Abstract
Introduction
Highly pathogenic Asian H5-subtype avian influenza viruses have been found in poultry and wild birds worldwide since they were first detected in southern China in 1996. Extensive control efforts have not eradicated them. Vaccination prevents such viruses infecting poultry and reduces the number lost to compulsory slaughter. The study showed the efficacy of inactivated H5 vaccine from the H5N8 virus against highly pathogenic H5N8 and H5N6 avian influenza viruses in chickens.
Material and Methods
Reverse genetics constructed an H5 vaccine virus using the HA gene of the 2014 H5N8 avian influenza virus and the rest of the genes from A/PR/8/34 (H1N1). The vaccine viruses were grown in fertilised eggs, partially purified through a sucrose gradient, and inactivated with formalin. Chickens were immunised i.m. with 1 μg of oil-adjuvanted inactivated H5 antigens.
Results
Single dose H5 vaccine recipients were completely protected from lethal infections by homologous H5N8 avian influenza virus and shed no virus from the respiratory or intestinal tracts but were not protected from lethal infections by heterologous H5N6. When chickens were immunised with two doses and challenged with homologous H5N8 or heterologous H5N6, all survived and shed no virus.
Conclusion
Our results indicate that two-dose immunisations of chickens with H5 antigens with oil adjuvant are needed to provide broad protection against different highly pathogenic H5 avian influenza viruses.
Keywords: chickens, avian influenza, H5N6, H5N8, vaccine
Introduction
Influenza viruses are members of Orthomyxoviridae and are classified into four types A, B, C, and D based on their nucleoprotein (NP) and matrix protein (MP) (6, 38). Influenza A viruses circulate in a variety of hosts such as humans, pigs, horses, dogs, and wild birds (38). Influenza B and C viruses mainly circulate in humans (38), and influenza D virus circulates in cattle, swine, camelids, and small ruminant populations (11, 12, 27). Among the 18 subtypes of haemagglutinin (HA) (24, 38), the H5 and H7 subtypes of avian influenza viruses can be converted into highly pathogenic (HP) viruses by obtaining polybasic amino acids such as arginine (R) and lysine (K) in the cleavage sites of HA proteins (17, 27).
Asian-origin HP H5 avian influenza viruses originated from the HA of A/goose/Guangdong/1/1996 (H5N1) isolated in China (41). In 1997, HP H5N1 influenza viruses jumped from poultry in the live bird markets in Hong Kong to 18 humans, and 6 of these infected humans died (4, 35, 36). Since 2003, these viruses have spread around the world despite intensive control measures, and they have reassorted with other avian influenza viruses, resulting in different subtypes (10, 19, 20, 21, 22, 23, 39). In 2014, huge outbreaks of HP H5N8 avian influenza viruses occurred in poultry in South Korea, resulting in the slaughter of thousands of birds (19, 34). HP H5N8 avian influenza viruses have been found in migratory birds and poultry in many countries such as the United States, Bangladesh, France, Italy, Japan, and the Netherlands (2, 9, 13, 28, 30, 31, 33). In 2016, another huge outbreak of HP H5N6 avian influenza virus occurred in poultry in South Korea, resulting in the loss of over 30 million birds (21, 22). HP H5N6 avian influenza viruses have been reported in poultry and migratory birds in many countries such as China, Japan, Laos, and the Netherlands (3, 7, 29, 42).
Attempts have been made to develop effective vaccines to control HP avian influenza viruses in poultry using the inactivated whole virus, recombinant viral vectors expressing HA proteins, and virus-like particles containing the HA protein (5, 15, 16, 18, 32, 40). In this study, we determined whether inactivated H5 antigens derived from HP H5N8 avian influenza virus (clade, 2.3.4.4) could protect immunised chickens from infections by HP H5N8 or H5N6 avian influenza viruses and whether one dose or two doses of vaccine were needed for the complete protection of chickens from lethal infections by H5N8 and H5N6 viruses.
Material and Methods
Viruses and animals. The HP avian influenza viruses, A/Waterfowl/S005/Korea/2014 (H5N8) (clade 2.3.4.4), and A/Waterfowl/Korea/S57/2016 (clade 2.3.4.4.) were isolated from faecal samples from wild birds in our laboratory. Ten-day-old fertilised eggs were used to amplify these viruses. The studies on HP avian influenza viruses were performed in a BSL-3 facility which was certified by the Korean Center for Disease Control and Prevention. Fertilised eggs from healthy chickens (White Leghorn) were purchased from a local farm in Korea and were hatched in our laboratory. Three-week-old grown chickens were used for starting vaccinations with the inactivated H5 antigens.
Generation of reassorted H5 vaccine virus. Reassorted vaccine viruses were generated according to methods by Hwang et al. (14). Robert G. Webster from St. Jude Children’s Research Hospital, Memphis, USA, provided plasmids of A/PR/8/34 (H1N1), PB2, PB1, PA, NP, NA, M, and NS. The HA genes which did not have polybasic amino acids were derived from A/Waterfowl/S005/Korea/2014 (H5N8) (clade 2.3.4.4). Vero cells were transfected with 1 μg of each plasmid for 48 h before 300 μL of supernatant was treated with L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK)–treated trypsin (1 μg/mL). The treated supernatants were injected into the allantoic cavity of 10-day-old fertilised eggs from healthy chickens. The reassorted viruses were identified by genetic analysis and haemagglutination inhibition (HI) assay. Regarding the vaccine strain, this reassorted virus was designated as Vaccine-H5N8-RG-CNUK4-2014 (H5RG).
Preparation and antigen measurement of H5RG inactivated vaccines. H5RG vaccine viruses were amplified in 10-day-old fertilised eggs (35°C, 60 h). The harvested fluid was reduced to 1/10 of its original volume by an Amicon concentrator apparatus (Merck Millipore, USA). A 20% to 75% continuous sucrose gradient was used for purification of virus-containing fluid (26,000 rpm, 4°C, 2 h). Formalin (0.01% per volume) was used to inactivate viruses (4°C, overnight). Virus inactivation was determined by injection into hens’ eggs. The H5RG protein amount was measured with the Bradford method (Bio-Rad, USA) using the standard formula of bovine serum albumin (BSA).
Inoculation of chickens with oil-adjuvanted H5RG antigens and infection of immunised chickens with HP H5N8 and H5N6 viruses Three-week-old chickens (n = 10 per group), negative for H5N8, H5N6, and H9N2 viruses, were injected intramuscularly (i.m.) with 300 μL of 1.0 μg/mL oil-adjuvant inactivated H5RG antigens in 70%:30% oil composition (Montanide IMS 1313 NPR, Seppic, France). The second boosting dose was inoculated into chickens three weeks after the first inoculation. The inoculated chickens were intranasally (i.n.) infected four weeks after vaccination with 500 μL of 105EID50/mL of A/Waterfowl/S005/Korea/2014 (H5N8) or A/Waterfowl/Korea/S57/2016 (H5H6) strains.
Antibody titre measurement in vaccinated chickens. An HI assay was performed. Receptor-destroying enzyme (RDE) (Denka Seiken, Japan) was used to treat sera from vaccinated chickens diluted twofold in PBS (pH 7.4) and pipetted into V-bottomed 96-well plates. Twenty microlitres (8 HA units) of A/Waterfowl/S005/Korea/2014 (H5N8) or A/Waterfowl/Korea/S57/2016 (H5N6) viruses was added and the plates were reacted for 15 min at room temperature. Red blood cells from turkeys (0.5%, 50 μL) were added next and then the plates were reacted for 40 min at room temperature. HI titre was measured by reciprocal dilutions which prevented haemagglutination.
Viral titre measurement in swab samples from the trachea, cloaca, and lung tissues. The swabs were taken from the trachea and cloaca of live chickens after challenge with HP H5N6 or H5N8 viruses. Swab samples were treated with PBS (pH 7.4) supplemented with Antibiotic-Antimycotic Solution (2×) (Sigma-Aldrich, USA). Lung tissues were collected from chickens three days post infection and were homogenised in PBS (1 g/mL). Tenfold serial dilutions of swab and lung samples were performed with PBS (pH 7.4) before they were injected into four 10-day-old fertilised eggs. Virus growth in the injected eggs was identified by haemagglutination assay. Log10 egg infectious dose 50/mL (log10 EID50/mL) was used for measurement of viral titres. The virus detection limit was over 1.0 log10 EID50/mL.
Histopathology of lung tissues. Lung tissues of chickens used for detecting viral titres were fixed in neutral buffered formalin (10%) and then embedded in paraffin. Sections (5 μm) were stained with haematoxylin and eosin (H&E) (1).
Analysis of data. The Mann–Whitney U test was used for determining statistical significance.
Results
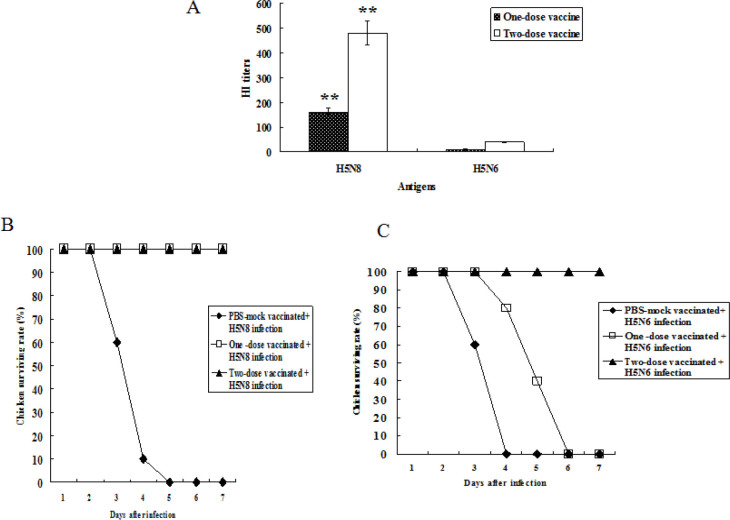
HI titres and survival rate of chickens vaccinated with oil-adjuvanted H5RG antigens. The mean HI titres for the one-dose vaccine against the homologous H5N8 influenza virus and heterologous H5N6 influenza virus were 160 and less than 10, respectively. The mean HI titres for the two-dose vaccine against the viruses were 480 and 40, respectively (Fig. 1A)
Fig. 1.
Antibody titres in immunised chickens and survival rate of chickens challenged with HP H5N8 and H5N6 avian influenza viruses. Three-week-old chickens (n = 10 per group) were immunised i.m. with 1.0 μg of oil-adjuvant inactivated H5RG in the pectoral muscle. The second dose was inoculated into chickens three weeks after the first inoculation. Three and four weeks after immunisation, sera were collected from the chickens and HI antibody titres were measured (A). Data were mean HI titre of immunised chickens ±standard deviation. Statistical analysis was performed using data from H5N8 and H5N6 HI titres. **P < 0.001. The immunised chickens (n = 10 per group) were infected i.n. with 105EID50/mL of A/Waterfowl/S005/Korea/2014 (H5N8) (B) or A/Waterfowl/Korea/S57/2016 (H5N6) (C) four weeks after vaccination. Survival was observed for seven days after challenge
The survival rate of vaccinated chickens differed between the group which received homologous and the group which received heterologous HP influenza viruses (Fig. 1B, 1C) The survival rate in the one-dose or two-dose vaccinated chickens infected with the homologous HP H5N8 was 100%, while all PBS mock-vaccinated chickens infected with H5N8 HP influenza virus died within five days (Fig. 1B) When the immunised chickens were challenged with the heterologous HP H5N6 influenza virus, no one-dose or PBS mock-immunised chickens survived, while all two-dose immunised chickens did (Fig. 1C)
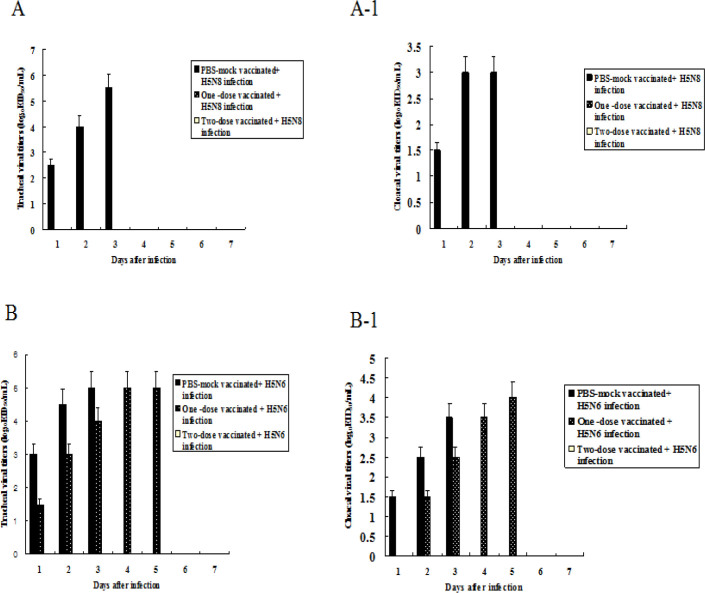
Viral detection in chickens vaccinated with oil-adjuvanted H5RG antigens. Viral titres in swabbed samples from the trachea and cloaca of surviving chickens were found for seven days after infection. Viruses were not detected in the trachea and cloaca of one-dose or two-dose vaccinated chickens that were infected i.n. with the homologous HP H5N8 influenza virus, while viral titres in tracheal and cloacal swab samples from PBS mock-immunised chickens infected with the virus were 5.5 and 3.0 EID50/mL three days after infection, respectively (Fig. 2A A-1). When the immunised chickens were challenged with heterologous HP H5N6 influenza virus, viruses were shed through the trachea and cloaca of one-dose immunised chickens, but were not shed this way by two-dose immunised birds. The viral titres in the tracheal and cloacal swab samples of one-dose immunised and infected chickens were 5.0 and 4.0 EID50/mL five days after infection, respectively, while those in the tracheal and cloacal swab samples from PBS mock-immunised chickens infected with the virus were 5.0 and 3.5 EID50/mL three days after infection, respectively (Fig. 2 B, B-1).
Fig. 2.
Viral titres in tracheal and cloacal swab samples in chickens infected with HP H5N8 or H5N6 avian influenza viruses. Viral titres were measured by log10EID50/mL. Data were mean viral titre of surviving chickens ±standard deviation. A – tracheal viral titres in chickens infected with A/Waterfowl/S005/Korea/2014 (H5N8); A-1 – cloacal viral titres in chickens infected with A/Waterfowl/S005/Korea/2014 (H5N8); B – tracheal viral titres in chickens infected with A/Waterfowl/Korea/S57/2016 (H5N6); B-1 – cloacal viral titres in chickens infected with A/Waterfowl/Korea/S57/2016 (H5N6)
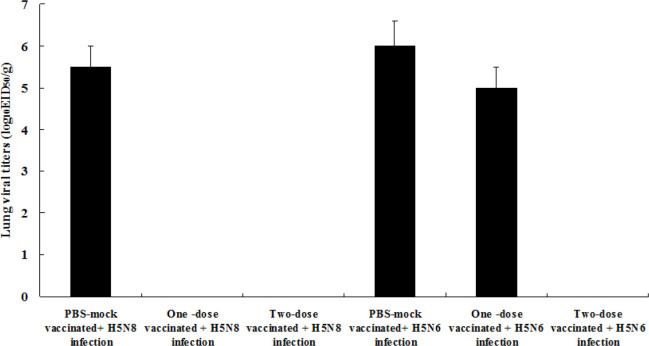
No virus was detected in the lung tissues of one-and two-dose vaccinated chickens infected with the homologous HP H5N8 influenza virus or in the tissues of two-dose immunised chickens challenged with the heterologous HP H5N6 influenza virus (Fig. 3). The mean viral titre in the lung tissues of one-dose vaccinated chickens challenged with the heterologous HP H5N6 influenza virus was 5.0 EID50/mL three days after the infection. The mean viral titres in the same tissues of PBS mock-vaccinated chickens infected with H5N8 and H5N6 viruses were 5.5 and 6.0 EID50/mL three days after the infection, respectively.
Fig. 3.
Viral titres in the lung tissues of chickens infected with HP H5N8 or H5N6 avian influenza viruses. Viral titres were measured by log10EID50/g. Data were mean viral titre of surviving chickens ± standard deviation
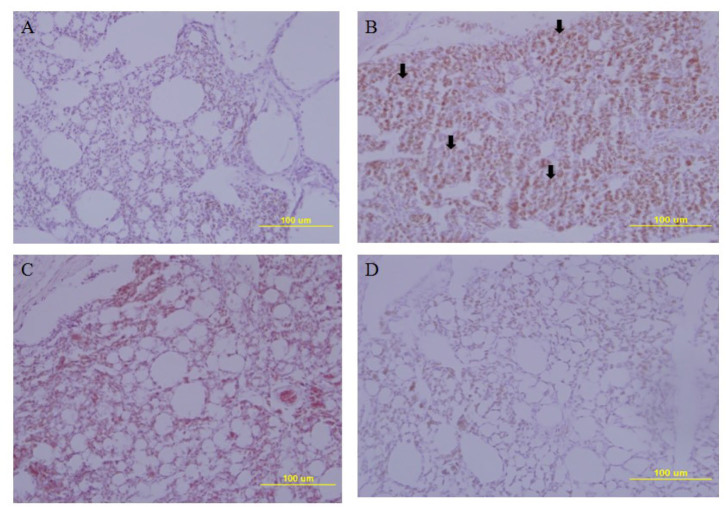
Lung pathology in chickens vaccinated with oil-adjuvanted H5RG antigens. No sign of pneumonia was observed in the lung tissues of one-dose or two-dose vaccinated chickens infected with homologous H5N8 HP influenza virus (Fig. 4) or in the lung tissues of two-dose vaccinated chickens challenged with heterologous HP H5N6 influenza virus (Fig. 5). When the chickens were challenged with the homologous HP H5N8 influenza virus (Fig. 4), the lung tissue of PBS mock-immunised infected chickens showed signs of severe interstitial pneumonia with many infiltrated inflammatory cells (Fig. 4B), while the lung tissues of uninfected chickens (Fig. 4A) one-dose immunised chickens (Fig. 4C) or two-dose immunised chickens (Fig. 4D) did not show any signs of pneumonia. When the chickens were infected with the heterologous HP H5N6 (Fig. 5), as with homologous H5N8, the lung tissues of PBS mock-immunised and infected chickens (Fig. 5B) or one-dose immunised and infected chickens (Fig. 5C) showed signs of severe interstitial pneumonia with many infiltrated inflammatory cells, while those of uninfected chickens (Fig. 5A) or two-dose immunised chickens (Fig. 5D) did not show any signs of the disease.
Fig. 4.
Lung pathology in chickens infected with HP H5N8 avian influenza virus. A – uninfected chicken lung tissue; B – H5N8-infected PBS mock-immunised chicken lung tissue; C – H5N8-infected one-dose immunised chicken lung tissue; D – H5N8-infected two-dose immunised chicken lung tissue; arrow – pneumonia
Fig. 5.
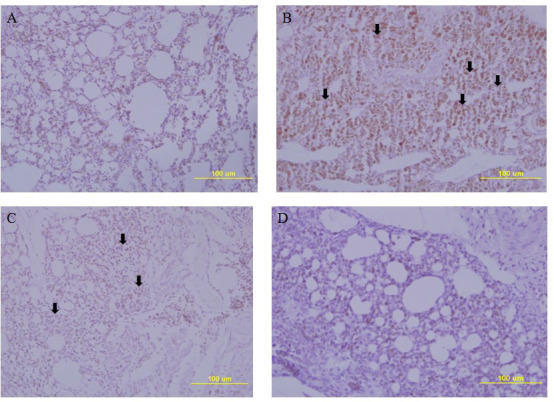
Lung pathology in chickens infected with HP H5N6 avian influenza virus. A – uninfected chicken lung tissue; B – H5N6-infected PBS mock-immunised chicken lung tissue; C – H5N6-infected one-dose immunised chicken lung tissue; D – H5N6-infected two-dose immunised chicken lung tissue; arrow – pneumonia
Discussion
Asian HP H5 avian influenza viruses have been circulating in poultry worldwide for over 20 years. Intensive monitoring and eradication efforts have not been successful. Infections of chickens and ducks by the viruses have resulted in huge economic losses for the poultry industry. The vaccination of poultry with H5 antigens could help to reduce the number of poultry that must be killed and limit the spread of H5 virus among poultry and from poultry to wild birds, as a complementary policy to slaughter to remove infected animals. In this study, we explored the efficacy of H5 antigens from the recent HP H5N8 (clade 2.3.4.4) avian influenza virus in chickens. The vaccinated chickens were protected from infections by both HP H5N8 and H5N6 avian influenza viruses in a dose-dependent manner.
Our results showed that an oil-adjuvant one-dose (1 μg) inactivated vaccination provided complete protection of chickens against infection with homologous H5N8 HP avian influenza virus (A/Waterfowl/S005/Korea/2014, clade 2.3.4.4), but it did not protect them against infection by heterologous H5N6 HP avian influenza virus (A/Waterfowl/Korea/S57/2016 (clade 2.3.4.4). The HP H5N8 and H5N6 avian influenza viruses used in this study belong to the same clade 2.3.4.4, but the similarity between amino acid sequences of the H5 HA protein was only 95%. The HI antibody titre against H5N6 virus was also less than 10 (Fig. 1A) It is regarded that HI antibody titres over 40 can be protective immunity against influenza viruses. A previous study on the inactivated vaccines in chickens showed that inactivated H5 antigens provided quite good protection against lethal challenge by H5N8 and H5N2 HP avian influenza viruses (15). Chickens receiving the inactivated HA antigens of A/gyrfalcon/Washington/2014 (H5N8, clade 2.3.4.4) were 100% protected against infections with A/gyrfalcon/Washington/2014 (H5N8, clade 2.3.4.4) and A/northern pintail/Washington/40964/2014 (H5N2, clade 2.3.4.4). The discrepancy in the cross-subtype protection rate between our study and this previous study may be due to differences in the extent of similarity between amino acid sequences of the H5 HA proteins. The HA nucleotide difference between A/Waterfowl/S005/Korea/2014 (H5N8, clade 2.3.4.4) and A/Waterfowl/Korea/S57/2016 (H5N6, clade 2.3.4.4) is about 5%, while that between A/gyrfalcon/Washington/2014 (H5N8, clade 2.3.4.4) and A/northern pintail/Washington/40964/2014 (H5N2, clade 2.3.4.4) is only about 1%.
Two doses of inactivated H5 antigens provided sterile protection of chickens from infections by both HP H5N8 and H5N6 avian influenza viruses. This result suggests that inactivated antigens could elicit broader protection immunity when chickens were immunised with a double dose of them. Considering that inactivated vaccines have been used to protect chickens from HP H5 avian influenza viruses in some countries such as China, Italy, and Vietnam (8, 37), the prime-boosting strategy in which chickens are immunised with inactivated H5 antigens on farms would be practical to protect them from infections by more diverse HP H5 avian influenza viruses.
The two-dose H5-antigen immunised chickens that were lethally infected with HP H5N8 and H5N6 avian influenza viruses did not shed viruses from the respiratory or intestinal tracts. This result suggests that oil-adjuvant two-dose H5 antigens could elicit strong immunity against the homologous and heterologous HP H5 avian influenza viruses. A previous study on Newcastle disease virus-based H5 antigen of A/chicken/Iowa/04-20/2015 (H5N2, clade, 2.3.4.4) demonstrated that immunised chickens were 100% protected from A/turkey/Minnesota/9845-4/2015 (H5N2, clade 2.3.4.4). However, some immunised chickens that were infected with the turkey isolate shed viruses through the respiratory tract (25).
In conclusion, two doses of H5 antigens from HP H5N8 avian influenza virus provided chickens with sterile immunity against both HP homologous H5N8 and heterologous H5N6 avian influenza viruses, suggesting that this vaccine could be useful to minimise the number of infected poultry and to prevent further transmission among poultry during outbreaks of HP H5 avian influenza viruses.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through the Livestock Disease Preparedness Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (grant no. 116125-3).
Animal Rights Statement:Chungnam National University (CNU) Internal Animal Use Committee approved the protocol for the chicken vaccine study and collection of clinical chicken samples.
References
- 1.Bancroft J.D., Stevens A. Theory and Practice of Histological Techniques, Churchill Livingstone. New York: 1996. [Google Scholar]
- 2.Beerens N., Heutink R., Bergervoet S.A., Harders F., Bossers A., Koch G.. Multiple reassorted viruses as cause of highly pathogenic avian influenza A(H5N8) virus epidemic, the Netherlands 2016. Emerg Infect Dis 2017. 23:1974–1981. doi: 10.3201/eid2312.171062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beerens N., Koch G., Heutink R., Harders F., Vries D.P.E., Ho C., Bossers A., Elbers A.. Novel highly pathogenic avian influenza A(H5N6) virus in the Netherlands, December. Emerg Infect Dis. 2017;2018:24. doi: 10.3201/eid2404.172124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender C., Hall H., Huang J., Klimov A., Cox N., Hay A., Gregory V., Cameron K., Lim W., Subbarao K.. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. doi: 10.1006/viro.1998.9529. [DOI] [PubMed] [Google Scholar]
- 5.Bertran K., Balzli C., Lee D.H., Suarez D.L., Kapczynski D.R., Swayne D.E.. Protection of White Leghorn chickens by U.S. emergency H5 vaccination against clade 2.3.4.4 H5N2 high pathogenicity avian influenza virus. Vaccine. 2017;35:6336–6344. doi: 10.1016/j.vaccine.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 6.Bouvier N.M., Palese P.. The biology of influenza viruses. Vaccine. 2008;26:49–53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler J., Stewart C.R., Layton D.S., Phommachanh P., Harper J., Payne J., Evans R.M., Valdeter S., Walker S., Harvey G., Shan S., Bruce M.P., Rootes C.L., Gough T.J., Rohringer A., Peck G.R., Fardy S.J., Karpala A.J., Johnson D., Wang J., Douangngeun B., Morrissy C., Wong F.Y., Bean A.G., Bingham J., Williams D.T.. Novel reassortant H5N6 influenza A virus from the Lao People's Democratic Republic is highly pathogenic in chickens. PLoS One. 2016;11:e0162375. doi: 10.1371/journal.pone.0162375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capua I., Marangon S.. The use of vaccination to combat multiple introductions of notifiable avian influenza viruses of the H5 and H7 subtypes between 2000 and 2006 in Italy. Vaccine 2007. 25:4987–4995. doi: 10.1016/j.vaccine.2007.01.113. [DOI] [PubMed] [Google Scholar]
- 9.El-Shesheny R., Barman S., Feeroz M.M., Hasan M.K., Jones-Engel L., Franks J., Turner J., Seiler P., Walker D., Friedman K., Kercher L., Begum S., Akhtar S., Datta A.K., Krauss S., Kayali G., McKenzie P., Webby R.J., Webster R.G.. Genesis of influenza A(H5N8) viruses. Emerg Infect Dis. 2017;23:1368–1371. doi: 10.3201/eid2308.170143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farah Z.E., Khatib O., Hamadeh S., Ahmad K., El Bazzal B., Zalloua P., Ammar W., Ghosn N.. Containment of highly pathogenic avian influenza A (H5N1) virus, Lebanon. Emerg Infect Dis 2018. 2016;24:374–376. doi: 10.3201/eid2402.171276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson L., Luo K., Olivier A.K., Cunningham F.L., Blackmon S., Hanson-Dorr K., Sun H., Baroch J., Lutman M.W., Quade B., Epperson W., Webby R., DeLiberto T.J., Wan X.F.. Influenza D virus infection in feral swine populations, United States. Emerg Infect Dis. 2018;24:1020–1028. doi: 10.3201/eid2406.172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn O., Gallagher C., Mooney J., Irvine C., Ducatez M., Hause B., McGrath G., Ryan E.. Influenza D virus in cattle, Ireland. Emerg Infect Dis. 2018;24:389–391. doi: 10.3201/eid2402.170759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusaro A., Monne I., Mulatti P., Zecchin B., Bonfanti L., Ormelli S., Milani A., Cecchettin K., Lemey P., Moreno A., Massi P., Dorotea T., Marangon S., Terregino C.. Genetic diversity of highly pathogenic avian influenza A (H5N8/H5N5) viruses in Italy, 2016–2017. Emerg Infect Dis. 2017;23:1543–1547. doi: 10.3201/eid2309.170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang S.D., Kim H.S., Cho S.W., Seo S.H.. Single dose of oil-adjuvanted inactivated vaccine protects chickens from lethal infections of highly pathogenic H5N1 influenza virus. Vaccine. 2011;29:2178–2186. doi: 10.1016/j.vaccine.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Kapczynski D.R., Pantin-Jackwood M.J., Spackman E., Chrzastek K., Suarez D.L., Swayne D.E.. Homologous and heterologous antigenic matched vaccines containing different H5 hemagglutinins provide variable protection of chickens from the 2014 U.S. H5N8, and H5N2 clade 2.3.4.4 highly pathogenic avian influenza viruses. Vaccine 2017. 35:6345–6353. doi: 10.1016/j.vaccine.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Kapczynski D.R., Sylte M.J., Killian M.L., Torchetti M.K., Chrzastek K., Suarez D.L.. Protection of commercial turkeys following inactivated or recombinant H5 vaccine application against the 2015U.S. H5N2 clade 2.3.4.4 highly pathogenic avian influenza virus. Vet Immunol Immunopathol 2017. 191:74–79. doi: 10.1016/j.vetimm.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kawaoka Y., Nestorowicz A., Alexander D.J., Webster R.G.. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: origin of a virulent turkey strain. Virology. 1987;158:218–227. doi: 10.1016/0042-6822(87)90256-x. [DOI] [PubMed] [Google Scholar]
- 18.Kilany W., Dauphin G., Selim A., Tripodi A., Samy M., Sobhy H., VonDobschuetz S., Safwat M., Saad M., Erfan A., Hassan M., Lubroth J., Jobre Y.. Protection conferred by recombinant turkey herpesvirus avian influenza (rHVT-H5) vaccine in the rearing period in two commercial layer chicken breeds in Egypt. Avian Pathol. 2014;43:514–523. doi: 10.1080/03079457.2014.966302. [DOI] [PubMed] [Google Scholar]
- 19.Ku K.B., Park E.H., Yum J., Kim J.A., Oh S.K., Seo S.H.. Highly pathogenic avian influenza A(H5N8) virus from waterfowl, South Korea. Emerg Infect Dis 2014. 2014;20:1587–1588. doi: 10.3201/eid2009.140390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C.C., Zhu H., Huang P.Y., Peng L., Chang Y.C., Yip C.H., Li Y.T., Cheung C.L., Compans R., Yang C., Smith D.K., Lam T.T., King C.C., Guan Y.. Emergence and evolution of avian H5N2 influenza viruses in chickens in Taiwan. J Virol. 2014;88:5677–5686. doi: 10.1128/JVI.00139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E.K., Song B.M., Lee Y.N., Heo G.B., Bae Y.C., Joh S.J., Park S.C., Choi K.S., Lee H.J., Jang I., Kang M.S., Jeong O.M., Choi B.K., Lee S.M., Jeong S.C., Park B.K., Lee H.S., Lee Y.J.. Multiple novel H5N6 highly pathogenic avian influenza viruses, South Korea. Infect Genet Evol 2017. 2016;51:21–23. doi: 10.1016/j.meegid.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Lee I.H., Jin S.Y., Seo S.H.. Genetic and pathogenic analysis of a novel reassortant H5N6 influenza virus isolated from waterfowl in South Korea in 2016. Arch Virol 2017. 162:3507–3510. doi: 10.1007/s00705-017-3488-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.S, Chenm L.H., Chenm Y.P., Liu Y.P., Li W.C., Lin Y.L., Lee F.. Highly pathogenic avian influenza viruses H5N2, H5N3, and H5N8 in Taiwan in 2015. Vet Microbiol 2016. 187:50–57. doi: 10.1016/j.vetmic.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay L.L., Plancarte M., Brenn-White M., Boyce W.M.. Complete genome sequences of the first reported california h16 influenza a viruses. Genome Announc. 2014;2:e00329–14. doi: 10.1128/genomeA.00329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J., Lee J., Liu H., Mena I., Davis A.S., Sunwoo S.Y., Lang Y., Duff M., Morozov I., Li Y., Yang J., García-Sastre A., Richt J.A., Ma W.. Newcastle disease virus-based H5 influenza vaccine protects chickens from lethal challenge with a highly pathogenic H5N2 avian influenza virus. NPJ Vaccines. 2017;2:33. doi: 10.1038/s41541-017-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekata H., Yamamoto M., Hamabe S., Tanaka H., Omatsu T., Mizutani T., Hause B.M., Okabayashi T.. Molecular epidemiological survey and phylogenetic analysis of bovine influenza D virus in Japan. Transbound Emerg Dis. 2018;65:e355–e360. doi: 10.1111/tbed.12765. [DOI] [PubMed] [Google Scholar]
- 27.Mulatti P., Zecchin B., Monne I., Vieira J.T., Dorotea T., Terregino C., Lorenzetto M., Piccolomini L.L., Santi A., Massi P., Bonfanti L., Marangon S.. H7N7 Highly pathogenic avian influenza in poultry farms in Italy in 2016. Avian Dis 2017. 61:261–266. doi: 10.1637/11540-112516-Case.1. [DOI] [PubMed] [Google Scholar]
- 28.Napp S., Majó N., Sánchez-Gónzalez R., Vergara-Alert J.. Emergence and spread of highly pathogenic avian influenza A (H5N8) in Europe in 2016–2017. Transbound Emerg Dis. 2018 doi: 10.1111/tbed.12861. [DOI] [PubMed] [Google Scholar]
- 29.Okamatsu M., Ozawa M., Soda K., Takakuwa H., Haga A., Hiono T., Matsuu A., Uchida Y., Iwata R., Matsuno K., Kuwahara M., Yabuta T., Usui T., Ito H., Onuma M., Sakoda Y., Saito T., Otsuki K., Ito T., Kida H.. Characterization of highly pathogenic avian influenza virus A (H5N6), Japan, November. Emerg Infect Dis 2017. 2016;23:691–695. doi: 10.3201/eid2304.161957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantin-Jackwood M.J., Costa-Hurtado M., Bertran K., DeJesus E., Smith D., Swayne D.E.. Infectivity, transmission, and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet Res. 2017;48:33. doi: 10.1186/s13567-017-0435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salaheldin A.H., El-Hamid H.S., Elbestawy A.R., Veits J., Hafez H.M., Mettenleiter T.C., Abdelwhab E.M.. Multiple introductions of influenza A (H5N8) virus into poultry, Egypt. Emerg Infect Dis. 2017;2018(5):24. doi: 10.3201/eid2405.171935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos J.J.S., Obadan A.O., Garcia S.C., Carnaccini S., Kapczynski D.R., Pantin-Jackwood M., Suarez D.L., Perez D.R.. Short- and long-term protective efficacy against clade 2.3.4.4 H5N2 highly pathogenic avian influenza virus following prime-boost vaccination in turkeys. Vaccine. 2017;35:5637–5643. doi: 10.1016/j.vaccine.2017.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scoizec A., Niqueux E., Thomas R., Daniel P., Schmitz A., Le Bouquin S.. Airborne detection of H5N8 highly pathogenic avian influenza virus genome in poultry farms, France. Front Vet Sci. 2018;5:15. doi: 10.3389/fvets.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song B.M., Lee E.K., Lee Y.N., Heo G.B., Lee H.S., Lee Y.J.. Phylogeographical characterization of H5N8 viruses isolated from poultry and wild birds during 2014–2016 in South Korea. J Vet Sci. 2017;18:89–94. doi: 10.4142/jvs.2017.18.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez D.L., Perdue M.L., Cox N., Rowe T., Bender C., Huang J., Swayne D.E.. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subbarao K., Klimov A., Katz J., Regnery H., Lim W., Hall H., Perdue M., Swayne D., Bender C., Huang J., Hemphill M., Rowe T., Shaw M., Xu X., Fukuda K., Cox N.. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 37.Swayne D.E.. Avian influenza vaccines and therapies for poultry. Comp Immunol Microbiol Infect Dis. 2009;32:351–363. doi: 10.1016/j.cimid.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Webster R.G., Bean W.J., Gorman O.T., Chambers T.M., Kawaoka Y.. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong F.Y., Phommachanh P., Kalpravidh W., Chanthavisouk C., Gilbert J., Bingham J., Davies K.R., Cooke J., Eagles D., Phiphakhavong S., Shan S., Stevens V., Williams D.T., Bounma P., Khambounheuang B., Morrissy C., Douangngeun B., Morzaria S.. Reassortant highly pathogenic influenza A(H5N6) virus in Laos. Emerg Infect Dis. 2015;21:511–516. doi: 10.3201/eid2103.141488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu P., Lu J., Zhang X., Mei M., Feng L., Peng D., Hou J., Kang S.M., Liu X., Tang Y.. Single dose of consensus hemagglutinin-based virus-like particles vaccine protects chickens against divergent H5 subtype influenza viruses. Front Immunol. 2017;8:1649. doi: 10.3389/fimmu.2017.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X., Subbarao K., Cox N.J., Guo Y.. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999. 261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z., Li R., Jiang L., Xiong C., Chen Y., Zhao G., Jiang Q.. The complexity of human infected AIV H5N6 isolated from China. BMC Infect Dis. 2016;16:600. doi: 10.1186/s12879-016-1932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]