Abstract
Introduction
Exposure to zoonotic factors in veterinary practice is closely related to the nature of the work. The main aim of the study was to determine the risk of selected zoonotic infections among the occupational group of veterinarians in Poland.
Material and Methods
Blood samples of 373 veterinarians (162 males and 211 females) from 12 provinces of Poland were collected by the venipuncture of a forearm for serological tests. Commercial immunoenzymatic tests (ELISA) were used for detection of specific IgG antibodies to Echinococcus granulosus, IgM and IgG to Leptospira spp., and IgM, IgA, and I and II phase IgG to Coxiella burnetii. Enzyme-linked fluorescence assays (ELFA) were used to detect IgM and IgG antibodies to Toxoplasma gondii.
Results
Positive results were found in 209 (56.0%) veterinarians for at least one of the examined diseases. The overall proportion of participants found to have specific Toxoplasma gondii antibodies in the IgM and/or IgG assays amounted to 44.5%. The presence of Coxiella burnetii antibodies was found in 16 (4.3%) subjects, while Leptospira spp. antibodies were detected in 63 (16.9%) veterinarians. Among the 373 veterinarians examined, no Echinococcus granulosus antibodies were found.
Conclusion
Results of the study seem to indicate a slightly elevated risk of Toxoplasma gondii infection and a moderate risk of infection with Leptospira spp. and Coxiella burnetii in veterinarians.
Keywords: veterinarians, Toxoplasma gondii, Leptospira spp, Coxiella burnetii, Echinococcus granulosus
Introduction
The risk of exposure to zoonotic factors in veterinary practice is closely related to the nature of the work. Infectious diseases occurring among veterinarians are underreported, hence practicing veterinarians should effectively protect their own health as well as that of staff. Many aetiological factors of infectious diseases are so far still poorly known, both concerning their occurrence in the environment and the transmission paths of diseases which jeopardise health.
Toxoplasma gondii (Nicolle and Manceaux, 1908) counts among the parasitic protozoa widespread in the environment and is the aetiological factor of toxoplasmosis. The course of toxoplasmosis in healthy people is usually subclinical, manifesting only with the presence of specific antibodies. T. gondii infection can be particularly dangerous in the case of primary invasion in pregnant women, due to the risk of invading the foetus. It can also have serious consequences for people with weakened immunity. Toxoplasmosis can also manifest in the form of inflammation of the eyes or neurological disorders, diagnosed even many years after the time of infection (5). Human infection is most often caused by ingestion of raw meat containing cysts of the parasite or consumption of food or water contaminated with oocysts (5, 26). An invasion may also result from direct contact with the faeces of an infected cat. The frequency of human infection in Poland based on serological studies is estimated at 40%–60%. Results of studies on the animal reservoir in Poland show a significant level of infection (pigs 10%– 15%, cattle up to 20%, and cats up to 75%) and indicate the risk of invasion for consumers of raw meat and for people who have direct contact with infected animals such as veterinarians (2, 23, 26).
The aetiological factor of Q fever is the Gram-negative Coxiella burnetii. The main bacterial reservoirs are domestic animals (sheep, goats, and cattle) usually latently infected (18). Ticks are an important factor in the circulation of C. burnetii in nature, but human Coxiella infections are rarely caused by tick bites. The most common sources of infection are animals, their secretions, meat, skin products, and definitely less often the dust-like faeces of an infected tick which can get into the body through the nose, mouth, or eyes. C. burnetii usually infects humans by inhalation or by the intestinal route (as a result of infected milk consumption). Infected animals can excrete the bacteria with urine, faeces, and milk, and the pathogen may be present in placenta, amniotic fluid, and aborted foetuses (19) The first symptoms of infection are high fever, chills, weakness, and muscle and head pain. In the later phase of the disease, pneumonia, liver damage, or endocarditis may occur. The people at the highest risk level are farmers of dairy animals and veterinarians (9).
Leptospirosis is a worldwide zoonotic disease caused by various species of Leptospira. The reservoir of Leptospira spp. is mainly dogs, cats, pigs, cattle, horses, and wild animals (31). These bacteria penetrate the body through damaged skin, conjunctiva, and mucous membranes (34). People can also be infected by the consumption of contaminated water or inhalation of an aerosol containing Leptospira (4). Leptospirosis may have different courses, from an asymptomatic form, through a mild influenza-like manifestation, to a serious multisystemic disease. In the severest cases multi-organ failure and death may occur (7).
Echinococcosis is a zoonotic parasitic disease that is caused by eggs of a tapeworm from the genus Echinococcus. Definitive hosts which have mature tapeworms are the most common species of canids: foxes, wolves, dogs, and jackals (35). Man can become an intermediate host due to infection with eggs released to the environment by infected animals. The invasion occurs after eating food or drinking contaminated water (12). Symptoms of echinococcosis in intermediate hosts may be different, depending on the affected organ and size and number of blisters located in it (28). Blisters can cause pressure on blood vessels and bile ducts, induce jaundice, congestive cholangitis, and also cause coughing, shortness of breath, haemorrhage, chest pain, and even death (35). The clinical course of echinococcosis involving the liver and lungs, is usually chronic (from ingestion of eggs to the onset of the first clinical symptoms may take from several to a dozen or more years), while the location of the parasite in the brain or eyeball manifests early and usually inflicts diseases with a severe course. There are two species of this genus: single-chambered (Echinococcus granulosus) and multi-chambered (Echinococcus multilocularis). The last one is usually harder to treat due to lack of a definite border between host and parasite tissues.
Many zoonoses (such as Q fever and leptospirosis) are regarded as neglected diseases which are properly diagnosed in Poland only in a small number of cases. However, they may be an important cause of occupational diseases, occurring mostly in farmers, veterinarians, butchers, and dairy workers. The main aim of the study was to determine the risk of selected zoonosis infections among occupational group of veterinarians in Poland.
Material and Methods
A total of 373 veterinarians working in district veterinary inspectorates and private veterinary clinics located in 12 provinces of Poland (Fig. 1) were examined from June to December 2017. The examined veterinarians comprised 162 males (43.4%) and 211 females (56.6%), and their age ranged from ≤30 to ≥61 years. Blood samples of the veterinarians were collected by the puncture of a forearm vein for later serological tests. Each of the persons signed an informed consent form, and the research was approved by the Bioethical Commission of the Institute of Rural Health (Permit No. 9/2017).
Fig. 1.
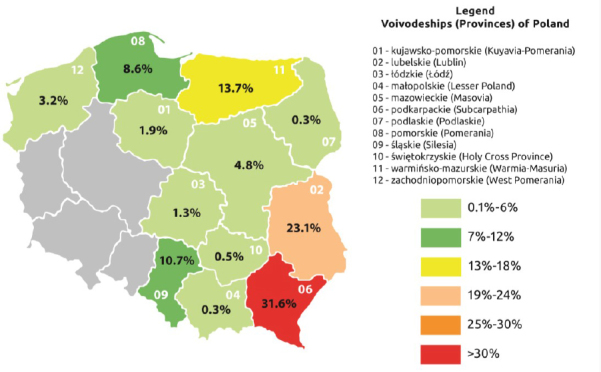
Percentages of veterinarians from particular provinces participating in the study
Commercial immunoenzymatic tests (ELISAs from Bordier Affinity Products SA, Switzerland) were used for testing serum samples for the presence of specific IgG antibodies to Echinococcus granulosus. The result was positive when the absorbance of the analysed sample was higher than the absorbance of weak positive serum. For II phase IgM, I phase IgG, and IgA to Coxiella burnetii assays (Institut Virion/Serion, Germany), the results were determined compared to absorbance of cut-off serum. Results were considered borderline when they fell in the range of 90%–110% of cut-off serum absorbance. In the IgM and IgG to Leptospira spp. and II phase IgG to Coxiella burnetii assays (Institut Virion/Serion, Germany), the mathematical curve fitting antibody quantification based on the 4-parameter logistic function was plotted with results expressed in U/mL. Borderline results of IgM antibodies to Leptospira ranged from 15 to 20 U/mL, and to C. burnetii they ranged from 10 to 15 U/mL for IgG and from 20 to 30 U/mL for II phase IgG. Enzyme-linked fluorescence assays (ELFA) (Vidas Toxo IgG and Vidas Toxo IgM, bioMérieux, France) were used to detect IgM and IgG Toxoplasma gondii antibodies. A Mini Vidas immunoanalyser device facilitated the tests (bioMérieux). An IgM index value <0.55 was considered a negative, ≥0.55 and <0.65 a borderline, and ≥0.65 a positive result. IgG class was given in IU/mL units where a result below 4 was considered a negative, ≥4 and <8 a borderline and ≥8 a positive result.
The performance of all tests and interpretation of results were carried out according to the manufacturer’s instructions.
The obtained qualitative data was subjected to statistical analysis with chi-squared (χ2) and Spearman’s tests using the Statistica 8.0 package (Statsoft Inc., USA).
Results
Results of serological tests for the detection of Toxoplasma gondii antibodies. The overall proportion of participants found to have specific antibodies in IgM and/or IgG anti-Toxoplasma gondii amounted to 44.5%. A statistically significant increase in the proportion of seropositive individuals was observed with age increase (from 25.42% in the age group below 30 to 78.85% in the group of people over 61) (Spearman’s rank correlation test =0.39; P < 0.001). Considering only positive results, they were most commonly found in people aged 51–60, in both the IgM (7.25%) and IgG (68.12%) classes. Significantly more frequent positive results occurred in the group of men (55.21%) than in the group of women (36.19%) (χ2 = 13.4; P < 0.001). All persons who were positive in the IgM class (eight participants) also had positive results in the IgG class (Table 1).
Table 1.
Seropositive results for toxoplasmosis, Q fever, and leptospirosis among veterinarians from Poland
| Parameter | a No. (%) | Toxoplasmosis No.b/(%) | Q fever No.b/(%) | Leptospirosis No.b/(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | Total | IgM phase II | IgA phase I | IgG phase II | IgG phase I | Total | IgM | IgG | Total | ||
| Age (years) | ||||||||||||
| 59 | 0 | 15 | 15** | 1 | 0 | 2 | 0 | 2 | 7 | 4 | 9 | |
| ≤ 30 | (15.82) | (0.00) | (25.42) | (25.42) | (1.69) | (0.0) | (3.39) | (0.0) | (3.39) | (11.86) | (6.78) | (15.25) |
| 125 | 0 | 35 | 35** | 2 | 0 | 2 | 1 | 3 | 17 | 11 | 27 | |
| 31–40 | (33.51) | (0.0) | (28.0) | (28.0) | (1.6) | (0.0) | (1.6) | (0.8) | (2.4) | (13.6) | (880) | (21.6) |
| 68 | 1 | 28 | 28** | 1 | 0 | 1 | 0 | 2 | 6 | 3 | 9 | |
| 41–50 | (18.23) | (1.47) | (41.18) | (41.18) | (1.47) | (0.0) | (1.47) | (0.0) | (2.94) | (8.82) | (4.41) | (13.24) |
| 69 | 5 | 47 | 47** | 0 | 5 | 4 | 3 | 6 | 5 | 9 | 14 | |
| 51–60 | (18.5) | (7.25) | (68.12) | (68.12) | (0.0) | (7.25) | (5.8) | (4.35) | (8.7) | (7.25) | (13.04) | (20.29) |
| 52 | 2 | 41 | 41** | 0 | 1 | 3 | 1 | 3 | 1 | 3 | 4 | |
| ≥ 61 | (13.94) | (3.85) | (78.85) | (78.85) | (0.0) | (1.92) | (5.77) | (1.92) | (5.77) | (1.92) | (5.77) | (7.69) |
| Gender | ||||||||||||
| 210 | 3 | 76 | 76** | 4 | 1 | 3 | 1 | 6 | 31 | 15 | 43* | |
| Women | (56.6) | (1.43) | (36.19) | (36.19) | (1.9) | (0.48) | (1.43) | (0.48) | (2.86) | (14.76) | (7.14) | (20.48) |
| 163 | 5 | 90 | 90** | 0 | 5 | 9 | 4 | 10 | 5 | 15 | 20* | |
| Men | (43.4) 373 | (3.07) 8 | (55.21) 166 | (55.21) 166 | (0.0) 4 | (3.07) 6 | (5.52) 12 | (2.45) 5 | (6.13) 16 | (3.07) 36 | (9.2) 30 | (12.27) 63 |
| Total | (100.0) | (2.14) | (44.5) | (44.5) | (1.07) | (1.61) | (3.22) | (1.34) | (4.29) | (9.65) | (8.04) | (16.89) |
P < 0.05; **P < 0.001; No.a – number of examined veterinarians; No.b – number of positive results.
Spearman’s rank correlation test (seroprevalence of T. gondii vs age), chi-squared test (seroprevalence of T. gondii vs gender, seroprevalence of Leptospira spp. vs gender)
Results of serological tests for the detection of Coxiella burnetii antibodies. The presence of specific Coxiella burnetii antibodies was found in 16 (4.29%) subjects. Most people with positive results were in the 51–60-year-old age group (8.7%). The highest percentage of affected veterinarians was diagnosed with II phase IgG (3.22%). In turn, the lowest percentage was people with positive results in the IgM class (1.07%), which may indicate a recent infection. C. burnetii antibodies were more frequently detected in the group of men than women (6.13% and 2.86%, respectively), but it was not statistically significant (P = 0.13). Similarly, there was no statistically significant relationship between occurrence of seropositive results and age (Table 1).
Results of serological tests for the detection of Leptospira spp. antibodies. The presence of specific Leptospira spp. antibodies was detected in 63 (16.89%) practitioners. Antibodies in the IgM class were found in 9.65% of veterinary workers, while positive results in the IgG class were detected in 8.04% of participants. The highest percentages of seropositive persons were found in the 31–40 (21.6%) and 51–60 age groups (20.29%). The lowest percentages of positive results were found in people over 61 years old (7.69%). There were no statistically significant correlations between the age of respondents and the incidence of positive results. Positive results were found more frequently in women than in men (20.48% and 12.27%, respectively) (χ2 = 4.4; P < 0.05) (Table 1).
Results of serological tests for the detection of Echinococcus granulosus antibodies. Among 373 examined veterinarians, no Echinococcus granulosus antibodies were found. However, ELISA results (OD value) in the case of 6 (1.6%) people were close to the border value for a positive result (cut-off). It was recommended that the test should be repeated approximately four weeks after the first blood sample was collected.
Among the investigated veterinarians, in 209 (56.0%) cases positive results were found for at least one of the examined diseases. Two samples (0.5%) had antibodies to Toxoplasma gondii, Leptospira spp., and Coxiella burnetii. In 24 (6.4%) participants the presence of T. gondii and Leptospira spp. antibodies was confirmed and in 8 (2.1%) cases the copresence of T. gondii and C. burnetii antibodies was found. Only in one case the co-occurrence of C. burnetii and Leptospira spp. antibodies (1.6%) was detected and the result was statistically significant (χ2 = 3.6; P < 0.05) (Fig. 2).
Fig. 2.
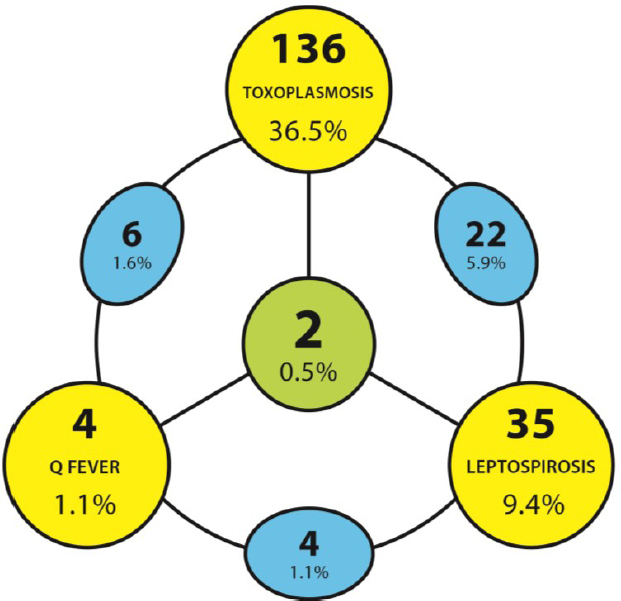
Co-infection positive results in studied veterinarians
Discussion
In Poland, seroprevalence of toxoplasmosis in people lies in the 40%–60% range, depending on the examined group (17). The present study showed an overall 44.5% seroprevalence of T. gondii infection among veterinarians, and this infection rate does not appear to be very high against the known range for the general population. To date, numerous studies have suggested preventive strategies against toxoplasmosis for people having close contact with animals, in mitigation of the unavoidable high risk such workers are. Unfortunately, data reported on toxoplasmosis among this group of employees are limited. Previous studies suggested that veterinary personnel have a higher risk of T. gondii infection since they frequently interact with cats (25). However, other authors reported no significant difference in the prevalence of T. gondii antibodies counterposing veterinary school students (5.6%) with undergraduate students of non-veterinary disciplines (2.4%) (21). Another study also observed no statistically significant difference in the infection rate between the veterinary laboratory science student group and the control group (22), and our results seem to correspond to this trend. In the present study, age was the only risk factor to have a significant impact on Toxoplasma seropositive status in tested persons, and those who were older were more likely to have T. gondii antibodies. Age was also considered a significant factor for seropositivity of vets in a study by Brandon-Mong et al. (2). Due to the high percentage of Toxoplasma gondii antibodies determined in the present study in veterinarians (about 45%), where this percentage reaches 25% in the group of people below 30 years of age, veterinary service workers should be periodically subject to precautionary serological tests for toxoplasmosis. In particular, prophylactic examinations should cover women of childbearing age due to the risk of congenital toxoplasmosis.
On the basis of the conducted research, it can be stated that the awareness of veterinarians of the risks associated with some of the zoonoses is quite high, which is evidenced by negative results in echinococcosis test. According to the annual bulletin “Infectious diseases and poisonings in Poland”, echinococcosis is recorded in Poland as one entity, and several dozen cases are registered annually (64 cases were reported in 2016 and 75 in 2017) (16). In Estonia, Echinococcus spp. seroprevalence in the general population was 3.3% and 0.5% of the cases were confirmed by Western blot test. Among veterinarians the prevalence of positive results was 2.0% and among animal caretakers it was 1.1% (11). In Turkey, sera from two out of 93 veterinary surgeons (2.15%) were found to be positive for Echinococcus IgG antibody (10). The negative results of diagnostic tests for Echinococcus granulosus obtained in the present study could be the effect of the awareness of veterinarians or should be correlated with environmental study results regarding the occurrence of the above-mentioned pathogen.
Higher seroprevalence of leptospirosis and Q fever has been determined in veterinarians where the percentages of seropositive individuals were 16.89% and 4.29%, respectively. In one case, the entire family of a seropositive veterinarian was examined, and one child aged 10 was found to have Coxiella burnetii antibodies, and the infection was most likely caused by ingestion (consumption of sheep’s milk cheeses). In Poland, the last epidemics occurred in 1983 in the Lublin region, and according to the annual report of the National Institute of Public Health – National Institute of Hygiene, only a single case of Q fever has been recognised since 2010. In the study of Fiecek et al. (8) on Coxiella burnetii infection, 4.4% seropositivity was found among cattle breeders and veterinarians. Q fever cases in Poland are statutorily recorded, and the zero figures for 2015, 2016, and 2017 indicate that there were no incidents of this disease in the country. The seroprevalence in veterinarians in this study (4.3%) is lower than in farm workers in Poland, reported between 15.23% and 39.07% by Szymańska-Czerwińska et al. (27). Results presented in this study are also much lower than obtained by Monno et al. (15) in Italy, concerning subjects exposed to farm animals (73.4%) and a control group (13.6%). Lower results can be explained by the risk factor of Q fever associated with C. burnetii infection rising with the number of hours of animal contact per week (30). Veterinarians examined in this study probably had less contact with cattle, sheep, and goats than did farmers. The prevalence of antibodies to C. burnetii among Dutch livestock veterinarians reached 65.1% in 2012, which could be explained by the Q fever epidemics in the Netherlands between 2007 and 2010 (30). A higher risk among veterinarians, people associated with veterinary medicine, and veterinary students has been also demonstrated in other countries and is shown in seroprevalences: 22.2% in the United States (33), 13.5% in Japan (1), 7% in Turkey (6), 26.3% in Taiwan (3), and 11.02% in Spain (29). The low seroprevalence among veterinarians from Poland in relation to other countries could be also explained by the use of animal vaccination since 2013.
The seroprevalence of Leptospira spp. in veterinarians from Poland (16.89%) is much higher than the 2.5% rate of leptospiral infection detected in veterinarians in the United States (32) or the 5.1% in New Zealand (24). A low frequency of leptospirosis was reported among veterinarians with a large animal practice (0.6%) in Argentina (14). However, in Mexico seropositivity for Leptospira spp. among swine-specialist veterinarians was 38.8% (20). No extensive epidemics of leptospirosis have occurred in Poland since the 1970s, and only a few cases of infection have been noted in the country. The prevalence of seropositive farmers noted in the study of Wasiński et al. (31) with field work conducted after a flood was low (3%), and insignificantly small compared to farmers from areas not affected by the flood (9.2%). Such high prevalence of Leptospira antibodies detected among Polish veterinarians is therefore unexpected. To minimise the risk of human leptospirosis, vaccination of farm livestock could be considered, as is New Zealand practice with a cattle vaccine (13).
Due to detection of anti Leptospira spp. and Coxiella burnetii antibodies at the levels of 17% and 4%, respectively, there is a need to implement educational and prophylactic activities to counter threats from these pathogenes. It seems particularly important to broaden the knowledge of veterinarians regarding potential sources of infection both in the veterinarian's work environment and outside it.
In conclusion, our results seem to indicate a slightly elevated risk of Toxoplasma gondii infection in veterinarians, although this hazard remaining close to the level of risk for the general population. The results also indicate a moderate risk of infection with Leptospira spp. and Coxiella burnetii, while low or no risk seems to occur with regard to Echinococcus granulosus.
Footnotes
Human Rights Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards approved by the Bioethical Commission of the Institute of Rural Health (Permit No. 9/2017).
Conflict of Interest Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was supported by the Ministry of Science and Higher Education/National Centre for Research and Development under a project coordinated by the Central Institute for Labour Protection – National Research Institute, Warsaw, Poland (agreement no. TP-54/2017/PW-PB project no. II.N.22: “Improving safety and working conditions”, 2017–2019).
Animal Rights Statement: None required.
References
- 1.Abe T., Yamaki K., Hayakawa T., Fukuda H., Ito Y., Kume H., Komiya T., Ishihara K., Hirai K.. A seroepidemiological study of the risks of Q fever infection in Japanese veterinarians. Eur J Epidemiol. 2001;17:1029–1032. doi: 10.1023/a:1020018907452. [DOI] [PubMed] [Google Scholar]
- 2.Brandon-Mong G.J., Che Mat Seri N.A., Sharma R.S., Andiappan H., Tan T.C., Lim Y.A., Nissapatorn V.. Seroepidemiology of toxoplasmosis among people having close contact with animals. Front Immunol. 2015;6:143. doi: 10.3389/fimmu.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang C.C., Lin P.S., Hou M.Y., Lin C.C., Hung M.N., Wu T.M., Shu P.Y., Shin W.Y., Lin J.H., Chen W.C., Wu H.S., Lin L.J.. Identification of risk factors of Coxiella burnetii (Q fever) infection in veterinary-associated populations in southern Taiwan. Zoonoses Public Health. 2010;57:95–101. doi: 10.1111/j.1863-2378.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 4.Doudier B., Garcia S., Quennee V., Jarno P., Brouqui P.. Prognostic factors associated with severe leptospirosis. Clin Microbiol Infect. 2006;12:299–300. doi: 10.1111/j.1469-0691.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 5.Dubey J.P.. The history of Toxoplasma gondii-the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 6.Ergonul O., Zeller H., Kilic S., Kutlu S., Kutlu M., Cavusoglu S., Esen B., Dokuzoğuz B.. Zoonotic infections among veterinarians in Turkey: Crimean-Congo hemorrhagic fever and beyond. Int J Infect Dis. 2006;10:465–469. doi: 10.1016/j.ijid.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Felzemburgh R.D., Ribeiro G.S., Costa F., Reis R.B., Hagan J.E., Melendez A.X., Fraga D., Santana F.S., Mohr S., dos Santos B.L., Silva A.Q., Santos A.C., Ravines R.R., Tassinari W.S., Carvalho M.S., Reis M.G., Ko A.I.. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLOS Negl Trop Dis. 2014;8:2927. doi: 10.1371/journal.pntd.0002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiecek B., Grochowalska A., Chmielewski T., Tylewska-Wierzbanowska S.. Leptospira spp. and Coxiella burnetii infections occurring in Radomskie District in people of selected professional groups. Przegl Epidemiol. 2012;66:605–610. [PubMed] [Google Scholar]
- 9.Hackert V.H., van der Hoek W., Dukers-Muijrers N., de Bruin A., Al Dahouk S., Neubauer H., Bruggeman C.A., Hoebe C.J.. Q fever: single-point source outbreak with high attack rates and massive numbers of undetected infections across an entire region. Clin Infect Dis. 2012;55:1591–1599. doi: 10.1093/cid/cis734. [DOI] [PubMed] [Google Scholar]
- 10.Kiliç S., Al F.D., Celebi B., Babür C.. The investigation of the seroprevalence of cystic echinococcosis in veterinary surgeons. Türkiye Parazitol Derg. 2007;31:109–111. [PubMed] [Google Scholar]
- 11.Lassen B., Janson M., Viltrop A., Neare K., Hütt P., Golovljova I., Tummeleht L., Jokelainen P.. Serological evidence of exposure to globally relevant zoonotic parasites in the Estonian population. PLoS One. 2016;11:0164142. doi: 10.1371/journal.pone.0164142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurimaa L., Davison J., Plumer L., Süld K., Oja R., Moks E., Keis M., Hindrikson M., Kinkar L., Laurimae T., Abner J., Remm J., Anijalg P., Saarma U.. Noninvasive detection of Echinococcus multilocularis tapeworm in urban area, Estonia. Emerg Infect Dis. 2015;21:163–164. doi: 10.3201/eid2101.140136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLean M., Ruscoe Q., Kline T., King C., Nesdale A.. A cluster of three cases of leptospirosis in dairy farm workers in New Zealand. N Z Med J. 2014;127:13–20. [PubMed] [Google Scholar]
- 14.Molineri A., Signorini M.L., Pérez L., Tarabla H.D.. Zoonoses in rural veterinarians in the central region of Argentina. Aust J Rural Health. 2013;21:285–290. doi: 10.1111/ajr.12054. [DOI] [PubMed] [Google Scholar]
- 15.Monno R., Fumarola L., Trerotoli P., Cavone D., Giannelli G., Rizzo C., Ciceroni L., Musti M.. Seroprevalence of Q fever, brucellosis and leptospirosis in farmers and agricultural workers in Bari, Southern Italy. Ann Agric Environ Med. 2009;16:205–209. [PubMed] [Google Scholar]
- 16.National Institute of Public Health – National Institute of Hygiene (NIZP-PZH) – Reports on cases of infectious diseases and poisonings in Poland. 2017. http://www.old.pzh.gov.pl/oldpage/epimeld/index_p.html
- 17.Paul M.. Potential risk factors for Toxoplasma gondii infection in cases with recently acquired toxoplasmosis. Przegl Epidemiol. 1998;52:447–454. [PubMed] [Google Scholar]
- 18.Porter S.R., Czaplicki G., Mainil J., Guattéo R., Saegerman C.. Q Fever: current state of knowledge and perspectives of research of a neglected zoonosis. Int J Microbiol. 2011;2011:248418. doi: 10.1155/2011/248418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quijada S.G., Terán B.M., Murias P.S., Anitua A.A., Cermeño J.L., Frías A.B.. Q fever and spontaneous abortion. Clin Microbiol Infect. 2012;18:533–538. doi: 10.1111/j.1469-0691.2011.03562.x. [DOI] [PubMed] [Google Scholar]
- 20.Rivera-Benitez J.F., Rosas-Estrada K., Pulido-Camarillo E., de la Peña-Moctezuma A., Castillo-Juárez H., Ramírez-Mendoza H.. Serological survey of veterinarians to assess the zoonotic potential of three emerging swine diseases in Mexico. Zoonoses Public Health. 2014;61:131–137. doi: 10.1111/zph.12055. [DOI] [PubMed] [Google Scholar]
- 21.Rosypal A.C., Houk A.E., Zajac A.M., Lindsay D.S.. Prevalence of IgG antibodies to Toxoplasma gondii in veterinary and undergraduate students at Virginia Tech, Blacksburg, Virginia. Zoonoses Publ Health. 2015;62:553–556. doi: 10.1111/zph.12184. [DOI] [PubMed] [Google Scholar]
- 22.Sadaghian M., Jafari R.. Prevalence of toxoplasma infection in veterinary laboratory sciences students comparing to ordinary people: a case-control study. J Parasit Dis. 2016;40:768–771. doi: 10.1007/s12639-014-0575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sang-Eun L., Hong S.H., Jeong Y.I., Lee J.H., Yoo S.J., Lim H.S., Lee W.J., Cho S.H.. Cross-sectional analysis of the seropositivity and risk factors of Toxoplasma gondii infection among veterinarians, in relation to their public professional activities. Vet Parasitol. 2014;203:29–34. doi: 10.1016/j.vetpar.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Sanhueza J.M., Heuer C., Wilson P.R., Benschop J., Collins-Emerson J.M.. Prevalence and risk factors for Leptospira exposure in New Zealand veterinarians. Epidemiol Infect. 2015;143:2116–2125. doi: 10.1017/S0950268815000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuhaiber S., Koren G., Boskovic R., Einarson T.R., Soldin O.P., Einarson A.. Seroprevalence of Toxoplasma gondii infection among veterinary staff in Ontario, Canada 2002 implications for teratogenic risk. BMC Infect Dis 2003. 3:8. doi: 10.1186/1471-2334-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sroka J., Wójcik-Fatla A., Szymańska J., Dutkiewicz J., Zając V., Zwoliński J.. The occurrence of Toxoplasma gondii infection in people and animals from rural environment of Lublin region – estimate of potential role of water as a source of infection. Ann Agric Environ Med. 2010;17:125–132. [PubMed] [Google Scholar]
- 27.Szymańska-Czerwińska M., Galińska E.M., Niemczuk K., Knap J.P.. Prevalence of Coxiella burnetii infection in humans occupationally exposed to animals in Poland. Vector Borne Zoonotic Dis. 2015;15:261–267. doi: 10.1089/vbz.2014.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touma D., Sersté T., Ntounda R., Mulkay J.P., Buset M., Van Laethem Y.. The liver involvement of the hydatid disease: a systematic review designed for the hepatogastroenterologist. Acta Gastroenterol Belg. 2013;76:210–218. [PubMed] [Google Scholar]
- 29.Valencia M.C., Rodriguez C.O., Punet O.G., de Blas Giral I.. Q fever seroprevalence and associated risk factors among students from the Veterinary School of Zaragoza, Spain. Eur J Epidemiol. 2000;16:469–476. doi: 10.1023/a:1007605414042. [DOI] [PubMed] [Google Scholar]
- 30.Van den Brom R., Schimmer B., Schneeberger P.M., Swart W.A., van der Hoek W., Vellema P.. Seroepidemiological survey for Coxiella burnetii antibodies and associated risk factors in Dutch livestock veterinarians. PLoS One. 2013;8:54021. doi: 10.1371/journal.pone.0054021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasiński B., Sroka J., Wójcik-Fatla A., Zając V., Cisak E., Knap J.P., Sawczyn A., Dutkiewicz J.. Seroprevalence of leptospirosis in rural populations inhabiting areas exposed and not exposed to floods in eastern Poland. Ann Agric Environ Med. 2012;19:285–288. [PubMed] [Google Scholar]
- 32.Whitney E.A., Ailes E., Myers L.M., Saliki J.T., Berkelman R.L.. Prevalence of and risk factors for serum antibodies against Leptospira serovars in US veterinarians. J Am Vet Med Assoc. 2009;234:938–944. doi: 10.2460/javma.234.7.938. [DOI] [PubMed] [Google Scholar]
- 33.Whitney E.A., Massung R.F., Candee A.J., Ailes E.C., Myers L.M., Patterson N.E., Berkelman R.L.. Seroepidemiologic and occupational risk survey for Coxiella burnetii antibodies among US veterinarians. Clin Infect Dis. 2009;48:550–557. doi: 10.1086/596705. [DOI] [PubMed] [Google Scholar]
- 34.Wynwood S.J., Graham G.C., Weier S.L., Collet T.A., McKay D.B., Craig S.B.. Leptospirosis from water sources. Pathog Glob Health. 2014;108:334–338. doi: 10.1179/2047773214Y.0000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W., Wang S., McManus D.P.. Echinococcus granulosus genomics: a new dawn for improved diagnosis, treatment, and control of echinococcosis. Parasite. 2014;21:66. doi: 10.1051/parasite/2014066. [DOI] [PMC free article] [PubMed] [Google Scholar]


