently increased retention of CD8+ memory T cells in the vagina (27). CD4+ T cells have also been shown to play a role in maintenance of memory CD8+ T cells. In the absence of CD4+ T cell help, memory T cells have been shown to become dysfunctional and diminished in number over a period of several weeks (31). In other studies, expansion of memory CD8+ T cells to a second encounter with antigen required CD4+ T cells at the priming stage (46). Similarly, the results of our current study show that depletion of CD4+ T cells in previously infected guinea pigs resulted in a reduction in the frequency of functional CD8+, HSV-specific IFN-γ SC. Because the quantification of HSV-specific cells is function dependent in the current study, it was not possible to distinguish between a loss of HSV-specific CD8+ T cells and a functional impairment of this population in the absence of CD4+ T cells. Given the presence of CD8+ TRM in the vaginal epithelium of humans and laboratory animals, the current results suggest that failure to maintain the HSV-specific CD8+ T cell population contributed, in part, to the diminished control of HSV-2 shedding in CD4-depleted animals.
Additional CD4+ T cell-dependent mechanisms exist that may play a role in control of HSV shedding. CD4+ T cells have been shown to be involved in migration and retention of innate immune cells to sites of infection (36), regulating the activation and effector function of natural killer (NK) cells (47, 48) and improving access for antiviral antibody to neuronal tissues (49). In the current studies, we detected no impact of CD4 depletion on production of serum antibody or the number of HSV-specific ASC in secondary lymph node tissue, including the bone marrow. While the role of HSV-specific antibody in control of HSV-2 is complex and not completely understood, the results of this study suggest that the frequency of virus shedding was increased by diminished cell-mediated activity despite the maintenance of high titers of HSV-specific antibody.
Understanding the immune basis for control of HSV shedding is important for development of effective therapeutic vaccines to prevent transmission of HSV-2. To our knowledge, this study represents the first specific manipulation of an adaptive immune response for analysis of immune control of HSV shedding in an animal model that experiences spontaneous HSV reactivations. The results strongly support a role for CD4+ T cells in controlling the frequency, but not the magnitude, of HSV-2 shedding. Moreover, CD4 depletion did not impact protection against development of recurrent disease. Our results demonstrating a requirement for CD4+ T cell help in maintenance of HSV-specific CD8+ memory T cell populations, but not HSV-specific antibody or ASC levels, suggest that the diminished control of HSV shedding results mainly from a loss of cell-mediated immune function. Establishment of a role for CD4+ T cells in immune control of HSV shedding also suggests that therapeutic vaccines designed to strongly enhance the HSV-specific CD4 response will prove effective in controlling virus shedding and preventing HSV transmission.
MATERIALS AND METHODS
Virus.
Working stocks of HSV-2 strain MS were prepared using Vero cell monolayers and stored at −80°C as previously described (50). Stocks of the replication-defective HSV-2 dI5-29 strain (51) (a kind gift from David Knipe, Harvard Medical School, Boston, MA) were prepared by infection of V529 cells as previously described (52).
Guinea pig model of genital herpes.
Female Hartley guinea pigs (Charles River Breeding Laboratories, Wilmington, MA) were housed in an AAALAC-approved vivarium and allowed to acclimate prior to experimentation. All studies were humanely conducted with the approval of the UTMB Institutional Animal Care and Use Committee with oversight of staff veterinarians.
Animals were inoculated intravaginally with 6.0 log10 PFU of HSV-2 strain MS, as described previously (53). Following HSV-2 inoculation, animals were examined daily, and the severity of primary disease and the frequency and severity of spontaneous recurrent disease (days 18 to 39 p.i.) were scored as previously described (37). Animals were administered five intraperitoneal injections of 1.5 mg sterile, purified anti-guinea pig CD4 MAb or, as a control, rat serum diluted to approximate the concentration of IgG in the MAb treatment, on days 18, 21, 25, 30, and 36 p.i.
Vaginal swab samples were collected from HSV-2-infected guinea pigs on days 21 to 39 p.i. into 1.0 ml of Dulbecco’s modified Eagle medium (Corning Life Sciences-Mediatech, Inc., Manassas, VA) supplemented with 2% (vol/vol) newborn calf serum (Life Technologies Incorporated, Carlsbad, CA) and 1% penicillin-streptomycin (P/S; 10,000 U/ml penicillin and 10,000 μg/ml streptomycin stock; Sigma-Aldrich, St. Louis, MO). The samples were vortexed, and 100 μl was added to 100 μl total RNA lysis buffer (Bio-Rad, Hercules, CA) containing 1% β-mercaptoethanol (BME; Sigma-Aldrich).
Selection of spontaneous IgG subclass switch variants.
The rat anti-guinea pig CD4-specific MAb H155 (54) secretes an IgG2a antibody that is not effective at depleting CD4+ T cells in vivo (H. Schäfer, unpublished results). To obtain MAb that could deplete CD4+ T cells in vivo, IgG2b subclass switch variants were isolated in a stepwise fashion from the parental rat IgG2a-secreting H155 hybridoma. Briefly, H155 hybridoma cells were plated at 500 cells/well in 96-well culture plates. Supernatants were harvested when cultures covered greater than 50% of the plate surface area and were tested for rat IgG1 by rat subclass-specific ELISA. Hybridoma cultures testing positive for IgG1 were cultured at lower cell density (100 cells/well) and grown to greater than 50% confluence, and supernatants were tested again for rat IgG1. Positive cultures were repeatedly subcultured (starting frequencies between 1 and 10 cells/well) until the majority of 96-well cultures were positive for the presence of rat IgG1. Selected IgG1-positive cultures were then cloned twice by limiting dilution and expanded. The rat IgG2b-secreting H155 (H155-IgG2b) used for depletion was isolated in the same fashion from the newly isolated IgG1-secreting H155 hybridoma by repeated rounds of dilution subculturing of IgG2b-positive cultures and selection for cultures containing IgG2b, culminating in isolation of rat IgG2b-secreting clones by limiting dilution. H155-IgG2b was expanded and cultured in serum-deficient medium, and anti-guinea pig CD4 MAb was purified from culture supernatants by ammonium sulfate precipitation and purification over protein AG columns as previously described (52).
Assays for HSV-specific antibody production.
Quantification of HSV-specific ASC from spleen, bone marrow, and ingLN was performed by ELISPOT assay, as previously described (55), on HSV-glycoprotein-coated plates, with ovalbumin (OVA)-coated plates used as a control. Background spots detected on OVA-coated wells were routinely subtracted from totals on HSV-2 glycoprotein-coated plates. For ELISA measurement of serum antibodies, HSV-2-specific IgG titers from immune serum were obtained by ELISA on HSV-2 recombinant glycoprotein D (rgD; Meridian Life Science, Inc., Memphis, TN)-coated plates. Serial dilutions of serum samples were plated in duplicate and incubated at 4°C overnight. IgG titers were detected by incubation with horseradish peroxidase-goat anti-guinea pig IgG (Abcam, Cambridge, MA) and developed as described previously (56). The optical density at 405 nm (OD405) was determined using SoftmaxPro 7.0.3 software. The endpoint titer was defined as the serum dilution resulting in an OD405 reading greater than 0.1 and greater than three standard deviations above blank medium values.
IFN-γ ELISPOT assay.
Splenic lymphocytes from CD4-depleted and control-treated HSV-2-infected animals were assayed to quantify HSV-specific IFN-γ-secreting cells by ELISPOT assay as previously described (52). To determine the frequency of HSV-specific, CD8+ IFN-γ SC in treated animals, CD8+ T cells were enriched using antibody-coated bead columns according to the manufacturer’s instructions (Miltenyi Biotech). Briefly, Fc receptors of splenocytes were blocked using Fc Block (BD Pharm), stained with fluorescein isothiocyanate (FITC)-conjugated anti-guinea pig CD8 antibody (Bio-Rad), incubated with anti-FITC microbeads, and passed over magnetically activated cell-sorting LS separation columns (Miltenyi Biotech). Unlabeled cells were washed from the column, and then labeled cells (CD8+ fraction) were eluted and passed over a second column to increase enrichment. CD8+-enriched lymphocyte populations were stimulated with either HSV-2 dl5-29-infected or medium-treated murine lymph node cells on IFN-γ ELISPOT plates, and spots representing IFN-γ SC were developed as described previously (52).
Flow cytometry.
For flow cytometry, single-cell suspensions were prepared from spleen and ingLN by dissociating tissue through 50-μm mesh screens. Genital tracts, including the perineum, vagina, and cervix, were dissected from individual anti-CD4- or control-treated animals and digested as described previously (52). To allow for the potential reexpression of surface CD4 molecules in MAb-treated animals, single-cell suspensions of genital tracts, spleen, and ingLN were washed extensively and cultured for 24 h in T cell medium (Iscove’s modified Dulbecco’s medium, 10% fetal bovine serum [FBS], 1.0% l-glutamine, 1.0% nonessential amino acids, and 2-mercaptoethanol). Red blood cells were removed from cultured cells by incubation in red blood cell lysis buffer (Sigma-Aldrich) and washed before resuspending the cells in fluorescence-activated cell-sorting (FACS) medium (10% FBS, 1% P/S, and 0.1% Na azide in RPMI). Cells were stained with Live/Dead blue (Invitrogen, Life Technologies Corporation, Eugene, OR), and Fc receptors were blocked by incubation of cells in 24G2 antibody (Fc Block; BD Biosciences, San Jose, CA) in FACS medium prior to staining with anti-CD8 FITC, anti-CD4 phycoerythrin, and anti-T lymphocyte allophycocyanin (Bio-Rad Antibodies, Hercules, CA). Cells were washed and fixed in 2% formaldehyde prior to analysis. Data were acquired on a BD FACSCanto II (BD Biosciences) at the UTMB Flow Cytometry Core Facility and analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistics.
For all groups, data were analyzed using Prism software (v6.0; GraphPad, La Jolla, CA). Comparisons between multiple groups were made by one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test. Comparisons between the means of two groups were made using an unpaired, 2-tailed Student's t test. Comparison of the slopes of cumulative virus shedding curves was made between groups using linear regression analysis. Comparison of shedding incidence was made using Fisher’s exact test. For all comparisons, a P value of 0.05 was used to designate significance.
ACKNOWLEDGMENT
This work was supported by grant AI107784 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One 10:e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mertz KJ, Trees D, Levine WC, Lewis JS, Litchfield B, Pettus KS, Morse SA, St Louis ME, Weiss JB, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald GA, Novotny J, Weisfuse I, Goldberg M, O'Donnell JA, Knaup R. 1998. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis 178:1795–1798. doi: 10.1086/314502. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Bailey G, Rahman M, Chen C, Ballard R, Moffat HJ, Kenyon T, Kilmarx PH, Totten PA, Astete S, Boily MC, Ryan C. 2005. Changes in the etiology of sexually transmitted diseases in Botswana between 1993 and 2002: implications for the clinical management of genital ulcer disease. Clin Infect Dis 41:1304–1312. doi: 10.1086/496979. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg SD, Stewart JA, Gerber AR, Byers RH, Lee FK, O'Malley PM, Nahmias AJ. 1988. Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. JAMA 259:1048–1050. doi: 10.1001/jama.1988.03720070048033. [DOI] [PubMed] [Google Scholar]
- 6.Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 7.Flewett TH, Parker RG, Philip WM. 1969. Acute hepatitis due to herpes simplex virus in an adult. J Clin Pathol 22:60–66. doi: 10.1136/jcp.22.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hull HF, Blumhagen JD, Benjamin D, Corey L. 1984. Herpes simplex viral pneumonitis in childhood. J Pediatr 104:211–215. doi: 10.1016/S0022-3476(84)80994-4. [DOI] [PubMed] [Google Scholar]
- 9.Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, Corey L. 1987. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med 317:1246–1251. doi: 10.1056/NEJM198711123172002. [DOI] [PubMed] [Google Scholar]
- 10.Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, Starr S, Jacobs R, Powell D, Nahmias A, Sumaya C, Edwards K, Alford C, Caddell G, Soong S-J. 1991. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med 324:450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Ives AM, Bertke AS. 2015. Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J Virol 89:8383–8391. doi: 10.1128/JVI.00468-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi M, Bertke AS, Patel A, Krause PR. 2011. Spread of herpes simplex virus to the spinal cord is independent of spread to dorsal root ganglia. J Virol 85:3030–3032. doi: 10.1128/JVI.02426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey L, Adams HG, Brown ZA, Holmes KK. 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 14.Schiffer JT, Wald A, Selke S, Corey L, Magaret A. 2011. The kinetics of mucosal herpes simplex virus-2 infection in humans: evidence for rapid viral-host interactions. J Infect Dis 204:554–561. doi: 10.1093/infdis/jir314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. 1992. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 16.Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, Winter C, Holmes KK, Corey L. 1985. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis 12:33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, Corey L. 2008. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, Diem K, Jin L, Stanaway J, Tronstein E, Kwok WW, Huang ML, Selke S, Fong Y, Magaret A, Koelle DM, Wald A, Corey L. 2014. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 88:4921–4931. doi: 10.1128/JVI.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L. 2013. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. 2012. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol 86:10587–10596. doi: 10.1128/JVI.01237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 23.Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, Carbone FR, Tscharke DC, Heath WR, Mueller SN, Mackay LK. 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 19:183–191. doi: 10.1038/s41590-017-0027-5. [DOI] [PubMed] [Google Scholar]
- 24.Shin H, Kumamoto Y, Gopinath S, Iwasaki A. 2016. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun 7:13346. doi: 10.1038/ncomms13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posavad CM, Zhao L, Dong L, Jin L, Stevens CE, Magaret AS, Johnston C, Wald A, Zhu J, Corey L, Koelle DM. 2017. Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal Immunol 10:1259–1269. doi: 10.1038/mi.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posavad CM, Zhao L, Mueller DE, Stevens CE, Huang ML, Wald A, Corey L. 2015. Persistence of mucosal T-cell responses to herpes simplex virus type 2 in the female genital tract. Mucosal Immunol 8:115–126. doi: 10.1038/mi.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. 2008. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med 205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. 2005. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis 192:2117–2123. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- 30.Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. 2012. A Vaxfectin-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 30:7046–7051. doi: 10.1016/j.vaccine.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JC, Williams MA, Bevan MJ. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol 5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milligan GN, Bernstein DI, Bourne N. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol 160:6093–6100. [PubMed] [Google Scholar]
- 33.Tang VA, Rosenthal KL. 2010. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol 87:39–44. doi: 10.1016/j.jri.2010.06.155. [DOI] [PubMed] [Google Scholar]
- 34.Bird MD, Chu CF, Johnson AJ, Milligan GN. 2007. Early resolution of herpes simplex virus type 2 infection of the murine genital tract involves stimulation of genital parenchymal cells by gamma interferon. J Virol 81:423–426. doi: 10.1128/JVI.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol 79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soudja SM, Chandrabos C, Yakob E, Veenstra M, Palliser D, Lauvau G. 2014. Memory-T-cell-derived interferon-gamma instructs potent innate cell activation for protective immunity. Immunity 40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valencia F, Veselenak RL, Bourne N. 2013. In vivo evaluation of antiviral efficacy against genital herpes using mouse and guinea pig models. Methods Mol Biol 1030:315–326. doi: 10.1007/978-1-62703-484-5_24. [DOI] [PubMed] [Google Scholar]
- 38.Stanberry LR. 1991. Evaluation of herpes simplex virus vaccines in animals: the guinea pig vaginal model. Rev Infect Dis 13:S920–S923. doi: 10.1093/clind/13.Supplement_11.S920. [DOI] [PubMed] [Google Scholar]
- 39.Schacker T, Zeh J, Hu HL, Hill E, Corey L. 1998. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis 178:1616–1622. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 40.Schiffer JT, Swan DA, Magaret A, Schacker TW, Wald A, Corey L. 2016. Mathematical modeling predicts that increased HSV-2 shedding in HIV-1 infected persons is due to poor immunologic control in ganglia and genital mucosa. PLoS One 11:e0155124. doi: 10.1371/journal.pone.0155124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A 104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson AJ, Chu CF, Milligan GN. 2008. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J Virol 82:9678–9688. doi: 10.1128/JVI.01159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milligan GN, Bernstein DI. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 45.Milligan GN, Dudley-McClain KL, Young CG, Chu CF. 2004. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J Reprod Immunol 61:115–127. doi: 10.1016/j.jri.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 47.Porichis F, Hart MG, Massa A, Everett HL, Morou A, Richard J, Brassard N, Veillette M, Hassan M, Ly NL, Routy JP, Freeman GJ, Dube M, Finzi A, Kaufmann DE. 2018. Immune checkpoint blockade restores HIV-specific CD4 T cell help for NK cells. J Immunol 201:971–981. doi: 10.4049/jimmunol.1701551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen B, Lee AJ, Chew MV, Ashkar AA. 2017. NK cells require antigen-specific memory CD4(+) T cells to mediate superior effector functions during HSV-2 recall responses in vitro. J Leukoc Biol 101:1045–1052. doi: 10.1189/jlb.4A0416-192R. [DOI] [PubMed] [Google Scholar]
- 49.Iijima N, Iwasaki A. 2016. Access of protective antiviral antibody to neuronal tissues requires CD4 T-cell help. Nature 533:552–556. doi: 10.1038/nature17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourne N, Ireland J, Stanberry LR, Bernstein DI. 1999. Effect of undecylenic acid as a topical microbicide against genital herpes infection in mice and guinea pigs. Antiviral Res 40:139–144. doi: 10.1016/S0166-3542(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 51.Da Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM. 2000. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol 74:7963–7971. doi: 10.1128/JVI.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia J, Veselenak RL, Gorder SR, Bourne N, Milligan GN. 2014. Virus-specific immune memory at peripheral sites of herpes simplex virus type 2 (HSV-2) infection in guinea pigs. PLoS One 9:e114652. doi: 10.1371/journal.pone.0114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis 187:542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 54.Schafer H, Burger R. 1991. Identification and functional characterization of guinea-pig CD4: antibody binding transduces a negative signal on T-cell activation. Immunology 72:261–268. [PMC free article] [PubMed] [Google Scholar]
- 55.Milligan GN, Bernstein DI. 1995. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology 206:234–241. doi: 10.1016/S0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 56.Milligan GN, Meador MG, Chu CF, Young CG, Martin TL, Bourne N. 2005. Long-term presence of virus-specific plasma cells in sensory ganglia and spinal cord following intravaginal inoculation of herpes simplex virus type 2. J Virol 79:11537–11540. doi: 10.1128/JVI.79.17.11537-11540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]