Abstract
Introduction
Immune-potentiating functions of Lactobacillus plantarum strains in the common carp were evaluated.
Material and Methods
Fourteen days of feeding fish dry diet supplemented with the bacteria provided parameters of nonspecific humoral immunity (lysozyme, ceruloplasmin, γ-globulin, total protein levels, and serum bactericidal activity) and cellular immunity (pinocytosis, respiratory burst activity, and potential killing activity of organ phagocytes), as well as the proliferative response of organ lymphocytes stimulated with mitogens. The resistance of fish to infection with Aeromonas hydrophila was also determined.
Results
Dietary supplementation with L. plantarum had a substantial influence on the activity of organ phagocytes, especially the potential killing activity of head kidney cells. A significant increase in the proliferative activity of LPS-stimulated B lymphocytes and in the levels of γ-globulins and total protein was observed. The supplemented diet conveyed higher resistance than the control diet as the cumulative fish mortalities after infection with A. hydrophila were 65% and 85%, respectively.
Conclusion
The results indicate that dietary supplementation with L. plantarum stimulates the antibacterial resistance of common carp and may reinforce defence against bacterial infections, but further studies need to be conducted.
Keywords: common carp, probiotics, Lactobacillus plantarum, immune response, disease resistance
Introduction
In a time of intensive fish rearing, bacterial diseases are becoming a serious problem in the aquaculture sector, particularly when complicated by increasing bacterial antibiotic resistance. Negative effects of antibiotics on fish, fish consumers, and the environment can be avoided if probiotics are used to protect fish from bacterial infections or, if disease symptoms appear, to serve as a viable alternative to antibiotic therapy. Probiotics can stimulate the immune system, improve resistance to bacterial and parasitic diseases, contribute to more rapid growth of fish and better feed efficiency by raising digestive system enzymatic activity, and can even improve the quality of water in fish ponds (8, 21). Among probiotic bacteria, lactic acid bacteria (LAB) constitute the most thoroughly investigated group, which includes inter alia Gram-positive bacilli from the Lactobacillus genus.
The research was conducted on Lactobacillus plantarum strains, which are widespread on plants, fermenting food, and in the digestive tract of humans and animals, including fish. These bacteria can survive in the digestive system, act antagonistically towards pathogenic bacteria, produce compounds which exert antimicrobiological effects, and adhere to the mucous membrane of the digestive tract, which facilitates their colonisation of and persistent presence in the intestines (12). The previous studies on L. plantarum strains confirm that they are able to produce such antimicrobial substances as plantaricin, active towards specific pathogens and to inhibit their growth (5).
Numerous investigations have been reported on the efficacy of administration of Lactobacillus spp. bacteria to different commercially cultivated and ornamental fish, for example rainbow trout (Oncorhynchus mykiss), turbot (Scophthalmus maximus), red sea bream (Pagrus major), orange spotted grouper (Epinephelus coioides), and basa fish (Pangasius bocourti) (7, 19, 26).
Common carp (Cyprinus carpio) is one of the most important cultured fish species in Poland, affected by bacterial diseases, particularly ones caused by mesophilous strains of Aeromonas hydrophila. Pathogenic changes occur mostly on the fish skin as motile aeromonad infection (MAI) and appear in the form of petechiae, ulcers, abscesses, and even deep lesions penetrating down to the muscle tissue. Less often, these bacteria cause generalised infection termed motile aeromonad septicaemia (MAS), which is accompanied by the fish’s emaciation, exophthalmia, and ruffled scales due to the accumulation of liquid in the body cavity. Internal organs are pale with visible petechiae (10).
The purpose of this study was to evaluate the immune-potentiating functions of L. plantarum strains in the common carp fingerling after two weeks sustenance with probiotic-supplemented feed, and substantiate or discount the potential for the bacterium to increase resistance of the fish to bacterial infection with A. hydrophila.
Material and Methods
Experimental fish. Common carp fingerlings of an average body weight of 45 g originated from the Experimental Fish Farm of the Stanisław Sakowicz Inland Fisheries Institute in Żabieniec, Poland. The fish were healthy, showed no signs of disease (examined through gross examination of samples of fins, skin, and gills), and had no previous history of parasitic infections.
The fish were acclimatised for two weeks prior to the experiment and maintained on a normal diet in one tank. After the acclimation period, the fish were randomly divided into two experimental groups: one fed a diet supplemented with L. plantarum (the LAB group) and the other fed a diet without supplementation (the control group). Each group was prepared in triplicate to make six tanks altogether. The tank capacity was 300 L, ~30% of the water was exchanged daily, and each tank contained 40 fish. The physical and chemical characteristics of the water were as follows: temperature 18 ± 0.5ºC, pH 7.2, dissolved oxygen concentration 6.2–8.0 mg L−1, and ammonia concentration 0.04–0.10 mg L−1. Fish were fed three times per day and the daily feed ration equalled 1.5% of body weight. Of the fish in each group, 20 were used in a challenge test and 20 were used to investigate the immune parameters.
Probiotic bacteria and experimental diets. The experiments involved five strains of L. plantarum: 225/1, 155/1, 211/1B, 226/1, and 274/1, obtained from the Department of Microbial Biochemistry of the Institute of Biochemistry and Biophysics, Polish Academy of Sciences in Warsaw. The strains were isolated from a sample of raw milk produced in the region of Warmia and Mazury, Poland and their phenotypic and genetic characteristics were determined. The ability of the strains to use 49 different sources of carbon was examined by the API 50CHL test (bioMérieux, France) and the data were processed with Apiweb software (bioMérieux). The enzymatic profile and ability of the strains to produce 19 enzymes was also determined by the API Zym test (bioMérieux).
L. plantarum strains were characterised for important properties such as ability to grow in the presence of 10% fish bile, tolerance to low pH, and antagonism in vitro towards pathogens dangerous for fish such as Aeromonas salmonicida, A. hydrophila, and Pseudomonas fluorescens, therefore meeting the criteria adopted for strains with probiotic properties (12).
The probiotic-supplemented diet (given to the LAB group) was prepared according to a slightly modified method of Harikrishnan et al. (11). The cultures of L. plantarum strains from overnight growth on de Man, Rogosa, and Sharpe agar (MRS) plates were inoculated into 10 mL volumes of MRS broth incubated at 29°C for 24 h, then centrifuged at 2,500 g for 20 min at 4°C, after which the cell pellets were washed twice and resuspended in 0.9% (w/v) saline. Next, the concentration was adjusted to 1010 cells mL−1 using a DR 3900 Hach-Lange spectrophotometer (Hach-Lange, Germany) and the suspension was mixed proportionally to obtain an experimental probiotic mixture of the five strains. The probiotic mixture was stirred thoroughly with 567 g of commercial carp feed (Aller Aqua, Denmark) to achieve a dose of ~108 cells g−1 of feed. The modified feed was stored in screw-top glass bottles at room temperature until required. To ensure a high probiotic level in the supplemented feed, fresh diets were prepared on a weekly basis (6). Fish in the control group were supplied with commercial feed only.
Sample collection. After 14 days of feeding, the fish were sacrificed with an overdose of anaesthetic (MS-222, Sigma-Aldrich, USA). Blood was collected from the caudal vein and transferred into Eppendorf tubes. Following centrifugation (2,000 g, 10 min, 4°C), serum was collected and stored at −20°C until use. Serum was separated and subjected to lysozyme, ceruloplasmin, total protein, and total immunoglobulin assays. The bactericidal activity of serum was also evaluated.
Evaluation of non-specific humoral immunity and biochemical parameters. The lysozyme activity in the plasma was measured with the turbidimetric assay presented by Siwicki and Anderson (24). The standard used was hen egg white lysozyme (Sigma-Aldrich) and Micrococcus lysodeikticus (Sigma-Aldrich) suspension in phosphate buffer.
The ceruloplasmin activity in the plasma was determined according to Siwicki et al. (25) modified for micro-methods. The plasma was incubated for 15 min on microplates in acetate buffer containing 0.2% p-phenylenediamine (PPD) (Sigma-Aldrich). After incubation, 140 μL of 0.02% sodium azide solution was added to stop the reaction. The ceruloplasmin activity was measured at 540 nm on the DR 3900 Hach-Lange spectrophotometer (Hach-Lange).
Total serum immunoglobulin levels were measured using the Lowry micro-method modified by Siwicki and Anderson (24). This method first requires precipitation of immunoglobulin out of serum with polyethylene glycol (10,000 kDa).
Total serum protein levels were determined spectrophotometrically according to the colorimetric Lowry micro-method described by Siwicki and Anderson (24).
Serum bactericidal activity. Before the tests, fish sera were pooled (four individuals) within the control and LAB groups (n = 5). The serum bactericidal activity towards A. hydrophila (pathogenic to carp) and Yersinia ruckeri (non-pathogenic to carp, but occasionally isolated from healthy fish) was determined using the spectrophotometric method described by Villamil et al. (27) with some modifications. In short, equal volumes of serum and bacterial suspension (A. hydrophila or Yersinia ruckeri, both bacteria adjusted to 1 × 108 cells mL−1) in PBS were mixed together in sterile Eppendorf tubes and incubated at room temperature for 1 and 3 h. Subsequently, 100 μL of each sample was transferred to wells of a microtiter plate and centrifuged at 200 g for 10 min. The supernatant was removed and 100 μL of MTT (3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma-Aldrich) solution (1 mg mL−1) in PBS was added to each well. After 15 min in the dark, the optical densities (OD) of samples were measured at 600 nm. Bacterial suspensions mixed with sterile PBS in the ratio 1:1 served as controls. The ODs of samples were compared to the ODs of the controls and expressed as a percentage of surviving bacteria.
Isolation of carp immune cells. Before cell isolation, carp head kidneys and spleens were pooled (four organs) within the control and LAB groups (n = 5). Following aseptic removal, pooled organs were passed through 100 μm nylon mesh and placed in RPMI-1640 medium with L-glutamin and sodium bicarbonate (Sigma-Aldrich). Then, the cell suspensions were layered over the Histopaque 1077 density gradient (Sigma-Aldrich) and centrifuged at 400 g for 40 min at 4°C. The interface cells were collected and washed three times in RPMI-1640 medium. The viability of isolated immune cells was evaluated by trypan blue exclusion. Live cells were suspended at a concentration of 1×106 cells mL−1 in RPMI-1640 medium supplemented with 10% foetal calf serum and 1% antibiotic-antimycotic solution (both reagents from Sigma-Aldrich), dispensed into 96-well microtiter plates (Nunc, Denmark), and cultured and incubated at 22°C. Samples obtained from each pool were tested in duplicate using the assays described in the following.
Evaluation of non-specific cellular immunity
Pinocytosis assay – neutral red uptake (NRU) assay. The pinocytosis assay was performed using a commercial TOX-4 kit from Sigma-Aldrich, according to the manufacturer’s instructions. Following overnight incubation, plates were washed to remove non-adherent cells, then phagocytes were incubated in serum-free medium containing 0.033% of neutral red for 3 h at 22°C in order to allow pinocytosis. Afterwards, cells were washed with PBS and 100 μL of solubilisation solution (1% acetic acid in 50% ethanol) was added to each well. After gentle shaking, the absorbance was measured at a wavelength of 540 nm with 690 nm as a reference wavelength. The results were compared to the OD of the baseline neutral red solution (cell-free wells containing 0.033% neutral red solution in 100 μL of solubilisation solution) and expressed as a percentage of ingested dye.
Respiratory burst activity (RBA) test. The metabolic activity of phagocytes was determined by the measurement of the intracellular RBA after stimulation with phorbol myristate acetate (PMA) (Sigma-Aldrich), as described previously (15). Following overnight incubation, the plates were washed to remove non-adherent cells and 100 μL of PMA (1 μg mL−1) in 0.1% nitroblue tetrazolium (NBT) (Sigma-Aldrich) solution was added to each well. The mixture was incubated for 60 min at 22ºC. Afterwards, supernatants were removed and the reaction was stopped by the addition of absolute ethanol. The formazan produced within the cells was dissolved in 120 μL of 2M KOH and 140 μL of dimethylsulphoxide (DMSO) (POCh, Poland) and the optical density was measured colorimetrically at 620 nm. The results were expressed as stimulation index (SI) values, which were calculated by dividing the mean OD of PMA-stimulated cells by the ODs of control, unstimulated cells.
Potential killing activity (PKA) test. The technique described earlier (15) was used to measure the PKA of phagocytes. Adherent cells were mixed with 100 μL of 0.1% NBT solution in PBS, containing A. hydrophila (1×108 cells mL−1) and incubated for 60 min at 22ºC. After incubation, the supernatants were removed and cells were fixed with absolute ethanol. A total of 120 μL of 2M KOH and 140 μL of DMSO were added to each well and the amount of extracted reduced NBT was measured colorimetrically at 620 nm. These results were also expressed as stimulation index values, which were calculated by dividing the mean OD of bacteria-stimulated cells by the ODs of control, unstimulated cells.
Proliferative response of lymphocytes – MTT reduction assay. The mitogenic response of carp lymphocytes was determined using the MTT colorimetric assay, described originally by Mosmann (16), with some modifications. Briefly, 100 μL aliquots of head kidney or spleen cell suspensions were cultured for 72 h at 22°C in growth medium containing mitogens: concanavalin A (ConA) as a T-cell mitogen or lipopolysaccharide (LPS) from Escherichia coli as a B-cell mitogen (both mitogens in concentrations of 10 μg mL−1, from Sigma-Aldrich). Control cells were maintained in RPMI medium without mitogens. Following incubation, 10 μL of MTT solution (10 mg mL−1) in PBS was added to each well and the plate was incubated for another 3 h. Then, the supernatants were removed and 100 μL of DMSO was added to each well. The optical density was measured at a wavelength of 570 nm with 640 nm as a reference wavelength using a Sunrise absorbance reader (Tecan, Austria). As in the previously described assays, the results were expressed as stimulation index values, derived from division of the mean OD of mitogen-stimulated cells by the ODs of control, unstimulated cells.
Challenge test. A. hydrophila originating from the Department of Fish Pathology and Immunology, Inland Fisheries Institute, Poland, and isolated from infected common carp was identified based on the phenotypic and biochemical characteristics, and its cultures were stored at −70°C in tryptic soy broth (TSB) containing 10% glycerol until further use. After two weeks of feeding, 20 fish were collected from each of the probiotic-supplemented and control tanks and distributed into six other tanks for a challenge study. The challenge was performed by intraperitoneally injecting 0.2 mL of A. hydrophila suspension (1 × 108 CFU). The challenged fish were kept under observation for two weeks and the dead fish were removed from the tanks and used for bacterial re-isolation. The mortality was recorded daily and the results are presented as cumulative rates.
Statistical analysis. Data were analysed statistically by Student’s t-test. Evaluation of the results was performed using GraphPadPrism software package (GraphPad Software, USA).
Results
Evaluation of non-specific humoral immunity and biochemical parameters. The serum lysozyme activity and the ceruloplasmin activity were not significantly different between the experimental groups (P > 0.05). The gammaglobulin level and total protein level in serum were significantly higher (P < 0.05) in the LAB group compared to the control group (Table 1).
Table 1.
Humoral-mediated immune parameters in common carp given L. plantarum supplemented feed (LAB) or commercial diet (control)
| Parameter | Control | LAB |
|---|---|---|
| Lysozyme activity in serum (mg L−1) | 0.73 ± 0.09 | 0.75 ± 0.07 |
| Ceruloplasmin activity in serum (IU) | 46.23 ± 1.94 | 48.37 ± 3.44 |
| Total protein level in serum (g L−1) | 25.59 ± 4.84 | 37.53 ± 5.72* |
| Total γ-globulin level in serum (g L−1) | 5.13 ± 1.68 | 9.95 ± 1.79* |
mean ± SD, n = 20, *mean values were significantly different, P < 0.05
Bactericidal activity of serum. Serum bactericidal activity against A. hydrophila and Y. ruckerii was not found in either of the groups. The results showed an increase in the level of live bacteria in the LAB group, and the increase was significant (P < 0.05) after 3 h incubation (Fig. 1).
Fig. 1.
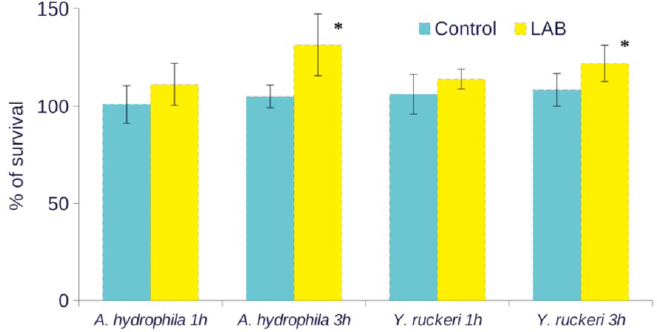
Serum bactericidal activity of common carp fingerlings against A. hydrophila and Y. ruckeri after two weeks of feeding L. plantarum strains supplemented (LAB) or non-supplemented (control) diets. Values represent percentages of surviving bacteria and are means with standard deviations represented by vertical bars for five pooled samples. * Mean values were significantly different, P < 0.05
Evaluation of non-specific cellular immunity. The probiotic treatment resulted in no significant differences (P > 0.05) in the pinocytic activity of spleen or head kidney phagocytes in the groups (Table 2). The respiratory burst activity of spleen and head kidney phagocytes in the LAB group was found not to differ significantly between the LAB and control groups (P > 0.05) (Table 2). The potential killing activity of spleen phagocytes was not marked by significant differences (P > 0.05) between the LAB and control groups, but the potential killing activity of head kidney phagocytes was significantly higher (P < 0.05) in the LAB group compared to the controls (Table 2).
Table 2.
Cell-mediated immune parameters in common carp fed L. plantarum supplemented feed (LAB) or commercial diet (control)
| Parameter | Control | LAB |
|---|---|---|
| Pinocytic (% of ingested activity NR) of spleen phagocytes | 7.78 ± 2.11 | 10.17 ± 2.36 |
| Pinocytic (% of ingested activity NR) of head kidney phagocytes | 52.49 ± 8.36 | 58.27 ± 9.98 |
| Respiratory burst activity of spleen phagocytes (SI) | 1.171 ± 0.152 | 1.327 ± 0.166 |
| Respiratory burst activity of head kidney phagocytes (SI) | 3.45 ± 0.599 | 3.714 ± 0.379 |
| Potential killing activity of spleen phagocytes (SI) | 1.317 ±0.110 | 1.392 ± 0.054 |
| Potential killing activity of head kidney phagocytes (SI) | 1.675 ±0.167 | 2.345 ± 0.369* |
| Proliferative response of spleen lymphocytes stimulated by ConA (SI) | 1.358 ± 0.186 | 1.445 ± 0.225 |
| Proliferative response of head kidney lymphocytes stimulated by ConA (SI) | 1.412 ± 0.164 | 1.537 ± 0.185 |
| Proliferative response of spleen lymphocytes stimulated by LPS (SI) | 1.154 ± 0.130 | 1.218 ± 0.212 |
| Proliferative response of head kidney lymphocytes stimulated by LPS (SI) | 1.216 ± 0.134 | 1.590 ± 0.204* |
mean ±SD, n = 20, *mean values were significantly different, P < 0.05
Proliferative response of lymphocytes – MTT reduction assay. In the proliferative response of common carp spleen and head kidney lymphocytes, there was found no significant difference between the LAB and control groups (P > 0.05) (Table 2). The proliferative response of carp head kidney lymphocytes stimulated by LPS was significantly higher (P < 0.05) in the LAB group than in the control group, whereas in the case of lymphocytes isolated from the spleen, there was no significant difference (P > 0.05) between the experimental groups (Table 2).
Challenge test. The dietary supplementation with L. plantarum led to mortality reduction after challenge with A. hydrophila (Fig. 2). A dramatic rise in mortality of common carp fingerlings infected with A. hydrophila occurred on days 5 to 7 post-challenge. In particular, the fish fed the probiotic-supplemented diet (the LAB group) seemed to have higher resistance than the fish fed the control diet (control group), and the cumulative mortality in the groups reached 65% and 85%, respectively. Typical symptoms of haemorrhagic septicaemia were observed in moribund or dead fish. Colonies of A. hydrophila were isolated from dead fish.
Fig. 2.
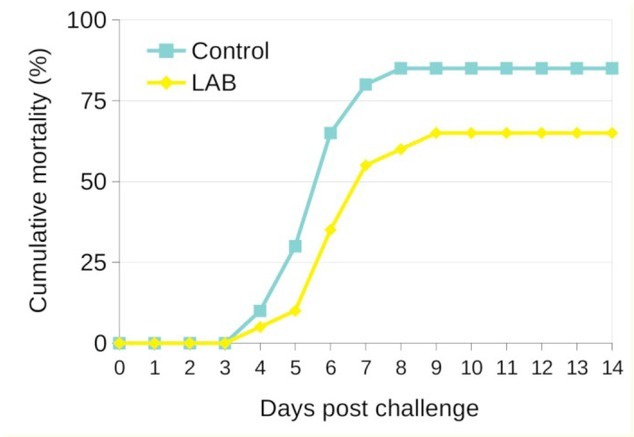
Effect of two weeks’ dietary administration of L. plantarum strains (LAB) compared to unsupplemented commercial feed provision (control) on the percentage of post-challenge cumulative mortality of common carp fingerlings after intraperitoneal injection of pathogenic A. hydrophila
Discussion
This research is an effort to stimulate the resistance in common carp, a commercially important fish species in Poland and the whole of Europe. Bacterial diseases are the main threat to carp aquaculture, particularly to carp fingerlings. Pathogenic lesions are most often the source of isolates of A. hydrophila, which cause infections in immunocompromised fish. Infections are most common after the wintering period when the fish’s energy reserves are depleted, or when fish are under stress (during catching, sorting, and transport). Other factors predisposing to disease are high water temperature fluctuations, oxygen deficit, and an increase in the level of nitrogen compounds.
Previous studies carried out on Cyprinidae such as roho labeo (Labeo rohita) and catla (Catla catla) demonstrated that administration of probiotics to fish favours an improvement in such immunological parameters as lysozyme activity, total protein content, and activity of RBA. Additionally, it improves the resistance of fish to infections with the bacteria A. hydrophila, Edwardsiella tarda, Vibrio parahaemolyticus, and Vibrio harveyi (6, 8). It is known that the protective role of probiotics arises from the modulation of the activity of gut-associated lymphoid tissues (GALT), and most studies on the immunostimulant action of probiotics in fish focus on the evaluation of non-specific resistance parameters (4). The reason is the phylogenetic position of fish. The specific immunological response of exothermic animals occurs at a much slower rate and is less important than in endotherms. The non-specific immunity factors act rapidly and effectively in a broader range of temperatures, while lymphocytes are more efficient at higher temperatures (14).
Among the analysed mechanisms of non-specific humoral immunity, the bacteria tested in this study had a beneficial effect only on the levels of immunoglobulins and total protein in serum, having no influence on the activity of lysozyme. Lysozyme is produced by phagocytic cells, including macrophages and neutrophils, and is a protein substance with enzymatic properties, which can decompose the cell wall mainly of gram-positive bacteria, and lysozyme is a component of non-specific humoral immunity. In our study, we did not observe differences in the activity of lysozyme in serum from group of fish fed the Lactobacillus supplemented feed and the control group. In other investigations, no significant differences were found in the activity of lysozyme attributable to probiotic feeds in such fish as brown trout (Salmo trutta) or rainbow trout (2, 3, 20) and such supplementation may even lead to depressed lysozyme activity (8).
Total protein is composed of all blood protein fractions (albumins, fibrinogen, immunoglobulins, and lysozyme) and is an important indicator of the physiological condition as well as a reflection of the potential of the innate resistance of an organism. In our investigations, we observed an increase in the total protein level in the group provided with a feed supplemented with Lactobacillus following 14 days of treatment. Al-Dohail et al. (1) detected a similar response in North African catfish (Clarias gariepinus) receiving a feed supplemented with Lactobacillus acidophilus. A similar effect was achieved also when rainbow trout, catla, and roho labeo were maintained on a feed with addition of Bacillus spp. bacteria (6, 17, 18).
The fish serum contains natural immunoglobulins, which protect an organism until the constituents of specific humoral immunity develop. The polyreactivities of the immunoglobulins enable them to react with the broad spectrum of antigens simultaneously and protect against them. In this study, the level of immunoglobulins was found to be nearly twice as high in the probiotic supplemented group as in the controls. Balcázar et al. (2) demonstrated an increase in the concentration of immunoglobulins in rainbow trout serum after administration of lactic acid bacteria, although the difference relative to the control was not significant. An elevated level of immunoglobulins has also been described in African catfish, rainbow trout, and roho labeo (1, 19); however, there are reports suggesting that the elevated level of immunoglobulins is only a transient effect associated with the administration of probiotics (8).
An important contributor to anti-bacterial immunity is the bactericidal activity of serum, distinguished by the rapid kinetics of immunological responses. This type of immunity has a multi-factorial nature and depends on the activity of lysozyme, the complement system and natural immunoglobulins as well as the properties of a given microorganism. In our study, we did not observe any bactericidal activity of serum in either the L. plantarum-supplemented or the control group against the pathogenic bacteria A. hydrophila or Y. ruckeri. These pathogenic bacteria were characterised by high resistance to the activity of common carp serum. Most Gram-negative bacteria are unaffected by lysozyme owing to the outer membrane which encapsulates their cells. In addition, A. hydrophila and Y. ruckeri are characterised by high variability of surface antigens, which aids their defence against the bactericidal action of serum. We determined a higher survivability of the analysed bacteria in the serum sampled from the fish fed the diet supplemented with L. plantarum, despite its higher level of immunoglobulins, which may be associated with a higher level of total protein in serum and suggest serum to be a more valuable substrate for multiplying bacteria. Other studies, however, have demonstrated the bactericidal activity of serum of roho labeo fed B. subtilis against the bacteria A. hydrophila (13).
It has been found that probiotics affect the cellular mechanisms of non-specific defence of an organism by stimulating the activity of phagocytic cells (monocytes/macrophages and neutrophils), serving as the first line of defence. These cells also stimulate the development of specific immunity, and a necessary condition underlying their efficient functioning is the proper course of metabolic transformations. The intracellular process of killing microorganisms is accompanied by the activation of several enzymes, which leads to the generation of reactive forms of oxygen and nitrogen.
The considerable increase in the PKA of phagocytes originating from the head kidney after stimulation with A. hydrophila evidenced in this study, may indicate an improved antibacterial defence in animals which receive the tested strains of bacteria in their feed. Elevated bactericidal activity of macrophages has also been observed in rainbow trout after feed supplementation with Kocuria SM1 and B. subtilis (18, 23). Another observation made in the current study was no increase in the ability of the head kidney phagocytes to undergo respiratory burst. However, scientific reports on this subject are frequently equivocal. There are studies implicating that application of probiotics does not have a significant influence on the metabolic activity of neutrophils and monocytes (17), while other investigations, carried out in both in vitro and in vivo conditions, point to a considerable elevation in RBA in many aquatic animals, including fish (8, 9, 19, 23, 26). Giri et al. (8) showed that the metabolic activity of phagocytes derived from the head kidney of roho labeo was significantly increased after 30 days of feeding the fish L. plantarum at a dose of 1010 CFU g−1, while in the fish receiving a dose of 108 CFU g−1 of the bacteria, the same parameter peaked after 60 days. Increased metabolic activity of phagocytes from the head kidney after PMA stimulation was also found in groupers following supplementation with L. plantarum (26) as well as in the fry of common carp fed Paenibacillus polymyxa (9). The differences in obtained results were possibly caused by the shorter period of feeding of common carp fingerling with the probiotic supplement in our study.
An increase in pinocytic activity is considered one of the most distinguishing features of macrophage activation. This increase in pinocytic activity (ability to absorb micromolecular particles of neutral red) of head kidney and spleen phagocytes was not observed in this study, which agrees with the results obtained by Pieters et al. (21) in rainbow trout maintained on a feed with A. sobria and Brochothrix thermosphacta.
Relatively little attention has been paid in the available literature to the influence of probiotics on fish lymphocytes, and the few references mostly discuss the issue in the context of production of selected cytokines (3). To the best of our knowledge, no studies have been completed that would evaluate the influence of probiotic bacteria on the proliferative response of fish lymphocytes. However, since quite numerous investigations on a mouse model implicate the modulating effect of probiotics on the mitogenic response of lymphocytes, we decided to determine this parameter as well.
In common carp fingerling fed the L. plantarum supplemented diet for 14 days, the proliferative activity of B lymphocytes stimulated with bacterial LPS was found to have increased significantly. A similar proliferative effect of various L. plantarum strains on the spleen lymphocytes in mice was previously described by Ren et al. (22). In a study by Bujalance et al. (4), this bacterium did not increase the proliferation of spleen B lymphocytes in healthy mice, but had an immunocorrective effect in immunocompromised animals, raising the inhibited proliferative response of B cells to LPS considerably. The humoral type of immune response prevails in the specific response of an organism to bacterial infections. This response is conditioned by the function of B lymphocytes and their production of antibodies, which bind and neutralise the antigen as well as activate several mechanisms leading to the pathogen removal. Thus, the effect observed in our research may suggest indirectly that L. plantarum has a stimulating influence not only on the non-specific but also on the specific antibacterial response in common carp.
Our investigations have also shown that dietary supplementation with L. plantarum lowers the mortality rate among common carp fingerlings infected with A. hydrophila. Harikrishnan et al. (11) observed a similar tendency and Giri et al. (8) determined the highest survival rate among roho labeo after infection with A. hydrophila in a group of fish fed a diet containing 108 CFU g−1 L. plantarum. Such enhancement in fish disease resistance can be due to the increase in immune factors and the antagonistic activity of L. plantarum towards bacterial pathogens (12).
The results of our research suggest that supplementation of a diet with L. plantarum stimulates antibacterial resistance in common carp and can be an efficient measure in the defence against bacterial infections. Administration of probiotic bacteria may help to improve the health of fish and be an alternative prophylactic approach, which inhibits the multiplication of pathogens while stimulating the host organism by raising its resistance to bacterial infections. However, the obtained results of this study suggest that there is a need to continue the research under conditions prevailing in the natural environment and on the fish farm.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was supported by the S-004 Project of the Stanislaw Sakowicz Inland Fisheries Institute, Olsztyn, Poland, and did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Animal Rights Statement: The research was in compliance with Polish animal welfare regulations and approved by the Local Ethics Committee for Animal Experimentation of the Stanislaw Sakowicz Inland Fisheries Institute in Olsztyn, Poland.
References
- 1.Al-Dohail M.A., Hashim R., Aliyu-Paiko M.. Evaluating the use of Lactobacillus acidophilus as a biocontrol agent against common pathogenic bacteria and the effects on the haematology parameters and histopathology in African catfish Clarias gariepinus juveniles. Aquac Res. 2011;42:196–209. [Google Scholar]
- 2.Balcázar J.L., de Blas I., Ruiz-Zarzuela I., Vendell D., Calvo A.C., Marquez I., Gironés O., Muzquiz J.L.. Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout Salmo trutta. Brit J Nutr. 2007;97:522–527. doi: 10.1017/S0007114507432986. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee G., Ray A.K.. The advancement of probiotics research and its application in fish farming industries. Res Vet Sci. 2017;115:66–77. doi: 10.1016/j.rvsc.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Bujalance C., Moreno E., Jimenez-Valera M., Ruiz-Bravo A.. A probiotic strain of Lactobacillus plantarum stimulates lymphocyte responses in immunologically intact and immunocompromised mice. Int J Food Microbiol. 2007;113:28–34. doi: 10.1016/j.ijfoodmicro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Cebeci A., Gurakan C.. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 2003;20:511–518. [Google Scholar]
- 6.Das A., Nakhro K., Chowdhury S., Kamilya D.. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla Catla catla. Fish Shellfish Immunol. 2013;35:1547–1553. doi: 10.1016/j.fsi.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Dawood M.A.O., Koshio S., Ishikawa M., Yokoyama S.. Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance, and immune response of red sea bream, Pagrus major. Aquaculture. 2015;442:29–36. [Google Scholar]
- 8.Giri S.S., Sukumaran V., Oviya M.. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 2013;34:660–666. doi: 10.1016/j.fsi.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A., Gupta P., Dhawan A.. Dietary supplementation of probiotics affects growth, immune response and disease resistance of Cyprinus carpio fry. Fish Shellfish Immunol. 2014;41:113–119. doi: 10.1016/j.fsi.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Guz L., Kozińska A.. Antibiotic susceptibility of Aeromonas hydrophila and A. sobria isolated from farmed carp Cyprinus carpio Bull Vet Inst Pulawy. 2004;48:391–395. [Google Scholar]
- 11.Harikrishnan R., Balasundaram C., Heo M.S.. Potential use of probiotic- and triherbal extract-enriched diets to control Aeromonas hydrophila infection in carp. Dis Aquat Organ. 2010;92:41–49. doi: 10.3354/dao02240. [DOI] [PubMed] [Google Scholar]
- 12.Kazuń B., Kazuń K., Żylińska J., Siwicki A.K.. In vitro study of Lactobacillus plantarum properties as a potential probiotic strain and an alternative method to antibiotic treatment of fish. Fish Aquat Life. 2018;26:49–57. [Google Scholar]
- 13.Kumar R., Mukherjee S.C., Ranjan R., Nayak S.K.. Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunol. 2008;24:168–172. doi: 10.1016/j.fsi.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Magnadóttir B.. Innate immunity of fish (overview) Fish Shellfish Immunol. 2006;20:137–151. doi: 10.1016/j.fsi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Małaczewska J., Siwicki A.K.. The in vitro effect of commercially available noble metal nanocolloids on the rainbow trout Oncorhynchus mykiss leukocyte and splenocyte activity. Pol J Vet Sci. 2013;16:77–84. doi: 10.2478/pjvs-2013-0011. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T.. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Nayak S.K., Swain P., Mukherjee S.C.. Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.) Fish Shellfish Immunol. 2007;23:892–896. doi: 10.1016/j.fsi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Newaj-Fyzul A., Adesiyun A.A., Mutani A., Ramsubhag A., Brunt J., Austin B.. Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout Oncorhynchus mykiss Walbaum) J Appl Microbiol. 2007;103:1699–1706. doi: 10.1111/j.1365-2672.2007.03402.x. [DOI] [PubMed] [Google Scholar]
- 19.Nikoskelainen S., Ouwehand A., Bylund G., Salminen S., Lilius E.M.. Immune enhancement in rainbow trout Oncorhynchus mykiss by potential probiotic bacteria Lactobacillus rhamnosus. Fish Shellfish Immunol. 2003;15:443–452. doi: 10.1016/s1050-4648(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 20.Panigrahi A., Kiron V., Puangkaew J., Kobayashi T., Satoh S., Sugita H.. The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture. 2005;243:241–254. [Google Scholar]
- 21.Pieters N., Brunt J., Austin B., Lyndon A.R.. Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliis skin infections in rainbow trout Oncorhynchus mykiss Walbaum) J Appl Microbiol. 2008;105:723–732. doi: 10.1111/j.1365-2672.2008.03817.x. [DOI] [PubMed] [Google Scholar]
- 22.Ren D., Li C., Qin Y., Yin R., Du S., Liu H., Zhang Y., Wang C., Rong F., Jin N.. Evaluation of immunomodulatory activity of two potential probiotic Lactobacillus strains by in vivo tests. Anaerobe. 2015;35:22–27. doi: 10.1016/j.anaerobe.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Sharifuzzaman S.M., Austin B.. Development of protection in rainbow trout Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum following use of the probiotic Kocuria SM1. Fish Shellfish Immunol. 2010;29:212–216. doi: 10.1016/j.fsi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Siwicki A.K., Anderson D.P. Immunostimulation in fish: measuring the effects of stimulants by serological and immunological methods. USFWS, IFI; Olsztyn: 1993. pp. 1–17. [Google Scholar]
- 25.Siwicki A.K., Zakęś Z., Trapkowska S., Terech-Majewska E., Czerniak S., Głąbski E., Kazuń K.. Nonspecific cellular and humoral defence mechanisms in pikeperch Sander lucioperca grown in an intensive system of culture. Arch Pol Fish. 2003;11:207–212. [Google Scholar]
- 26.Son V.M., Chang C.C., Wu M.C., Guu Y.K., Chiu C.H., Cheng W.. Dietary administration of the probiotic, Lactobacillus plantarum enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol. 2009;26:691–698. doi: 10.1016/j.fsi.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Villamil L., Figueras A., Novoa B.. Immunomodulatory effects of nisin in turbot Scophthalmus maximus L.) Fish Shellfish Immunol. 2003;14:157–169. doi: 10.1006/fsim.2002.0425. [DOI] [PubMed] [Google Scholar]


