Abstract
Pancreatic cancer (PC) is a common malignancy with a poorly understood pathogenesis. Currently, the efficacy of anti-PC therapies is insufficient, partially due to the chemoresistance of cancer cells. The present study aimed to elucidate the role of miR-183 in the proliferation, apoptosis, and chemosensitivity to 5-fluorouracil and gemcitabine of human PC cells and the associated mechanisms. PANC-1 cells were transfected with microRNA (miR)-183 inhibitors, and the effect of miR-183 on cell proliferation was evaluated via MTT assay. Apoptosis and cell cycle distribution were determined by flow cytometry. In vivo tumor xenograft models of PANC-1 cells were generated in BALB/c nude mice to examine the effect of miR-183 downregulation on tumor growth. Furthermore, components of the phosphatase and tensin homolog deleted on chromosome ten (PTEN)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway were examined via reverse transcription-quantitative polymerase chain reaction and western blotting in the collected cells. Finally, PANC-1 cells were treated with 5-fluorouracil or gemcitabine and transfected with miR-183 inhibitors, and the viability of cells was determined by MTT assay. The results demonstrated that knockdown of miR-183 could significantly decrease proliferation and promote apoptosis of PANC-1 cells. The cells transfected with miR-183 inhibitors were significantly arrested at the G1 phase (P<0.01). Furthermore, miR-183 downregulation led to significant decreases in the mRNA levels of PI3K, Akt and B cell lymphoma-2 (Bcl-2) expression (P<0.001), and significant increases in PTEN and Bcl-2 associated X protein expression in PANC-1 cells (P<0.001). Knockdown of miR-183 was able to significantly increase the chemosensitivity of PANC-1 cells to 5-fluorouracil and gemcitabine. These results indicate that downregulation of miR-183 can inhibit the growth of PC cells in vitro and in vivo, and increase cell sensitivity to 5-fluorouracil and gemcitabine through regulating the PTEN/PI3K/Akt signaling pathway.
Keywords: microRNA-183, phosphatase and tensin homolog deleted on chromosome ten, chemosensitivity, pancreatic cancer
Introduction
Pancreatic cancer (PC) is a common aggressive malignancy, and one of the leading causes of cancer-related mortality (1). Based on previous reports, >227,000 deaths are caused by PC worldwide each year (2). At present, the pathogenesis of PC has remained unclear, and the prognosis continues to be poor (5-year survival rate, <2%) (3). Gemcitabine and 5-fluorouracil are the most commonly used anti-PC agents; however, the efficacy of current anti-PC therapies remains unsatisfactory, in part due to the chemoresistance of cancer cells (4). Previous studies have indicated that genetics may serve important roles in the tumorigenesis of PC, through the activation of oncogenes and the silencing of tumor-suppressor genes (5–7). Therefore, it is essential to identify key molecules associated with the pathogenesis of PC that may serve as potential therapeutic targets.
In recent years, the roles of microRNAs (miRNA or miRs) in cancer have been investigated intensively. miRNAs are small, non-coding RNAs (~22 nucleotides) that can bind to the 3′-untranslated region (UTR) of one or more target genes and negatively regulate the expression of those genes (8). Aberrant expression of miRNAs has been observed in tissue and serum samples from PC patients, as well as PC cell lines, suggesting that miRNAs may participate in the pathogenesis of PC (9–13).
miR-183 has been reported to have important roles in many types of cancer. Previous studies demonstrated that miR-183 may participate in various cellular and molecular events during the process of tumorigenesis and development (14–16). miR-183 has been demonstrated to be aberrantly expressed in human breast cancer, colorectal cancer and esophageal cancer, and the role of miR-183 either as a tumor-suppressor or an oncomiR has been discussed previously (17–20). A previous study demonstrated that miR-183 was overexpressed in breast cancer, and that it participated in the processes of cancer development through promoting the proliferation and metastasis of cancer cells (17); conversely, in cervical cancer, miR-183 has been reported as a tumor-suppressor that may inhibit the invasion and metastasis of cancer cells (21).
It has been reported that the expression of miR-183 is upregulated in PC (22); however, the biological characteristics and targets of miR-183 are not well understood. In the present study, the role of miR-183 in PC was explored, paying special attention to the effects of miR-183 on cancer cell proliferation, apoptosis, and chemosensitivity to 5-fluorouracil and gemcitabine in the human pancreatic carcinoma cell line PANC-1.
Materials and methods
Cell lines
The human normal pancreatic cell line H6C7 was purchased from GuangZhou Jennio Biological Technology Co., Ltd. (Guangzhou, China), and cultured in keratinocyte serum-free medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS). The human pancreatic carcinoma cell line PANC-1 was purchased from Shanghai Meixuan Biological Technology Ltd. (Shanghai, China), and cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. Cultures were maintained in a humidified atmosphere at 37°C with 5% CO2. H6C7 cells were used to determine the miR-183 expression in normal pancreatic cells and PANC-1 cells were used in all other experiments.
Cell transfection
The miR-183 inhibitor (5′-AGUGAAUUCUACCAGUCCCAUA-3′) and inhibitor control (5′-CAGUACUUUUGUGUAGUACAA-3′) (22) were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). Transfections were performed using Lipofectamine® RNAiMAX transfection reagent (Invitrogen; Thermo Fisher scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. Further analyses were performed following a 48-h incubation at 37°C with 5% CO2. PANC-1 cells were divided into the following three groups: Untransfected (NC), cells transfected with inhibitor control, and cells transfected with miR-183 inhibitor.
Cell proliferation assay
Cell proliferation was determined using an MTT Cell Proliferation and Cytotoxicity Assay kit (Beyotime Institute of Biotechnology, Haimen, China) 48 h following transfection, according to the manufacturer's protocol. DMSO was used to dissolve the purple formazan in cells, which was measured at a wavelength of 490 nm using a SynergyTM 2 Multi-function Microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). Representative images of the MTT assay were captured using an inverted microscope (CKX31; Olympus Corporation, Tokyo, Japan; magnification, ×100).
Cell apoptosis
Cells were stained using an Annexin V/Propidium Iodide (PI) Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ, USA) 48 h following transfection. The cells were seeded into 6-well plates (4×105 cells/well). Following transfection, the cells were collected, washed with PBS, and resuspended in 500 µl HEPES buffer solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Subsequently, 5 µl Annexin V-FITC and 5 µl PI were added to the buffer and incubated at room temperature for 15 min in the dark. The apoptosis rate was analyzed using a FACSVerse™ flow cytometer with the FACSCanto II FACP Array™ software (version 3.0; BD Biosciences).
Cell cycle assay
The cell cycle assay was performed using the Cell Cycle and Apoptosis Analysis kit (Beyotime Institute of Biotechnology). Cells were seeded into 6-well plates (4×105 cells/well). Following transfection, the cells were collected and washed with PBS. Subsequently, 100 µl RNase A solution was added, then the cells were incubated at 37°C for 30 min. Finally, 400 µl PI was added and incubated at room temperature for 30 min. The DNA content was detected with a FACSVerse™ flow cytometer with FACSCanto II; FACP Array™ software (version 3.0; BD Biosciences).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA samples from the cells were isolated using TRIzol® reagent (Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The expression of miR-183 was quantified using a Maxima® SYBR Green/ROX qPCR Master Mix (2X; Thermo Fisher Scientific, Inc.), with U6 as the internal control. The expression levels of phosphoinositide 3-kinase (PI3K), serine/threonine-protein kinase B (Akt), phosphatase and tensin homolog deleted on chromosome ten (PTEN), B cell lymphoma-2 (Bcl-2) and Bcl-2 associated X protein (Bax) were determined by performing RT with a Revert Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) and qPCR analysis using a StepOne™ Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. GAPDH was applied as the internal control. The sequences of the primers used were as follows: miR-183, forward 5′-CGGTATGGCACTGGTAGAATTCACT-3′ and reverse, 5′-GCTTTCCAATGCACTGACCATT-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; PI3K forward, 5′-TATTTGGACTTTGCGACAAGACT-3′ and reverse, 5′-TCGAACGTACTGGTCTGGATAG-3′; Akt forward, 5′-TCCTCCTCAAGAATGATGGCA-3′ and, reverse 5′-GTGCGTTCGATGACAGTGGT-3′; PTEN forward, 5′-TTTGAAGACCATAACCCACCAC-3′ and reverse, 5′-ATTACACCAGTTCGTCCCTTTC-3′; Bcl-2 forward, 5′-GGTGGGGTCATGTGTGTGG-3′ and reverse, 5′-CGGTTCAGGTACTCAGTCATCC-3′; Bax forward, 5′-CCCGAGAGGTCTTTTTCCGAG-3′ and reverse, 5′-CCAGCCCATGATGGTTCTGAT-3′; and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. The thermocycling conditions were as follows: Initial denaturation at 95°C for 2 min; denaturation at 95°C for 30 sec; annealing at 59°C for 45 sec and elongation at 72°C for 60 sec for 30 cycles, followed by elongation at 72°C for 7 min.
Western blot analysis
Total cell protein was extracted using a radioimmunoprecipitation assay lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) according to the manufacturer's protocol. The concentration of protein was determined using a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein (30 µg/lane) were separated using 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were then blocked with 5% non-fat milk at 4°C for 2 h, and incubated overnight at 4°C with primary antibodies (anti-PI3K, 1:1,000, ab151549; anti-Akt, 1:10,000, ab179463; anti-PTEN, 1:10,000, ab32199; anti-Bcl-2 1:1,000, ab32124; anti-Bax, 1:5,000, ab32503; and anti-β-actin, 1:200, ab115777; all purchased from Abcam, Cambridge, MA, USA). The following day, the membranes were washed and incubated at room temperature with the horseradish peroxidase-conjugated secondary antibody (1:1,000; A0208; Beyotime Institute of Biotechnology) for 45 min. Finally, membranes were incubated with BeyoECL Plus (Beyotime Institute of Biotechnology), and detected using a ChemiDoc™ XRS+imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). -actin was applied as the internal control.
Gemcitabine and 5-fluorouracil chemosensitivity assay
PANC-1 cells were treated with various concentrations of gemcitabine (0.01, 0.1, 1, 10 and 100 µM; cat. no. 95058-81-4; Shanghai Xinyu Biotechnology Pharmaceutical Co., Ltd., Shanghai, China) or 5-fluorouracil (0.01, 0.1, 1, 10 and 100 µM; cat. no. 51-21-8; WuHan Dong Kang Yuan Technology Co., Ltd., Wuhan, China) at 37°C with 5% CO2 for 1.5 h, and the percentage of viable cells was determined using an MTT assay, as aforementioned.
In vivo tumor xenograft model
In vivo tumor xenograft assays were conducted using 4–6-week-old male BALB/c nude mice (weight, 15–20 g) purchased from the Animal Center of Nanjing Medical University (Nanjing, China). All animal research protocols were approved by the Institutional Animal Care and Use Committee at Shanghai Pudong Hospital (Pudong, China). The mice were randomly divided into three groups (5 per group) and maintained in a pathogen-free environmentally controlled room (temperature, 22°C±2°C; humidity, 50–65%; 12 h light/dark cycle) with free access to standard animal feed and filtered tap water for 7 days to acclimatize to their new environment. Subsequently, PANC-1 cells (untransfected or transfected with the miR-183 inhibitor or inhibitor control) were subcutaneously injected into separate nude mice (5×106 cells per mouse), who still received free access to food and water during the experiment. All mice were euthanized at 30 days following injection and tumor sizes were measured. Tumor size was calculated using the following equation: Tumor volume=tumor length × tumor width × tumor height/2. Tumor weight was measured using a Sartorius Electronic Balance (Suzhou Science Instrument Co., Ltd., Suzhou, China).
Dual-luciferase reporter assay
To assess potential miR-183-related target genes, the miRanda database (www.microrna.org) was used to predict possible targets. The results revealed that PTEN was a potential target of miR-183. To confirm this prediction, the luciferase activity assay was performed in 96-well plates. The pmirGLO plasmid was obtained from Promega Corporation (Madison WI, USA). PANC-1 cells were co-transfected with PTEN 3′UTR pmirGLO plasmid (containing wild-type or mutant PTEN 3′UTRs; Guangzhou RiboBio Co., Ltd., Guangzhou, China) and a miR-183 inhibitor or inhibitor control (NC) vector using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The 3′UTR-wild type (WT) comprised PANC-1 cells containing the wild-type PTEN 3′UTR pmirGLO plasmid. The 3′UTR-mutant (MUT) group comprised PANC-1 cells containing the mutant PTEN 3′UTR pmirGLO plasmid. As a control, the vector containing Renilla luciferase was co-transfected for normalization. At 48 h following transfection, firefly and Renilla luciferase were assayed using the Dual-Luciferase Reporter System (Promega Corporation) according to the manufacturer's instructions. The relative luciferase activity was reported following the normalization of firefly luminescence to that of Renilla.
If the miR-183 seed sequence was matched to the PTEN 3′UTR, then the miR-183 seed sequence had activated the PTEN 3′UTR to decrease the expression of the luciferase gene. The decreased luciferase reacted with the substrate to produce a weak fluorescence, which was detected by the Dual-Luciferase Reporter System to demonstrate that the miR-183 seed sequence could matched the PTEN 3′UTR.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation. Statistical significance was determined using a Student's t-test between two groups of data sets. Comparisons among multiple groups were evaluated using one-way analysis of variance followed by a post-hoc LSD test. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-183 is upregulated in PANC-1 cells
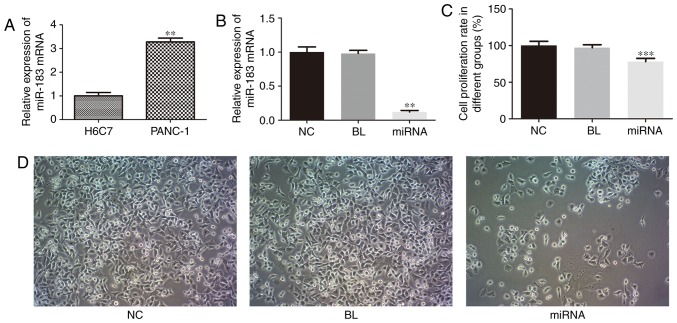
RT-qPCR was performed to examine the expression level of miR-183 in PC cells. As presented in Fig. 1A, the expression of miR-183 in PANC-1 cells was significantly upregulated compared with that in H6C7 cells (P<0.01). These results indicated that miR-183 may be a potential marker associated with PC.
Figure 1.
miR-183 knockdown inhibits cell proliferation in pancreatic cancer cells. (A) Relative miR-183 expression in H6C7 and PANC-1 cells. (B) Relative miR-183 expression in PANC-1 cells following transfection. (C) Cell proliferation rate measured by an MTT assay. (D) Representative images of the MTT assay showing the effect of miR-183 transfection on PANC-1 cell proliferation (magnification, ×100). **P<0.01 vs. NC and ***P<0.001 vs. NC. miR, microRNA; NC, untransfected group; BL, inhibitor control group; miRNA, miR-183 inhibitor group.
miR-183 knockdown inhibits cell proliferation in PANC-1 cells
To investigate the biological functions of miR-183 in PANC-1 cell proliferation, apoptosis and cell cycle, which are associated with tumorigenesis, PANC-1 cells were transfected with a miR-183 inhibitor, and the effect on cell proliferation was evaluated using an MTT assay. As presented in Fig. 1B, miR-183 expression was markedly decreased in the miR-183 inhibitor group compared with untransfected cells (P<0.01). As presented in Fig. 1C, compared with the untransfected cells, knockdown of miR-183 induced a significant decrease in cell proliferation (P<0.001), thus suggesting that miR-183 is a positive regulator of PANC-1 cell proliferation. As presented in Fig. 1D, the number of PANC-1 cells was markedly decreased and the morphology was markedly altered, with the demolishment of membrane-cytoskeleton, when compared with untransfected cells.
Knockdown of miR-183 promotes apoptosis and affects the cell cycle of PANC-1 cells in vitro
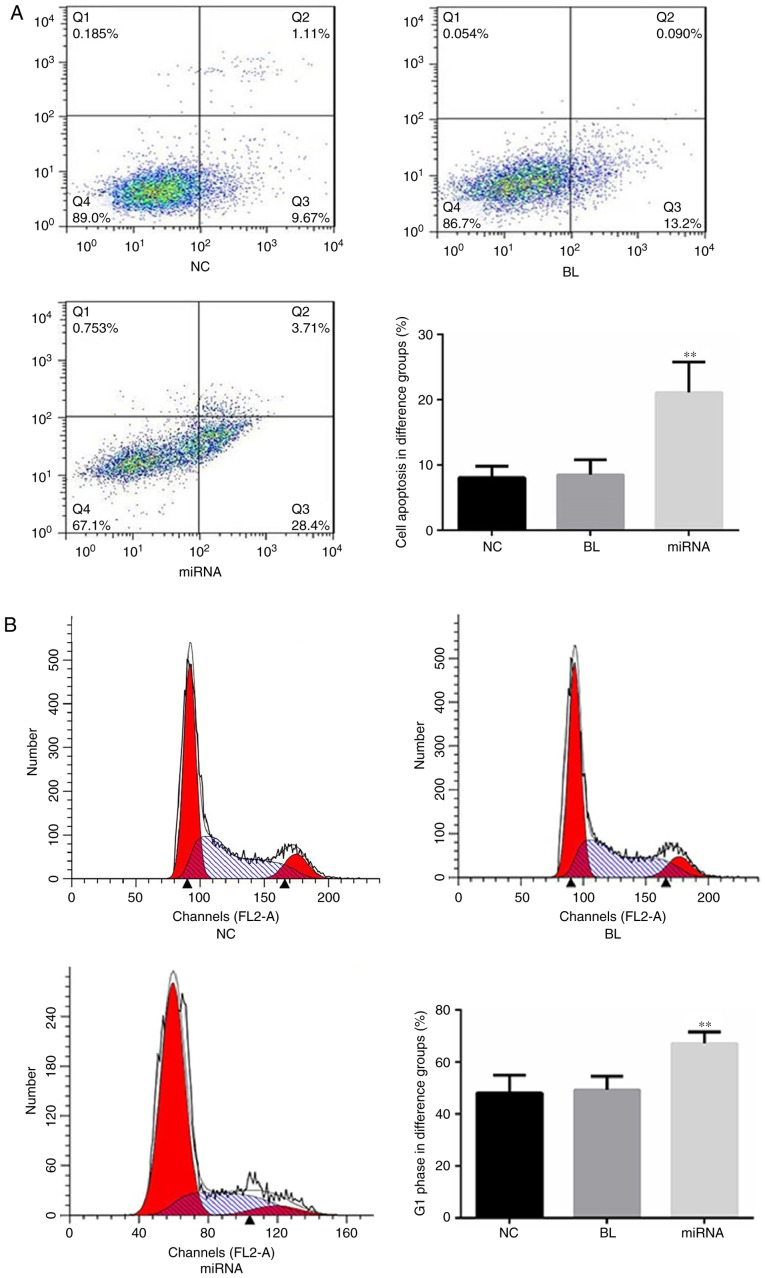
The role of miR-183 in apoptosis and the cell cycle of PANC-1 cells was evaluated using flow cytometry. As presented in Fig. 2A, cells transfected with miR-183 inhibitors demonstrated a significant increase in cell apoptosis (P<0.01) compared with the untransfected group. Furthermore, compared with the untransfected cells, the percentage of cells in the G1 phase was significantly increased in the miR-183 inhibitor-transfected group (P<0.01; Fig. 2B).
Figure 2.
Knockdown of miR-183 promotes apoptosis and arrests the cell cycle of PANC-1 cells in vitro. (A) Effect of miR-183 on cell apoptosis in PANC-1 cells. (B) Effect of miR-183 on cell cycle in PANC-1 cells. **P<0.01 vs. NC. miR, microRNA; NC, untransfected group; BL, inhibitor control group; miRNA, miR-183 inhibitor group.
Knockdown of miR-183 delays tumor growth
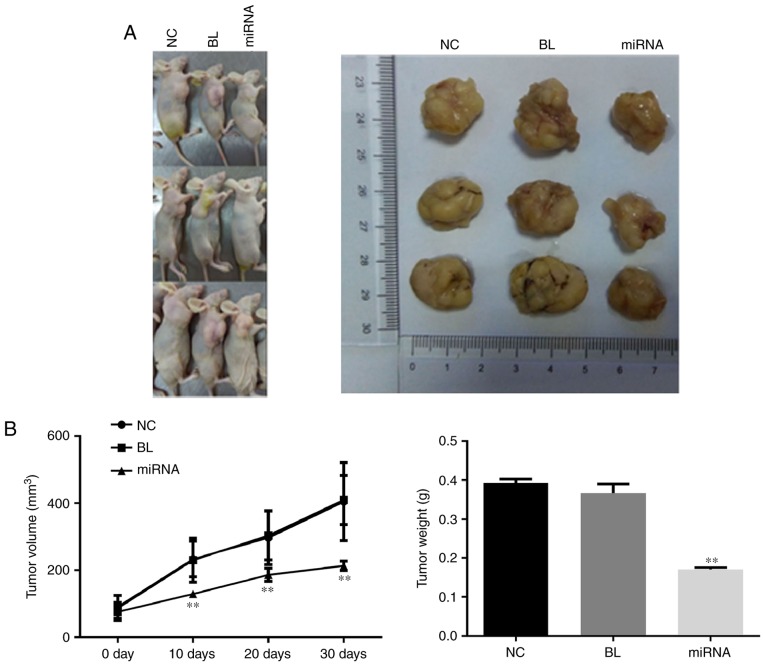
To confirm the tumor growth inhibitory effects of miR-183 downregulation, in vivo tumor xenograft models were generated by implanting PANC-1 cells into nude mice. As presented in Fig. 3A, the tumor size in the miR-183 inhibitor-transfected group was markedly smaller than that in the untransfected group, which was consistent with the results of the cell proliferation assay. The tumor growth curve and results of tumor weight measurement indicated that knockdown of miR-183 significantly delays tumor growth (P<0.01; Fig. 3B).
Figure 3.
Knockdown of miR-183 delays tumor growth. (A) Representative photographs of dissected tumors in nude mice. (B) Tumor growth curve and tumor weights in different groups. **P<0.01 vs. NC. NC, untransfected group; BL, inhibitor control group; miRNA, microRNA-183 inhibitor group.
miR-183 may affect the PTEN/PI3K/Akt signaling pathway in PANC-1 cells
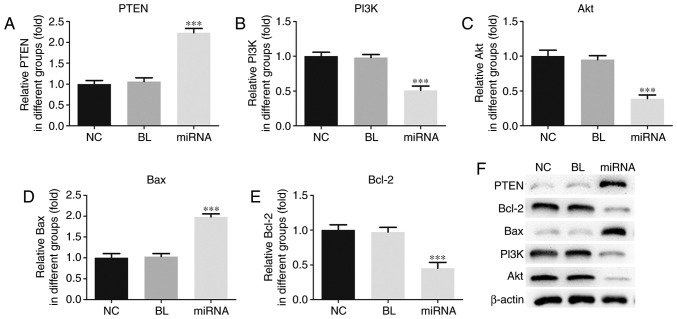
The PI3K pathway is known to regulate metabolism, cell growth and cell survival (23). Therefore, to further explore the underlying mechanisms of the effects of miR-183 on PANC-1 cells, RT-qPCR and western blot analyses were performed to investigate the effect of miR-183 on the PTEN/PI3K/Akt signaling pathway. As presented in Fig. 4, compared with untransfected cells, knockdown of miR-183 induced significant increases in the mRNA expression levels of PTEN and Bax, and significant decreases in the mRNA expression of PI3K, Akt and Bcl-2 in PANC-1 cells (P<0.001) (Fig. 4A-E). Similar marked differences were observed at the protein level (Fig. 4F).
Figure 4.
miR-183 affects the PTEN/PI3K/Akt signaling pathway in PANC-1 cells. mRNA levels of (A) PTEN, (B) PI3K, (C) Akt, (D) Bax and (E) Bcl-2 were assessed by reverse transcription-quantitative polymerase chain reaction. (F) Protein levels were assessed by western blotting. ***P<0.001 vs. NC. miR, microRNA; PTEN, phosphatase and tensin homolog deleted on chromosome ten; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; Bcl-2, B cell lymphoma-2; Bax, Bcl-2 associated X protein; NC, untransfected group; BL, inhibitor control group; miRNA, miR-183 inhibitor group.
PTEN is a direct target of miR-183
As presented in Fig. 5A, the miR-183 seed sequence matched the PTEN 3′-UTR. A dual-luciferase reporter assay was performed to verify this. The results demonstrated that miR-183 significantly inhibited the luciferase activity of the reporter containing the wild-type PTEN 3′-UTR compared with the control group (P<0.05) (Fig. 5B). No significant effect was observed in the cells transfected with the reporter containing the mutant-type 3′-UTR of PTEN. These results indicated that PTEN is a direct target of miR-183 in PANC-1 cells.
Figure 5.
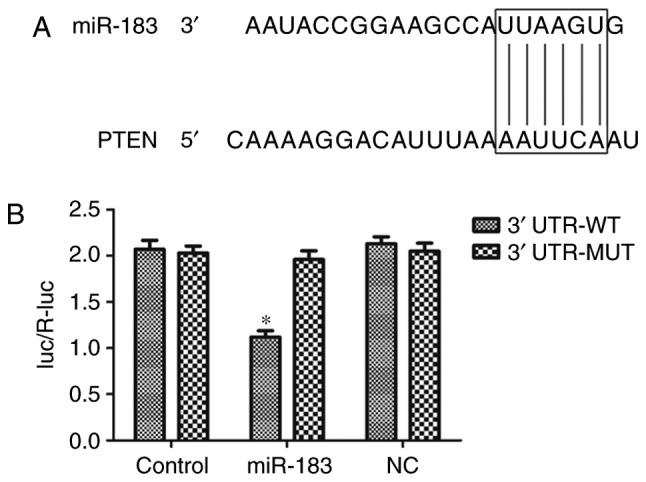
PTEN is a direct target of miR-183. (A) The 3′-UTR of PTEN mRNA was observed to contain a sequence complementary to miR-183, using miRanda. (B) Overexpression of miR-183 significantly suppressed the luciferase activity of reporters containing the WT 3′-UTR of PTEN, but not of the reporters containing the mutant-type MT 3′-UTR of PTEN. *P<0.05 vs. NC. PTEN, phosphatase and tensin homolog deleted on chromosome ten; miR, microRNA; UTR, untranslated region; WT, wild type; MT, mutant type; luc/R-luc, luciferase/Renilla luciferase; NC, inhibitor control transfected group, Control, untransfected group.
Knockdown of miR-183 increases the chemosensitivity PANC-1 cells to 5-fluorouracil and gemcitabine in vitro
Finally, the effect of miR-183 on chemosensitivity to 5-fluorouracil and gemcitabine in PANC-1 cells was examined using an MTT assay. The findings indicated indicated that knockdown of miR-183 significantly increased the chemosensitivity of PANC-1 cells to both 5-fluorouracil and gemcitabine (P<0.05; Fig. 6).
Figure 6.
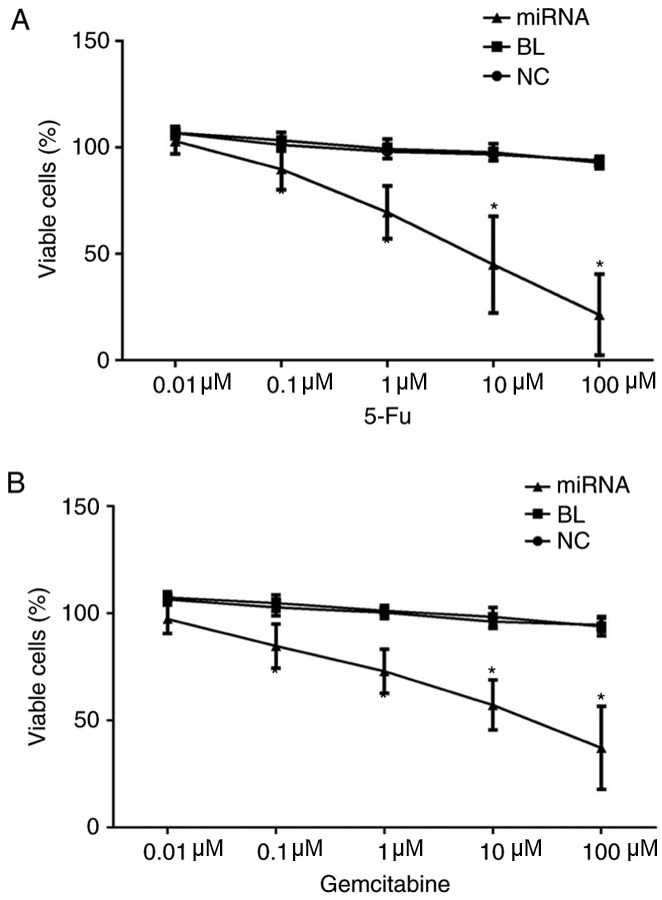
Knockdown of miR-183 increases the chemosensitivity of PANC-1 cells to 5-fluorouracil and gemcitabine in vitro. (A) Effect of 5-fluorouracil treatment on PANC-1 cell proliferation following transfection. (B) Effect of gemcitabine treatment on PANC-1 cell proliferation following transfection. *P<0.05 vs. NC. miR, microRNA; NC, untransfected group; BL, inhibitor control group; miRNA, miR-183 inhibitor group.
Discussion
The roles of miR-183 in PC have been discussed previously. Using a microarray assay, Yu et al (24) demonstrated that miR-183 was upregulated in tissue samples from patients with pancreatic intraepithelial neoplasms, suggesting that miR-183 may serve an important role in the pathogenesis of PC. Zhou et al (25) observed low expression of miR-183 in pancreatic ductal adenocarcinoma (PDAC) tissue samples and cell lines, and the results indicated that miR-183 levels were associated with the severity of the disease, as well as with the poor survival and prognosis of the patients. Furthermore, it was demonstrated that miR-183 could affect the proliferation and apoptosis of PDAC cells through targeting B cell-specific Moloney murine leukemia virus insertion site 1. Lu et al (26) reported that miR-183 could regulate the proliferation, apoptosis and invasion of the PC cell line SW1990 through targeting PDCD4, which indicates that miR-183 has the potential for use as a therapeutic target for PC treatment. In a recent study, Miao et al (27) demonstrated that miR-183-5p could not only promote proliferation, invasion and metastasis, but also inhibited the apoptosis of human pancreatic adenocarcinoma cells by downregulating the expression of suppressor of cytokine signaling 6. In the present study, PANC-1 cells were transfected with miR-183 inhibitors, and it was discovered that silencing miR-183 induced a significant decrease in the proliferation, and a marked increase in the apoptosis of PANC-1 cells; furthermore, transfection with miR-183 inhibitors also resulted in a significant increase in the percentage of cells at the G1 phase. These results are consistent with previous findings, suggesting that miR-183 can regulate the proliferation, apoptosis and cell cycle of PANC-1 cells in vitro.
The role of PTEN as a tumor-suppressor has been well investigated. Previous findings suggested that PTEN is downregulated in pancreatic cancer, colon cancer and hepatocellular carcinoma, and PTEN has been presented to regulate the proliferation, apoptosis, migration and invasion of cancer cells, including PC cells (28–31). PI3K and Akt are downstream kinases of PTEN. In cancer, the downregulation of PTEN leads to activation of the PI3K/Akt signaling pathway, which can partially contribute to the genesis and development of tumors (32–34). Results from previous studies suggested that miRNAs can regulate the proliferation and apoptosis of cancer cells through targeting PTEN/PI3K/Akt signaling (32–34). In the present study, it was initially demonstrated that knockdown of miR-183 induced increases in the expression of PTEN and Bax, and decreases in the expression of PI3K, Akt and Bcl-2 in PANC-1 cells at both the mRNA and protein levels, as compared with untransfected cells. These results indicated that miR-183 can affect the PTEN/PI3K/Akt signaling pathway in PANC-1 cells.
At present, chemotherapeutic agents for treating PC are limited. The most common anti-PC drugs are the DNA-damaging compounds 5-fluorouracil and gemcitabine. However, patients may develop drug resistance over the course of such therapies, leading to unsatisfactory efficacy (35,36). In recent years, the roles of miRNAs in regulating the drug sensitivity of PC cells have been investigated. Ma et al (37) recently reported that miR-223 can regulate the epithelial-mesenchymal transition in gemcitabine-resistant PC cells; Liang et al (38) demonstrated that miR-33a can enhance the gemcitabine sensitivity of human PC cells through suppressing the nuclear translocation of β-catenin; Wei et al (39) reported that overexpression of miR-21 in human PC cells may increase 5-fluorouracil resistance by inhibiting the expression of PTEN and PDCD4; Wang et al (40) discovered that miR-320a can increase chemoresistance by targeting PDCD4 in human PC cells. In the present study, an MTT assay was performed to investigate the role of miR-183 in the chemosensitivity to 5-fluorouracil and gemcitabine of human PC cells. The present findings indicated that silencing miR-183 could increase chemosensitivity to both 5-fluorouracil and gemcitabine in PANC-1 cells.
In conclusion, the results of the present study demonstrated that inhibition of miR-183 decreased proliferation and promoted apoptosis in PANC-1 cells, as well as enhanced the chemosensitivity of the cells to 5-fluorouracil and gemcitabine, via regulating the PTEN/PI3K/Akt signaling pathway. These findings suggest that the miR-183 has potential to become a therapeutic target for the treatment of PC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Academic Leaders Training Program of Pudong Health Bureau of Shanghai (grant no. PWRd2016-04), Zhejiang Natural Science Foundation (grant no. LY16H160045) and the Hangzhou Science and Technology Development Project (grant no. 20150733Q16).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XY and BY conceptualized and designed the current study, and XY performed the majority of the experiments. XY, WW and XZ constructed the in vivo tumor xenograft model and performed the follow-up anatomical experiment. QZ and LC cultured the cells and performed cell transfections. XY, QZ and LC acquired and interpreted the data and wrote the manuscript. XY and BY made comments, suggested appropriate modifications and made corrections. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal research protocols were approved by the Institutional Animal Care and Use Committee at Shanghai Pudong Hospital (Pudong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Basha R, Connelly SF, Sankpal UT, Nagaraju GP, Patel H, Vishwanatha JK, Shelake S, Tabor-Simecka L, Shoji M, Simecka JW, El-Rayes B. Small molecule tolfenamic acid and dietary spice curcumin treatment enhances antiproliferative effect in pancreatic cancer cells via suppressing Sp1, disrupting NF-κB translocation to nucleus and cell cycle phase distribution. J Nutr Biochem. 2016;31:77–87. doi: 10.1016/j.jnutbio.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louvet C, Philip PA. Accomplishments in 2007 in the treatment of metastatic pancreatic cancer. Gastrointest Cancer Res. 2008;2:S37–S41. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee C, He H, Jiang Y, Di Y, Yang F, Li J, Jin C, Fu D. Elevated expression of tumor miR-222 in pancreatic cancer is associated with Ki67 and poor prognosis. Med Oncol. 2013;30:700. doi: 10.1007/s12032-013-0700-y. [DOI] [PubMed] [Google Scholar]
- 4.Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–362. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Zhao S, Jiang X, Zhang E, Hu G, Hu B, Zheng P, Xiao J, Lu Z, Lu Y, et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016;371:314–325. doi: 10.1016/j.canlet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Li HL, Li JL, Shi BL, Chen F. MicroRNA-296 targets AKT2 in pancreatic cancer and functions as a potential tumor suppressor. Mol Med Rep. 2017;16:466–472. doi: 10.3892/mmr.2017.6602. [DOI] [PubMed] [Google Scholar]
- 7.Zhu JH, Wu J, Pei XC, Tan ZJ, Shi JQ, Lubman DM. Annexin A10 is a candidate marker associated with the progression of pancreatic precursor lesions to adenocarcinoma. PLoS One. 2017;12:e0175039. doi: 10.1371/journal.pone.0175039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berindan-Neagoe I, Calin GA. Molecular pathways: microRNAs, cancer cells, and microenvironment. Clin Cancer Res. 2014;20:6247–6253. doi: 10.1158/1078-0432.CCR-13-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WY, Liu WJ, Zhao YP, Zhou L, Zhang TP, Chen G, Shu H. Induction, modulation and potential targets of miR-210 in pancreatic cancer cells. Hepatobiliary Pancreat Dis Int. 2012;11:319–324. doi: 10.1016/S1499-3872(12)60168-4. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Ou Y, Wu K, Chen Y, Sun W. miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour Biol. 2012;33:1863–1870. doi: 10.1007/s13277-012-0446-8. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T, Hirajima S, et al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108:361–369. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Y, Dang X, Li W, Ma Q. miR-133a functions as a tumor suppressor and directly targets FSCN1 in pancreatic cancer. Oncol Res. 2013;21:353–363. doi: 10.3727/096504014X14024160459122. [DOI] [PubMed] [Google Scholar]
- 13.Zhao G, Zhang JG, Liu Y, Qin Q, Wang B, Tian K, Liu L, Li X, Niu Y, Deng SC, Wang CY. miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKα1. Mol Cancer Ther. 2013;12:83–93. doi: 10.1158/1535-7163.MCT-12-0534-T. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Li SG, Chen JY, Xiao M. MiR-183 maintains canonical Wnt signaling activity and regulates growth and apoptosis in bladder cancer via targeting AXIN2. Eur Rev Med Pharmacol Sci. 2018;22:4828–4836. doi: 10.26355/eurrev_201808_15618. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Xu Y, Zhang S, Li X, Wang Y, Zhang Y, Zhao X, Li Y, Wang Y. MicroRNA-183 suppresses the vitality, invasion and migration of human osteosarcoma cells by targeting metastasis-associated protein 1. Exp Ther Med. 2018;15:5058–5064. doi: 10.3892/etm.2018.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XL, Xu G, Zhou Y, YAN JJ. MicroRNA-183 promotes the proliferation and metastasis of renal cell carcinoma through targeting Dickkopf-related protein 3. Oncol Lett. 2018;15:6003–6008. doi: 10.3892/ol.2018.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Xiang G, Meng Y, Dong R. MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod Biol. 2016;16:225–233. doi: 10.1016/j.repbio.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Huangfu L, Liang H, Wang G, Su X, Li L, Du Z, Hu M, Dong Y, Bai X, Liu T, et al. miR-183 regulates autophagy and apoptosis in colorectal cancer through targeting of UVRAG. Oncotarget. 2016;7:4735–4745. doi: 10.18632/oncotarget.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M, Liu R, Li X, Liao J, Pu Y, Pan E, Yin L, Wang Y. miRNA-183 suppresses apoptosis and promotes proliferation in esophageal cancer by targeting PDCD4. Mol Cells. 2014;37:873–880. doi: 10.14348/molcells.2014.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Quan H, Wang S, Li X, Che X. MiR-183 promotes growth of non-small cell lung cancer cells through FoxO1 inhibition. Tumour Biol. 2015;36:8121–8126. doi: 10.1007/s13277-015-3550-8. [DOI] [PubMed] [Google Scholar]
- 21.Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X, Jin X, Tian X. MicroRNA-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol Oncol. 2016;141:166–174. doi: 10.1016/j.ygyno.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Hanci V, Yurdakan G, Yurtlu S, Turan IÖ, Sipahi EY. Protective effect of dexmedetomidine in a rat model of α-naphthylthiourea-induced acute lung injury. J Surg Res. 2012;178:424–430. doi: 10.1016/j.jss.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Han B, Jiang P, Li ZX, Yu Y, Huang T, Ye X, Li X. Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Akt and mitochondrial-associated apoptotic pathway. Phytomedicine. 2018;48:152–160. doi: 10.1016/j.phymed.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18:981–992. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Zhang WG, Wang DS, Tao KS, Song WJ, Dou KF. MicroRNA-183 is involved in cell proliferation, survival and poor prognosis in pancreatic ductal adenocarcinoma by regulating Bmi-1. Oncol Rep. 2014;32:1734–1740. doi: 10.3892/or.2014.3374. [DOI] [PubMed] [Google Scholar]
- 26.Lu YY, Zheng JY, Liu J, Huang CL, Zhang W, Zeng Y. miR-183 induces cell proliferation, migration, and invasion by regulating PDCD4 expression in the SW1990 pancreatic cancer cell line. Biomed Pharmacother. 2015;70:151–157. doi: 10.1016/j.biopha.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Miao F, Zhu J, Chen Y, Tang N, Wang X, Li X. MicroRNA-183-5p promotes the proliferation, invasion and metastasis of human pancreatic adenocarcinoma cells. Oncol Lett. 2016;11:134–140. doi: 10.3892/ol.2015.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni S, Wang H, Zhu X, Wan C, Xu J, Lu C, Xiao L, He J, Jiang C, Wang W, He Z. CBX7 suppresses cell proliferation, migration, and invasion through the inhibition of PTEN/Akt signaling in pancreatic cancer. Oncotarget. 2017;8:8010–8021. doi: 10.18632/oncotarget.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi X, Gu HT, Lin SB, Zhang Y, Yang J, Qian CJ. Abnormal expression of PTEN and PIK3CA in pemetrexed-resistant human pancreatic cancer cell line Patu8988. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15036991. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Yu ZR, Ren SY, Song JK, Wang JH, Du GH. Metabolic reprogramming in colon cancer reversed by DHTS through regulating PTEN/AKT/HIF1α mediated signal pathway. Biochim Biophys Acta Gen Subj. 2018;1862:2281–2292. doi: 10.1016/j.bbagen.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Long H, Wu Y, Li H, Dong L, Zhong JL, Liu Z, Yang X, Dai X, Shi L, et al. HRD1-mediated PTEN degradation promotes cell proliferation and hepatocellular carcinoma progression. Cell Signal. 2018;50:90–99. doi: 10.1016/j.cellsig.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Lim HJ, Wang X, Crowe P, Goldstein D, Yang JL. Targeting the PI3K/PTEN/AKT/mTOR pathway in treatment of sarcoma cell lines. Anticancer Res. 2016;36:5765–5771. doi: 10.21873/anticanres.11160. [DOI] [PubMed] [Google Scholar]
- 33.Lu XX, Cao LY, Chen X, Xiao J, Zou Y, Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016:2476842. doi: 10.1155/2016/2476842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang LL, Hao S, Zhang S, Guo LJ, Hu CY, Zhang G, Gao B, Zhao JJ, Jiang Y, Tian WG, et al. PTEN/PI3K/AKT protein expression is related to clinicopathologic features and prognosis in breast cancer with axillary lymph node metastases. Hum Pathol. 2017;61:49–57. doi: 10.1016/j.humpath.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 35.Mynhardt C, Damelin LH, Jivan R, Peres J, Prince S, Veale RB, Mavri-Damelin D. Metformin-induced alterations in nucleotide metabolism cause 5-fluorouracil resistance but gemcitabine susceptibility in oesophageal squamous cell carcinoma. J Cell Biochem. 2018;119:1193–1203. doi: 10.1002/jcb.26291. [DOI] [PubMed] [Google Scholar]
- 36.Amponsah PS, Fan P, Bauer N, Zhao Z, Gladkich J, Fellenberg J, Herr I. microRNA-210 overexpression inhibits tumor growth and potentially reverses gemcitabine resistance in pancreatic cancer. Cancer Lett. 2017;388:107–117. doi: 10.1016/j.canlet.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, Wang Z. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740–1749. doi: 10.18632/oncotarget.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang C, Wang Z, Li YY, Yu BH, Zhang F, Li HY. miR-33a suppresses the nuclear translocation of beta-catenin to enhance gemcitabine sensitivity in human pancreatic cancer cells. Tumour Biol. 2015;36:9395–9403. doi: 10.1007/s13277-015-3679-5. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, Wang W, Wang L, Zhang Y, Zhang X, Chen M, Wang F, Yu J, Ma Y, Sun G. MicroRNA-21 induces 5-fluorouracil resistance in human pancreatic cancer cells by regulating PTEN and PDCD4. Cancer Med. 2016;5:693–702. doi: 10.1002/cam4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Zhao L, Wei X, Wang L, Liu S, Yang Y, Wang F, Sun G, Zhang J, Ma Y, et al. MicroRNA-320a promotes 5-FU resistance in human pancreatic cancer cells. Sci Rep. 2016;6:27641. doi: 10.1038/srep27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.