Abstract
Background/Aim: Clonogenicity is a key feature of stem/progenitor cells. The present study aimed to enrich stem/progenitor cells from dental pulp cells by means of culturing the cells at a low clonal density with spatial separation and the evaluate differentiation potential of the surviving cells. Materials and Methods: Pulp cells derived from wisdom teeth were seeded into wells of a 96-plate at a mean density of 1 cell/well and cultured for 2 weeks. Surviving cells were harvested, pooled together and subjected to differentiation into adipocytes, osteoblasts and neurons using respective inducing conditions for 3 weeks. The former two types of cells were examined by staining with Oil Red O and Alizarin Red, respectively. Neuron-like cells were inspected for their morphology and immunostained for microtubule-associated protein 2 and β-tubulin III. Results: Vital cells were obtained in eight wells of a 96-well plate, corresponding to a survival rate of 8%. Since fewer than two wells would be expected to contain more than four cells at seeding, the majority of surviving cells likely grew from 1-3 cells, which is a very low density. These cells differentiated into functional adipocytes and osteoblasts, and morphologically neuron-like cells. Conclusion: Low-density seeding with spatial separation enables statistical estimation of cell number in wells and provides an effective strategy for enriching stem/progenitor cells and for isolating clonal dental pulp cells.
Keywords: Stem cells, progenitor cells, clonogenicity, dental pulpcells, differentiation, Iow-density seeding, cell enrichment
For cell-based therapies in regenerative medicine, dental pulp cells provide a valuable source (1,2). These cells can be obtained from erupting primary teeth or extracted teeth. The procedure is non-invasive unlike the case of aspirating bone marrow. Human dental pulp cells are reported to contain cells which are clonogenic, grow rapidly and exhibit multi-lineage differentiation potential (3-6). Since dental pulp cells originate from the neural crest (7), they have a sort of innate neurogenic potential which may make them especially valuable for therapies for neurodegenerative diseases (1,2,8).
Clonogenicity is a major feature of stem/progenitor cells and has also been reported for dental pulp cells. According to standard protocol for colony formation, 1,000 or more cells are seeded in culture wells or flask and the colonies of cells are counted manually 2-4 weeks later (3,9). These studies postulate that all cells in one colony are derived from a single mother cell. However, it is also possible that one colony may be derived from more than one mother cell. Furthermore, two or more close colonies may also fuse into one (10).
For enriching stem/progenitor cells, sorting with flow-cytometry is a well-established method, wherein surface markers are used which should be specific for stem/progenitor cells. However, this method is laborious, expensive and requires a large number of cells. In addition, a flow cytometer is often not available for many laboratories. Furthermore, specificities of markers for stem/progenitor cells are often controversial and may vary depending on cell passage, cultural conditions and donors of the specimens from which the cells are derived.
In the present study, we therefore tried an alternative simple and inexpensive strategy for enriching stem/progenitor cells from dental pulp cells. The basic idea was to seed pulp cells at a low clonal density, for example, 1 cell/well into wells of a 96-plate. Physical separation of the wells enables a statistical estimation of the cell number in each well. Assuming that the distribution of cell numbers follows the Poisson pattern, fewer than 2% of the wells will contain more than three cells (Table I). Therefore, vital cells in a well will most likely originate from one to three cells. Since a high self-renewal potential is a key feature of stem/progenitor cells, cells surviving from such low-density seeding may represent enriched stem/progenitor cells. These cells were further evaluated for their potential in adipo-, osteogenic and neural differentiation.
Table I. Estimated Poisson distribution of wells containing the given numbers of cells, with mean number of cells/well=1.
Materials and Methods
Wisdom teeth were used for culturing dental pulp cells. The teeth were recovered from the bio-waste of teeth extraction, and anonymized before they were transported to the laboratory for the study. According to the local privacy protection regulation, the study protocol was reported to the corresponding local authority. The patients were informed about the study and all of them have given written consent for use of their tissues. For culturing dental pulp cells, the outgrowth method was used. Briefly, the teeth were broken using a hammer, the pulp tissues taken out, cut into small pieces and left to attach onto the cultural surface before standard Dulbecco’s modified Eagle’s medium (DMEM) was added. Five to seven days later, cells grew from the explants.
Upon subconfluence, pulp cells were harvested, and living cells were used to prepare a 20 cell/ml suspension. To each well of a 96-well plate, 50 μl of the suspension was added, giving an average of 1 cell/well. Two weeks later, each well of the plates was inspected under a microscope to find wells with vital cells or cell clusters. All vital cells were harvested and pooled together for the subsequent differentiation assays.
For inducing adipo-differentiation, cells were cultured in DMEM/Hams F12 with 10% human serum, 1 μM dexamethasone, 0.5 mM IBMX, 0.2 mM indomethacin and 10 μM insulin.
For inducing osteogenic differentiation, cells were cultured in DMEM/Hams F12 with 5% human serum, 50 μM ascorbic acid-2-phosphate and 10 mM β-glycerophosphate.
For inducing neural differentiation, cells were cultured in Neurobasal® medium with B27 supplement, 2 nM human heregulin, 0.5nM IBMX, 5 μM forskolin and 10 ng/ml basic fibroblast growth factor. Heregulin was a generous gift from Dr. Jody Longo and Dr. Steven Carroll of the Medical University of South California, USA.
Differentiation was continued for 3 weeks and half of the medium was refreshed twice a week. Cells induced to adipo-differentiation were stained with Oil Red to visualize oil droplets. Cells induced to osteogenic differentiation were stained with Alizarin Red to visualize mineral sedimentation. Neural differentiated cells were examined for their neuron-like morphology. Immunofluorescence staining for neurons was carried out using antibodies against microtubule-associated protein 2 (MAP2) and β-tubulin III.
Patch clamp measurement was attempted to examine the electrophysiological features of expected neurons. The bath solution used for the measurement contained 135 mM NaCl, 5 mM KCl, 1 mM CaCl2 and MgCl2,10 mM glucose and 10 mM HEPES at pH 7.0.
Results
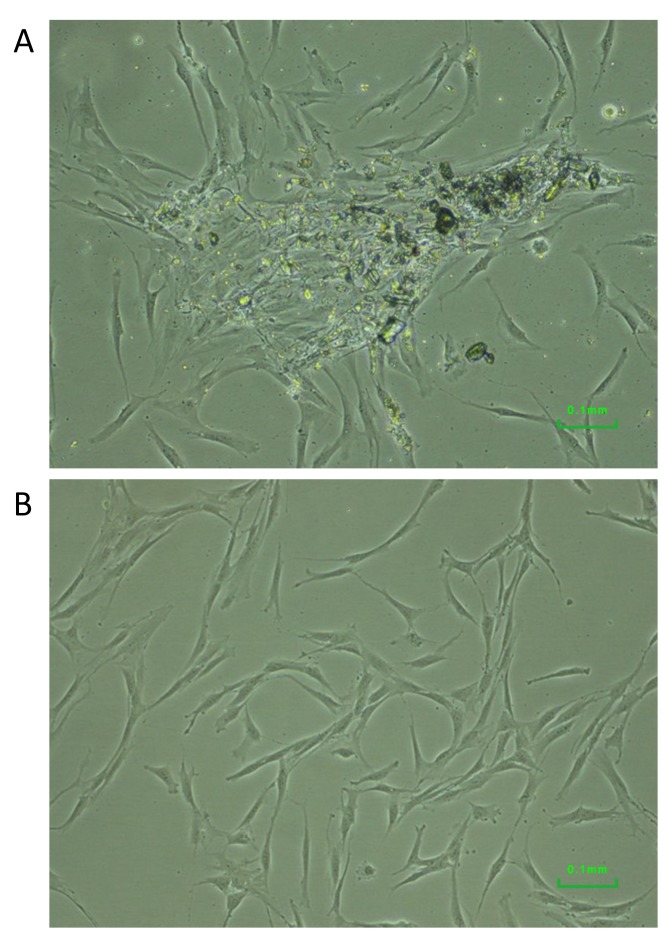
Dental pulp cells and low-density culture. Using the explant and outgrowth method, we successfully obtained dental pulp cells with a success rate of approximately 60%. Cells migrating from the explants were visible in 1 to 2 weeks (Figure 1A). Dental pulp cells exhibited a bipolar and fibroblastic-like morphology (Figure 1B).
Figure 1. Dental pulp cells migrating from an explant (A) and cells exhibiting fibroblast-like morphology (B).
Pulp cells from the first passage were harvested and seeded at a clonal density of 1 cell/well into wells of a 96-plate. After 2 weeks, vital cells were visible in eight wells, corresponding to a rate of 8%. Cells surviving low-density seeding were harvested, pooled together and examined for their potential in multilineage differentiation.
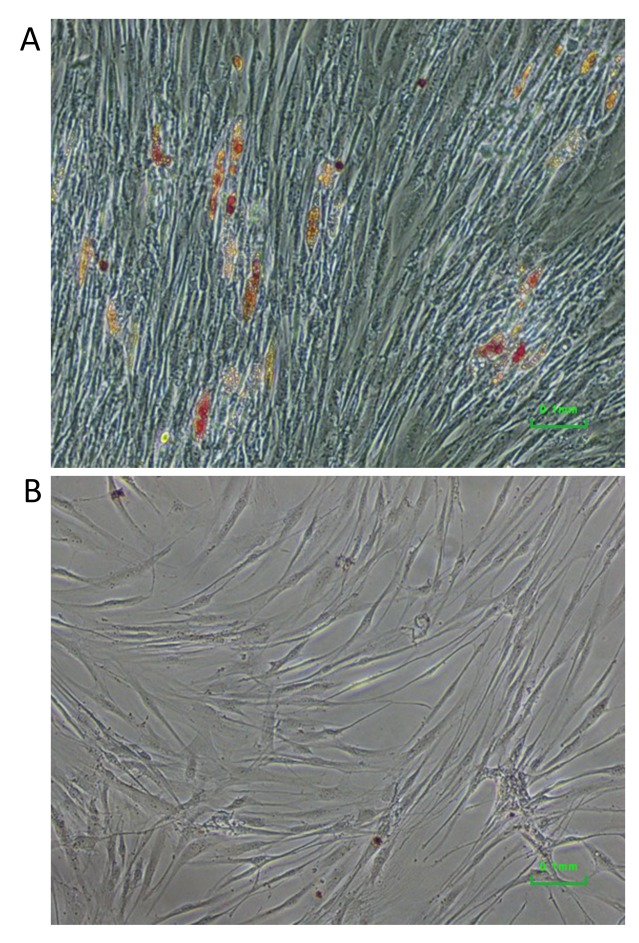
Adipo-differentiation. Cells cultured under conditions for adipo-differentiation started to form adipocytes and produced lipid droplets 10 days later. After 3 weeks of differentiation, lipid droplets were more clearly visible and stained with Oil Red O (Figure 2A). No such staining nor lipid droplets were observed in control cells cultured in standard medium (Figure 2B).
Figure 2. Adipo-differentiation of dental pulp cells surviving low-density seeding. Lipid droplets stained with Oil Red O were visible in cells induced for such differentiation (A), but not in the control cells (B).
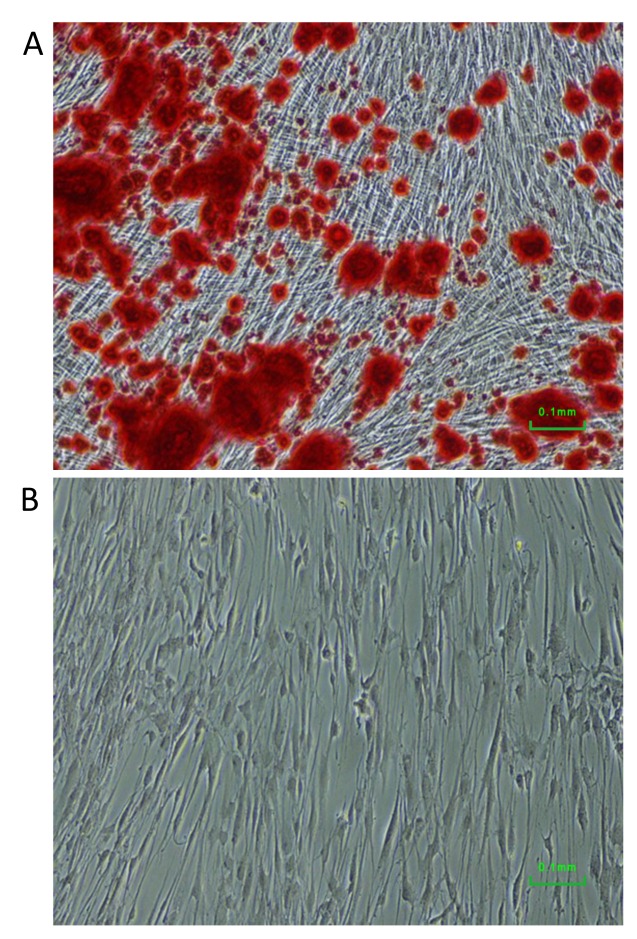
Osteogenic differentiation. Osteogenic differentiation was reached by culturing the enriched pulp stem/progenitor cells under osteogenic inducing condition. After 3 weeks of differentiation, large amount of deposits of mineralized extracellular matrix were visible by staining with Alizarin Red (Figure 3A). For comparison, control cells cultured under standard condition did produce such mineral deposits (Figure 3B).
Figure 3. Osteogenic differentiation of dental pulp cells surviving low-density seeding. Mineral sediments were stained with Alizarin Red in cells induced for osteogenic differentiation (A), but not in the control cells (B).
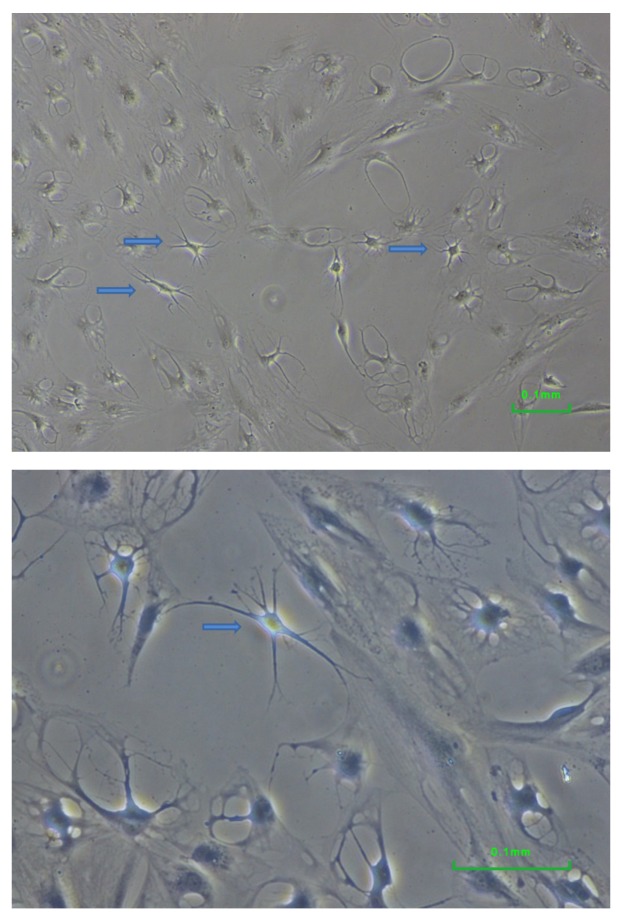
Neural differentiation. When cultured under neural differentiation condition, cells underwent remarkable change in their appearance (Figure 4). Multiple neurite-like extensions were visible. For comparison, no cells with such morphology were observed when cultured under standard conditions nor when induced to adipo- or osteogenic differentiation (Figures 2B and 3B).
Figure 4. Neural differentiation of dental pulp cells surviving low-density seeding. Multiple neurites were visible in some cells (arrows). The appearance of the cells differed from that of the control cells and from that of adipo- and osteo-differentiated cells.
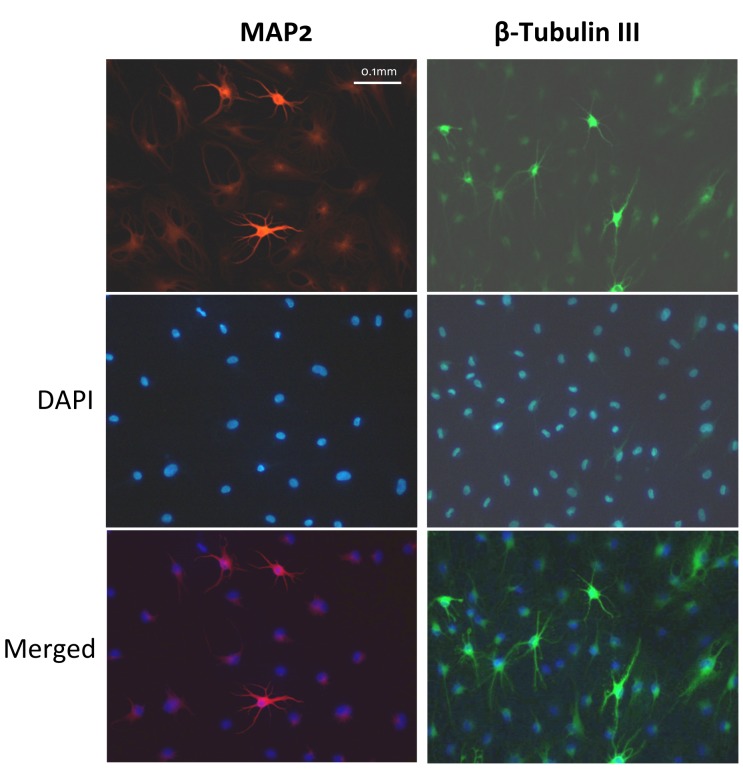
Approximately 10% of the neural-differentiated cells were positive for MAP2 and β-tubulin III (Figure 5), both are markers for mature neurons. Unstained negative cells were also visible in the same visual fields for both markers, indicating specificity of the staining.
Figure 5. Approximately 10% of the neural-differentiated cells were positive for MAP2 (red) and β-tubulin III (green), both are markers for mature neurons. Nuclei were stained in blue with 4’,6-diamidino-2-phenylindole, dihydrochloride (DAPI). The presence of negative cells in the same visual field argues against non-specific staining.
Patch clamp for measuring action potential of neuron-like cells was not possible because the cells died quickly in the required medium.
Discussion
Clonogenicity or the survival potential at very low density is a key feature of stem/progenitor cells. In the present study, we used this feature to enrich stem/progenitor cells from dental pulp cells. At a density of 1 cell/well, we obtained vital cells in eight wells on a 96-well plate. Statistically, fewer than two wells from a 96-well plate are expected to have more than four cells (Table I). Therefore, the cells surviving in the eight wells most likely grew from an initial 1-3 cells, which is a very low density. The surviving cells therefore may represent cells with stem/progenitor features.
Indeed, the dental pulp cells which survived from low-density culture exhibited multi-differentiation potential. Adipo- and osteogenic differentiation were confirmed directly by detection of lipid droplets and mineral sedimentation, respectively. Functional assessment for neurons failed due to lack of optimal conditions. However, cells cultured under the respective conditions exhibited distinct neuron-like morphology which was not present in cells without such induction. In addition, positive staining for MAP2 and β-tubulin III suggested successful neural differentiation, although the proportion of positively stained cells was only around 10%. Since MAP2 and β-tubulin III stain mature neurons, it is possible that there were also neural progenitor cells and cells which had differentiated into other subsets of neural cells, for example, glial cells.
Previous studies usually seeded more than 1,000 cells onto a larger cultural surface and counted the number of colonies later (3,9). They presumed that each colony originated from a single cell. However, this presumption lacks direct supporting evidence (10). In contrast, seeding cells into spatially separated smaller wells in a 96-well plate enables for statistical estimation of wells with single and multiple cells, and prevents fusing of colonies. In addition, colonies can be easily recovered and further characterized or used for specific differentiation (10).
In summary, we demonstrated the feasibility of a simple and inexpensive method for enriching stem/progenitor cells from pulp cells by means of culturing them at a low clonal density. Spatial separation using 96-well plates provides a strategy of preventing possible fusion of colonies and enables further comparative characterization of each colony in subsequent studies.
Acknowledgements
The Authors thank Mrs. Jane Rehberg for her technical assistance. Y. Zhao and Y. Zheng are supported by China Scholarship Council Grant No. 201608080159 and No. 201608080075, respectively.
References
- 1.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 2.Chalisserry EP, Nam SY, Park SH, Anil S. Therapeutic potential of dental stem cells. J Tissue Eng. 2017;8:1–17. doi: 10.1177/2041731417702531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 5.Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015;9:1205–1216. doi: 10.1002/term.1899. [DOI] [PubMed] [Google Scholar]
- 6.Bakopoulou A, About I. Stem cells of dental origin: Current research trends and key milestones towards clinical application. Stem Cells Int. 2016;2016:4209891–4209891. doi: 10.1155/2016/4209891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Noce M, Paino F, Spina A, Naddeo P, Montella R, Desiderio V, De Rosa A, Papaccio G, Tirino V, Laino L. Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. J Dent. 2014;42:761–768. doi: 10.1016/j.jdent.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Young F, Sloan A, Song B. Dental pulp stem cells and their potential roles in central nervous system regeneration and repair. J Neurosci Res. 2013;91:1383–1393. doi: 10.1002/jnr.23250. [DOI] [PubMed] [Google Scholar]
- 9.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–J81. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 10.Rennerfeldt DA, Van Vliet KJ. Concise review: When colonies are not clones: Evidence and implications of intracolony heterogeneity in mesenchymal stem cells. Stem Cells. 2016;34:1135–1141. doi: 10.1002/stem.2296. [DOI] [PubMed] [Google Scholar]