Abstract
Receptor for advanced glycation end products (RAGE) is a major proinflammatory receptor and its role in atherosclerosis has only been emphasized recently. Increasing evidence has demonstrated an association between RAGE and the susceptibility to atherosclerosis development. Therefore, the role of RAGE in atherogenesis and the possible impact of genetic variations in RAGE on the atherosclerotic process in subjects with coronary artery disease (CAD) was investigated in the present study. The RAGE expression in carotid specimens was analyzed by immunohistochemistry and sequence variations of the RAGE gene selected from the Hapmap database were also screened. The plasma levels of S100 calcium binding protein B (S100B) were determined by ELISA. Immunohistochemical staining of tissue samples demonstrated an increased RAGE expression in atherosclerotic carotid plaques compared with that in normal arteries. Furthermore, compared with the corresponding wild-type genotype, the rs2269422 single-nucleotide polymorphism of RAGE was associated with the susceptibility of patients with CAD to atherosclerosis. Furthermore, reverse transcription polymerase chain reaction and western blot analyses indicated increased coronary artery RAGE mRNA levels and protein expression, respectively, in CAD patients vs. control subjects. Furthermore, the plasma levels of S100B in CAD patients that were carriers of the AA/AT genotype of the rs2269422 variant of RAGE was increased compared with that in TT genotype carriers; as this was also identified in control subjects, it may not be CAD-specific. The RAGE rs2269422 variant is therefore significantly associated with an increased occurrence of CAD in the present Han Chinese population. Thus, RAGE variants significantly impact the risk of CAD in Han Chinese subjects.
Keywords: receptor for advanced glycation end products, coronary artery disease, single nucleotide polymorphism
Introduction
In developed countries, cardiovascular disease is the primary cause of mortality and disability worldwide, and its incidence is increasing. Among these, coronary artery disease (CAD) has an important role and is a frequent cause of death. Numerous environmental factors, including obesity and hyperlipemia, have been confirmed to lead to the development of CAD (1). However, it remains a challenge to fully elucidate the pathogenesis of CAD and determine risk factors (1,2).
Identification of individuals at risk and the rapid and accurate diagnosis of CAD are warranted to further enhance the success of preventative or therapeutic procedures. A decrease in the morbidity of CAD and the associated mortality may be attributed to primary and secondary care and prevention programs (3).
Certain studies have indicated an association between genetic variants and the occurrence of CAD (4,5). S100 calcium binding protein B (S100B), a ligand for the receptor for advanced glycation end products (RAGE), has a crucial role in the development and progression of atherosclerosis (6–8). RAGE interacting with its ligand, S100B, induces lymphocytes to enter atherosclerotic plaque lesions through the damaged endothelial barrier, leading to the accumulation of inflammatory cells in coronary artery atherosclerotic plaques, thus contributing to the development of atherosclerosis. Furthermore, enhanced RAGE expression has been observed in atherosclerotic lesions from apolipoprotein E-deficient mice (9–12). It has also been reported that variants of RAGE and its ligand are associated with susceptibility to type 2 diabetes (7,9,13). Therefore, it is vital to elucidate the functions of RAGE and thereby enhance the current understanding of the pathogenesis of CAD.
To the best of our knowledge, only few studies have reported an association between RAGE variants and the occurrence of CAD in the Han Chinese population. Therefore, the association between RAGE tag single nucleotide polymorphisms (tagSNPs) and the risk of cardiovascular disease was explored in the present study. The present study aimed to increase the understanding of the effect of RAGE polymorphisms on atherogenesis in subjects with CAD.
Patients and methods
Study participants
A total of 1,719 Han Chinese subjects who were treated at Shenyang Military General Hospital (Shenyang, China) between July 2014 and August 2016 were included in the present study. These included 852 patients with CAD and 867 control subjects without any cardiovascular disease. In these participants, the presence of conventional cardiovascular risk factors according to the Guidelines for Assessment and Management of cardiovascular risk from 2007 (14) were determined by the treating doctor using a standardized interview, which included the history of smoking, hypertension and diabetes, to assess the patient's symptoms. The body weight (kg) divided by height (m)2 was used to determine the body mass index. The conventional criteria for diagnosis of hypertension and type 2 diabetes mellitus were based on the World Health Organization guidelines. Hypertension was diagnosed according to either a diastolic pressure of ≥90 mmHg or arterial blood pressure ≥140 mmHg according to the Seventh Report of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (15). All participants underwent coronary angiography at the Shenyang Military General Hospital to evaluate the suspected or established CAD. Patients with CAD recruited for the present study were those who had undergone coronary angiography after being diagnosed with CAD based on an angiography examination (>70% stenosis affecting at least one coronary vessel). Among the 852 CAD patients, they were the patients with mixed acute coronary syndrome. The participants with a normal coronary angiogram or major coronary artery stenosis of ≤20% according the results of coronary arteriography were defined as the control group. Furthermore, the control group did not have a history of CAD or ultrasonic cardiogram abnormalities (13). The patients with CAD were a mixed group of patients with acute coronary syndrome (ACS) and stable CAD patients. ACS based on the pathological basis of rupture or invasion of atherosclerotic plaque and subsequent complete or incomplete occlusive thrombosis included the acute T-segment elevation myocardial infarction, acute non-ST-segment elevation myocardial infarction and unstable angina (16). Cardiac syndrome X, also known as microvascular angina, is defined as angina pectoris caused by abnormalities of the small coronary arteries, and is characterized by the patient experiencing chest pain during exercise and evidence of myocardial ischemia with a non-invasive stress test (17). With these patients the coronary angiography can appear normal (18). This is usually diagnosed based on chest pain typical for angina, exercise-induced significant ST-segment depression and normal coronary angiograms (19). Syndrome X is diagnosed based on chest pain, exercise-induced ST-segment depression and the absence of either significant epicardial coronary stenosis or vasospasm (20). Certain patients with syndrome X exhibit pathologic changes around microvessels themselves (21). As control subjects, those patients with a normal coronary angiogram and suspected syndrome X were subjected to the treadmill exercise test. If the test was negative, CAD was excluded and the subjects were enrolled in the control group.
Patients with active inflammatory disease, autoimmune disease, severe heart failure, hemodynamic instability, suspected myocarditis or pericarditis, diseases of the hematopoietic system, extension of kidney or liver disease, malignant disease, intake of immunosuppressive drugs and renal or hepatic diseases were excluded from this study.
Prior to any invasive procedures or medical therapies, peripheral blood samples of the control and CAD patients were drawn at the time-point of admission to the hospital. The blood was collected in pyrogen-free EDTA-containing tubes, and after isolation, the plasma samples were immediately paced on ice and then stored at −80°C.
Selection of tagSNPs
The Tiangen DNA extraction kit (Beijing, China) was used to extract DNA from peripheral blood. The Han population Hapmap data (http://www.hapmap.org) was used to identify TagSNPs that covered the entire region of the RAGE gene, from 10,000 bp upstream to 4,000 bp downstream (22). Lewontin's D′ statistics and r2 correlation statistics were used to assess the pairwise linkage disequilibrium difference. The variants with a frequency at a cut-off of 0.05 and an r2 threshold of 0.8 were further investigated. A total of 25 tagSNPs of RAGE (rs10754558, rs10925025, rs12048215, rs12143966, rs12564791, rs1800624, rs2027432, rs204993, rs204994, rs2071290, rs2269421, rs2269422, rs2269423, rs2282659, rs3006476, rs3014880, rs3014885, rs3130349, rs3134943, rs3134946, rs3738448, rs4772, rs4925648, rs7528887 and rs7529058) were selected from different haplotype regions.
Genotyping
The 25 tagSNPs of the RAGE gene in samples from the CAD and control patients were analyzed by matrix-assisted laser desorption time of flight (MALDI-TOF) mass spectrometry. Polymerase chain reaction (PCR) was generally adopted (23); however, the phosphorylation state was also analyzed using shrimp alkaline phosphatase (Hoffman-La Roche, Basel, Switzerland) (24). All analyses were performed using a silicon chip made of a 384-well porous plate. PCR products were put onto a MALDI matrix and were analyzed using the Bruker Biflex III MALDI-TOF SpectroReader mass spectrometer (Bruker Corp., Billerica, MA, USA) (24). The mass spectra were analyzed using SpectroTyper software (Sequenom, Inc., San Diego, CA, USA).
Collection of carotid artery tissues
A total of 10 carotid and coronary arteries, with and without atherosclerotic lesions, were collected from eight donors. Atherosclerotic carotid and coronary arteries were acquired from four patients who died of atherosclerotic heart disease during autopsy. Normal carotid and coronary arteries were acquired from four subjects that were healthy prior to death during autopsy. The donors' relatives provided written informed consent.
Immunohistochemistry and immunofluorescence
H&E staining and Masson staining was performed to observe the morphological features of the carotid tissues collected. Immunohistochemistry and immunofluorescence were performed to determine RAGE and CD68 expression in the carotid tissue. In brief, carotid arteries (formalin-fixed, paraffin-embedded) were serially sectioned (4 µm; ≥3 sections per sample). Subsequently, anti-RAGE (cat. no. ab37647; Abcam, Cambridge, MA, USA) and anti-CD68 (cat no. GMO81404; Dako, Glostrup, Denmark) antibodies were used to analyze protein expression in the serial sections. The samples were incubated with the primary antibodies (dilution, 1:100) at 4°C overnight, the unbound antibodies were then washed away. Goat anti-rabbit Alexa Fluor 488 (cat. no. 11034) and donkey anti-mouse Alexa Fluor 555 secondary antibodies (cat. no. 31570; both; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) were incubated at room temperature for 2 h to detect the bound primary antibodies. Avidin-biotin-peroxidase was incubated at room temperature for 15 sec to detect the bound antibodies (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Staining with 3,3′-diaminobenzidine (cat. no. E2886; Sigma-Aldrich; Merck KGaA) was performed for 2 h at 4°C to detect immunoreactivity. Images were obtained using a light microscope and a confocal laser scanning microscope (FV500; Olympus, Tokyo, Japan).
Semi-quantitative reverse transcription (RT)-PCR analysis of RAGE
RNA from the coronary artery tissues from the CAD and control patients was isolated using the Eastep™ total RNA extraction kit (cat. no. LS1030; Promega Corp., Madison, WI, USA). The RNA from the CAD and control groups was reverse-transcribed using the First Strand complementary (c)DNA Synthesis kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's protocol. A PCR analysis was then used to amplify the cDNA of RAGE using the Superscript II kit (Takara Bio, Inc.) with Taq polymerase and the primers listed in Table I, which also states the thermocycling conditions and product lengths. The amplified cDNA was resolved using 2% agarose gel electrophoresis and visualized with ethidium bromide. ImageJ (version 1.8.0; National Institutes of Health, Bethesda, MD, USA) software was used to analyze the results as previous described (25).
Table I.
Primer sequences used for the genotyping analysis of RAGE and GAPDH variations.
| Name | Primer sequence (5′-3′) | Size of PCR product (bp) | Thermocycling protocol |
|---|---|---|---|
| RAGE | F: AGGTGAGTGGAGAAAGCCAG | 121 | 94°C for 3 min; |
| R: ATGTGTCAGGTGTTTAATCA | 35 cycles of 94°C for 30 sec, 56°C for | ||
| 30 sec and 72°C for 30 sec; | |||
| 72°C for 7 min; | |||
| 4°C ∞ | |||
| 94°C for 3 min; | |||
| GAPDH | F: AGGATGGTGTGGCTCCCTTG | 105 | 35 cycles of 94°C for 30 sec, 58°C for |
| R: GCAGGGCTGAGACAGCTTCC | 25 sec and 72°C for 25 sec; | ||
| 72°C for 7 min; | |||
| 4°C ∞ |
RAGE, receptor for advanced glycation end products; F, forward; R, reverse; PCR, polymerase chain reaction.
Western blot analysis
Tissues were lysed in lysis buffer containing freshly added protease inhibitor (cat. no. 78425; Invitrogen; Thermo Fisher Scientific, Inc.). After centrifugation at 12,000 × g for 10 min at 4°C, the protein in the supernatant was subjected to western blot analysis. Protein concentrations were determined using the bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Proteins were resolved by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking with 5% skimmed milk, the membranes were incubated with anti-RAGE (1:1,000 dilution; cat. no. ab37647) and anti-GAPDH antibody (1:1,000 dilution; cat no. ab9484; both Abcam) overnight at 4°C. Specific binding was detected by subsequent incubation with horseradish peroxidase-conjugated secondary antibodies at 4°C for 2 h and an enhanced chemiluminescence kit (cat. no. NCI4106; Pierce; Thermo Fisher Scientific, Inc.). The blots were quantified using a Bio-Rad Gel system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ELISA
The plasma levels of S100B in CAD and control patients were assessed using S100B ELISA kits (cat. no. DY1820-05; R&D Systems, Minneapolis, MN, USA). The absorbance of each well was detected at 450 nm and the plasma concentration of S100B was determined using a standard curve according to the manufacturer's protocol. When determining whether the S100B plasma levels were associated with the genotype of RAGE rs2269422, the rare AA homozygote carriers were grouped together with the AT heterozygotes due to the small number of AA homozygotes present in the control and CAD groups (26).
Statistical analysis
Values are expressed as the mean ± standard error of the mean and were analyzed using SPSS software version 21.0 (IBM Corp., Armonk, NY, USA). Student's t-test or the Mann-Whitney U-test was used to compare among the different groups, and multiple comparisons between groups were assessed by one-way analysis of variance (ANOVA) followed by Tukey's Honest Significant Difference post-hoc test analysis. The difference in the plasma levels of S100B between CAD and control groups were also analyzed by ANOVA. The distribution of genotype and allele frequencies between the CAD and control groups were analyzed by determining the Hardy-Weinberg equilibrium. The CAD risk was evaluated by the P-values, 95% confidence intervals (95% CIs) and odds ratios (ORs). The Bonferroni adjustment method was used for multiple comparisons among the 25 tagSNPs and a significance level of <0.002 (0.05/25=0.002) was required.
Results
Clinical characteristics of the study participants
The demographic data of the study population, including 852 CAD and 867 control subjects, are presented in Table II. In the control group, the distribution of genotypes was in a Hardy-Weinberg equilibrium. The CAD group was characterized by a higher prevalence of traditional atherosclerotic risk factors compared with that in the control group. The conventional vascular risk factors, including cigarette smoking, hypertension and diabetes mellitus had a higher prevalence in patients with CAD compared with the control subjects (P<0.05). In addition, increased levels of triglycerides, as well as low-density and very low-density lipoprotein-cholesterol, and lower levels of high-density lipoprotein-cholesterol were detected in the CAD patients compared with those in the control subjects (Table II).
Table II.
Clinical Characteristics of CAD patients and control subjects.
| Characteristic | Control group (n=867) | CAD group (n=852) | P-value |
|---|---|---|---|
| Age (years) | 58.3±7.9 | 59.8±8.2 | 0.001 |
| Sex (M/F) | 477/390 | 440/412 | 0.161 |
| BMI (kg/m2) | 24.8±4.7 | 25.2±4.2 | 0.001 |
| DM | 104 (12.0) | 266 (31.2) | P<0.001 |
| Hypertension | 432(49.8) | 514 (60.3) | P<0.001 |
| Smoking | 268 (30.9) | 369 (43.3) | P<0.001 |
| TG (mmol/l) | 1.74±1.38 | 2.41±1.48 | P<0.001 |
| TC (mmol/l) | 4.07±1.03 | 4.79±1.14 | P<0.001 |
| LDL-C (mmol/l) | 1.92±0.62 | 2.61±0.81 | P<0.001 |
| HDL-C (mmol/l) | 1.81±0.39 | 1.29±0.35 | P<0.001 |
Values are expressed as the mean ± standard error of the mean or n (%). CAD, coronary artery disease; BMI, body mass index; DM, diabetes mellitus; F, female; M, male; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Association of tagSNPs with the risk of CAD
All study participants were genotyped for the RAGE tagSNP rs2269422. The primary data for the tagSNPs are listed in Table III. The frequency distribution of the 25 tagSNPs in the controls was in accordance with the Hardy-Weinberg equilibrium (P≥0.05). Table III presents the genotype and allele frequencies for the 25 tagSNPs of RAGE in the cases and controls. It was revealed that the A allele of the RAGE SNP rs2269422 was associated with an enhanced risk of CAD (P<0.001, OR=0.505, 95% CI, 0.409–0.625) in a Han Chinese population. Thus, it was indicated that this tagSNP of RAGE is closely associated with the risk of CAD in this population. Of note, the allele or genotype frequencies for the remaining tagSNPs of RAGE were not significantly different between the CAD and the control subjects (Table III).
Table III.
Genotypic and allelic frequencies of receptor for advanced glycation end products polymorphisms in the control (n=867) and CAD group (n=852).
| Polymorphism | Genotypes/alleles | Control | Patients with CAD | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| Rs10754558 | Genotypes | ||||
| CC | 229 (26.4) | 265 (31.1) | 1.00 | ||
| CG | 459 (52.9) | 439 (51.5) | 0.089 | 0.826 (0.663–1.030) | |
| GG | 179 (20.6) | 148 (17.4) | 0.019 | 0.714 (0.540–0.946) | |
| Alleles | |||||
| C | 917 (52.9) | 969 (56.9) | 1.00 | ||
| G | 817 (47.1) | 735 (43.1) | 0.019 | 0.851 (0.744–0.974) | |
| Rs10925025 | Genotypes | ||||
| AA | 156 (18.0) | 156 (18.3) | 1.00 | ||
| AG | 455 (52.5) | 405 (47.5) | 0.379 | 0.890 (0687–1.153) | |
| GG | 256 (29.5) | 291 (34.2) | 0.367 | 1.137 (0.861–1.501) | |
| Alleles | |||||
| A | 767 (44.2) | 717 (42.1) | 1.00 | ||
| G | 967 (55.8) | 987 (57.9) | 0.202 | 1.091 (0.953–1.249) | |
| Rs12048215 | Genotypes | ||||
| AA | 477 (54.9) | 456 (53.5) | 1.00 | ||
| AG | 344 (39.6) | 354 (41.5) | 0.462 | 1.076 (0.885–1.310) | |
| GG | 46 (5.5) | 42 (4.9) | 0.955 | 0.955 (0.617–1.479) | |
| Alleles | |||||
| A | 1,298 (74.9) | 1,266 (74.3) | 0.019 | 0.844 (0.733–0.973) | |
| G | 436 (25.1) | 438 (25.7) | |||
| Rs12143966 | Genotypes | ||||
| AA | 177 (20.4) | 201 (23.6) | 1.00 | ||
| AG | 204 (23.5) | 211 (24.7) | 0.837 | 0.955 (0.617–1.479) | |
| GG | 486 (56.1) | 440 (51.6) | 0.064 | 0.797 (0.627–1.013) | |
| Alleles | |||||
| A | 558 (32.2) | 613 (35.9) | 1.00 | ||
| G | 1,176 (67.8) | 1,091 (64.0) | 0.091 | 0.827 (0.718–0.953) | |
| Rs12564791 | Genotypes | ||||
| CC | 633 (73.0) | 639 (75.0) | 1.00 | ||
| CT | 222 (25.6) | 189 (22.2) | 0.134 | 0.843 (0.675–11.054 | |
| TT | 12 (1.4) | 24 (2.8) | 0.052 | 1.981 (0.982–3.996) | |
| Alleles | |||||
| C | 1,488 (85.8) | 1,467 (86.1) | 0.814 | 0.977 (0.806–1.185) | |
| T | 246 (14.2) | 237 (13.9) | |||
| Rs1800624 | Genotypes | ||||
| AA | 588 (67.8) | 594 (69.7) | 1.00 | ||
| AT | 261 (30.1) | 234 (27.5) | 0.265 | 0.887 (0.719–1.095) | |
| TT | 18 (2.1) | 24 (2.8) | 0.380 | 1.320 (0.709–2.456) | |
| Alleles | |||||
| A | 1,437 (82.9) | 1,422 (83.5) | 0.682 | 0.960 (0.802–1.147) | |
| T | 297 (17.1) | 237 (16.5) | |||
| Rs2027432 | Genotypes | ||||
| GG | 747 (86.2) | 732 (85.9) | 1.00 | ||
| AG | 117 (13.5) | 111 (13.0) | 0.820 | 0.968 (0.732–1.280) | |
| AA | 3 (0.3) | 9 (1.1) | 0.078 | 3.061 (0.826–11.353) | |
| Alleles | |||||
| A | 123 (7.1) | 129 (7.6) | |||
| G | 1,611 (92.9) | 1,575 (92.4) | 0.601 | 0.932 (0.721–1.205) | |
| Rs204993 | Genotypes | ||||
| AA | 312 (36.0) | 294 (34.5) | 1.00 | ||
| AG | 435 (50.2) | 408 (47.9) | 0.965 | 0.995 (0.808–1.227) | |
| GG | 120 (13.8) | 150 (17.6) | 0.054 | 1.327 (0.994–1.769) | |
| Alleles | |||||
| A | 123 (7.1) | 129 (7.6) | 1.00 | ||
| G | 1,611 (92.9) | 1,575 (92.4) | 0.118 | 1.115 (0.973–1.278) | |
| Rs204994 | Genotypes | ||||
| CC | 554 (63.9) | 528 (62.0) | 1.00 | ||
| CT | 289 (33.3) | 288 (33.8) | 0.665 | 1.046 (0.854–1.280) | |
| TT | 24 (2.8) | 36 (4.2) | 0.091 | 1.574 (0.926–2.674) | |
| Alleles | |||||
| C | 1,397 (80.5) | 1,344 (78.9) | 1.00 | ||
| T | 337 (19.4) | 360 (21.1) | 0.710 | 1.110 (0.637–1.322) | |
| Rs2269421 | Genotypes | ||||
| CC | 809 (93.2) | 816 (95.8) | 1.00 | ||
| CG | 55 (6.5) | 33 (3.8) | 0.020 | 0.595 (0.382–0.926) | |
| GG | 3 (0.3) | 3 (0.4) | 0.990 | 0.991 (0.200–4.927) | |
| Alleles | |||||
| C | 1,674 (96.4) | 1,685 (97.7) | 1.00 | ||
| T | 62 (3.6) | 39 (2.3) | 0.026 | 0.625 (0.416–0.938) | |
| Rs2071290 | Genotypes | ||||
| CC | 804 (92.7) | 798 (93.7) | 1.00 | ||
| CT | 63 (7.3) | 51 (6.0) | 0.295 | 0.816 (0.557–1.195) | |
| TT | 0 (0.0) | 3 (0.4) | 0.082 | 1.004 (1.000–1.008) | |
| Alleles | |||||
| C | 1,674 (96.4) | 1,685 (97.7) | 1.00 | ||
| G | 62 (3.6) | 39 (2.3) | 0.710 | 0.918 (0.637–1.322) | |
| Rs2269422 | Genotypes | ||||
| TT | 723 (83.4) | 598 (70.2) | 1.00 | ||
| AT | 138 (15.9) | 239 (28.1) | <0.001 | 2.094 (1.654–2.651 | |
| AA | 6 (0.7) | 15 (1.8) | 0.017 | 3.023 (1.166–7.838) | |
| Alleles | |||||
| A | 150 (8.7) | 269 (15.8) | <0.001 | 0.505 (0.409–0.625) | |
| T | 1,584 (91.3) | 1,435 (84.2) | |||
| Rs2269423 | Genotypes | ||||
| AA | 78 (9.0) | 81 (9.5) | 1.00 | ||
| AC | 351 (40.5) | 363 (42.6) | 0.981 | 0.996 (0.706–1.404) | |
| CC | 438 (50.5) | 408 (47.9) | 0.530 | 0.897 (0.639–1.259) | |
| Alleles | |||||
| A | 507 (29.2) | 525 (30.8) | 1.00 | ||
| T | 1,277 (70.8) | 1,179 (69.2) | 0.315 | 0.928 (0.802–1.074) | |
| Rs300647 | Genotypes | ||||
| CC | 693 (79.9) | 687 (80.6) | 1.00 | ||
| AC | 168 (19.4) | 165 (19.4) | 0.016 | 0.991 (0.780–1.259 | |
| AA | 6 (0.7) | 0 (0.0) | 0.015 | 0.991 (0.985–0.998) | |
| Alleles | |||||
| A | 180 (79.8) | 165 (9.7) | 1.00 | ||
| C | 1,554 (20.2) | 1,539 (90.3) | 1.080 | 0.532 (0.799–1.251) | |
| Rs2282659 | Genotypes | ||||
| AA | 552 (63.7) | 514 (60.3) | 1.00 | ||
| AG | 279 (32.2) | 301 (35.3) | 0.154 | 1.159 (0.949–1.419) | |
| GG | 36 (4.2) | 37 (4.3) | 0.683 | 1.104 (0.687–1.774) | |
| Alleles | |||||
| A | 1,383 (79.8) | 1,329 (78.0) | 1.00 | ||
| G | 351 (20.2) | 375 (22.0) | 0.210 | 1.113 (0.944–1.311) | |
| Rs3014880 | Genotypes | ||||
| CC | 702 (81.0) | 684 (80.3) | 1.00 | ||
| CG | 159 (18.3) | 168 (19.7) | 0.510 | 1.084 (0.852–1.380) | |
| GG | 6 (0.7) | 0 (0.0) | 0.016 | 0.992 (0.985–0.998) | |
| Alleles | |||||
| C | 1,563 (90.1) | 1,329 (78.0) | 1.00 | ||
| G | 171 (9.9) | 375 (22.0) | 0.042 | 1.000 (0.799–1251) | |
| Rs3014885 | Genotypes | ||||
| CC | 690 (79.6) | 681 (79.9) | 1.00 | ||
| CT | 171 (19.7) | 171 (20.1) | 0.914 | 1.013 (0.799–1,284) | |
| TT | 6 (0.7) | 0 (0.0) | 0.015 | 0.991 (0.985–0.998) | |
| Alleles | |||||
| C | 1,551 (89.4) | 1,533 (90.0) | 1.00 | ||
| T | 183 (10.6) | 171 (10.0) | 0.998 | 0.933 (0.749–1.163) | |
| Rs3130349 | Genotypes | ||||
| TT | 645 (74.4) | 624 (73.2) | 1.00 | ||
| CT | 216 (24.9) | 213 (25.0) | 0.864 | 1.019 (0.890–1.269) | |
| CC | 6 (0.7) | 15 (1.8) | 0.043 | 2.584 (0.996–6.073) | |
| Alleles | |||||
| C | 228 (13.1) | 243 (14.3) | 1.00 | ||
| T | 1,506 (86.9) | 1,461 (85.7) | 0.129 | 0.910 (0.749–1.106) | |
| Rs3134943 | Genotypes | ||||
| CC | 699 (80.6) | 663 (77.8) | 1.00 | ||
| CT | 153(17.6) | 171 (20.1) | 0.185 | 1.178 (0.924–1.502) | |
| TT | 15 (1.7) | 18 (20.1) | 0.505 | 1.265 (0.632–0.531) | |
| Alleles | |||||
| C | 1,551 (89.4) | 1,497 (87.9) | 1.00 | ||
| T | 183 (10.6) | 207 (12.1) | 0.346 | 1.172 (0.949–1.448) | |
| Rs3134946 | Genotypes | ||||
| CC | 684 (78.9) | 663 (77.8) | 1.00 | ||
| CG | 177 (20.4) | 171 (20.1) | 0.978 | 0.997 (0.787–1.262) | |
| GG | 6 (0.7) | 18 (20.1) | 0.012 | 3.095 (1.221–7.885) | |
| Alleles | |||||
| C | 1,545 (89.4) | 1,505 (88.3) | 1.00 | ||
| G | 189 (10.6) | 199 (11.7) | 0.484 | 1.081 (0.875–1.335) | |
| Rs3738448 | Genotypes | ||||
| GG | 558 (64.5) | 574 (67.4) | 1.00 | ||
| CT | 264 (30.4) | 241 (28.3) | 0.265 | 0.887 (0.719–1.095) | |
| TT | 45 (5.2) | 37 (4.3) | 0.329 | 0.799 (0.570–1.254) | |
| Alleles | |||||
| G | 1,380 (79.6) | 1,389 (81.5) | 0.155 | 0.884 (0.747–1.047) | |
| T | 354 (20.4) | 315 (18.5) | |||
| Rs4772 | Genotypes | ||||
| AA | 693 (79.9) | 690 (81.0) | 1.00 | ||
| G | 168 (19.4) | 159 (18.7) | 0.680 | 0.951 (0.747–1.210) | |
| GG | 6 (0.7) | 3 (0.3) | 0.322 | 0.502 (0.125–2.016) | |
| Alleles | |||||
| A | 1,544 (89.6) | 1,539 (90.3) | 1.00 | ||
| G | 180 (10.4) | 165 (9.7) | 0.532 | 1.767 (1.452–2.150) | |
| Rs4925648 | Genotypes | ||||
| CC | 576 (64.4) | 570 (66.9) | 1.00 | ||
| CT | 267 (30.8) | 246 (28.9) | 0.501 | 0.931 (0.756–1.147) | |
| TT | 24 (2.8) | 36 (4.2) | 0.121 | 1.516 (0.893–2.573) | |
| Alleles | |||||
| A | 1,419 (81.8) | 1,386 (81.3) | 1.00 | ||
| T | 315 (18.2) | 318 (18.7) | 0.708 | 1.034 (0.870–1.228) | |
| Rs7528887 | Genotypes | ||||
| AA | 465 (53.6) | 450 (52.8) | 1.00 | ||
| AG | 330 (38.0) | 351 (41.2) | 0.351 | 1.099 (0.901–1.340) | |
| GG | 72 (8.3) | 51 (6.0) | 0.108 | 0.732 (0.500–1.072) | |
| Alleles | |||||
| A | 1,260 (72.7) | 1,251 (73.4) | 1.00 | ||
| G | 474 (27.3) | 453 (26.6) | 0.963 | 0.963 (0.828–1.119) | |
| Rs7529058 | Genotypes | ||||
| CC | 234 (27.0) | 228 (26.8) | 1.00 | ||
| CG | 407 (46.9) | 390 (45.8) | 0.887 | 0.983 (0.782–1.237) | |
| GG | 226 (26.1) | 234 (27.5) | 0.645 | 1.063 (0.821–1.376) | |
| Alleles | |||||
| C | 875 (50.5) | 846 (49.6) | 1.00 | ||
| G | 859 (49.5) | 858 (50.4) | 0.657 | 1.033 (0.904–1.181) |
OR, odds ratio; CI, confidence interval; CAD, coronary artery disease.
RAGE expression in atherosclerotic carotid arteries
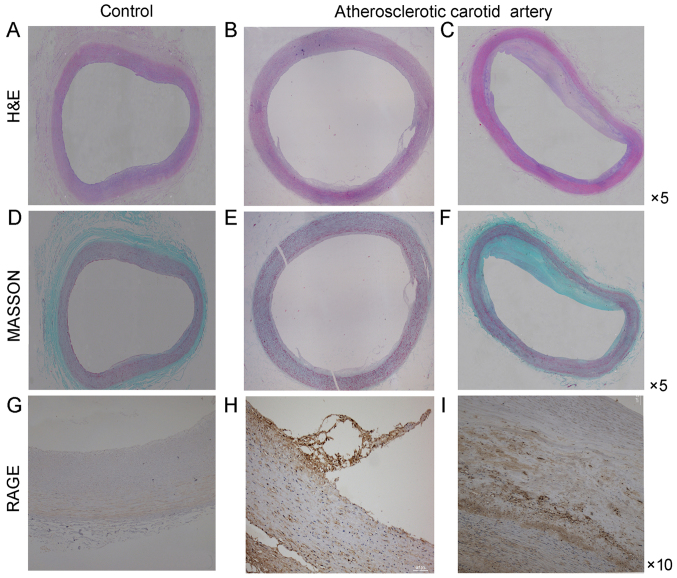
Sections stained with H&E and Masson demonstrated the basic morphological features of human carotid arteries (Fig. 1). The basic morphology of normal carotid artery and the morphologic change of atherosclerotic carotid artery were revealed. Endothelial cells in normal carotid arteries displayed a continuous single layer distribution and smooth muscle cells lined the vascular wall. On the contrary, endothelial cells in atherosclerotic carotid arteries did not have a continuous single layer distribution and smooth muscle cells were disorganized. At the same time macrophages infiltrated the atherosclerotic plaque.
Figure 1.
RAGE-positive structures in atherosclerotic lesions. Histology images of atherosclerotic plaques in the human carotid artery. (A-C) H&E staining, (D-F) Masson staining and (G-I) immunohistochemical staining for RAGE in human carotid artery sections. (A, D and G) Control carotid artery (magnification, ×5 or ×10). (B, C, E, F, H and I): The initial atherosclerotic stage of the human carotid artery and thick fibrous cap atheroma of the human carotid artery. The middle panel represents the early stage and the right-hand panel represents the thick fibrous cap atheroma in the late stage. RAGE, receptor for advanced glycation end products.
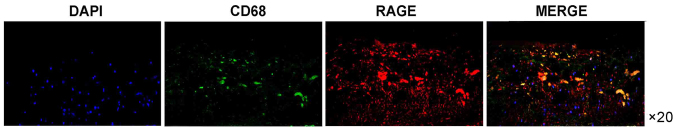
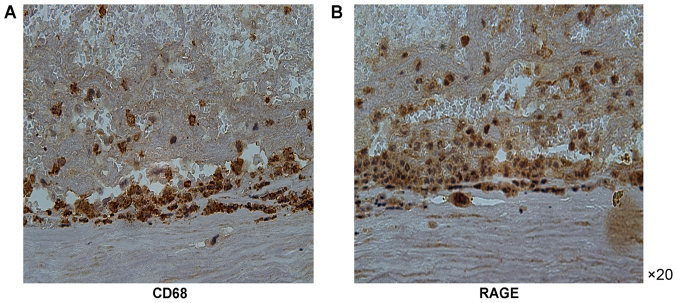
Immunohistochemical analysis of cross-sections of the carotid artery tissue specimens was performed to detect RAGE expression. The expression of RAGE was more prominent in the atherosclerotic carotid artery plaque specimens compared with that in the control carotid artery specimens (Fig. 1). Furthermore, the RAGE expression was more prominent in the atherosclerotic carotid tissue specimens containing CD68-positive macrophages compared with that in the carotid artery specimens of the control group. RAGE expression was also increased in areas of macrophage infiltration (Fig. 2). Of note, immunofluorescence analysis of RAGE indicated that it was closely colocalized with the CD68-positive regions. RAGE-positive regions were also positive for CD68 (Figs. 1–3).
Figure 2.
Double immunofluorescence analysis of RAGE- and CD68-expressing cells (macrophages) in the atherosclerotic lesion. A section from an atherosclerotic lesion was stained for RAGE (red) and macrophage marker CD68 (green), and the nuclei were stained with DAPI (blue) (magnification, ×20). RAGE, receptor for advanced glycation end products.
Figure 3.
Macrophages in atherosclerotic lesions expressing RAGE. Sections of formaldehyde-fixed, paraffin-embedded tissue blocks of atherosclerotic coronary arteries were subjected to immunohistochemical staining for CD68 and RAGE. (A) CD68 expression was identified in the coronary atherosclerotic plaques. (B) RAGE expression was observed in the same region of coronary atherosclerotic plaques (magnification, ×20). RAGE, receptor for advanced glycation end products.
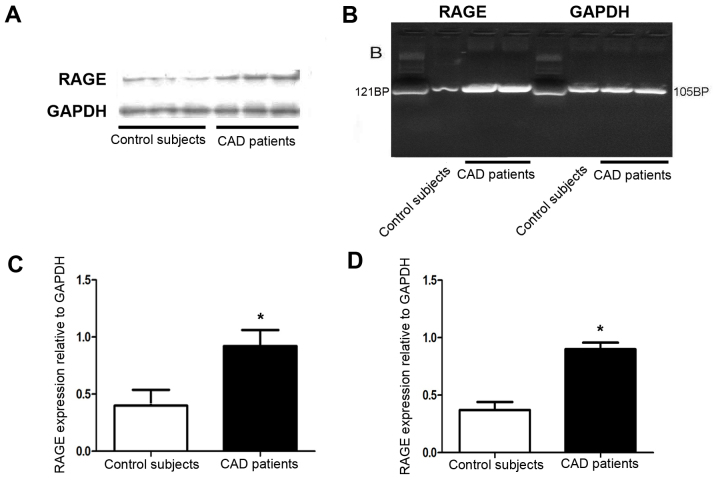
Furthermore, RT-PCR and western blot analysis respectively indicated that the mRNA and protein expression of RAGE was increased in the atherosclerotic coronary artery compared with that in the normal coronary artery tissues (Fig. 4A-D).
Figure 4.
Analysis of the surface RAGE expression in coronary artery tissues. Representative western blot(A) and RT-PCR(B) for the detection of RAGE protein and mRNA in subjects with CAD and controls. Quantified expression levels of RAGE at (C) the protein and (D) the mRNA level in coronary artery tissues. *P<0.05 vs. control subjects. RAGE, receptor for advanced glycation end products; CAD, coronary artery disease.
Plasma S100B levels in RAGE genotype subsets
The calcium-binding protein S100B binds to RAGE, which leads to activation of the RAGE-dependent signal transduction pathway, resulting in atherogenesis. Therefore, potential differences in plasma S100B levels between CAD patients and control subjects were assessed. The plasma S100B levels in the CAD patients were upregulated in comparison with those in the control group (243.97±48.29 vs. 102.4 ±25.16 pg/ml; P<0.05). Of note, patients with the RAGE rs2269422 AA/AT variant had higher plasma levels of S100B compared with those of the TT genotype in the CAD and control groups (286.69±63.91 vs. 201.24±32.68 pg/ml and 129.43±21.34 vs. 95.52±28.98 pg/ml, respectively; P<0.05; Table IV). Furthermore, exclusion of diabetic patients from the analysis did not significantly affect the results, and it was also indicated that the RAGE s2269422 variant was associated with the occurrence of CAD and that genetic variations of RAGE have a significant impact on the risk of CAD in the present Han Chinese population.
Table IV.
Plasma S100B levels according to the TT and AA/AT variants of the rs2269422 polymorphism of receptor for advanced glycation end products.
| S100B (pg/ml) | ||
|---|---|---|
| Group | TT | AA/AT |
| Control (n=90) | 95.52±28.98 | 129.43±21.34 |
| CAD (n=70) | 201.24±32.68a | 286.69±63.91a |
P<0.05 vs. the control group. Values are expressed as the mean ± standard deviation. S100B, S100 calcium binding protein B; CAD, coronary artery disease.
Discussion
CAD is an atherosclerotic inflammatory disease, and oxidative stress and inflammation have an important role in its pathogenesis (1). RAGE, as a transmembrane receptor, binds to cellular ligands, which induces signal transduction and cellular dysfunction, including the activation of nuclear factor κB and cyclic adenosine monophosphate pathways, leading to the amplification of atherosclerotic inflammatory responses (27–29). The present study demonstrated an increase in RAGE expression in atherosclerotic carotid plaques compared with that in normal arteries. Furthermore, RT-PCR and immunohistochemistry revealed increased RAGE mRNA levels and protein expression in coronary arteries of CAD patients vs. that in normal controls. It was further indicated that compared with the corresponding wild-type genotype, the rs2269422 SNP of RAGE was associated with an increased susceptibility to atherosclerosis. Furthermore, S100B expression in subjects with the AA/AT genotype of the RAGE SNP rs2269422 was higher compared with that in the TT genotype carriers; as this was also identified in control subjects, it may not be CAD-specific.
Previous studies have established the association between RAGE, the pathogenesis of atherosclerosis and the occurrence of CAD, highlighting that RAGE has a central role in the formation of unstable atherosclerotic plaques and the pathogenesis and progression of CAD (6,30). However, at present, the pathogenesis of CAD, including the association between the tagSNPs of RAGE and the occurrence of CAD, remains to be fully elucidated. In the present study, the association between 25 tagSNPs of RAGE and CAD was determined, and it was revealed that the presence of the A allele of the rs2269422 SNP of RAGE significantly increases the risk of CAD.
The results of the present study suggested that macrophages in atherosclerotic plaques of the carotid artery express more RAGE than the normal carotid tissues. Furthermore, the RAGE rs2269422 AA allele carriers had a 1.9-fold increased risk of CAD compared with that of the TT allele carriers. Of note, CAD patients also had increased plasma S100B levels compared with the control patients. Furthermore, the plasma S100B levels in RAGE rs2269422 AA/AT genotype carriers were also significantly increased in comparison with those in the RAGE rs2269422 TT genotype carriers, which were demonstrated in the control group as well as the CAD group.
Several studies have investigated the effect of RAGE polymorphisms on atherosclerotic disease. However, the effect of tagSNPs of RAGE on CAD has remained to be fully elucidated. Watson et al (31) indicated that RAGE p.Gly82Ser (G82S) mutation have a major effect on the susceptibility of type 2 diabetes mellitus patients to CAD. Subjects with the SS genotype of the G82S variant of RAGE had an increased risk of cardiovascular disease compared with that in the control group (6,30). To date, only a small number of candidate genes have been identified to be associated with CAD in Han Chinese populations. To the best of our knowledge, the association between the RAGE rs2269422 TT genotype with the progression and development of CAD has not been previously reported.
In the present study, an increased expression of RAGE was identified in macrophages, which may be activated and lead to the production of proteins, including S100B and pro-inflammatory cytokines, promoting inflammation and cytotoxicity. Upon S100B binding to RAGE, a series of cellular signaling pathways is activated to induce a massive release of inflammatory factors into the blood. The expression of RAGE by inflammatory cells attracts macrophages into the lesion region, followed by accumulation of excess cholesterol in the macrophages, contributing to the formation of foam cells in atherosclerotic lesions and then enhancing the severity of the coronary artery lesion (6,32). Thus, RAGE-positive macrophages and their ligands, including S100B, have an intermediary part in regulating cell proliferation, differentiation and apoptosis in the development of atherosclerotic plaques.
The rs2269422 SNP located in the intron region of the RAGE gene has not been previously reported to be associated with the occurrence of CAD in Han Chinese populations. The present study identified that the intronic rs2269422 SNP either directly or indirectly impaired the function of RAGE. An increasing number of studies have indicated that variations in the intron region influence gene splicing. Furthermore, the rs2269422 RAGE variant did not obviously affect gene translation, but it may indirectly influence the RAGE mRNA splicing function. Of note, relative to the control group, the plasma S100B levels of patients with CAD were also significantly higher and this requires further investigation.
The present study has several limitations that require to be addressed. First, the subjects enrolled in the study were from a homogenous Northern Han Chinese population. Thus, the results of the present case-control study should be verified in other populations. Furthermore, the 1,719 patients included in the present study may be too few to extend this conclusion to the entire Han Chinese population. In addition, an investigation of changes in potential novel signal transduction pathways, such as the RAGE-MAPK-NF-κB signaling pathway, caused by variations in RAGE and its ligands is required in future studies.
In conclusion, the present study indicates a correlation between the RAGE rs2269422 SNP and the occurrence of CAD in a Han Chinese population, and suggests that RAGE may contribute to the formation of coronary atherosclerotic plaques in patients with CAD. The rs2269422 variant of RAGE may thus be a genetic risk factor for the occurrence of CAD.
Acknowledgements
The authors would like to thank Miss Li Na and Miss Jun-Yan Du (Department of Cardiology, Shenyang Military General Hospital, Shenyang, China) for their efforts in collecting the data for the preliminary study.
Funding
The present study was supported by grants from the National Key Research and Development Program of China for the 13th five-year plan (grant no. 2016YFC1301300), the National Science Foundation of China (grant no. 81670340) and the Liaoning Science and Technology Project (grant no. 2015010400-301).
Availability of data and materials
Data from the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ, MC, FT and XS collected and statistically processed the clinical data. XY and MC performed the immunohistochemistry and immunofluorescence assays. XZ and FT performed the RT-PCR assay. XZ and XS performed the ELISA.
Ethics approval and consent to participate
The present study complied with the Declaration of Helsinki and informed consent had been obtained from the subjects or their relatives. The Shenyang Military General Hospital Ethics Committee approved the study protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests regarding this study.
References
- 1.Kriszbacher I, Koppan M, Bodis J. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;353:429–430. doi: 10.1056/NEJM200507283530425. [DOI] [PubMed] [Google Scholar]
- 2.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abaci A. Management of cardiovascular risk factors for primary prevention: Evaluation of Turkey results of the EURIKA study. Turk Kardiyol DernArs. 2012;40:135–142. doi: 10.5543/tkda.2012.01827. [DOI] [PubMed] [Google Scholar]
- 4.Arbab-Zadeh A, Fuster V. The myth of the ‘vulnerable plaque’: Transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–855. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorkegren JLM, Kovacic JC, Dudley JT, Schadt EE. Genome-wide significant loci: How important are they? Systems genetics to understand heritability of coronary artery disease and other common complex disorders. J Am Coll Cardiol. 2015;65:830–845. doi: 10.1016/j.jacc.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Deng L, Wang Y, Shi Y, Xiao X, Zheng X, Ren H, Xu D. Relationship between RAGE gene polymorphisms and cardiovascular disease prognosis in the Chinese Han population. Mol Genet Genomics. 2017;292:1139–1149. doi: 10.1007/s00438-017-1341-1. [DOI] [PubMed] [Google Scholar]
- 7.Kajikawa M, Nakashima A, Fujimura N, Maruhashi T, Iwamoto Y, Iwamoto A, Matsumoto T, Oda N, Hidaka T, Kihara Y, et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care. 2015;38:119–125. doi: 10.2337/dc14-1435. [DOI] [PubMed] [Google Scholar]
- 8.Katakami N. Can soluble receptor for advanced glycation end-product (sRAGE) levels in blood be used as a predictor of cardiovascular diseases? Atherosclerosis. 2017;266:223–225. doi: 10.1016/j.atherosclerosis.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Nam MH, Son WR, Lee YS, Lee KW. Glycolaldehyde-derived advanced glycation end products (glycol-AGEs)-induced vascular smooth muscle cell dysfunction is regulated by the AGES-receptor (RAGE) axis in endothelium. Cell Commun Adhes. 2015;22:67–78. doi: 10.1080/15419061.2016.1225196. [DOI] [PubMed] [Google Scholar]
- 10.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Reichert S, Triebert U, Santos AN, Hofmann B, Schaller HG, Schlitt A, Schulz S. Soluble form of receptor for advanced glycation end products and incidence of new cardiovascular events among patients with cardiovascular disease. Atherosclerosis. 2017;266:234–239. doi: 10.1016/j.atherosclerosis.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Rowisha M, El-Batch M, El Shikh T, El Melegy S, Aly H. Soluble receptor and gene polymorphism for AGE: Relationship with obesity and cardiovascular risks. Pediatr Res. 2016;80:67–71. doi: 10.1038/pr.2016.55. [DOI] [PubMed] [Google Scholar]
- 13.Ligthart S, Sedaghat S, Ikram MA, Hofman A, Franco OH, Dehghan A. EN-RAGE: A novel inflammatory marker for incident coronary heart disease. Arterioscler Thromb Vasc Biol. 2014;34:2695–2699. doi: 10.1161/ATVBAHA.114.304306. [DOI] [PubMed] [Google Scholar]
- 14.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery. Fleisher LA, Beckman JA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13–e118. doi: 10.1016/j.jacc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Elisaf M, Tzouvelekis E, Nikas N, Greek EURIKA Investigators Primary prevention of cardiovascular disease in Greece: Greek results of the EURIKA study. Hellenic J Cardiol. 2014;55:217–226. [PubMed] [Google Scholar]
- 16.Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J. 2018:1–12. doi: 10.1093/eurheartj/ehy529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong P, Athanasiadis A, Sechtem U. Treatment of angina pectoris associated with coronary microvascular dysfunction. Cardiovasc Drugs Ther. 2016;30:351–356. doi: 10.1007/s10557-016-6676-z. [DOI] [PubMed] [Google Scholar]
- 18.Loffler AI, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and management. Curr Cardiol Rep. 2016;18:1. doi: 10.1007/s11886-015-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezhumalai B, Ananthakrishnapillai A, Selvaraj RJ, Satheesh S, Jayaraman B. Cardiac syndrome X: Clinical characteristics revisited. Indian Heart J. 2015;67:328–331. doi: 10.1016/j.ihj.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhabra L, Kowlgi NG. Low incidence of diabetes mellitus in coronary microvascular dysfunction: An intriguing association. JACC Cardiovasc Interv. 2016;9:395–396. doi: 10.1016/j.jcin.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H. Different definition of microvascular angina. Eur J Clin Invest. 2015;45:1360–1366. doi: 10.1111/eci.12552. [DOI] [PubMed] [Google Scholar]
- 22.Morrison AC, Fu YP, O'Donnell CJ, Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium Subclinical Atherosclerosis and CHD Working Working Working Group: Variants in ANGPTL4 and the risk of coronary artery disease. N Engl J Med. 2016;375:2303. doi: 10.1056/NEJMc1607380. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Tian X, Li Y, Liu D, Liu M, Zhang X, Zhang Q, Yan C, Han Y. Up-Regulation of CREG expression by the transcription factor GATA1 inhibits high glucose- and high palmitate-induced apoptosis in human umbilical vein endothelial cells. PLoS One. 2016;11:e0154861. doi: 10.1371/journal.pone.0154861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai W, Tao J, Zhang X, Tian X, Liu T, Feng X, Bai J, Yan C, Han Y. Contribution of homeostatic chemokines CCL19 and CCL21 and their receptor CCR7 to coronary artery disease. Arterioscler Thromb Vasc Biol. 2014;34:1933–1941. doi: 10.1161/ATVBAHA.113.303081. [DOI] [PubMed] [Google Scholar]
- 25.Okon I, Ding Y, Zou MH. Ablation of interferon regulatory factor 3 promotes the stability of atherosclerotic plaques. Hypertension. 2017;69:407–408. doi: 10.1161/HYPERTENSIONAHA.116.08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang M, Han Y, Zhang X, Pei F, Deng J, Kang J, Yan C. An intron polymorphism in the CXCL16 gene is associated with increased risk of coronary artery disease in Chinese Han population: A large angiography-based study. Atherosclerosis. 2010;210:160–165. doi: 10.1016/j.atherosclerosis.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhao D, Tong L, Zhang L, Li H, Wan Y, Zhang T. Tanshinone II A stabilizes vulnerable plaques by suppressing RAGE signaling and NF-κB activation in apolipoprotein-E-deficient mice. Mol Med Rep. 2016;14:4983–4990. doi: 10.3892/mmr.2016.5916. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Yu Y, Wang L, Delguste F, Durand A, Guilbaud A, Rousselin C, Schmidt AM, Tessier F, Boulanger E, Neviere R. Advanced glycation end products receptor RAGE controls myocardial dysfunction and oxidative stress in high-fat fed mice by sustaining mitochondrial dynamics and autophagy-lysosome pathway. Free Radic Biol Med. 2017;112:397–410. doi: 10.1016/j.freeradbiomed.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Muniyappa R, Srinivas PR. Dicarbonyl stress and atherosclerosis: Is it all RAGE? Diabetes. 2014;63:3587–3589. doi: 10.2337/db14-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma WQ, Qu QR, Zhao Y, Liu NF. Association of RAGE gene Gly82Ser polymorphism with coronary artery disease and ischemic stroke: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e5593. doi: 10.1097/MD.0000000000005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson AM, Li J, Samijono D, Bierhaus A, Thomas MC, Jandeleit-Dahm KA, Cooper ME. Quinapril treatment abolishes diabetes-associated atherosclerosis in RAGE/apolipoprotein E double knockout mice. Atherosclerosis. 2014;235:444–448. doi: 10.1016/j.atherosclerosis.2014.05.945. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhu J, Chen L, Hu W, Wang M, Li S, Gu X, Tao H, Zhao B, Ma G, Li K. Genetic predisposition to ischaemic stroke by RAGE and HMGB1 gene variants in Chinese Han population. Oncotarget. 2017;8:100150–100164. doi: 10.18632/oncotarget.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the current study are available from the corresponding author on reasonable request.