Abstract
Monoamine oxidases (MAOs) regulate local levels of neurotransmitters such as dopamine, norepinephrine, and serotonin and thus have been targeted by drugs for the treatment of certain CNS disorders. However, recent studies have shown that these enzymes are upregulated with age in nervous and cardiac tissues and may be involved in degeneration of these tissues, since their metabolic mechanism releases hydrogen peroxide leading to oxidative stress. Thus, targeting these enzymes may be a potential anti-aging strategy. The purpose of this study was to compare the MAO inhibition and selectivity of selected dietary phenolic compounds, using a previously validated assay that would avoid interference from the compounds. Kynuramine metabolism by human recombinant MAO-A and MAO-B leads to formation of 4-hydroxyquinoline, with Vmax values of 10.2±0.2 and 7.35±0.69 nmol/mg/min, respectively, and Km values of 23.1±0.8 μM and 18.0±2.3 μM, respectively. For oral dosing and interactions with the gastrointestinal tract, curcumin, guaiacol, isoeugenol, pterostilbene, resveratrol, and zingerone were tested at their highest expected luminal concentrations from an oral dose. Each of these significantly inhibited both enzymes except for zingerone, which only inhibited MAO-A. The IC50 values were determined, and selectivity indices (MAO-A/MAO-B IC50 ratios) were calculated. Resveratrol and isoeugenol were selective for MAO-A, with IC50 values of 0.313±0.008 and 3.72±0.20 μM and selectivity indices of 50.5 and 27.4, respectively. Pterostilbene was selective for MAO-B, with IC50 of 0.138±0.013 μM and selectivity index of 0.0103. The inhibition of resveratrol (MAO-A) and pterostilbene (MAO-B) was consistent with competitive time-independent mechanisms. Resveratrol 4'-glucoside was the only compound which inhibited MAO-A, but itself, resveratrol 3-glucoside, and pterostilbene 4'-glucoside failed to inhibit MAO-B. Additional studies are needed to establish the effects of these compounds on MAO-A and/or MAO-B in humans.
1. Introduction
Monoamine oxidases (MAO) A and B are enzymes found in the mitochondria of the liver and other tissues, metabolizing neurotransmitters such as dopamine, serotonin, and norepinephrine [1]. Several approved therapeutic MAO inhibitors have long been used for the treatment of anxiety and depression and more recently for Parkinson's disease [2]. MAO reaction products include hydrogen peroxide, aldehydes, and ammonia; these may exhibit toxic effects in various tissues. Notably, expression of MAO increases with age by 6-fold for cardiac MAO-A and 4-fold for neuronal MAO-B [3]. As a result, both MAO-A and MAO-B have been investigated for their roles in oxidative stress, aging, and degenerative disease in the heart and the brain [1, 3–6].
In addition to FDA-approved drugs, natural products have also been investigated for their potential MAO inhibition. Some phenolic dietary compounds such as curcumin, eugenol, piperine, quercetin, and resveratrol are not substrates for MAO, but they have inhibitory effects on MAO [7–12]. Curcumin inhibits both MAO-A and MAO-B in mouse brain after p.o. administration [7]. Piperine and paeonol are reversible inhibitors for both MAO-A and MAO-B in rat brain. The mode of inhibition with piperine on MAO-A and MAO-B is mixed and competitive inhibition giving Ki values of 35.8 μM and 79.9 μM, respectively [9]. Paeonol has Ki values of 51.1 μM and 38.2 μM on MAO-A and MAO-B with non-competitive and competitive inhibition, respectively [9]. Emodin shows mixed mode inhibition on MAO-B with Ki values of 15.1 μM in rat brain [9]. Quercetin is a previously established inhibitor of MAO-A [3, 10, 11] and MAO-B [13]. Resveratrol is a potent inhibitor of MAO-A in rat brain with Ki of 2.5 μM [12]. Eugenol can competitively inhibit both human recombinant MAO-A and MAO- B with Ki of 26 μM and 211 μM [8]. Kaempferol is a selective MAO-A inhibitor [14]; furthermore, certain flavonoid structures have been established as reversible and competitive inhibitors, while glycosidation of these structures decreases or abolishes their MAO inhibition [15]. These phenolic compounds all lack amine groups and therefore MAO inhibition is unexpected and not immediately explained.
The drug-drug interactions between many oral sympathomimetic amines and monoamine oxidase (MAO) inhibitors have been well studied in the literature. The most common adverse effect is high blood pressure. Other adverse effects include headache, chest pain, cardiac arrhythmias, and circulation insufficiency [16]. MAO inhibitors inhibit presystemic and systemic metabolism of some sympathomimetic amines, which are substrates for MAO, resulting in the elevated level of these sympathomimetic amines in circulation [16]. Furthermore, the metabolism of exogenous and endogenous sympathomimetic amines in circulation and tissues could be inhibited by systemic exposure to MAO inhibitors.
Sympathomimetic amines can be divided into two types: direct and indirect acting amines. Indirect acting sympathomimetic amines stimulate the release of noradrenaline from the storage in the sympathetic nerve terminals to interact with postsynaptic adrenergic receptors. MAO inhibitors can increase the level of noradrenaline stored in the nerve terminals. These effects from sympathomimetic amines and MAO inhibitors cause the adverse interaction [16, 17]. Direct acting sympathomimetic amines bind directly to adrenergic receptors. Elimination of these direct acting sympathomimetics from interacting with adrenergic receptors occurs via metabolism by MAO, catechol-O-methyl transferase, and reuptake into presynaptic neurons. Therefore, MAO inhibitors can affect indirectly acting sympathomimetic amines more than directly acting sympathomimetic amines such as phenylephrine [17].
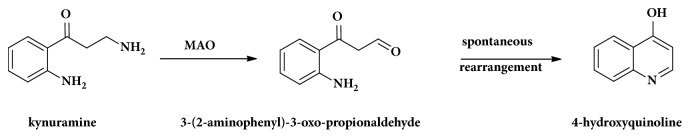
The purpose of this study was to compare the MAO inhibition potential and selectivity of selected phenolic compounds which may have utility in oral dietary supplement products. Since phenolic compounds can act as antioxidants and thus interfere with assay methods depending on the detection of peroxidase activity, a direct chromatographic method measuring the MAO product should yield more reliable results [18]. Therefore kynuramine, a typical substrate of both MAO-A and MAO-B, was used to test if these phenolic compounds can inhibit MAO-A or MAO-B followed by fluorescent HPLC analysis. The metabolite of kynuramine produced by MAO enzymes (3-(2-aminophenyl)-3-oxo-propionaldehyde) rapidly and spontaneously rearranges (by the Schiff base reaction) to the commercially available 4-hydroxyquinoline (shown in Figure 1), which has strong fluorescence for sensitive detection [13].
Figure 1.
Kynuramine converted to 4-hydroxyquinoline via 3-(2-aminophenyl)-3-oxo-propionaldehyde.
2. Materials and Methods
2.1. Chemicals and Reagents
Curcumin (mixture of curcumin, demethoxycurcumin, and bisdemethoxycurcumin) was purchased from Acros Organics (New Jersey, USA). Guaiacol and isoeugenol were purchased from TCI America (Portland, OR). 4-Hydroxyquinoline, zingerone, and trifluoroacetic acid were purchased from Alfa Aesar (Ward Hill, MA). Kynuramine dihydrobromide was purchased from Sigma-Aldrich (St. Louis, MO). Pterostilbene was purchased from ChromaDex (Irvine, CA). Resveratrol was purchased from Beta Pharma, Inc. (New Haven, CT). Piceid (resveratrol 3-glucoside) and vanillin were purchased from TCI America (Portland, OR); α-mangostin was purchased from Indofine (Hillsborough Township, NJ); gnetin-C was donated by Hosoda Nutritional (Fukui City, Japan). Silybin was purchased from Cayman Chemicals (Ann Arbor, MI) and chrysin was purchased from Hawkins Pharmaceutical (Roseville, MN). Acetonitrile was purchased from Avantor Performance Materials, Inc. (Center Valley, PA). Dimethyl sulfoxide, perchloric acid (70%), sodium hydroxide, and triethylamine were purchased from Fisher Scientific (Fair Lawn, NJ). Potassium phosphate monobasic was purchased from Sigma (St. Louis, MO). Potassium phosphate dibasic was purchased from J.T. Baker (Phillipsburg, NJ). Recombinant human MAO-A and MAO-B were purchased from BD Biosciences (San Jose, CA) or Corning Discovery Labware (Woburn, MA). Resveratrol 4'-glucoside and pterostilbene 4'-glucoside were synthesized as previously described [19, 20]. All test compounds were dissolved in DMSO.
2.2. Chromatographic Conditions and Detection
The chromatographic experiments were conducted by HPLC systems including Waters 2695 separation module, Waters 2487 dual λ absorbance detector, and Waters 2475 multi λ fluorescence detector (Waters Corporation, Milford, MA). The HPLC method was developed to simultaneously detect and quantify kynuramine and 4-hydroxyquinoline to monitor the enzymatic reaction of recombinant MAO-A/B. The analytical method used was similar with some modifications to those already published and validated [21, 22]. A Microsorb MV C18 column (100 × 4.6 mm, 3 μm, Agilent Technologies) was used at 30°C to separate kynuramine and 4-hydroxyquinoline. The gradient elution was applied at a flow rate of 1 mL/min with 6.5 mM triethylamine and 13 mM trifluoroacetic acid in water as mobile phase A and acetonitrile as mobile phase B, starting at 10% B and increasing to 50%. Kynuramine was detected by UV at 364 nm, and 4-hydroxyquinoline was detected by fluorescence (excitation 316 nm, emission 357 nm). Further details on the HPLC method are in Supplemental Data (available here).
2.3. MAO Enzyme Kinetic Assay and Km Determination
The optimized incubation time and human recombinant MAO concentration were selected in the linear range from the time-dependent and MAO concentration-dependent studies (Supplemental Data). Briefly, samples were prepared in potassium phosphate buffer (100 mM, pH 7.4) with a final concentration of MAO in the reaction solution of 0.01 mg/mL, incubated at 37°C for 15 min. Saturation of kynuramine metabolism with MAO-A/B was carried out at concentrations of 2, 5, 10, 25, 50, 100, 250, 500 μM. The enzymatic reactions (200μL) were stopped by 2 N NaOH (75 μL) followed with 70% perchloric acid (25 μL). The samples were vortexed and centrifuged for 5 min at 10,000×g. The supernatant was analyzed by HPLC as described above.
2.4. Inhibition Screening and IC50 Determination
According to the Km value determined in the experiment described above, the final concentration of kynuramine was set at 10 μM for the inhibition assay, which was less than the Km values for MAO-A and MAO-B. The incubation time was 15 min and MAO concentration was 0.01 mg/mL. The concentration used to screen the inhibitors of MAO-A/B for curcumin, guaiacol, isoeugenol, pterostilbene, resveratrol, and zingerone was 140, 435, 110, 270, 94, and 51 μM, respectively. If the compounds at these concentrations significantly decrease the formation of 4-hydroxyquinoline, further study would be accomplished to determine their IC50 for the inhibition of MAO-A/B. Additionally, clorgyline and selegiline were tested as positive controls for MAO-A and MAO-B inhibition [23, 24], while several other natural phenolic compounds were tested for MAO inhibition.
2.5. Inhibition Mechanism Studies
To describe the inhibition mechanism for resveratrol or pterostilbene, competition and time dependence were determined. For competition, MAO-A or MAO-B was incubated with varying concentrations of kynuramine (2-450μM) for 30 minutes in the absence (control) or presence of resveratrol (1μM; MAO-A) or pterostilbene (0.45μM). To determine time dependence, the protocol by Obach et al. was followed [25]. Briefly, the enzymes were incubated directly with the inhibitor at 10-fold higher than a quarter of their IC50 values determined herein for resveratrol and pterostilbene for times from 0 to 60 minutes, before diluting 10-fold with kynuramine at a concentration (20μM) approximating its Km values. Tranylcypromine was used as a positive control for time-dependent inhibition [24], and DMSO (0.9% v/v) was used as a negative control [23]. After adding kynuramine, the enzymes were incubated for 30 minutes before processing as described above.
2.6. Data Analysis
GraphPad Prism 5 was applied to fit a Michaelis-Menten model to the data to obtain the Km values in the saturation experiments. In the screening experiments, significant differences between control and treated group were determined by a one-way ANOVA followed by Dunnett's posttest (p < 0.05). The condition of the IC50 study was incubation of kynuramine (10 μM) and a broad concentration range of inhibitors with MAO-A/B (0.01 mg/mL) for 15 min. GraphPad Prism 5 was applied to fit the data to obtain IC50 values by using the concentration-response equation as follows:
| (1) |
This equation included the Hill coefficient as the parameter and could help to characterize the inhibition. If the 95% confidence interval of the Hill coefficient did include 1, the concentration-response equation with the Hill coefficient fixed at 1 was fitted to the data again by the following equation:
| (2) |
The selectivity index was calculated as a ratio of MAO-B IC50/MAO-A IC50 for each compound. The calculated solubility values for phenolic dietary compounds (all unionized at pH values ranging from 1 to 7) are from SciFinder [26]. The maximum single doses are from published sources [27–32]. The relevant GI concentration is the lesser of either solubility or the concentration after a single dose.
Time depended data were analyzed by fitting either straight line or one-phase exponential decay models to the data in GraphPad Prism v5.
3. Results
3.1. Optimized Enzyme Kinetic Assay and Km Determination
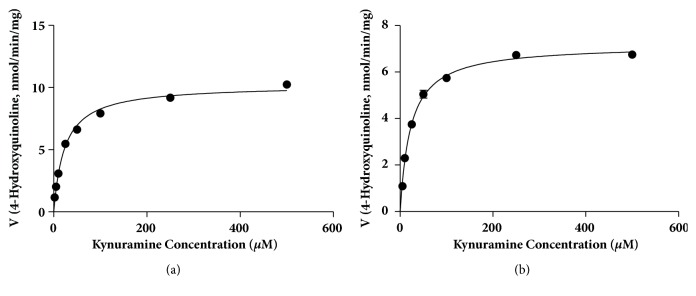
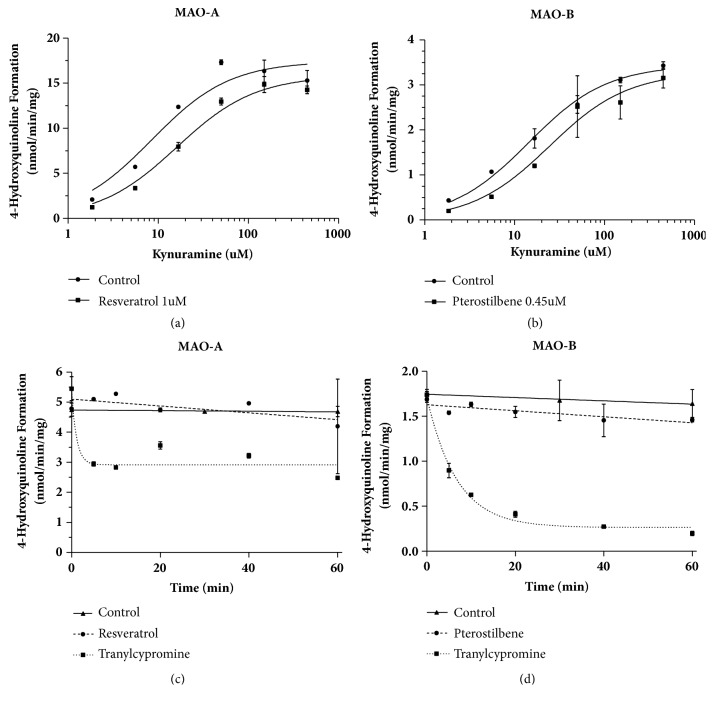
The concentration dependence for oxidative deamination of kynuramine with MAO-A and MAO-B is shown in Figure 2. Kynuramine (2 to 500 μM) was incubated in 200 μL potassium phosphate buffer (100 mM, pH 7.4) for 15 min with MAO-A or MAO-B (0.01 mg/mL). Michaelis-Menten model was used to fit the data by GraphPad Prism 5. The experiments were conducted 3 times in triplicate. Single representative experiments are shown. For MAO-A, Km and Vmax were 23.1 ± 0.8 μM and 10.2 ± 0.2 nmol/min/mg (mean ± SEM), respectively. For MAO-B, Km and Vmax were 18.0 ± 2.3 μM and 7.35 ± 0.69 nmol/min/mg (mean ± SEM), respectively. From these data, the concentration of kynuramine was set at 10 μM for both MAO-A and MAO-B for the following studies so that the kynuramine concentration was < Km.
Figure 2.
Concentration dependence of oxidative deamination of kynuramine by MAO-A (a) or MAO-B (b). Kynuramine was incubated with MAO-A (a) or MAO-B (b) (0.01mg/ml) for 15 minutes. The formation of 4-hydroxyquinoline (mean ± SD) was determined in three experiments in triplicate; a representative experiment is shown. The Michaelis-Menten model was fitted to the data, represented by the curve.
3.2. Inhibition Screening and IC50 Determination
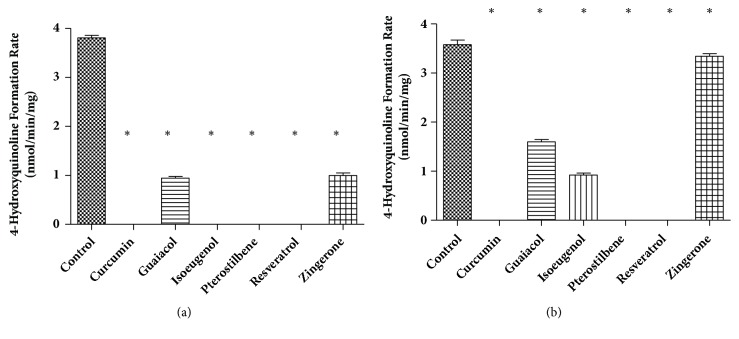
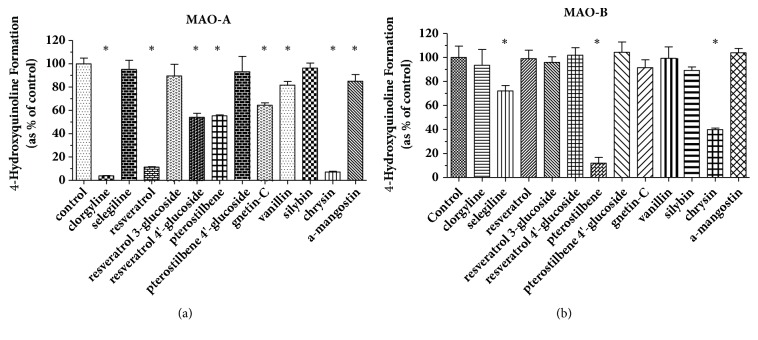
The inhibition screening for oxidative deamination of kynuramine with MAO-A or MAO-B is shown in Figure 3. Kynuramine (10 μM) was incubated in 200 μL potassium phosphate buffer (100 mM, pH 7.4) for 15 min with MAO-A or MAO-B (0.01 mg/mL) and one of these phenolic dietary compounds. The control was the incubation with kynuramine but without any dietary compounds. The numbers are expressed as means ± SD and the significant differences were analyzed between the control treatment (with no inhibitor) and treatments in presence of phenolic dietary compounds using one-way ANOVA analysis followed by Dunnett's post hoc test in GraphPad Prism 5. All the phenolic compounds tested in the experiments showed significant inhibition of MAO-A activity with p < 0.05. All the phenolic compounds tested in the experiments showed significant inhibition of MAO-B activity with p < 0.05. However, zingerone showed less than 10% inhibition at its GI concentration (51 μM) expected from maximum single dose. Therefore zingerone was not investigated further with MAO-B.
Figure 3.
The inhibition of MAO-A (a) or MAO-B (b) activity by phenolic dietary compounds. The inhibition screening for oxidative deamination of kynuramine with MAO-A (a) or MAO-B (b) was conducted with kynuramine (10 µM) incubated with either enzyme (0.01 mg/mL) and one of these phenolic dietary compounds. Concentrations tested: curcumin 140μM, guaiacol 435μM, isoeugenol 110μM, pterostilbene 270μM, resveratrol 94μM, and zingerone 51μM. The numbers are expressed as means ± SD; ∗ indicates that the significant differences were analyzed between the control (no inhibitor) and phenolic compounds. Absent bars indicate that the formation of 4-hydroxyquinoline was below the quantification limit.
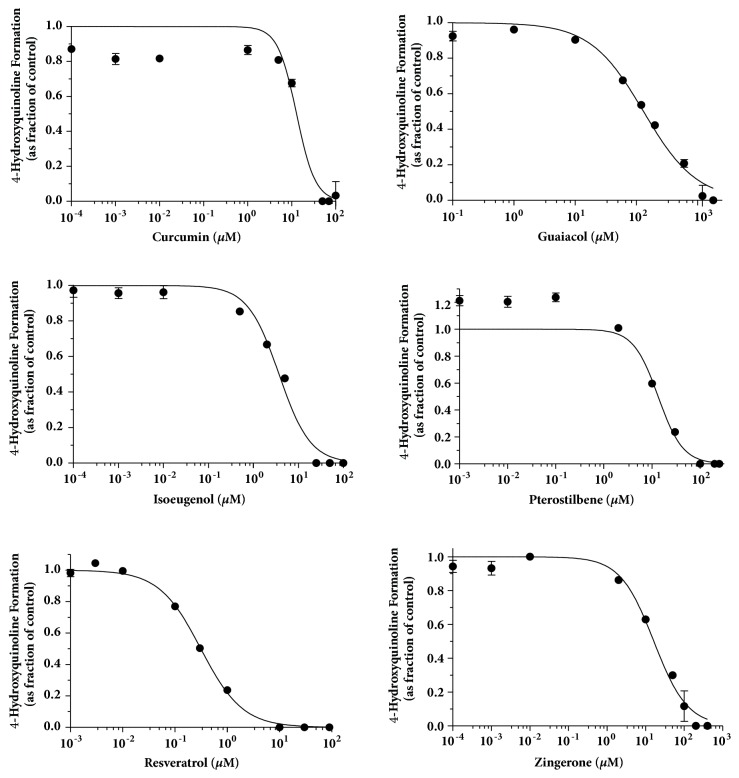
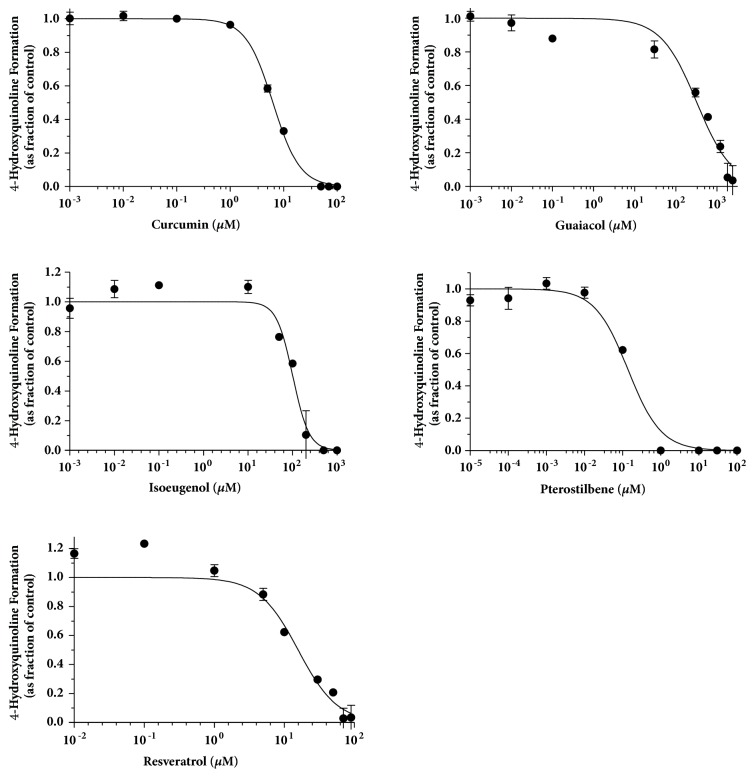
The IC50 curves for the inhibitors for kynuramine oxidative deamination with MAO-A are shown in Figures 4 and 5. MAO activities were measured by the formation of 4-hydroxyquinoline with inhibitors in a broad range of concentrations (at least 104-fold) for 15 min incubation of kynuramine with MAO-A or MAO-B. The fractional activity is the value divided by the control (in absence of inhibitor). The formation of 4-hydroxyquinoline was under the lower limit of detection when incubating kynuramine with the negative control for MAO activity. IC50 values, Hill coefficients, and selectivity indices are shown in Table 1.
Figure 4.
Determination of IC 50 for curcumin, guaiacol, isoeugenol, pterostilbene, resveratrol, and zingerone on MAO-A activity. MAO-A activity was measured by the formation of 4-hydroxyquinoline with inhibitors in a broad range of concentrations (at least 104-fold) for 15 min. The Y-axis is expressed as fraction of the control (in absence of inhibitor) and all points on the curves are expressed as means ± SD.
Figure 5.
Determination of IC 50 for curcumin, guaiacol, isoeugenol, pterostilbene, and resveratrol on MAO-B activity. MAO-B activity was measured by the formation of 4-hydroxyquinoline with inhibitors in a broad range of concentrations (at least 104-fold) for 15 min. The Y axis is expressed as fraction of the control (in absence of inhibitor) and all points on the curves are expressed as means ± SD.
Table 1.
Selectivity of phenolic compounds for MAO-A and MAO-B.
| MAO-A | MAO-B | Selectivity Index | |||
|---|---|---|---|---|---|
| IC50 (µM) | Hill coefficient | IC50 (µM) | Hill coefficient | ||
| curcumin | 12.9 ± 1.3 | 2.0 ± 0.4 | 6.30 ± 0.11 | 1.7 ± 0.1 | 0.488 |
| guaiacol | 131 ± 6 | 1.0 | 322 ± 27 | 1.0 | 2.46 |
| isoeugenol | 3.72 ± 0.20 | 1.2 ± 0.1 | 102 ± 5 | 2.4 ± 0.3 | 27.4 |
| pterostilbene | 13.4 ± 1.5 | 1.7 ± 0.3 | 0.138 ± 0.013 | 1.0 | 0.0103 |
| resveratrol | 0.313 ± 0.008 | 1.1 ± 0.0 | 15.8 ± 1.3 | 1.6 ± 0.2 | 50.5 |
| zingerone | 16.3 ± 1.1 | 1.0 | |||
In Figure 6, Clorgyline or selegiline selectively inhibited MAO-A or MAO-B as expected [24]. Among the glucosides, only resveratrol 4'-glucoside inhibited MAO-A while MAO-B was not inhibited. As previously reported [23], chrysin inhibited both enzymes.
Figure 6.
MAO inhibition by natural phenolic compounds. Recombinant human monoamine oxidases A (a) and B (b) (0.01mg/ml) were exposed to kynuramine dihydrobromide (10μM) for 15 minutes in the absence (control; n=6) or presence (n=3) of clorgyline (1μM), selegiline (0.5μM), or 15 μM of resveratrol, resveratrol 3-glucoside, resveratrol 4'-glucoside, pterostilbene, pterostilbene 4'-glucoside, gnetin-C, vanillin, silybin, chrysin, or α-mangostin. Comparisons were made by one-way ANOVA with Dunnett's posttest; ∗ indicates p<0.05. Control rates for MAO-A and MAO-B were 0.357 ± 0.018 and 0.105 ± 0.010 nmol/min/mg protein, respectively.
3.3. Inhibition Mechanism
Figure 7 showed that in the absence or presence of resveratrol (1 μM), the Vmax and Km values for MAO-A were 17.4 ± 0.7 or 15.8 ± 0.4 nmol/min/mg protein and 8.46 ± 1.56 or 16.1 ± 1.8 μM, respectively. For MAO-B, in the absence or presence of pterostilbene (0.45 μM), the Vmax and Km values were 3.44 ± 0.07 or 3.28 ± 0.19 nmol/min/mg protein and 14.4 ± 1.2 or 24.4 ± 5.4 μM, respectively. MAO-A activity showed dependence upon preincubation time with tranylcypromine (1 μM), with a preincubation half-life of 1.1 minutes, and a plateau showing 48% inhibition. However, resveratrol (1.6μM) preincubation revealed a time profile not significantly different from the control. MAO-B preincubation with tranylcypromine (0.5μM) also showed time dependence, with a half-life of 4.7 minutes, and a plateau at 85% inhibition, while preincubation with pterostilbene (0.7μM) revealed a time profile not significantly different from the control.
Figure 7.
Mechanism of resveratrol and pterostilbene inhibition of MAO enzymes. Kynuramine (2-450μM) and MAO-A or MAO-B (0.01mg/ml) were incubated for 15 minutes in the absence or presence of resveratrol (1μM) (a) or pterostilbene (0.45μM) (b). The Michaelis-Menten equation was fit to the data by nonlinear regression to compare any changes in Km or Vmax. (c) MAO-A was preincubated with tranylcypromine (1μM), resveratrol (1.6μM), or DMSO (control) for 0-60 minutes before the addition of kynuramine (20μM). (d) MAO-A was preincubated with tranylcypromine (0.5μM), pterostilbene (0.7μM), or DMSO (control) for 0-60 minutes before the addition of kynuramine (20μM). All samples were incubated at 37°C for 30 minutes. One-phase decay or straight line equations were fit to the data.
4. Discussion
Previously validated HPLC methods [21, 23] were adapted to simultaneously quantitate kynuramine and the product of its metabolism by MAO 4-hydroxyquinoline. We applied this assay to facilitate the quantitation and comparison of several phenolic compounds on human MAO enzymes. Interestingly, some of these compounds showed considerable selectivity toward MAO-A or MAO-B. A previous study shows that resveratrol had similar potency for inhibiting MAO-A and MAO-B [33]; however, our study showed that resveratrol had higher potency and selectivity for MAO-A. The reason for the difference is unknown, but may be due to differences in assay methods, i.e., Amplex Red vs. kynuramine. The interference with peroxidase-based assays has been previously established [18].
From the optimization studies (Supplementary Materials), the formation of 4-hydroxyquinoline was linear over 60 min with the protein concentration range of 0.003 mg/mL–0.03 mg/mL, which was comparable with the results from the paper published by Herraiz et al. in 2006 [21]. The Km values of kynuramine oxidative deamination by MAO-A and MAO-B were 23 μM and 18 μM, respectively, which indicated MAO-A has similar affinity toward kynuramine, compared to MAO-B. In the literature, the Km values of kynuramine for human MAO-A and MAO-B were reported as 42 μM and 26 μM [34]. Another study obtained the Km values of MAO-A and MAO-B with kynuramine as 44.1 and 90.0 μM, respectively [35]. Km values reported here were similar to the values in literature, although differences in methods or recombinant enzyme sources may account for differences in reported Km or Vmax values. The concentration of kynuramine for the inhibition study with phenolic compounds was set at 10 μM, which was below the Km value for both MAO-A and MAO-B.
Nonlinear regression revealed Hill coefficients of unity for guaiacol and zingerone showing 1-to-1 apparent binding to MAO-A, and guaiacol and pterostilbene followed 1-to-1 apparent binding to MAO-B. The Hill coefficient of resveratrol with MAO-A was 1.08, while curcumin, isoeugenol, and pterostilbene had Hill coefficients larger than 1, suggesting positive cooperativity, multiple active sites, or non-ideal inhibition behavior [36]. Positive cooperativity could be a possible reason. The binding of the inhibitor to one active site on the enzyme may increase the binding affinity of the inhibitor to other active sites [36]. Another possibility is that the complete inhibition of an enzyme can be achieved by binding of more than one molecule of inhibitor to the enzyme [36]. Further study is required to investigate the mechanism of inhibition which leads to the Hill coefficient larger than 1, including possible allosterism.
Among these tested phenolic dietary compounds, the inhibitory effects on MAO-A and MAO-B in animal models were reported in the literature previously [7, 12]. However, in this study, human recombinant MAO-A and MAO-B enzymes were used as models to test these phenolic compounds. Curcumin can inhibit MAO-A and MAO-B in mouse brain after p.o. administration [7]. We also found curcumin inhibited both MAO-A and MAO-B with IC50 values of 12.89 μM and 6.30 μM, respectively. In this study, resveratrol was the most potent inhibitor for MAO-A with IC50 as 0.31 μM; its inhibition was consistent with a competitive mechanism, as previously demonstrated by Ryu et al. [12]. Resveratrol is a potent competitive inhibitor of MAO-A in rat brain with IC50 of 2 μM and Ki of 2.5 μM [12]. Resveratrol was also previously reported to be an inhibitor of MAO-A but did not significantly inhibit MAO-B up to 10μM [18], which is consistent with our study in which resveratrol showed a relatively high IC50 value for MAO-B of 15.8μM (Table 1). Pterostilbene has never been reported as an MAO inhibitor, but it was the most potent MAO-B inhibitor in our study, with an IC50 of 0.138 μM.
Compared to the GI concentration converted from the maximum single dose, the IC50 values of all phenolic inhibitors on MAO-A and MAO-B are smaller than the maximum concentration in GI tract. The most potent inhibitor for MAO-A was resveratrol followed by isoeugenol, curcumin, pterostilbene, zingerone, and guaiacol in descending order of potency. The most potent inhibitor for MAO-B was pterostilbene followed by curcumin, resveratrol, isoeugenol, and guaiacol in descending order of potency.
Additionally, the selectivity indices expressed as the ratios of MAO-B/MAO-A IC50 values (Table 1) showed that resveratrol and isoeugenol are selective MAO-A inhibitors, while pterostilbene is a selective MAO-B inhibitor. These data are especially intriguing, considering that only two methyl groups differentiate resveratrol and pterostilbene. Besides resveratrol, isoeugenol showed the next highest level of potency and selectivity for MAO-A. Isoeugenol has a methylated catechol moiety (as do zingerone and guaiacol) and also a hydrophobic side chain, which may contribute to its MAO-A inhibition exceeding those of zingerone and guaiacol. Furthermore, among the glucosides of resveratrol and pterostilbene tested herein, MAO-A was inhibited only by resveratrol 4'-glucoside, while MAO-B was not inhibited. This is consistent with the loss of inhibitory activity by glycosides of kaempferol [15].
Phenolic compounds are substrates for neither MAO-A or MAO-B and, unlike other MAO, substrates and inhibitors are devoid of any nitrogen atoms. The mechanism of the inhibition of phenolic compounds on MAO is not clear, but none of them was reported to have irreversible inhibition on MAO-A or MAO-B [8, 9]. The researchers found that they are reversible inhibitors with various mode of inhibition such as competitive inhibition, non-competitive, or mixed-type inhibition [8, 9]. Furthermore, several flavonoids have been established as reversible and competitive inhibitors [15].
Published studies show that these phenolic MAO-A inhibitors all have low oral bioavailability in animal models, although this has not been determined in humans. Curcumin has poor bioavailability after oral administration in humans even after a high dose of 12 g/day, which leads to low plasma concentrations [37]. At doses of 4 g, 6 g, and 8 g, the maximum concentration of curcumin in plasma is 0.51 μM, 0.64 μM, and 1.77 μM, respectively [38]. After gavage administration, the absolute bioavailability of isoeugenol in female and male rats is 19% and 10%, respectively. The low bioavailability of isoeugenol was also observed in mice as 28% for male mice and 31% for female mice after gavage bolus [39]. The peak plasma concentration of resveratrol in humans is very low after oral dosing [40, 41]. At 25 mg, 50 mg, 100 mg, and 150 mg doses, the maximum plasma concentrations of resveratrol are 1.48 ng/mL, 6.59 ng/mL, 21.4 ng/mL, and 24.8 ng/mL, respectively [40]. At higher doses of 0.5 g, 1.0 g, 2.5 g, and 5.0 g, the corresponding peak plasma concentrations of resveratrol are 72.6 ng/mL, 117.0 ng/mL, 268.0 ng/mL, and 538.8 ng/mL [41]. The oral bioavailability in rats was determined as 12.5% after 10 mg/kg gavage administration by Lin et al. [42]. After giving rats 56 or 168 mg/kg/day for pterostilbene by gavage for 14 continuous days, the oral bioavailability is 80% [43]. The reason why these phenolic compounds have such low bioavailability is that they all undergo extensive presystemic metabolism and are converted to their metabolites before going to the systemic circulation [41, 43–51].
As described above, in the dose range of 25 mg to 5.0 g, and considering the plasma protein binding of 91% for resveratrol [52], the unbound maximal peak plasma concentrations range from 0.584 to 212 nM. With the IC50 values of 0.313 and 15.8 μM for MAO-A and MAO-B and assuming competitive inhibition of resveratrol on human MAO-A and MAO-B, the values of Ki would be 0.218 and 10.2 μM for MAO-A and MAO-B, respectively. The drug-drug interaction index (unbound Cmax/Ki [53]) for MAO-A is in the range of 2.7 × 10−3 to 0.97. Thus at high resveratrol doses (5.0 g), inhibition of MAO-A may occur. However, the drug-drug interaction index for MAO-B is in the range of 5.7 × 10−5 to 0.021, making clinically relevant MAO-B interactions with resveratrol unlikely. As with many other natural phenolic compounds, human plasma concentration data and absolute oral bioavailability for oral doses of pterostilbene are lacking [54], making predictions of gastrointestinal or systemic MAO-B interactions speculative. A human clinical study with pterostilbene shows that 125mg twice daily orally for 6-8 weeks reduces both systolic and diastolic blood pressure, although it slightly raised plasma low-density lipoproteins [55]. Another clinical study with pterostilbene 50mg in combination with nicotinamide riboside 250mg for two months also reduced diastolic blood pressure [56]. Acute, severe, systemic MAO inhibition is expected to increase blood pressure and result in serotonin syndrome [2]; however, such adverse reactions were not reported in these studies. As with many dietary compounds, the absolute oral bioavailability of pterostilbene has not been reported, but animal studies show that it is much greater than that of resveratrol [43]. Additional studies are required to determine the absolute oral bioavailability of pterostilbene and other potentially beneficial phenolic compounds and to establish their antiaging effects on human cardiac and neuronal tissues.
Since these phenolic compounds all have relatively low bioavailability, the inhibition occurring after first-pass metabolism is likely to be limited. Most inhibitory effects on MAO-A and MAO-B would be limited to GI tract and liver. Nishimura et al. established the mRNA levels of MAO-A and MAO-B in various human tissues [57]. From their data, the MAO-A mRNA/MAO-B mRNA ratio in the liver is 0.727, whereas the ratio in the small intestine is 4.41, suggesting that MAO-A and MAO-B expression may be similar in the liver, but MAO-A predominates in the intestine. Thus, any effects of MAO-A inhibition in the intestine could be concerning. When other dietary compounds are substrates of MAO and their presystemic metabolism is blocked resulting in higher systemic exposure, MAO inhibition may be problematic. One example is tyramine. Interactions between food constituents and drugs are complicated with various conditions and difficult to predict. When patients take irreversible MAO inhibitors, tyramine-rich foods are to be avoided.
Other assays for MAO activity are available, including peroxidase- and luciferase-based assays. However, antioxidant phenolics can interfere with peroxidase-based assays by quenching the hydrogen peroxide formed [58]. In our lab, resveratrol strongly quenched luminesce in the MAO-glo assay (Promega, Madison, WI; Tamoor Hassan, unpublished data). The metabolic production of 4-hydroxyquinoline could avoid these interferences, but only for compounds which do not interfere with its detection. Thus, the kynuramine-based assay with chromatographic separation offers a solution to avoid interferences. Assays for kynuramine and 4-hydroxyquinoline have been previously reported, and Herraiz et al. showed that antioxidants can indeed interfere with Amplex Red assays, while this interference is avoided by HPLC analysis of 4-hydroxyquinoline formation [18]. The quantitative analysis could be simply achieved by fluorometric assay [59]. The phenolic compounds tested in this study have very strong fluorescence, which may interfere with the fluorescent signal from 4-hydroxyquinoline if measured in a microplate reader. Therefore, a fluorometric microplate assay may not be selective for the detection of 4-hydroxyquinoline and thus chromatographic separation of 4-hydroxyquinoline and the phenolic compounds are required. Other analyses are accomplished by HPLC with UV and fluorescence detection as well as LC-MS/MS [21, 34, 60, 61]. Since 4-hydroxyquinoline has very good fluorescence and kynuramine can be detected by UV detection, HPLC methods with UV and fluorescence detectors are found to be quite adequate for analysis in in vitro enzyme kinetic studies. In order to avoid analytical interferences, Herraiz et al. developed a reversed-phase HPLC method by gradient elution with 50 mM ammonium phosphate buffer at pH 3 and 20% of this buffer in acetonitrile [21, 60]. Also, in their HPLC method for 4-hydroxyquinoline, Parikh et al. used a mobile phase containing 0.2mM perchloric acid [23]. In order to avoid the potential for damage to our HPLC system, we modified the mobile phase as discussed in the method section.
The HPLC method for quantitative analysis of kynuramine and 4-hydroxyquinoline used 6.5 mM triethylamine and 13 mM trifluoroacetic acid in water as its aqueous phase, which has a pH value around 2. The estimated most basic pKa value for kynuramine is 8.4, making it cationic in the mobile phase [26]. The estimated most acidic and most basic pKa values for 4-hydroxyquinoline are 4.3 and 11.1, respectively [26]. Hence 4-hydroxyquinoline is also cationic in the mobile phase. At high concentration, trifluoroacetic acid can act as an ion-pairing agent for cations, which can improve kynuramine and 4-hydroxyquinoline retention. When using the aqueous mobile phase with only TFA at 0.05%, there was a tailing problem with the peak shape. This can be caused by the ions like sodium and potassium bound to silanol exchanging with ionized basic analytes at low pH. As an additive in the mobile phase, triethylamine can fix the tailing problem on the column. Excess triethylamine in the mobile phase can replace the ions instead of basic analytes. Therefore, triethylamine can reduce the peak tailing [62].
5. Conclusions
In conclusion, we applied a previously validated kynuramine-based MAO activity assay with HPLC separation and fluorescence detection for determining the inhibition and selectivity of several phenolic compounds. Among the compounds tested, resveratrol was potent and selective for MAO-A inhibition, while pterostilbene was potent and selective for MAO-B inhibition. Both compounds appeared to be competitive, time-independent inhibitors. Our calculations suggest that high doses of resveratrol have the potential to inhibit MAO-A in the gastrointestinal tract. Human pharmacokinetic studies with oral dosing of pterostilbene will facilitate future predictions of its clinical potential to interact with MAO-B in the gastrointestinal tract, liver, or systemic circulation. The anti-aging potential of these compounds is worth further investigations.
Acknowledgments
This work was submitted by Zhenxian Zhang as a part of her PhD dissertation at the VCU School of Pharmacy. This study was supported by the VCU School of Pharmacy, the VCU Graduate School Dissertation Enhancement Award, and Pfizer Consumer Healthcare (Investigator-Initiated Research Program). The authors acknowledge Dr. Jurgen Venitz for helpful discussions regarding this project. They also acknowledge Hosoda Nutritional for their kind gift of gnetin-C.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
Supplementary materials are available describing the HPLC method and the optimization of the enzyme incubation conditions.
References
- 1.Bortolato M., Chen K., Shih J. C. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Advanced Drug Delivery Reviews. 2008;60(13-14):1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunton L. L., Chabner B. A., Knollman B. C. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill; 2011. [Google Scholar]
- 3.Maggiorani D., Manzella N., Edmondson D. E., et al. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxidative Medicine and Cellular Longevity. 2017;2017:8. doi: 10.1155/2017/3017947.3017947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martín-Fernández B., Gredilla R. Mitochondrial oxidative stress and cardiac ageing. Clínica e Investigación en Arteriosclerosis: publicacion oficial de la Sociedad Espanola de Arteriosclerosis. 2018;30(2):74–83. doi: 10.1016/j.arteri.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Aluf Y., Vaya J., Khatib S., Loboda Y., Finberg J. P. Selective inhibition of monoamine oxidase A or B reduces striatal oxidative stress in rats with partial depletion of the nigro-striatal dopaminergic pathway. Neuropharmacology. 2013;65:48–57. doi: 10.1016/j.neuropharm.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Hauptmann N., Grimsby J., Shih J. C., Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Archives of Biochemistry and Biophysics. 1996;335(2):295–304. doi: 10.1006/abbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y., Ku B.-S., Yao H.-Y., et al. The effects of curcumin on depressive-like behaviors in mice. European Journal of Pharmacology. 2005;518(1):40–46. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Tao G., Irie Y., Li D.-J., Wing M. K. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorganic & Medicinal Chemistry. 2005;13(15):4777–4788. doi: 10.1016/j.bmc.2005.04.081. [DOI] [PubMed] [Google Scholar]
- 9.Kong L. D., Cheng C. H. K., Tan R. X. Inhibition of MAO a and B by some plant-derived alkaloids, phenols and anthraquinones. Journal of Ethnopharmacology. 2004;91(2-3):351–355. doi: 10.1016/j.jep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Yoshino S., Hara A., Sakakibara H., et al. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition Journal. 2011;27(7-8):847–852. doi: 10.1016/j.nut.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Bandaruk Y., Mukai R., Kawamura T., Nemoto H., Terao J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. Journal of Agricultural and Food Chemistry. 2012;60(41):10270–10277. doi: 10.1021/jf303055b. [DOI] [PubMed] [Google Scholar]
- 12.Ryu S. Y., Han Y. N., Han B. H. Monoamine oxidase-A inhibitors from medicinal plants. Archives of Pharmacal Research. 1988;11(3):230–239. doi: 10.1007/BF02861314. [DOI] [Google Scholar]
- 13.Carradori S., D'Ascenzio M., Chimenti P., Secci D., Bolasco A. Selective MAO-B inhibitors: A lesson from natural products. Molecular Diversity. 2014;18(1):219–243. doi: 10.1007/s11030-013-9490-6. [DOI] [PubMed] [Google Scholar]
- 14.Gidaro M. C., Astorino C., Petzer A., et al. Kaempferol as Selective Human MAO-A Inhibitor: Analytical Detection in Calabrian Red Wines, Biological and Molecular Modeling Studies. Journal of Agricultural and Food Chemistry. 2016;64(6):1394–1400. doi: 10.1021/acs.jafc.5b06043. [DOI] [PubMed] [Google Scholar]
- 15.Carradori S., Gidaro M. C., Petzer A., et al. Inhibition of Human Monoamine Oxidase: Biological and Molecular Modeling Studies on Selected Natural Flavonoids. Journal of Agricultural and Food Chemistry. 2016;64(47):9004–9011. doi: 10.1021/acs.jafc.6b03529. [DOI] [PubMed] [Google Scholar]
- 16.Sjöqvist F. Psychotropic drugs (2). Interaction between monoamine oxidase (MAO) inhibitors and other substances. Proceedings of the Royal Society of Medicine. 1965;58(11):967–978. doi: 10.1177/003591576505811P206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rand M. J., Trinker F. R. The mechanism of the augmentation of responses to indirectly acting sympathomimetic amines by monoamine oxidase inhibitors. British Journal of Pharmacology. 1968;33(2):287–303. doi: 10.1111/j.1476-5381.1968.tb00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herraiz T., Flores A., Fernández L. Analysis of monoamine oxidase (MAO) enzymatic activity by high-performance liquid chromatography-diode array detection combined with an assay of oxidation with a peroxidase and its application to MAO inhibitors from foods and plants. Journal of Chromatography B. 2018;1073:136–144. doi: 10.1016/j.jchromb.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Uesugi D., Hamada H., Shimoda K., Kubota N., Ozaki S., Nagatani N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Bioscience, Biotechnology, and Biochemistry. 2016;81(2):226–230. doi: 10.1080/09168451.2016.1240606. [DOI] [PubMed] [Google Scholar]
- 20.Sato D., Shimizu N., Shimizu Y., et al. Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Bioscience, Biotechnology, and Biochemistry. 2014;78(7):1123–1128. doi: 10.1080/09168451.2014.921551. [DOI] [PubMed] [Google Scholar]
- 21.Herraiz T., Chaparro C. Analysis of monoamine oxidase enzymatic activity by reversed-phase high performance liquid chromatography and inhibition by β-carboline alkaloids occurring in foods and plants. Journal of Chromatography A. 2006;1120(1-2):237–243. doi: 10.1016/j.chroma.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Yan Z., Caldwell G. W., Zhao B., Reitz A. B. A high-throughput monoamine oxidase inhibition assay using liquid chromatography with tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2004;18:834–840. doi: 10.1002/rcm.1415. [DOI] [PubMed] [Google Scholar]
- 23.Parikh S., Hanscom S., Gagne P., Crespi C., Patten C. A fluorescent-based, high-throughput assay for detecting inhibitors of human monoamine oxidase A and B. Drug Metabolism Reviews. 2002;34:p. 164. [Google Scholar]
- 24.Tipton K. F., Davey G., Motherway M. Monoamine Oxidase Assays. Current Protocols in Pharmacology. 2000;9(1):3.6.1–3.6.42. doi: 10.1002/0471141755.ph0306s09. [DOI] [PubMed] [Google Scholar]
- 25.Obach R. S., Fahmi O. A., Walsky R. L. Inactivation of human cytochrome P450 enzymes and drug-drug interactions. In: Pang K. S., Rodrigues A. D., Peter R. M., editors. Enzyme- and Transporter-Based Drug-Drug Interactions: Progress and Future Challenges. New York, NY, USA: Springer; 2010. pp. 473–495. [Google Scholar]
- 26.SciFinder. Chemical Abstracts Service. 2019, https://www.cas.org/products/scifinder.
- 27.Kuge T., Shibata T., Willett M. S. Wood Creosote, the Principal Active Ingredient of Seirogan, an Herbal Antidiarrheal Medicine: A Single-Dose, Dose-Escalation Safety and Pharmacokinetic Study. Pharmacotherapy. 2003;23(11):1391–1400. doi: 10.1592/phco.23.14.1391.31940. [DOI] [PubMed] [Google Scholar]
- 28.Ogata N., Matsushima N., Shibata T. Pharmacokinetics of wood creosote: Glucuronic acid and sulfate conjugation of phenolic compounds. Pharmacology. 1995;51(3):195–204. doi: 10.1159/000139335. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration. Substances Added to Food (formerly EAFUS) Updated 10/10/2018, U.S. Department of Health and Human Services, https://www.accessdata.fda.gov/scripts/fdcc/?set=FoodSubstances.
- 30.U.S. Food and Drug Administration. GRAS Notices. Updated 12/20/2018, U.S. Department of Health and Human Services, https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices.
- 31.Bobka M. S. The 21 CFR (Code of Federal Regulations) online database: Food and Drug Administration regulations full-text. Medical Reference Services Quarterly. 1993;12:7–15. doi: 10.1300/J115V12N04_02. [DOI] [PubMed] [Google Scholar]
- 32.Burdock G. Fenaroli's Handbook of Flavor Ingredients. 5th. Boca Raton, FL, USA: CRC Press; 2005. [Google Scholar]
- 33.Yáñez M., Fraiz N., Cano E., Orallo F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochemical and Biophysical Research Communications. 2006;344(2):688–695. doi: 10.1016/j.bbrc.2006.03.190. [DOI] [PubMed] [Google Scholar]
- 34.Yan Z., Caldwell G. W., Zhao B., Reitz A. B. A high-throughput monoamine oxidase inhibition assay using liquid chromatography with tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2004;18(8):834–840. doi: 10.1002/rcm.1415. [DOI] [PubMed] [Google Scholar]
- 35.Naoi M., Nomura Y., Ishiki R., Suzuki H., Nagatsu T. 4‐(O‐Benzylphenoxy)‐N‐Methylbutylamine (Bifemelane) and Other 4‐(O‐Benzylphenoxy)‐N‐Methylalkylamines as New Inhibitors of Type A and B Monoamine Oxidase. Journal of Neurochemistry. 1988;50(1):243–247. doi: 10.1111/j.1471-4159.1988.tb13256.x. [DOI] [PubMed] [Google Scholar]
- 36.Copeland R. A. Evaluation Of Enzyme Inhibitors In Drug Discovery: A Guide For Medicinal Chemists And Pharmacologists. Wiley-Interscience: City; 2005. [DOI] [PubMed] [Google Scholar]
- 37.Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. Bioavailability of curcumin: problems and promises. Molecular Pharmaceutics. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 38.Cheng A. L., Hsu C. H., Lin J. K., et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 39.Hong S. P., Fuciarelli A. F., Johnson J. D., et al. Toxicokinetics of Isoeugenol in F344 rats and B6C3F. Xenobiotica. 2013;43(11):1010–1017. doi: 10.3109/00498254.2013.790576. [DOI] [PubMed] [Google Scholar]
- 40.Almeida L., Vaz-da-Silva M., Falcão A., et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Molecular Nutrition & Food Research. 2009;53(1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 41.Boocock D. J., Faust G. E. S., Patel K. R., et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(6):1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 42.Lin H. S., Yue B. D., Ho P. C. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomedical Chromatography. 2009;23(12):1308–1315. doi: 10.1002/bmc.1254. [DOI] [PubMed] [Google Scholar]
- 43.Kapetanovic I. M., Muzzio M., Huang Z., Thompson T. N., McCormick D. L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemotherapy and Pharmacology. 2011;68(3):593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcea G., Berry D. P., Jones D. J. L., et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(1):120–125. [PubMed] [Google Scholar]
- 45.Ireson C., Orr S., Jones D. J. L., et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2production. Cancer Research. 2001;61(3):1058–1064. [PubMed] [Google Scholar]
- 46.Ireson C. R., Jones D. J. L., Orr S., et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(1):105–111. [PubMed] [Google Scholar]
- 47.Badger D. A., Smith R. L., Bao J., Kuester R. K., Sipes I. G. Disposition and metabolism of isoeugenol in the male Fischer 344 rat. Food and Chemical Toxicology. 2002;40(12):1757–1765. doi: 10.1016/S0278-6915(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 48.Vitaglione P., Sforza S., Galaverna G., et al. Bioavailability of trans-resveratrol from red wine in humans. Molecular Nutrition & Food Research. 2005;49(5):495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 49.Aumont V., Krisa S., Richard T., et al. Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Archives of Biochemistry and Biophysics. 2001;393(2):281–289. doi: 10.1006/abbi.2001.2496. [DOI] [PubMed] [Google Scholar]
- 50.Miksits M., Maier-Salamon A., Aust S., et al. Sulfation of resveratrol in human liver: Evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica. 2005;35(12):1101–1119. doi: 10.1080/00498250500354253. [DOI] [PubMed] [Google Scholar]
- 51.Remsberg C. M., Yáñez J. A., Ohgami Y., Vega-Villa K. R., Rimando A. M., Davies N. M. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytotherapy Research. 2008;22(2):169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- 52.Burkon A., Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides—two novel resveratrol metabolites in human plasma. Molecular Nutrition & Food Research. 2008;52(5):549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 53.Huang S.-M., Temple R., Throckmorton D. C., Lesko L. J. Drug interaction studies: Study design, data analysis, and implications for dosing and labeling. Clinical Pharmacology & Therapeutics. 2007;81(2):298–304. doi: 10.1038/sj.clpt.6100054. [DOI] [PubMed] [Google Scholar]
- 54.Wang P., Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors. 2018;44(1):16–25. doi: 10.1002/biof.1410. [DOI] [PubMed] [Google Scholar]
- 55.Riche D. M., McEwen C. L., Riche K. D., et al. Analysis of Safety from a Human Clinical Trial with Pterostilbene. Journal of Toxicology. 2013;2013:5. doi: 10.1155/2013/463595.463595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dellinger R. W., Santos S. R., Morris M., et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. npj Aging and Mechanisms of Disease. 2017;3(17) doi: 10.1038/s41514-017-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimura M., Naito S. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metabolism and Pharmacokinetics. 2006;21(5):357–374. doi: 10.2133/dmpk.21.357. [DOI] [PubMed] [Google Scholar]
- 58.Carpéné C., Hasnaoui M., Balogh B., et al. Dietary Phenolic Compounds Interfere with the Fate of Hydrogen Peroxide in Human Adipose Tissue but Do Not Directly Inhibit Primary Amine Oxidase Activity. Oxidative Medicine and Cellular Longevity. 2016;2016:15. doi: 10.1155/2016/2427618.2427618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto T., Suzuki O., Furuta T., et al. A sensitive fluorometric assay for serum monoamine oxidase with kynuramine as substrate. Clinical Biochemistry. 1985;18(2):126–129. doi: 10.1016/S0009-9120(85)80094-1. [DOI] [PubMed] [Google Scholar]
- 60.Herraiz T., Chaparro C. Human monoamine oxidase enzyme inhibition by coffee and β-carbolines norharman and harman isolated from coffee. Life Sciences. 2006;78(8):795–802. doi: 10.1016/j.lfs.2005.05.074. [DOI] [PubMed] [Google Scholar]
- 61.Jones C. K., Bubser M., Thompson A. D., et al. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson's disease. The Journal of Pharmacology and Experimental Therapeutics. 2012;340(2):404–421. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snyder L. R., Kirkland J. J., Glajch J. L. Practical HPLC Method Development. John Wiley & Sons; 2012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials are available describing the HPLC method and the optimization of the enzyme incubation conditions.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.