Abstract
Background: Single-agent carboplatin at area under the curve 10 (AUC10) is an effective treatment for metastatic seminoma. As far as we are aware of, there have been no studies reporting its effects on short-term quality of life. The objective was to study the efficacy, safety and tolerability, using health-related quality of life, of carboplatin AUC10 chemotherapy in patients with metastatic seminoma. Patients and Methods: Forty-four patients with metastatic seminoma treated at Mount Vernon Cancer Centre with carboplatin AUC10 were included in this study. Response to treatment was determined by radiological imaging (Response Evaluation Criteria in Solid Tumors v 1.1) and serum tumour markers. Toxicities were evaluated using the Common Terminology Criteria for Adverse Events Version 4.0. Quality of life treatment-related toxicities were assessed during treatment at pre-chemotherapy assessments. After treatment, toxicity was assessed using a defined telephone questionnaire consisting of four questions relating to hair loss, hearing impairment, days absent from work, and neuropathy. Results: At a median follow-up of 27.5 (range=4-84) months, no patient had experienced relapse. Grade 3/4 neutropenia was seen in 15 (35%) patients, nine (21%) required prophylactic granulocyte colony-stimulating factor, 13 (30%) patients had grade 3/4 thrombocytopenia. Commonest non-haematological toxicities were fatigue in 28 (65%) and nausea 14 (33%) patients. They were grade 1 in 82% and 92% of cases, respectively. Six out of 44 (14%) had residual tinnitus. One patient had residual grade 1 peripheral neuropathy. Ten patients continued to work throughout treatment and two patients were retired. Of the remaining patients, 16 (37%), took fewer than 5 days off work. Conclusion: Carboplatin AUC10 is a safe and effective treatment for stage II/III seminoma with better health-related quality of life than experienced with combination cisplatin-based chemotherapy.
Keywords: Seminoma, bone marrow, carboplatin, work functioning, toxicity
Germ cell tumours of the testis are classified as seminoma (approximately 50%) or non-seminomatous tumours (embryonal, teratoma, choriocarcinoma, yolk sac tumours and mixed histology). Around 85% of men with seminoma present with stage 1 and 10% with stage II disease (1). Of men with stage I seminoma, 15% will experience relapse following orchidectomy. Active surveillance is generally recommended for patients with stage I disease as a strategy to minimize treating the majority who are already cured by surgery alone (2,3). Risk factors potentially indicating a higher risk of relapse in stage I include tumour size >4 cm, lymphovascular invasion and involvement of the rete testes (4).
Cisplatin-based combination chemotherapy (bleomycin, cisplatin and etoposide; BEP) has been considered the main treatment strategy for metastatic seminoma. More recently it has been shown that 3-4 cycles of single-agent carboplatin [at area under the curve 10 (AUC10)] (5-11), has a similar efficacy. As treatment for metastatic seminoma is now so successful and most patients will become long-term survivors, the impact of short- and long-term toxicities of this treatment on patient quality of life has become paramount. Other factors such as length of treatments, convenience and cost also play an increasingly important role in patient’s decision making, if offered a choice between combination cisplatin-based therapy and single-agent carboplatin.
The most important short-term toxicity of carboplatin is haematological toxicity, with a reported incidence of 70%, 54% and 26% of grade 3/4 neutropenia, thrombocytopenia and anaemia, respectively (9). Obvious immediate effects of chemotherapy range from changes in personal appearance (hair loss) to fatigue, which may have an impact on employment and social life. Adverse effects such as neuropathy and tinnitus can also be very debilitating and more so perhaps for young adults commonly affected by seminoma, whose peers are all likely to be in good health. Approximately 10% will suffer significant psychosocial problems (12). Although many studies have looked into long-term psychosocial aspects of chemotherapy, none have explored short-term toxicities and effects on social life during chemotherapy (13-15).
Here we studied the efficacy, safety and tolerability of carboplatin AUC10 chemotherapy and explored health-related quality of life in patients with metastatic seminoma during and immediately following their chemotherapy.
Patients and Methods
All patients with metastatic seminoma treated with carboplatin AUC10 between 2010 and 2017 were identified from treatment databases at our centre. Data were collected retrospectively and staging assessed using the seventh edition of the TNM classification (16) with computed tomographic (CT) imaging of the chest, abdomen and pelvis [Response Evaluation Criteria in Solid Tumours (RECIST) 1.1] (17) and serum tumour markers alpha foeto-protein (AFP), human chorionic gonadotropin hormone (β-HCG) and lactate dehydrogenase (LDH) when available. Appropriate approvals were received from the hospital Ethics Committee (Audit number 15541).
Carboplatin dosing (AUC10) was calculated using the Calvert formula [total dose in mg=target AUC (glomerular filtration rate ml/min+25)]. An accurate estimation of glomerular filtration rate was obtained for every patient before treatment using 51Cr-labelled ethylenediamine tetra-acetic acid (EDTA) clearance (18). The doses were not capped for the initial treatment. Treatment was administered as a single dose every 21 days for three or four cycles.
Response to treatment was determined by radiological imaging and serum tumour markers. Toxicities were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.1 Quality of life (QoL) and intermediate treatment-related toxicities were assessed post treatment using a self-designed telephone questionnaire consisting of four questions about toxicity, which included hair loss, hearing impairment, days absent from work, and neuropathy.
Response to treatment was assessed after three cycles of carboplatin AUC10. If complete response to treatment was achieved, no further treatment was given. In the case of partial response, a fourth cycle of carboplatin was administered. Residual para-aortic lymphadenopathy was considered for subsequent retroperitoneal lymph node dissection (RPLND).
Dose reduction was considered based on the grade of haematological toxicity and the clinical assessment. No prophylactic G-CSF was routinely used with the first cycle; however, it was added to second and subsequent cycles if there were dose delays or greater than grade 1 neutropenia on day 21. Blood product support was available as required according to local protocols. No dose recalculation of carboplatin was performed after the initial dosing based on EDTA.
All patients underwent full blood count, liver function tests, urea, electrolyte, AFP and HCG determinations prior to each cycle. A CT scan of the chest, abdomen and pelvis was performed prior to starting treatment and after cycle 3. Repeat CT imaging of the abdomen was considered 6 weeks following treatment completion in those who only obtained partial responses after three cycles, prior to recommending RPLND.
If serum tumour markers were not elevated prior to treatment, response was determined according to RECIST criteria alone. Complete response (CR) was defined as complete resolution of disease on imaging (reduction of any enlarged lymph nodes to short axis of under 1 cm) and normalization of serum tumour markers. Partial response was defined as more than 30% reduction in the target lesions; progressive disease was determined by a combination of progressive disease on imaging with/without a rise in serum tumour markers (19). Toxicities were evaluated using CTCAE version 4.1 and recorded during treatment but data were collected retrospectively (20).
Patients were followed-up every 3 months in the first year, every 4 months in the second year, 6-monthly in the third year and annually thereafter. A full blood count, renal function, liver function tests with serum tumour markers (AFP, HCG, LDH) were performed at each visit. CT imaging of the abdomen (retroperitoneum) alone, unless other sites of disease were documented prior to treatment, was carried out at the end of treatment and annually for 2 years post chemotherapy.
Short and intermediate follow-up QoL was assessed using a locally designed questionnaire. based on the CTCAE v 1.0 toxicity assessments. Questions were put directly to patients on treatment at the clinic and, where possible, when attending for follow-up appointments. To complete the dataset, however, some patients were questioned over the telephone if the follow-up QoL data were missing.
Results
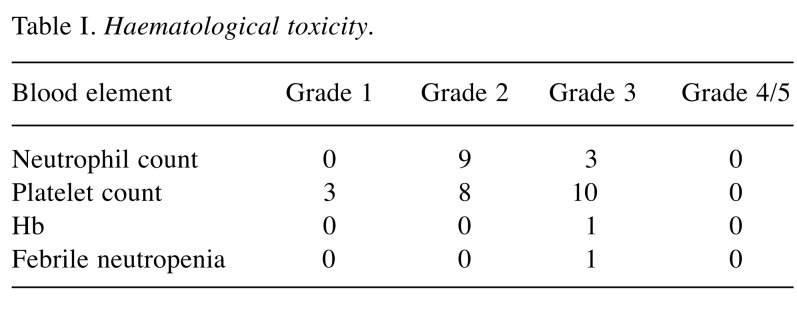
A total of 44 patients, with a median age of 44 (range=26-77) years, were treated at Mount Vernon Cancer Centre between 2010 and 2017. Twenty patients had stage IIA, 17 stage IIB, five stage IIC and two stage III disease. Of these, 17 (39%) patients had stage 1 disease at diagnosis. The average dose of carboplatin administered per cycle was 1240 mg (range=800-1,800 mg). The tumour markers were as follows- LDH elevated in 17 (39%), HCG in nine (21%) patients, one patient had persistently raised AFP, which was unrelated to his disease. Carboplatin was given in two, three and four cycles to 4.3%, 87% and 8.7% patients, respectively. The four patients who required a fourth cycle of carboplatin all had stage IIc disease. Following this, two patients who had their initial diagnosis made on node biopsies underwent RPLND, with histology showing necrosis, and two required close surveillance of residual para-aortic lymphadenopathy, which resolved on follow-up. There was no evidence of disease relapse at a median follow-up of 27.5 (range=4-84) months. Data regarding haematological toxicity were available for all patients and are reported in Table I. Grade 3 and 4 neutropenia and thrombocytopenia were experienced by 3 (7%) and 11 (23%) patients respectively. Nine (21%) required prophylactic G-CSF in total, with four after their first cycle and five after cycle 2. Thirteen patients (30%) had grade 3/4 thrombocytopenia. There was only one hospital admission for neutropenic sepsis.
Table I. Haematological toxicity.
Five patients required a single cycle of chemotherapy to be delayed by 1 week, four of these as a result of haematological toxicity. Overall, 10 patients needed reductions in chemotherapy doses for neutropenia/thrombocytopenia, by 10% in six patients and 20% for another five, following which there were no further dose reductions or treatment delays. A single patient required a blood transfusion and only two patients were admitted, one for intravenous anti-emetics and the other for neutropenic sepsis.
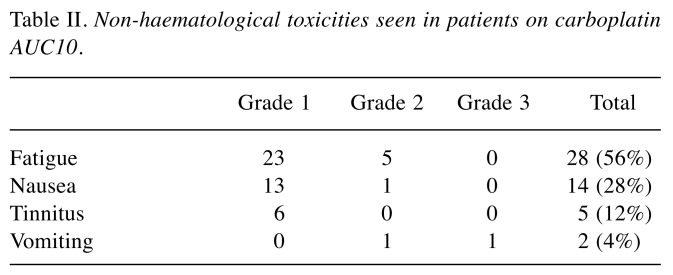
The most common non-haematological toxicity was fatigue, which was seen in 65% of the treated patients, with grade 1 fatigue seen in 23/28 cases (Table II). Nausea was less common, seen in 14 (33%) patients, with grade 1 symptoms in 13/14 cases. Mild Grade 1 tinnitus affected six (14%) patients, and one suffered grade 3, while only one patient suffered mild hearing loss. Forty out of 44 (93%) patients had no residual tinnitus. Regrettably, hearing loss was not formally tested in this cohort of patients, but is not very common in relation to carboplatin, unlike cisplatin treatment, with a reported incidence of 20-50% hearing loss (21-23). Two patients had minimal hair loss during their treatment. One patient had residual grade 1 peripheral neuropathy. Out of the 44 patients, 10 (23%) patients continued to work throughout their treatment, not having to take any full days off at all, including their treatment day. Sixteen (37%) took fewer than 5 days off work, four (9%) patients took between 5-21 days and 13 patients more than 21 days (range 21 to 90 days), including two patients who were already retired.
Table II. Non-haematological toxicities seen in patients on carboplatin AUC10.
Discussion
Whilst radiation had been the standard treatment for early-stage seminoma for many years, growing concerns about long-term toxicities in patients with this highly curative disease prompted additional treatment strategies to be pursued in order to minimize long-term morbidity and mortality attributable to treatment (24,25).
Platinum-based chemotherapy has gained gradual popularity both in early and advanced disease based on increasing evidence that this is more effective than radiotherapy in stage II seminoma and associated with an improved toxicity profile. Originally, Horwich et al. reported a study of 70 patients with metastatic seminoma treated with single-agent carboplatin at 400 mg/m2 (analogous to AUC6) with 3-year survival rate of 91%, but 22% patients experienced relapse and required salvage therapy (5). By contrast, the Spanish Germ Cell Cancer Group Study reported treating stage IIA or IIB seminoma with four cycles of cisplatin and etoposide or three cycles of BEP chemotherapy in 2008 and showed no relapses among 72 patients (26). Subsequently two randomized controlled trials failed to demonstrate equivalence or superiority of single-agent carboplatin AUC7 compared to cisplatin-based regimens (8). The Medical Research Council UK trial, although underpowered to show equivalence, demonstrated no statistically significant differences in major survival endpoints when comparing single-agent carboplatin at a dose of 400 mg/m2 to etoposide and cisplatin (27,28). Krege et al. also investigated the use of single-agent carboplatin at a dose of AUC7, but as an alternative to radiotherapy in stage IIA/B disease in a prospective phase II trial. Again, an overall relapse rate of 18%, with a median time to relapse of 6 months failed to better relapse rates seen with radiotherapy but did demonstrate a significantly lower toxicity profile (29,30). The findings of these trials, particularly the inability of single-agent carboplatin at AUC7 to improve on radiotherapy or cisplatin combinations, resulted in BEP becoming the standard treatment for patients with metastatic seminoma, as well as good prognosis for those with teratoma/mixed germ cell tumour.
In vitro work suggested that there may be a dose–response effect with carboplatin in seminoma (31). This information and the significant toxicity related to BEP, led the Orchid Clinical Trials collaborative group to support a pilot study where patients with good prognosis metastatic seminoma were offered even higher carboplatin dose at AUC10 (27). This successfully reported a failure rate of only 5%, but in a study of only in 20 patients. Validation of these superior results in 61 further patients treated with carboplatin at AUC10, with a 3-year overall survival) of 96.2% and progression-free survival) of 93.2%, confirmed significantly less toxicity compared to both combination regimens and radiotherapy (9).
Despite the numerous studies detailed above, no publication has reported detailed toxicity data for these patients. We demonstrate that carboplatin at AUC10 is a safe regimen as reflected by the absence of an adverse haematological profile.
In our cohort of patients, G-CSF prophylaxis was required in 20% of the patients and there was only one episode of neutropenic sepsis. This is very similar to toxicity data published by Tookman et al., where 23% of patients required prophylactic G-CSF, with a single admission for febrile neutropenia (9).
Most importantly, patients were able to preserve their physical and work functioning, with 19% being able to work throughout their treatment and nearly 60% of patients taking fewer than 5 days off work, in the 63-day treatment. Temporary hair loss was seen in two patients, which was reversed after treatment stopped, in keeping with the published data (32).
The study does have several limitations. This was a retrospective study, involving carboplatin at AUC10 at a single centre. The small sample size and bias, especially ascertainment bias, which is introduced in any cohort study, limit this study. Although it is known that carboplatin does not affect fertility compared to BEP (33), this study did not look at the fertility outcomes in these patients. Future studies should also incorporate long-term cardiovascular events in carboplatin-treated patients.
Conclusion
With no relapses detected so far, this retrospective review has shown that single-agent carboplatin at AUC10 should be considered a standard treatment for patients with metastatic seminoma even at stage III. Although some relapses are to be expected with larger numbers and longer follow-up, the great majority of relapses will be salvaged by combination platinum-based chemotherapy. The excellent tolerability and efficacy of this outpatient treatment should lead to its international acceptance as first-line therapy, without the need for a large randomised trial.
Funding
The study was supported by Cancer Treatment Research Trust UK (CTRT) and CRUK grant to AS.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Conflicts of Interest
None to declare.
Consent for Publication
Informed consent was obtained and is available for review by the editor.
References
- 1.Khan O, Protheroe A. Testis cancer. Postgrad Med J. 2007;83:624–632. doi: 10.1136/pgmj.2007.057992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De La Pena H, Sharma A, Glicksman C, Joseph J, Subesinghe M, Traill Z, Verrill C, Sullivan M, Redgwell J, Bataillard E, Pintus E, Dallas N, Gogbashian A, Tuthill M, Protheroe A, Hall M. No longer any role for routine follow-up chest x-rays in men with stage I germ cell cancer. Eur J Cancer. 2017;84:354–359. doi: 10.1016/j.ejca.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, von der Maase H. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 4.Bamberg M, Schmidberger H, Meisner C, Classen J, Souchon R, Weinknecht S, Schorcht J, Walter F, Engenhart-Cabillic R, Schulz U, Born H, Flink M. Radiotherapy for stages I and IIA/B testicular seminoma. Int J Cancer. 1999;83:823–827. doi: 10.1002/(sici)1097-0215(19991210)83:6<823::aid-ijc22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Horwich A, Dearnaley DP, A’Hern R, Mason M, Thomas G, Jay G, Nicholls J. The activity of single-agent carboplatin in advanced seminoma. Eur J Cancer. 1992;28A:1307–1310. doi: 10.1016/0959-8049(92)90505-v. [DOI] [PubMed] [Google Scholar]
- 6.de Wit R, Stoter G, Kaye SB, Sleijfer DT, Jones WG, ten Bokkel Huinink WW, Rea LA, Collette L, Sylvester R. Importance of bleomycin in combination chemotherapy for good-prognosis testicular nonseminoma: A randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. J Clin Oncol. 1997;15:1837–1843. doi: 10.1200/JCO.1997.15.5.1837. [DOI] [PubMed] [Google Scholar]
- 7.Saxman SB, Finch D, Gonin R, Einhorn LH. Long-term follow-up of a phase III study of three versus four cycles of bleomycin, etoposide and cisplatin in favorable-prognosis germ-cell tumors: The Indian University experience. J Clin Oncol. 1998;16:702–706. doi: 10.1200/JCO.1998.16.2.702. [DOI] [PubMed] [Google Scholar]
- 8.Bokemeyer C, Kollmannsberger C, Stenning S, Hartmann JT, Horwich A, Clemm C, Gerl A, Meisner C, Ruckerl CP, Schmoll HJ, Kanz L, Oliver T. Metastatic seminoma treated with either single.agent carboplatin or cisplatin-based combination chemotherapy: A pooled analysis of two randomised trials. B J Cancer. 2004;91:683–687. doi: 10.1038/sj.bjc.6602020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tookman L, Rashid S, Matakidou A, Phillips M, Wilson P, Ansell W, Jamal-Hanjani M, Chowdhury S, Harland S, Sarwar N, Oliver T, Powles T, Shamash J. Carboplatin AUC 10 for IGCCCG good prognosis metastatic seminoma. Acta Oncol. 2013;52:987–993. doi: 10.3109/0284186X.2012.714078. [DOI] [PubMed] [Google Scholar]
- 10.Gholam D, Fizazi K, Terrier-Lacombe MJ, Jan P, Culine S, Theodore C. Advanced seminoma--treatment results and prognostic factors for survival after first-line, cisplatin-based chemotherapy and for patients with recurrent disease: A single-institution experience in 145 patients. Cancer. 2003;98:745–752. doi: 10.1002/cncr.11574. [DOI] [PubMed] [Google Scholar]
- 11.Mencel PJ, Motzer RJ, Mazumdar M, Vlamis V, Bajorin DF, Bosl GJ. Advanced seminoma: treatment results, survival and prognostic factors in 142 patients. J Clin Oncol. 1994;12:120–126. doi: 10.1200/JCO.1994.12.1.120. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich A, Hofmann R. Quality-of-life issues in the treatment of testicular cancer. World J Urol. 1999;17:230–238. doi: 10.1007/s003450050138. [DOI] [PubMed] [Google Scholar]
- 13.Arai Y, Kawakita M, Hida S, Terachi T, Okada Y, Yoshida O. Psychosocial aspects in long-term survivors of testicular cancer. J Urol. 1996;155:574–578. [PubMed] [Google Scholar]
- 14.Carpentier MY, Fortenberry JD, Ott MA, Brames MJ, Einhorn LH. Perceptions of masculinity and self-image in adolescent and young adult testicular cancer survivors: implications for romantic and sexual relationships. Psychooncology. 2011;20:738–745. doi: 10.1002/pon.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpentier MY, Fortenberry JD. Romantic and sexual relationships, body image and fertility in adolescent and young adult testicular cancer survivors: A review of the literature. J Adolesc Health. 2010;47:115–125. doi: 10.1016/j.jadohealth.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS, British Nuclear Medicine S. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;25:759–769. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP, Bruner DW, Cleeland CS, Sloan JA, Chilukuri R, Baumgartner P, Denicoff A, St Germain D, O’Mara AM, Chen A, Kelaghan J, Bennett AV, Sit L, Rogak L, Barz A, Paul DB, Schrag D. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106(9):dju244–dju244. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, Kanz L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, Tattersall MH. Ototoxicity in patients receiving cisplatin: Importance of dose and method of drug administration. Cancer Treat Rep. 1982;66:19–23. [PubMed] [Google Scholar]
- 23.Kopelman J, Budnick AS, Sessions RB, Kramer MB, Wong GY. Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope. 1988;98:858–864. doi: 10.1288/00005537-198808000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Zagars GK, Ballo MT, Lee AK, Strom SS. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22:640–647. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 25.Horwich A, Fossa SD, Huddart R, Dearnaley DP, Stenning S, Aresu M, Bliss JM, Hall E. Second cancer risk and mortality in men treated with radiotherapy for stage I seminoma. Br J Cancer. 2014;110:256–263. doi: 10.1038/bjc.2013.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-del-Muro X, Maroto P, Guma J, Sastre J, Lopez Brea M, Arranz JA, Lainez N, Soto de Prado D, Aparicio J, Piulats JM, Perez X, Germa-Lluch JR. Chemotherapy as an alternative to radiotherapy in the treatment of stage IIA and IIB testicular seminoma: A Spanish Germ Cell Cancer Group Study. J Clin Oncol. 2008;26:5416–5421. doi: 10.1200/JCO.2007.15.9103. [DOI] [PubMed] [Google Scholar]
- 27.Matakidou A, Mutsvangwa K, Ansell W, Lim L, Powles TB, Oliver RT, Shamash J. Single-agent carboplatin AUC10 for metastatic seminoma with IGCCCG good prognosis disease; a feasibility study of the Orchid Clinical Trials Group. Ann Oncol. 2010;21:1730–1731. doi: 10.1093/annonc/mdq300. [DOI] [PubMed] [Google Scholar]
- 28.Horwich A, Oliver RT, Wilkinson PM, Mead GM, Harland SJ, Cullen MH, Roberts JT, Fossa SD, Dearnaley DP, Lallemand E, Stenning SP, MRC Testicular Tumour Working Party A medical research council randomized trial of single-agent carboplatin versus etoposide and cisplatin for advanced metastatic seminoma. MRC Testicular Tumour Working Party. Br J Cancer. 2000;83:1623–1629. doi: 10.1054/bjoc.2000.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krege S, Boergermann C, Baschek R, Hinke A, Pottek T, Kliesch S, Dieckmann KP, Albers P, Knutzen B, Weinknecht S, Schmoll HJ, Beyer J, Ruebben H, German Testicular Cancer Study Group Single-agent carboplatin for CS IIA/B testicular seminoma. A phase II study of the German Testicular Cancer Study Group (GTCSG) Ann Oncol. 2006;17:276–280. doi: 10.1093/annonc/mdj039. [DOI] [PubMed] [Google Scholar]
- 30.Domont J, Massard C, Patrikidou A, Bossi A, de Crevoisier R, Rose M, Wibault P, Fizazi K. A risk-adapted strategy of radiotherapy or cisplatin-based chemotherapy in stage II seminoma. Urol Oncol. 2013;31:697–705. doi: 10.1016/j.urolonc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Oliver RTD, Powles T, Ell PJ, Somasundram U, Shamash J. 22-Year phase 1/2 study of single agent carboplatin in metastatic seminoma: Potential for acceleration by a new surrogate end point, 72 hr PET scan response. J Clin Oncol. 2006;24:14565–14565. [Google Scholar]
- 32.de Jonge ME, Mathot RA, Dalesio O, Huitema AD, Rodenhuis S, Beijnen JH. Relationship between irreversible alopecia and exposure to cyclophosphamide, thiotepa and carboplatin (CTC) in high-dose chemotherapy. Bone Marrow Transplant. 2002;30:593–597. doi: 10.1038/sj.bmt.1703695. [DOI] [PubMed] [Google Scholar]
- 33.Ghezzi M, Berretta M, Bottacin A, Palego P, Sartini B, Cosci I, Finos L, Selice R, Foresta C, Garolla A. Impact of BEP or carboplatin chemotherapy on testicular function and sperm nucleus of subjects with testicular germ cell tumor. Front Pharmacol. 2016;7:122. doi: 10.3389/fphar.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.