Abstract
Background/Aim: The hypoglycemic drug metformin (MET) and the anti-epileptic drug valproic acid (VPA) have individually shown anti-tumor effects in prostate cancer in vitro. The present study intended to investigate the efficacy of the combination of MET and VPA in prostate cancer treatment in a pre-clinical xenograft model. Materials and Methods: Prostate cancer cell lines (LNCaP and PC-3) were inoculated under the skin of BALB/c nude mice. The mice were treated with 200 μl/ml MET and/or 0.4% (w/v) VPA diluted in drinking water, or with vehicle control, and were monitored until the tumor volume reached 2,000 mm3. Evaluation of toxicity of the drug combination was determined in liver and kidney by histology. Results: In both LNCaP and PC-3 xenografts, MET combined with VPA significantly reduced tumor growth during the first 4 weeks following treatment, and delayed the time-to-tumor volume of 2,000 mm3 by 90 days, as compared to MET or to VPA alone, and to vehicle control. There was no significant difference in total mouse weight, liver or kidney morphology in response to combination treatment (MET+VPA) compared to MET or VPA alone and vehicle control. Conclusion. The combination treatment of MET with VPA is more effective at slowing prostate tumor growth in vivo compared to either drug alone, in mouse xenografts. These pre-clinical results support previous in vitro data and also demonstrate the low toxicity of the combination of these drugs, suggesting that this may be a potential new therapy to be investigated in clinical trials for prostate cancer.
Keywords: Metformin, valproic acid, prostate cancer chemotherapy, xenograft, nude mice
Prostate cancer (PCa) represents the highest incidence of cancer among male patients in the Western world (1-3). Early stages of the disease can be treated with active surveillance, radical prostatectomy, and radiation therapy (external-beam radiation or low dose-rate brachytherapy), while the advanced or relapsing disease needs either hormonal therapy or chemotherapy (4,5). The management of patients that develop castrate-resistant prostate cancer (CRPC) following hormonal therapy remains a challenge with a low median survival time of 12-15 months (6). Current chemotherapies only extend the survival time of CRPC by a mean of 3-6 months (7-10) and are associated with significant cytotoxic effects (9,11). Therefore, it is clear that new cancer therapies are required for more robust cancer control, preferably with minimal toxicity.
Metformin (MET) is the first-line therapy for controlling glucose levels in blood and has been widely used in diabetic patients (12). The pleiotropic beneficial effects of MET have been evident in the polycystic ovarian syndrome (13), in non-alcoholic fatty liver disease (14), in premature puberty (15), as well as in cancer prevention (16,17). Inhibition of the mammalian target of rapamycin (mTOR) (18-21) and of cyclin D1 contributes to the anti-cancer effect of MET (18). A recent epidemiological study has demonstrated that overall PCa risk decreases in diabetic patients treated with MET (HR=1.44, 95% CI=1.09-1.91) (22), while a single-arm phase II clinical trial has found that MET can delay the progression of metastatic PCa, as measured by prostate-specific antigen (PSA) doubling time (23). However important these findings may be, they have been obtained after only a short period of treatment with no control arm included in the studies.
Valproic acid (VPA) is mainly indicated for epilepsy treatment (24). VPA has been shown to have potential as an anti-cancer therapeutic drug through its broad range activity as a histone deacetylase (HDAC) inhibitor targeting HDAC class I and II (IIa) enzymes (25,26). The antineoplastic effects of VPA may be attributed to its regulation of cellular activities that are important in cancer cell growth including cell cycle control, cell differentiation, DNA repair, and apoptosis (27-30). However, the only phase II clinical trial of VPA for prostate cancer failed to achieve the optimal pharmacological level due to its toxicity (31).
Combination therapy targeting multiple neoplastic pathways is likely to be more effective than monotherapy (32). Both MET and VPA appear promising as anti-cancer agents, but at doses required for anti-cancer effects they both exhibit limitations related to toxicity (31,33). However, MET and VPA act via different molecular biological pathways even though they both induce cell-cycle arrest, and have anti-proliferative and anti-apoptotic effects (18,25). Under this scope, we hypothesized that the combination of MET with VPA may be more effective as an anti-cancer therapy than either drug alone, potentially allowing for lower drug doses to be effective in the combination treatment.
We recently reported that MET and VPA in combination synergistically reduced the proliferation of prostate cancer cell lines in vitro (PC-3 and LNCaP), with minimal adverse effects in normal prostatic epithelial cells (34). This combination also synergistically induced cell apoptosis in the presence of p53 and the androgen signaling pathway (34). An additional in vitro report has demonstrated that MET combined with VPA act synergistically as anti-proliferative and pro-apoptotic agents in two clear cell renal cell carcinoma cell lines, but no mechanistic data were included in this study (35). There is, however, in vivo evidence showing that treatment exclusively with MET or VPA alone delays the growth of prostate cancer xenografts (18,36). Here, we demonstrate that the combination of MET and VPA at doses that do not cause any obvious liver or kidney damage, induce a greater anti-tumor effect compared to MET or VPA alone in prostate cancer cell line xenografts. These results suggest that chronic administration of MET combined with VPA may provide an effective low toxicity therapy for prostate cancer patients.
Materials and Methods
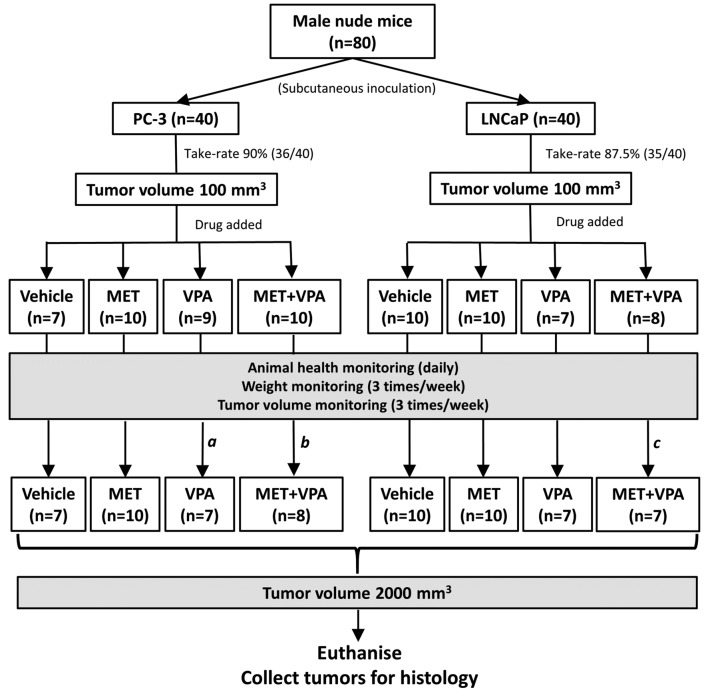
In vivo experimental design. The overall experimental design is shown in Figure 1 for LNCaP and PC-3 cells. This study was approved by the Flinders University Animal Welfare Committee (AWC Approval 893/15). Briefly, female nude (nu/nu) mice were obtained at 5 weeks of age and allowed to acclimate in the animal facility for one week. The mice were injected with the appropriate number of LNCaP or PC-3 cells determined in pilot studies (unpublished data) and the tumors allowed to grow to a volume of 100 mm3. The mice were then randomized into 4 different groups (n=10/group), which received one of the following in normal drinking water for a total period of 8 weeks: i) drinking water; ii) 200 μg/ml MET; iii) 0.4% (w/v) VPA in water; iv) 200 μg/ml MET + 0.4% (w/v) VPA. When the tumors reached a volume of 2,000 mm3, peripheral blood was collected from the submandibular vein to assess plasma levels of MET and VPA, and then the mice were euthanized and the tumors were harvested for further analysis.
Figure 1. Study design summary of metformin (MET) plus valproic acid (VPA) treatment in mice with PC-3 or LNCaP xenografts. Six-week-old nude mice (n=80) were randomly assigned into two groups and inoculated with either LNCaP (n=40) or PC-3 (n=40) cells. Once the xenograft tumor reached 100 mm3, mice were randomly assigned into one of 4 groups (n=10/group), treated with i) vehicle control, ii) 200 μg/ml MET alone, iii) 0.4% (w/v) VPA alone, and iv) 200 μg/ml MET + 0.4% (w/v) VPA. The xenograft tumors were allowed to grow until a maximum volume of 2,000 mm3 was reached. aTwo mice were excluded from the study, one due to fighting lesions on day 3 and the other due to chylothorax on day 14 of treatment. bOne mouse was excluded from the study due to fighting lesions on day 2 and one mouse was censored on day 58 of treatment due to tumor ulceration. cOne mouse was censored on day 32 of treatment due to tumor affecting mouse movement.
In the PC-3 xenograft group, one mouse was euthanized on day 2 (MET+VPA group) and one on day 3 (VPA group) of treatment, due to lesions sustained from fighting, while one mouse in the VPA group was euthanized at day 14 due to chylothorax (cause unknown). One mouse with LNCaP xenograft in the MET+VPA treatment group was euthanized at day 32 due to a tumor-associated motility problem, and one mouse with a PC-3 xenograft in the MET+VPA treatment group was euthanized due to tumor ulceration at day 58. These mice were not included in our analyses. The experimental design and final numbers of animals analyzed are summarized in Figure 1.
Cell culture and chemicals. LNCaP (clone FGC) (CRL-1730) and PC-3 (CRL-1435) prostate cancer cell lines were newly obtained from the American Type Culture Collection (ATCC®, Manassas, Virginia, USA). Metformin (PHR1084-500MG) and valproic acid (P4543-10G) were purchased from Sigma-Aldrich (Castle Hill, New South Wales, AU) and stocks (15 mg/ml for each drug) were made in distilled water and were sterilized using a 0.22 μm filter. Cancer cell lines were grown in RPMI 1640 medium (ThermoFisher, Melbourne, Victoria, AU) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 5% fetal bovine serum (FBS; Bovogen, Melbourne, Victoria, AU). When cells reached 80-90% confluence, they were harvested using 0.05% Trypsin/0.48 mM EDTA (ThermoFisher).
Establishment of tumor xenografts. Balb/c nude male mice (BALB/c-Fox1nu/Arc) were obtained from the Animal Resources Centre (Perth, Australia). LNCaP and PC-3 were grown up to 80-90% confluence and were harvested on the day of inoculation. The cells were washed briefly with PBS and were then centrifuged at 160 × g for 5 min at room temperature. Cell pellets were resuspended in 50 μl PBS and the same volume of Corning® Matrigel® Matrix (In Vitro Technologies, Melbourne, Victoria, AU) was added to the cell suspension to obtain 1×106 cells for the PC-3 and 3×106 cells for the LNCaP line in a final volume of 100 μl at 4˚C. Cell suspensions were kept on ice until they were injected subcutaneously into the right hind flank. All animals were subsequently checked on a daily basis for their general health condition, including faecal consistency, evidence of dehydration, general movement and breathing. Mouse weight and tumor size were measured 3 times per week throughout the study. Tumor volume was calculated using the formula (π/6) × A × B2 where A was the larger tumor diameter and B was the smaller tumor diameter (18,37). When the tumor reached a volume of 2,000 mm3, the mice were euthanized and the tumor, liver and kidneys were harvested. Gross necropsy of the animals was performed to ensure that there were no confounding health conditions.
Administration of MET and VPA. MET and VPA were added to the drinking water and this was replaced with a fresh solution every 3.5 days (36,38). The drug concentrations of MET at 200 μg/ml and VPA at 0.4% (w/v) in water were based on previous studies where MET and VPA on their own were shown to reduce LNCaP xenograft growth (18,36).
Plasma concentrations of MET and VPA using mass spectrometry. An initial pilot study was performed (n=3 for each treatment group) to verify the presence of MET and VPA in peripheral blood plasma using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS). The protocols were adapted for mice. Peripheral blood (50-100 μl) was collected from the submandibular vein of mice. The plasma was isolated from total blood by centrifuging at 1,000 × g for 10 min at 4˚C, which was then stored at –20˚C for subsequent analysis. Plasma samples (20 μl) for MET detection were injected onto a Phenomenex Kinetex HILIC column (2.1×100 mm, 2.6 μm) and were analyzed using an AcquityTM Ultra Performance LC (Waters, Rydalmere, New South Wales, AU). For VPA detection, UPLC-MS analysis was performed using an AcquityTM Ultra Performance LC system (Waters) coupled to a Premier qToF mass spectrometer (Waters). The mass spectrometer was operated on negative ionisation mode with a capillary voltage at 2.6 kV, a source temperature at 100˚C, a desolvation temperature 300˚C, a sample cone voltage of 26 V and a collision energy of 6 V. VPA was detected on tandem mass spectrometry mode by pseudo multiple reaction monitoring at the parent ion mass [M-H]-=143.1 Da.
Evaluation of liver and kidney histopathology. Liver and kidney tissues were fixed in 10% formalin (Sigma-Aldrich) overnight, and were then processed using an STP 120 Spin Tissue Processor (ThermoFisher). The processed tissues were embedded in paraffin (#Paraplast®, Surgipath®, Melbourne, Victoria, AU) using a Histostar™ Embedding Workstation (ThemoFisher). Hematoxylin (#II500JJ, ThermoFisher) and Eosin (#HT110116, Sigma-Aldrich) (H&E) staining was performed using the routine diagnostic protocol of the Pathology Department, Flinders Medical Centre, Adelaide, AU. In brief, paraffin embedded tissues were cut into 5-μm sections using a Microtome (Leica, Melbourne, Victoria, AU). The sections were mounted on APES (3-aminopropyltriethoxysilane) coated slides. The remaining paraffin was removed by placing the slides in an oven at 70˚C for 20 min, and washing the slides twice with the Histochoice® Clearing Agent (#H2779, Sigma-Aldrich) for 2 min with shaking. The sections were rehydrated by washing twice each in 100%, 95%, 70% and 50% ethanol for 1 min per wash with shaking, followed by a final wash in tap water. The slides were quickly dipped in acid ethanol and ammonia water before staining with Eosin for 2 min followed by Haematoxylin stain for 2 min. The slides were rehydrated serially in tap water, ethanol, and Histochoice® Clearing Agent, allowed to dry, and then mounted with Leica CV Mount (Leica, Melbourne, Victoria, AU) and imaged using an Olympus microscope (BX63). The histological score adapted from Chen et al. (39), was used to evaluate the glomerular changes (hypercellularity, hypertrophy, cellular crescent formation, thrombotic changes, fibrinoid material deposition), tubular dilation, necrosis, and inflammation. The toxicity of the liver was investigated using the histological score adapted from Mendler et al. (40) to evaluate portal fibrosis, lobular inflammation or necrosis, Mallory bodies, hepatocyte ballooning, peri-sinusoidal fibrosis, and indications of fatty liver diseases.
Statistical analysis. IBM SPSS 23 was used for statistical analyses. Differences in tumor volumes in vivo were analyzed using a two-way ANOVA test. Kaplan-Meier analysis was used to plot the time-to-tumor volume of up to 2,000 mm3 in the different treatment models.
Results
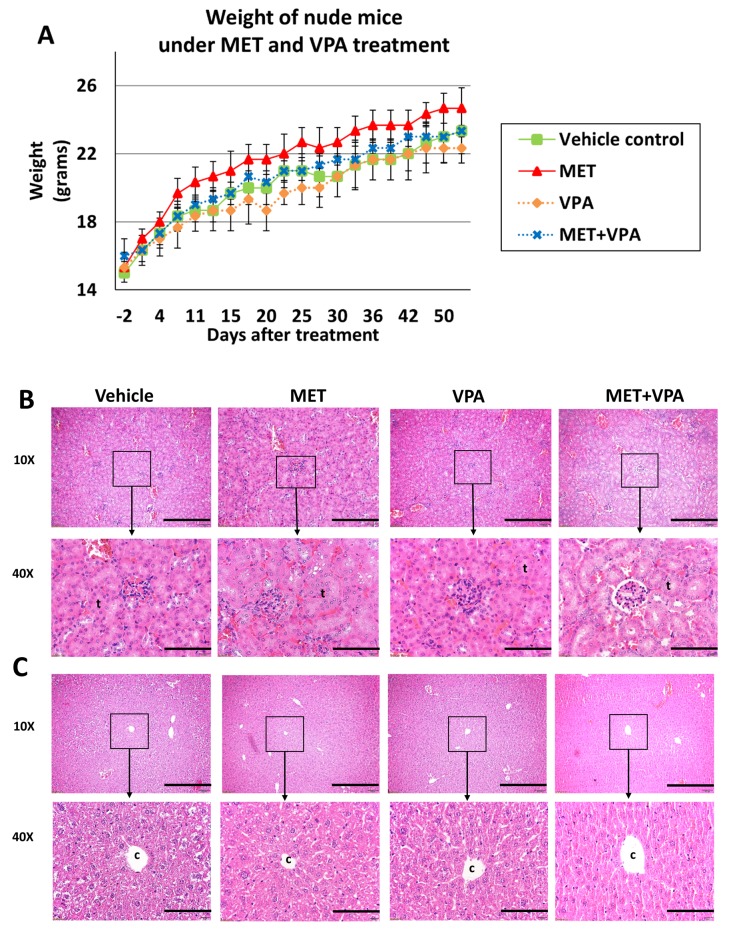
Chronic administration of MET combined with VPA in drinking water does not cause significant adverse effects. A pilot study was conducted to investigate possible adverse effects in nude mice in the absence of tumor xenografts in response to an 8-week treatment with either vehicle, 200 μg/ml MET alone, 0.4% (w/v) VPA alone, or 200 μg/ml MET + 0.4% (w/v) VPA treatment (n=3 in each group). The mice exhibited no side-effects as a result of any treatment. There was no significant variation in mouse weight observed between the vehicle, MET alone, VPA alone, and combination treatment (MET+VPA) groups throughout the course of experiment (p>0.05) (Figure 2A). Histological scoring of the kidney (Figure 2B) and liver (Figure 2C) showed no damage (score=0) to either organ in any of the treatment groups.
Figure 2. The weight of nude mice (mean ± SE) was evaluated before and after treatment with MET alone, VPA alone, MET plus VPA, or vehicle control (n=3). There was no significant difference in weight between the groups over 8 weeks of treatment (p>0.05) (A). Representative hematoxylin and eosin staining images (10Χ and 40Χ) of the kidney (B) and the liver (C), during week 8 of treatment. The histological scoring of the kidney (B) showed no tubule (t) changes, thrombosis, dilation, necrosis, or inflammation (score 0). The histological scoring of the liver (C) showed no signs of portal fibrosis around central vein (c), lobular inflammation or necrosis, Mallory bodies, hepatocyte ballooning, perisinusoidal fibrosis, or fatty changes (score 0). Scale bar 4 mm for 10Χ images, and 80 μm for 40Χ images.
Drinking consumption of MET and VPA in the nude mice was evaluated. The presence of MET and/or VPA in peripheral blood plasma was confirmed in all animals at 4 and at 8 weeks of treatment. As the drinking water containing the drugs was provided ad libitum, and the assays were semi-quantitative, it was not possible to accurately determine the exact drug concentrations in the blood.
MET combined with VPA causes greater reduction of tumor growth in both PC-3 and LNCaP xenografts than either drug alone. In order to investigate the effect of MET and VPA in tumor growth of PC-3 and LNCaP xenografts, 6-week-old male mice were inoculated with LNCaP (n=40) or PC-3 (n=40), then they were randomly assigned into vehicle control (water), MET, VPA, or MET+VPA treatment groups (n=10/treatment group/cell line). Once the xenograft tumor reached 100 mm3, mice were treated with either the vehicle control, 200 μg/ml MET alone, 0.4% (w/v) VPA alone, or 200 μg/ml MET + 0.4% (w/v) VPA. There were no significant differences in the time taken for the tumors to reach 100 mm3 in any of the four treatment groups and for either cell line. The tumor-take rate in this study was 87.5% (35/40) for LNCaP and 90% (36/40) for PC-3 cells. No spontaneous tumor regression was observed in the study. Tumor volumes were compared after each treatment was added, using the cut-off time of when the first PC-3 or LNCaP xenograft tumor reached 2,000 mm3, which was 28 days for PC-3 and 21 days for LNCaP xenografts. Mice euthanized before the cut-off time (one mouse in the VPA and one in the MET+VPA group both with PC-3 xenografts and one mouse in the VPA group with LNCaP xenografts) were excluded from analysis and mice euthanized after the cut-off time were removed from the Kaplan-Meier analysis (one mouse in the MET+VPA group with PC-3 and one in the same group with LNCaP xenografts).
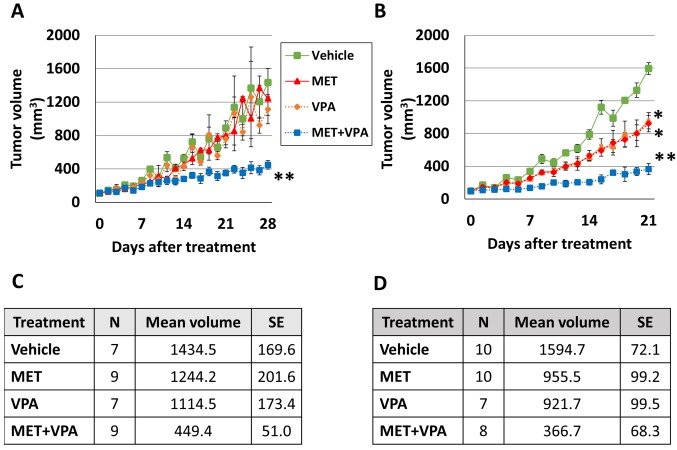
In the PC-3 xenograft group, there was no significant difference in tumor volume in mice treated with MET alone, VPA alone, or with vehicle alone (p>0.05). The combination of MET with VPA significantly inhibited the volume of the PC-3 tumor xenograft (86.7% decrease, p=0.001), whereas the MET alone (63.9% decrease, p=0.005), and VPA alone (59.7% decrease, p=0.04) (Figure 3A and C) had a less robust effect, as compared to the vehicle alone model on day 28 of the treatment.
Figure 3. Tumor growth in response to metformin (MET) and/or valproic acid (VPA) treatment. Nude mice at 6 weeks of age were inoculated subcutaneously with PC-3 (A) or LNCaP (B) cells. The xenograft tumors were allowed to grow to 100 mm3 before treating the mice with either vehicle control, 200 μg/ml MET alone, 0.4% (w/v) VPA alone, or 200 μg/ml MET+0.4% (w/v) VPA. The sample size (N), mean tumor volume (mm3) and standard error (SE) of PC-3 xenografts on day 28 of treatment and LNCaP xenografts on day 21 of treatment are presented in tables C and D, respectively. *p<0.05 compared to the vehicle, MET alone, and VPA alone. **p< 0.005 compared to the vehicle, MET alone, VPA alone, and MET+VPA (7≤n≤10).
In the LNCaP xenograft group, MET alone inhibited tumor growth compared to vehicle treatment (40.1% decrease, p<0.001), as did VPA alone (42.2% decrease, p<0.001) (Figure 3B and D). MET combined with VPA significantly inhibited tumor growth (77% decrease, p<0.001), with less dramatic changes in the MET alone (61.6% decrease, p<0.001), and the VPA alone (60.2% decrease, p<0.001) treatment, as compared to the vehicle alone model on day 21 of the treatment (Figure 3B and D).
Chronic administration of MET combined with VPA delays time-to-maximum tumor volume in LNCaP and PC-3 xenografts treated with MET and VPA. Throughout this study, there was no tumor regression observed in the absence of treatment. Mice were euthanized when the tumor volume reached 2,000 mm3. Most of the tumor xenografts reached 2,000 mm3 before day 90 following drug treatment initiation, with the exception of one LNCaP xenograft tumor which decreased to an undetectable size in the MET plus VPA group and one PC-3 xenograft in the MET plus VPA group which only reached 350 mm3. These mice were culled on day 102 and 114, respectively, as they exhibited no changes in tumor size for 2 weeks prior to euthanasia.
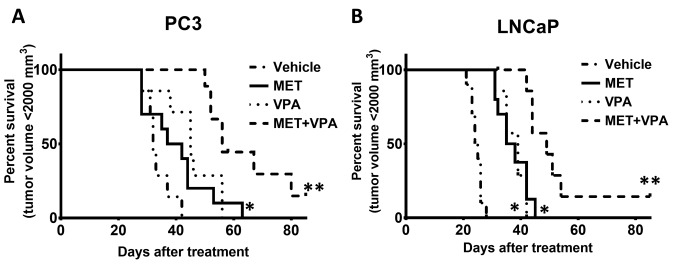
Analysis of cumulative survival using the Kaplan-Meier method showed that MET combined with VPA significantly delayed the time-to-maximum tumor volume in PC-3 xenografts compared to vehicle treatment (92.2% increase, p<0.001), MET alone (60.5% increase, p=0.002), and VPA alone (43.7% increase, p=0.01). Treatment of nude mice bearing PC 3 xenografts with VPA alone also delayed the time-to-maximum tumor volume compared to the vehicle treatment (32.8% increase, p=0.006), whereas the difference was not significant for the MET alone compared to the vehicle treatment group (p=0.08) (Figure 4A).
Figure 4. Kaplan-Meier analysis of percentage of animals reaching a tumor volume of 2,000 mm3 during the treatment with metformin (MET) and valproic acid (VPA). Mice bearing tumor xenografts with either PC-3 or LNCaP were treated with vehicle control, MET alone, VPA alone, or MET plus VPA. The mice were euthanized when the tumor volume reached 2,000 mm3. One mouse bearing a PC-3 xenograft in the MET+VPA treatment group only reached a tumor size of 350 mm3 and was euthanized on day 114 of treatment, while the tumor of one mouse bearing a LNCaP xenograft in the MET+VPA treatment group was reduced to an undetectable size and was euthanized on day 102 of treatment. *p< 0.05 compared to vehicle treatment; **p< 0.005 compared to vehicle treatment, MET alone, and VPA alone (7≤n≤10).
In the LNCaP xenograft group, the time-to-maximum tumor volume in response to MET plus VPA was significantly delayed compared to MET alone (44.2% increase, p=0.002), VPA alone (43.9% increase, p=0.001), and vehicle treatment (120% increase, p< 0.001). Treatment with either MET or VPA alone significantly delayed the time-to-tumor volume of 2,000 mm3 compared to vehicle treatment (52.4% increase, p< 0.001 and 52.8% increase, p< 0.001, respectively) (Figure 4B).
On necropsy, only one abdominal lymph node metastasis was identified in a mouse with a PC-3 xenograft (1/36) in the vehicle treatment group (day 33 after treatment), while no metastases of LNCaP xenografts (0/35) were observed.
Discussion
Our previous in vitro findings have demonstrated that the combination treatment of MET and VPA induces a synergistic anti-proliferative response in LNCaP and PC-3 cell lines with no significant side-effects in normal prostatic epithelial cells (PrEC) (34). A significant synergistic apoptotic response was observed in vitro in LNCaP, but not in PC-3 cells. MET combined with VPA reduced proliferation and induced apoptosis in human prostate tumor biopsy explants (34). The presence of p53 and the androgen signaling pathway were shown to play an important role in the synergistic apoptosis of prostate cancer cell lines in response to MET+VPA (34,41). Here, we performed an in vivo study to investigate the combination of MET and VPA in PC-3 and LNCaP xenografts in nude mice to support these in vitro findings and provide preliminary safety evidence for the use of this drug combination in clinical studies. In our xenograft experiments 200 μg/ml of MET and 0.4% (w/v) of VPA were diluted in drinking water, which relates to human equivalent doses of 2.0-4.1 mg/kg/day for MET (42,43) and 40.6-79.4 mg/kg/day for VPA (36). These doses are in the therapeutic range and are already being used for non-cancer purposes in humans with minimal adverse effects (44).
The combination of MET with VPA did not cause any kidney or liver toxicity. In previously published xenograft studies mice were administered MET alone, or VPA alone in drinking water, at the same doses as used here, with no health-related issues (18,36). However, there are currently no reports on tissue toxicity in response to the combination of MET and VPA. The mice in our study showed no general side-effects during the 8 weeks of receiving 200 μg/ml MET, 0.4% (w/v) VPA or both drugs combined in their drinking water. Gross necropsy indicated no tissue toxicity and histopathological analyses of liver and renal tissue (major metabolic sites of MET and VPA, respectively) showed no evidence of damage. These results suggest that chronic administration of MET and VPA can be combined at the doses used here with possibly minimal side-effects to the liver or kidney.
The combined treatment of MET with VPA inhibits tumor growth. There was no natural tumor regression observed after tumors reached 100 mm3 in our pilot study, therefore drug treatment was commenced at this point. Our current study used Matrigel® for inoculating the cancer cell lines and the tumor take-rate was 87.5 % for the LNCaP and 90% for the PC-3 xenograft. These results show a stronger effect than the one reported by Davoodpour et al. (45) who used culture medium for inoculating cancer cells in the xenograft, but are comparable to those of Gustavsson et al. (46), who, similarly to us, used Matrigel®. In the study presented here, subcutaneous injections of PC-3 and LNCaP cells were performed and a low metastatic rate at 2.8% (1/36) was shown in PC-3 xenografts, while no metastases in LNCaP xenografts (0/35), in line with the literature (36,37,39,40). No significant differences in tumor growth of PC-3 xenografts were observed in response to either MET or VPA alone compared to the vehicle treatment over the first 4 weeks of treatment. Our findings differ from the report by Shabbeer et al. (2007), where the administration of 0.4% (w/v) VPA in drinking water of nude mice for 4 weeks significantly reduced the PC3 xenograft tumor volume by 57% compared to the vehicle treatment (p=0.05, n=8) (47). This inconsistency could be attributed to the different time-point for treatment initiation, which was 2 weeks after inoculation in the study of Shabbeer et al. (2007) versus 41.2±9.4 days after inoculation (when the tumor reached 100 mm3) in our study. Although no anti-tumoral effect of VPA alone was observed at 4 weeks, long-term administration of VPA up to 90 days delayed the tumor progression by 32.8%. Importantly, the combination of MET with VPA, compared to MET alone, VPA alone or vehicle treatment, inhibited the tumor growth of PC-3 xenografts by 59-86% in both the first 4 weeks of treatment and further extended the time-to-tumor volume of 2,000 mm3 by 43-92% in long term treatment. There was only one PC-3 xenograft that did not reach a final tumor size of 2,000 mm3 during the study and this was in the MET+VPA treatment group. PC-3 has the characteristics of a small cell neuroendocrine carcinoma, which is highly aggressive and hormone refractory (48). The response of the PC-3 xenografts to MET and VPA combination therapy in our study highlights the potential of this combination treatment as an effective therapy even in castration-resistant prostate cancer.
In LNCaP xenografts, MET or VPA alone reduced the tumor volume by approximately 40% in the first 4 weeks after treatment and prolonged the time-to-tumor volume of 2000 mm3 by approximately 44% compared to the vehicle treatment. Our results agree with previous findings, where the addition of MET or VPA alone in the drinking water in nude mice bearing LNCaP xenografts reduced the tumor volume growth after 3-4 weeks of treatment (55% and 40% reduction, respectively) (18,36). Combining MET with VPA induced a stronger inhibition of initial tumor growth by 60-77% in the short-term (4 weeks) while the long term treatment further extended the time-to-tumor-volume of 2,000 mm3 by 42-120% at day 90, indicating the effectiveness of MET plus VPA combination therapy over the MET or VPA monotherapies.
LNCaP cells have normal p53 expression and are responsive to androgen receptor (AR), similar to early-stage localized PCa, while PC-3 cells have no p53 expression and are AR-independent, thus they behave more like CRPC and possibly represent PCa at different stages (48). We found greater anticancer effects using the combination of MET plus VPA in both initial and long-term treatments in LNCaP xenografts, compared to MET alone, VPA alone or vehicle treatment. There was one LNCaP xenograft in the present study which completely regressed and this was in the MET plus VPA treatment group. These findings suggest that the combination treatment of MET with VPA may be more effective in earlier stages of prostate cancer where p53 expression and AR responsiveness is more common (49).
In conclusion, long-term administration of MET and VPA in combination, at doses lower than the human therapeutic dose of MET for diabetes and at the same human therapeutic dose of VPA for epilepsy, could be a relatively non-toxic and efficient systemic therapy at different stages of prostate cancer. Based on these findings, our group has recently commenced a phase I clinical trial investigating the combination of MET with VPA as a neoadjuvant therapy prior to radical prostatectomy (ACTRN 12616001021460).
Acknowledgements
This research was supported by a Flinders Medical Centre Foundation Seeding grant, and an Australia Awards PhD scholarship to LNKT (ID ST0009TM3).
References
- 1.Australian Institute of Health Welfare & Australasian Association of Cancer Registries Cancer in Australia: An overview 2012. Cancer series. 2012;74(CAN 70) [Google Scholar]
- 2.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S. Eau-estro-siog guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD. Eau-estro-siog guidelines on prostate cancer. Part ii: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Denmeade SR, Isaacs JT. Development of prostate cancer treatment: The good news. The Prostate. 2004;58(3):211–224. doi: 10.1002/pros.10360. [DOI] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (cou-aa-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncol. 2015;16(2):152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S. Enzalutamide in metastatic prostate cancer before chemotherapy. New Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Widakowich C, de Castro G, De Azambuja E, Dinh P, Awada A. Review: Side effects of approved molecular targeted therapies in solid cancers. The Oncologist. 2007;12(12):1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (ukpds 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 13.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: Systematic review and meta-analysis. BMJ. 2003;327(7421):951–953. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchesini G, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358(9285):893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 15.Ibáñez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab. 2006;91(8):2888–2891. doi: 10.1210/jc.2006-0336. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti J-F, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27(25):3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 19.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (MTOR signaling. J Biol Chem. 2002;277(27):23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 20.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG. Minireview: The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 22.Haring A, Murtola TJ, Talala K, Taari K, Tammela TL, Auvinen A. Antidiabetic drug use and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Scand J Urol. 2017;1-11:1–11. doi: 10.1080/21681805.2016.1271353. [DOI] [PubMed] [Google Scholar]
- 23.Rothermundt C, Hayoz S, Templeton AJ, Winterhalder R, Strebel RT, Bärtschi D, Pollak M, Lui L, Endt K, Schiess R. Metformin in chemotherapy-naive castration-resistant prostate cancer: A multicenter phase 2 trial (sakk 08/09) Eur Urol. 2014;66(3):468–474. doi: 10.1016/j.eururo.2013.12.057. [DOI] [PubMed] [Google Scholar]
- 24.Mesdjian E, Ciesielski L, Valli M, Bruguerolle B, Jadot G, Bouyard P, Mandel P. Sodium valproate: Kinetic profile and effects on GABA levels in various brain areas of the rat. Prog Neuro-Psychopharmacol Biol Psychiatry. 1982;6(3):223–233. doi: 10.1016/s0278-5846(82)80172-3. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury C, Khanim F, Hayden R, Bunce C, White D, Drayson M, Craddock C, Turner B. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia. 2005;19(10):1751–1759. doi: 10.1038/sj.leu.2403910. [DOI] [PubMed] [Google Scholar]
- 26.Ververis K, Rodd AL, Tang MM, El-Osta A, Karagiannis TC. Histone deacetylase inhibitors augment doxorubicin-induced DNA damage in cardiomyocytes. Cell Mol Life Sci. 2011;68(24):4101–4114. doi: 10.1007/s00018-011-0727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1. Cancer Res. 2003;63(13):3637–3645. [PubMed] [Google Scholar]
- 28.Savickiene J, Borutinskaite V-V, Treigyte G, Magnusson K-E, Navakauskiene R. The novel histone deacetylase inhibitor BML-210 exerts growth inhibitory, proapoptotic and differentiation stimulating effects on the human leukemia cell lines. Eur J Pharmacol. 2006;549(1):9–18. doi: 10.1016/j.ejphar.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Martirosyan A, Leonard S, Shi X, Griffith B, Gannett P, Strobl J. Actions of a histone deacetylase inhibitor nsc3852 (5-nitroso-8-quinolinol) link reactive oxygen species to cell differentiation and apoptosis in MCF-7 human mammary tumor cells. J Pharmacol Exp Ther. 2006;317(2):546–552. doi: 10.1124/jpet.105.096891. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Pan RL, Zhang XL, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Induction of hepatic differentiation of mouse bone marrow stromal stem cells by the histone deacetylase inhibitor VPA. J Cell Mol Med. 2009;13(8b):2582–2592. doi: 10.1111/j.1582-4934.2008.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma S, Symanowski J, Wong B, Dino P, Manno P, Vogelzang N. A phase II clinical trial of oral valproic acid in patients with castration-resistant prostate cancers using an intensive biomarker sampling strategy. Transl Oncol. 2008;1(3):141–147. doi: 10.1593/tlo.08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology patient and health systems opportunities. Nature Reviews Clinical Oncology. 2015;12(12):732–742. doi: 10.1038/nrclinonc.2015.169. [DOI] [PubMed] [Google Scholar]
- 33.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967–CD002967. doi: 10.1002/14651858.CD002967.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran LN, Kichenadasse G, Butler LM, Centenera MM, Morel KL, Ormsby RJ, Michael MZ, Lower KM, Sykes PJ. The combination of metformin and valproic acid induces synergistic apoptosis in the presence of p53 and androgen signaling in prostate cancer. Mol Cancer Ther. 2017;16(12):2689–2700. doi: 10.1158/1535-7163.MCT-17-0074. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zhang X, Huang T, Geng J, Liu M, Zheng J. Combination of metformin and valproic acid synergistically induces cell cycle arrest and apoptosis in clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(3):2823–2828. [PMC free article] [PubMed] [Google Scholar]
- 36.Xia Q, Sung J, Chowdhury W, Chen C-l, Höti N, Shabbeer S, Carducci M, Rodriguez R. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006;66(14):7237–7244. doi: 10.1158/0008-5472.CAN-05-0487. [DOI] [PubMed] [Google Scholar]
- 37.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 38.Sharma VK, Nautiyal V, Goel KK, Sharma A. Assessment of thermal stability of metformin hydrochloride. Asian J Chem. 2010;22:3561–3566. [Google Scholar]
- 39.Chen S-m, Mukoyama T, Sato N, Yamagata S-I, Arai Y, Satoh N, Ueda S. Induction of nephrotoxic serum nephritis in inbred mice and suppressive effect of colchicine on the development of this nephritis. Pharmacol Res. 2002;45(4):319–324. doi: 10.1006/phrs.2002.0948. [DOI] [PubMed] [Google Scholar]
- 40.Mendler MH, Kanel G, Govindarajan S. Proposal for a histological scoring and grading system for non-alcoholic fatty liver disease. Liver International. 2005;25(2):294–304. doi: 10.1111/j.1478-3231.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 41.Tran L, Kichenadasse G, Sykes P. Combination therapies using metformin and/or valproic acid in prostate cancer: Possible mechanistic interactions. Curr Cancer Drug Targets. 2018;18:1–14. doi: 10.2174/1568009618666180724111604. [DOI] [PubMed] [Google Scholar]
- 42.Food and Drug Administration Guidance for industry – estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Pharmacol Toxicol. 2005:7–14. [Google Scholar]
- 43.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. The FASEB Journal. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 44.Burke J, Thenot J. Determination of antiepileptic drugs. J Chromatogr B Biomed Sci Appl. 1985;340:199–241. doi: 10.1016/0378-4347(85)80198-5. [DOI] [PubMed] [Google Scholar]
- 45.Davoodpour P, Landström M, Welsh M. Reduced tumor growth in vivo and increased c-abl activity in PC3 prostate cancer cells overexpressing the shb adapter protein. BMC Cancer. 2007;7(1):161–161. doi: 10.1186/1471-2407-7-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustavsson H, Tešan T, Jennbacken K, Kuno K, Damber J-E, Welén K. ADAMTS1 alters blood vessel morphology and tsp1 levels in LNCaP and LNCaP-19 prostate tumors. BMC Cancer. 2010;10(1):288–298. doi: 10.1186/1471-2407-10-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shabbeer S, SQ Kortenhorst M, Kachhap S, Galloway N, Rodriguez R, Carducci MA. Multiple molecular pathways explain the anti-proliferative effect of valproic acid on prostate cancer cells in vitro and in vivo. The Prostate. 2007;67(10):1099–1110. doi: 10.1002/pros.20587. [DOI] [PubMed] [Google Scholar]
- 48.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J. PC3 is a cell line characteristic of prostatic small cell carcinoma. The Prostate. 2011;71(15):1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]