Abstract
Background: Amrubicin is usually administered on days 1-3 every 3 weeks by intravenous infusion. However, it causes severe hematological toxicity, especially febrile neutropenia. It was reported that weekly administration confers higher dose intensity, less severe adverse events, and anti-tumor activity that is as effective as that of treatment with a conventional schedule. Patients and Methods: Weekly amrubicin was administered at a dose of 60 mg/m2 on days 1 and 8 every 3 weeks. The primary endpoint was overall response rate. Results: A total of 33 patients were enrolled. The overall response rate was 6.1% (95% confidence interval(CI)=0.7-20.2%) and the disease control rate after 2 months was 51.5%. The median progression-free survival was 2.93 months. Febrile neutropenia was observed in only two patients. Conclusion: The primary endpoint was not met in this study. However, weekly amrubicin achieved a high disease control rate and good tolerability.
Keywords: Amrubicin, non-small cell lung cancer, refractory, relapsed, phase II
Amrubicin is a potent topoisomerase II inhibitor and promising agent for both small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Therapeutic strategy has been mainly developed for SCLC and amrubicin using 40 mg/m2 on days 1-3 every 3 weeks (standard amrubicin) is widely used for SCLC patients for second-line setting or later.
In NSCLC, the late-phase II trial reported a response rate of 26.7% for patients treated with prior chemotherapy (1,2). However, amrubicin did not demonstrate its greater efficacy compared to docetaxel for patients with NSCLC treated in the second-line setting (3). Furthermore, standard amrubicin causes severe, occasionally fatal, hematological toxicity, especially febrile neutropenia (FN).
For administration method, amrubicin was initially investigated at a dose of once a day every 3 weeks (recommended dose was 100 mg/m2) in the phase I study (4), then divided into then doses on day 1-3 (1). However, in actual clinical application of standard amrubicin, the treatment schedule tends to be delayed due to myelosuppression from the previous course of amrubicin treatment, and the total dose tends to be lower than expected due to the discontinuation of amrubicin because of myelosuppression. It is suggested that toxicity may be kept low and total dosage may be increased by performing weekly administration and weekly administration of other chemotherapeutic reagents, such as paclitaxel, has been reported in a clinical trial (5).
Thus, we conducted a phase I study of weekly amrubicin and the recommended dose was 60 mg/m2 weekly on days 1 and 8 every 3 weeks (6). Here we conducted a phase II trial to evaluate the efficacy and toxicity of weekly amrubicin for advanced NSCLC.
Patients and Methods
Study design. This was an open-label, multicenter, phase II study. Primary inclusion criteria were histologically/cytologically-proven NSCLC, tumor progression or refractory disease after performing one or two chemotherapy regimens (excluding gefitinib or erlotinib), age ≥20 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, and adequate main organ functions. Primary exclusion criteria were contraindication for amrubicin, carcinomatous pleuritis, pericarditis, peritonitis and other metastasis with local therapy indication, in a course of receiving radiotherapy, serious medical complication, and infection.
This study was conducted in accordance with the Declaration of Helsinki and Guideline for Good Clinical Practice. The study protocol was reviewed and approved by the individual institutional review board, and written informed consent was obtained from all patients. This study has been registered in the UMIN Clinical Trials Registry (UMIN-CTR, URL: http://www.umin.ac.jp/ctr/), number UMIN000002570.
Amrubicin was administered at the dose of 60 mg/m2 weekly (on days 1 and 8 every 3 weeks) until disease progression or intolerable toxicity was confirmed. Adverse events (AEs) were monitored and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (7).
The primary endpoint was the tumor response rate. The response rate was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (8). Secondary endpoints were adverse events, disease control rate (DCR) after 2 months of initial administration of amrubicin, progression-free survival (PFS) and overall survival (OS).
Statistical methods. Based on the results of second-/third-line regimens for advanced NSCLC (9,10), the threshold response rate and the expected response rate were set as 10% and 30%, respectively in expectation of an effect higher than with those regimens. Calculating the required number of cases based on Simon's two-stage design under the condition of significance level of 5% (one-sided) and detection power of 85%, 14 cases were required in the first stage. Moreover, an additional 13 cases would be required if one or more cases were judged as partial response (PR) or more by the attending doctor. In consideration of inappropriate cases and drop-out cases, the target number of cases was 32 for the whole study.
Analysis was performed of cases in which amrubicin was administered one or more times in this study, and the response rate and its exact 95% confidence interval (CI) were estimated. For time-to-event data, the Kaplan–Meier method was used to illustrate the survival curve and estimate the median survival time. For adverse events of grade 3 or more, the incidence rate was calculated.
Results
A total of 33 patients were enrolled during the period from August 16, 2010 through May 11, 2012. The patients participated from two centers in Japan. Data cutoff was conducted on September 14, 2013. The median follow-up time was 9.27 months (0.93-36.8 months). Patient characteristics are shown in Table I. The median age was 67 years, two-thirds of the patients were male, 72% of them had adenocarcinoma, and 75.8% of the patients had received two or more prior chemotherapies. A median of three cycles (range=1->23) were administered.
Table I. Patient demographics.
*One EGFR-TKI regimen was included. ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
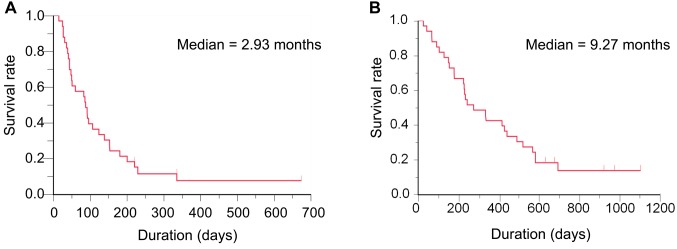
The ORR was 6.1% (95% CI=0.7-20.2%), which was lower than threshold response rate of 10% (Table II). The DCR after 2 months was 51.5%. Thus, the primary endpoint was not met in this study. In terms of secondary endpoints, grade 3 or 4 hematological AEs were neutropenia (51.6%), leukopenia (36.4%), anemia (15.2%) and thrombocytopenia (6.1%). FN was seen in two patients (6.1%). Regarding major grade 3 or 4 non-hematological AEs, anorexia (15.2%), malaise (9.1%), hypoxia (9.1%) and nausea (6.1%) were observed. Grade 3 pneumonitis occurred in one patient (3.0%) and no treatment-related death was reported (Table III). The median PFS was 2.93 months and median OS was 9.27 months (Figure 1).
Table II. Tumor response.
CR, Complete response; PR, partial response; SD, stable disease; PD, progression; NE, not evaluable.
Table III. Adverse events experienced by patients on weekly amrubicin.
No treatment-related deaths were reported.
Figure 1. Survival of patients on weekly amrubicin. A: Progression-free survival; B: overall survival.
Subsequent treatment of patients on weekly amrubicin is shown in Table IV.
Table IV. Subsequent treatment of patients on weekly amrubicin.
Discussion
In this study, the ORR of weekly amrubicin was 6.1% (95% CI=0.7-20.2%) and the primary endpoint was not met in patients with refractory or relapsed NSCLC. Docetaxel is the standard second-line cytotoxic agent for NSCLC, with ORR of 8.8%, stable disease rate of 46.4%, DCR of 55.0% and PFS of 2.9 months (9). Given that 75.8% of patients in this trial had received two or three prior chemotherapies, with a PFS of 2.93 months and DCR of 51.5%, weekly amrubicin may be an alternative candidate for use as second-line salvage therapy. As for as safety is concerned, the frequency of FN was acceptable (6.1%), considering that it was 12.7% in the trial using docetaxel (9).
There are several limitations in this trial. Firstly, it might be inadequate to choose the ORR as the primary endpoint, since three-quarters of patients had received two or three prior chemotherapies. PFS would have been better as the primary endpoint. Secondly, immune checkpoint inhibitors are administered in first-/second-line settings in current therapeutic strategies. However, no patients received immune checkpoint inhibitors in this trial and therefore we were unable to analyze the efficacy of weekly amrubicin after therapy with immune checkpoint inhibitors.
In conclusion, the primary endpoint was negative in this trial. However, this regimen showed comparable PFS and lower rates of FN. Weekly amrubicin can be an alternative regimen for salvage settings after second-line therapy.
Acknowledgements
The Authors thank all of the patients, their families, institutions and investigators participating in this study.
References
- 1.Sugiura T, Ariyoshi Y, Negoro S, Nakamura S, Ikegami H, Takada M, Yana T, Fukuoka M. Phase I/II study of amrubicin, a novel 9-aminoanthracycline, in patients with advanced non-small-cell lung cancer. Invest New Drugs. 2005;23:331–337. doi: 10.1007/s10637-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 2.Hanada M, Noguchi T, Murayama T. Profile of the anti-tumor effects of amrubicin, a completely synthetic anthracycline. Nihon Yakurigaku Zasshi. 2003;122:141–150. doi: 10.1254/fpj.122.141. [DOI] [PubMed] [Google Scholar]
- 3.Yoshioka H, Katakami N, Okamoto H, Iwamoto Y, Seto T, Takahashi T, Sunaga N7, Kudoh S, Chikamori K, Harada M, Tanaka H, Saito H, Saka H, Takeda K, Nogami N, Masuda N, Harada T, Kitagawa H, Horio H, Yamanaka T, Fukuoka M, Yamamoto N, Nakagawa K. A randomized, open-label, phase III trial comparing amrubicin versus docetaxel in patients with previously treated non-small-cell lung cancer. Ann Oncol. 2017;28:285–291. doi: 10.1093/annonc/mdw621. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Ogawa M, Horikoshi N, Mukaiyama T, Itoh Y, Imajoh K, Ozeki H, Nagamine D, Shinagawa K. Phase I and pharmacokinetic study of SM-5887, a new anthracycline derivative. Invest New Drugs. 1989;7:213–218. doi: 10.1007/BF00170860. [DOI] [PubMed] [Google Scholar]
- 5.Belani CP, Ramalingam S, Perry MC, LaRocca RV, Rinaldi D, Gable PS, Tester WJ. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:468–473. doi: 10.1200/JCO.2007.13.1912. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa C, Saka H, Kajikawa S, Mori K, Oki M, Suzuki R. Phase I and pharmacologic study of weekly amrubicin in patients with refractory or relapsed lung cancer: Central Japan Lung Study Group (CJLSG) 0601 trial. Cancer Chemother Pharmacol. 2012;69:1379–1385. doi: 10.1007/s00280-011-1812-8. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0. 2018 http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed on 10 October 2018. [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 10.Nokihara H, Lu S, Mok TSK, Nakagawa K, Yamamoto N, Shi YK, Zhang L, Soo RA, Yang JC, Sugawara S, Nishio M, Takahashi T, Goto K, Chang J, Maemondo M, Ichinose Y, Cheng Y, Lim WT, Morita S, Tamura T. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer) Ann Oncol. 2017;28:2698–2706. doi: 10.1093/annonc/mdx419. [DOI] [PMC free article] [PubMed] [Google Scholar]