Abstract
The present study determined the expression of microRNA (miR)-378 in the peripheral blood and lung tissues of children with asthma, and investigated its effect and mechanism of action on the biological functions of airway smooth muscle cells. A total of 23 asthmatic children and 15 healthy children were included in the study. Peripheral blood and tissues were obtained from asthmatic children. Healthy children provided peripheral blood. Quantitative real-time polymerase chain reaction was used to determine the expression of miR-378. Airway smooth muscle cells were isolated and cultured in vitro. The cells were transfected with miR-378 mimics or miR-378 inhibitor. Following transfection, proliferation of the cells was determined using the CCK-8 assay. In addition, flow cytometry was used to detect the cell cycles and apoptosis of smooth muscle cells. Western blotting was performed to determine the expression of extracellular matrix proteins in smooth muscle cells. Furthermore, bioinformatics was used to predict potential target genes of miR-378 and their downstream signaling pathways. Results indicated that the expression of miR-378 in peripheral blood and lung tissues from asthmatic children was increased compared with that in healthy children. Serum from asthmatic children promoted the proliferation of smooth muscle cells in vitro by affecting the cell cycle, and enhanced apoptotic resistance of smooth muscle cells. Notably, overexpression of miR-378 increased the proliferation of smooth muscle cells by affecting the cell cycle, and this upregulated apoptotic resistance of smooth muscle cells and enhanced the expression of extracellular matrix-related proteins in smooth muscle cells. However, downregulation of miR-378 expression reversed the promoting effect of serum from asthmatic children on the biological functions of smooth muscle cells. These findings suggested that miR-378 possibly affects the proliferation, apoptosis and motility of airway smooth muscle cells via downstream signaling pathways. To conclude, the present study demonstrated that miR-378 expression was elevated in the peripheral blood and lung tissues from children with asthma. Furthermore, miR-378 promoted the biological functions of extracellular matrix-related proteins of smooth muscle cells, and possibly exerts its effect via its target genes through downstream signaling pathways.
Keywords: microRNA-378, asthma, airway smooth muscle cells
Introduction
Bronchial asthma is a clinically common respiratory chronic inflammatory disease, which mainly involves inflammatory cells and structural cells (such as eosinophils, mast cells, lymphocytes, neutrophils, smooth muscle cells and airway epithelial cells), as well as relevant cellular components (1,2). Without effective control, chronic inflammation induced by asthma will lead to airway hyperresponsiveness, which further causes reversible airflow limitation, as well as recurrent gasp for breath, tachypnea, chest tightness or cough (3). Clinical studies show that the number of asthma patients around the world is as high as 300 million, and the prevalence of the disease varies from 1 to 18% across different countries (4,5). In China, the number of asthma patients is more than 30 million, and the mortality caused by asthma is the highest in the world (6,7). Of note, childhood asthma attracts more and more concern in medical practice, because this disease affects children and is easy to cause death (8). At present, long-term inhalation of corticosteroids is the main clinical treatment for children with asthma, and it greatly alleviates the illness of the children (9). However, long-term use of corticosteroids causes hormone resistance or dependence, greatly affects the growth and development of children, and induces related complications, such as necrosis of femoral head (9). Therefore, it is of important clinical value to explore the molecular mechanism of childhood asthma, to discover potential drug targets and to find effective treatment strategies. Existing studies have shown that chronic airway inflammation, airway hyperresponsiveness, and airway remodeling are the three pathological stages of asthma in children. Among these stages, airway remodeling phase is irreversible, and directly related to the prognosis of affected children (10). Airway smooth muscle cells play an important role in this process (11), but the molecular mechanisms are still unclear. It is reported that proteins in extracellular matrix play important roles in airway remodeling induced by asthma, including type I collagen (COL-I) and fibronectin (FN). The secretion of COL-I and FN leads to thickening of basilar membrane and subcutaneous fibrosis of the airway, eventually causing airway remodeling (12).
microRNA (miRNA or miR) molecules are a class of small non-encoding RNA molecules with 18–25 nucleotides. They form silencing complexes by binding with the 3′-untranslated region (UTR) of target genes to inhibit translation and reduce expression of the target proteins (13,14). It is shown that miRNA molecules exist widely in tissue cells and body fluids, participate in nearly all pathophysiological processes, and have important clinical values (15). miRNA molecules regulate the biological functions of immune cells, smooth muscle cells and epithelial cells through a variety of target genes (16). For example, miR-425 regulates the differentiation of Th17 cells by targeting the Foxo1 gene and participates in intestinal inflammation (17). miR-135a promotes the inflammatory response of rat vascular smooth muscle cells by targeting Foxo1 gene (18). In addition, the expression of miR-221 in fibrotic airway epithelial cells is obviously upregulated and it can target the expression of ATF6 (19). However, the molecular mechanisms by which miRNA molecules regulates the occurrence of asthma are not clear yet.
miR-378 was first discovered in tumor tissues and cells, and it plays important roles in biological processes of tumor cells, such as proliferation, apoptosis, and drug resistance (20,21). Depending on the types of tumors, miR-378 can play a role in promoting cancer or inhibiting cancer (20,21). It is reported that miR-378 is closely related to the differentiation, hypertrophy and metabolism of cardiomyocytes, and is involved in the pathological changes of the heart, suggesting that miR-378 may participate in the regulation of biological functions of myocytes (22). As a main component in respiratory tract remodeling, airway smooth muscle cells participate in the initiation and development of airway remodeling. As structural cells, airway smooth muscle cells not only directly participate in the thickening of airway wall through its own hypertrophy and proliferation, but also promote airway remodeling by phenotypic transformation, changes in migration function, and secretion of inflammatory factors and extracellular matrix (23,24). In the present study, we determine the expression of miR-378 in children with asthma, investigate its effect on the biological functions of smooth muscle cells, and try to provide experimental basis for understanding the pathogenesis of childhood asthma.
Patients and methods
Patients
A total of 23 asthmatic children who underwent biopsy by bronchoscopy at our hospital between January 2014 and January 2017 were included in the present study. In addition, 15 healthy children were included into control group. Sputum samples were obtained from all patients and healthy children. Peripheral blood (5 ml) was collected from patients and healthy subjects. All patients had mild to moderate asthma and this was their first treatment for asthma. None of the patients had used hormones within four weeks before admission to our hospital. All procedures were approved by the Ethics Committee of Maternity and Child Health Care Hospital of Zibo City (Zibo, China). Written informed consents were obtained from all patients or their families.
Cells
Lung tissues were obtained from non-asthmatic patients undergoing lobectomy, and washed with phosphate-buffered saline (PBS) thoroughly. Smooth muscle layer was separated from middle bronchoalveolar membrane, cut into small pieces (about 1 mm3), placed on the bottom of culture flasks containing DMEM medium supplemented with 20% fetal bovine serum, and cultured under 37°C and 5% CO2. Medium was replaced every two days, and smooth muscle cells that migrated out of the tissues were monitored under a light microscope. When reaching 70–80% confluency, smooth muscle cells were passaged at a ratio of 1:3 and DMEM supplemented with 10% fetal bovine serum was used for cell culture.
To transfect smooth muscle cells with miR-378 mimics, the cells (2×105) in logarithmic growth were seeded onto 24-well plates one day before transfection, and cultured in antibiotics-free DMEM medium supplemented with 10% fetal bovine serum until reaching 70% confluency. In the first vial, 1.25 µl miR-negative control (NC), miR-378 mimics or miR-378 inhibitor (20 pmol/µl; Hanbio Biotechnology Co., Ltd., Shanghai, China) was mixed with 50 µl Opti Mem medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). In the second vial, 1 µl Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) was mixed with 50 µl Opti Mem medium. After standing still for 5 min, the two vials were combined for additional waiting at room temperature for 20 min. Then, the mixtures were added onto cells in respective groups. Six hours later, the medium was replaced with DMEM containing 10% fetal bovine serum. After cultivation for 48 h, the cells were collected for further assays.
To test the effect of serum from asthmatic children on smooth muscle cells, the cells (2×105) in logarithmic growth phase were seeded onto 24-well plates, and cultured in DMEM supplemented with 10% fetal bovine serum. On the next day, 250 µl serum from healthy children (NC group) or asthmatic children (asthma serum group) was mixed with 250 µl DMEM, and then added onto smooth muscle cells. The cells were cultured for 7 days, during which the medium was replaced every two days.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Sputum samples (0.2 ml) were mixed with saline at a ratio of 1:10, and centrifuged at 12,000 × g and 4°C for 10 min to collect sediments. Then, the sediments were lysed using 1 ml TRIzol reagent following the manufacturer's manual (Thermo Fisher Scientific, Inc.). Total RNA was extracted using phenol chloroform method. The concentration and quality of RNA was measured using ultraviolet spectrophotometry (Nanodrop ND2000; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Then, cDNA was obtained by reverse transcription from 1 µg RNA and stored at −20°C. Reverse transcription of miRNA was carried out using miScript II RT kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's manual.
The expression of miR-378 was determined by miScript SYBR-Green PCR kit (Qiagen GmbH), using U6 as internal reference. The sequences of miR-378 primers were forward, 5′-CTCCTGACTCCAGGTCCTGTGT-3′ and reverse, 5′-ACTGGACTTGGAGTCAGAAGGC-3′; and the sequences of U6 primers were forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The reaction system (30 µl) contained 10 µl RT-qPCR-Mix, 1 µl upstream primer, 1 µl downstream primer, 5 µl cDNA and 13 µl ddH2O. The reaction protocol was: Initial denaturation at 95°C for 5 min; 40 cycles of denaturation at 95°C for 30 sec and annealing at 60°C for 30 sec (iQ5; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCt method was used to calculate the relative expression of miR-378 against U6. Each sample was tested in triplicate.
Immunohistochemistry
Smooth muscle cells (1×105) were seeded onto cell slides. When reaching 90% confluency, medium was discarded, and the cells were washed with PBS twice. After fixing with 4% formaldehyde at room temperature for 10 min, the cells were washed with PBS twice again. Then, the cells were incubated with α-smooth muscle actin (α-SMA) antibody (1:5 dilution) at s4°C overnight. On the next day, the cells were mixed with 3% H2O2 to block the activity of peroxidase. Then, horseradish peroxidase-labelled secondary antibody was added and the cells were incubated at room temperature for 2 h to develop color.
CCK-8 assay
Cells were seeded at a density of 2,000/well in 96-well plates. At 0, 24, 48 and 72 h, 20 µl CCK-8 (5 g/l) was added onto the cells. After incubation at 37°C for 2 h, the absorbance of each well was measured at 490 nm for plotting cell proliferation curves. Each group was tested in 3 replicate wells and the values were averaged.
Flow cytometry
At 24 h after transfection, smooth muscle cells (1×106) in each group were washed with pre-cooled PBS twice and subjected to flow cytometry using Cell Cycle Assay kit (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer's manual to detect cell cycle. The data were analyzed using ModFit software (Verity Software House, Topsham, ME, USA).
At 24 h after being treated with serum from healthy children or asthmatic children, or at 24 h after transfection with miR-378 mimics, smooth muscle cells (1×106) in each group were washed with pre-cooled PBS twice and subjected to flow cytometry using Annexin V FITC Apoptosis DTEC kit I (BD Biosciences) following the manufacturer's manual to detect cell apoptosis. Cells with Annexin V-positive values were early apoptotic cells, those with PI-positive values were necrotic cells, and those with double positive values were late apoptotic cells.
Western blot analysis
Before lysis, tissues were ground into powder, and cells were trypsinized and collected. Then, tissue samples or cells were lysed with precooled Radio-Immunoprecipitation Assay (RIPA) lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for 30 min on ice. The mixture was centrifuged at 12,000 rpm and 4°C for 10 min. The supernatant was used to determine protein concentration by bicinchoninic acid (BCA) protein concentration determination kit (RTP7102; Real-Times Biotechnology Co., Ltd., Beijing, China). The samples were then mixed with 5× sodium dodecyl sulfate loading buffer before denaturation in boiling water bath for 10 min. Afterwards, the samples (5 µl) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (250 mA, 1 h) and blocked with 50 g/l skimmed milk at room temperature for 1 h. Then, the membranes were incubated with mouse anti-human COL-I (1:1,000; ab90395; Abcam, Cambridge, UK), FN (1:1,000; MAB1918; R&D Systems, Minneapolis, MN, USA) or GAPDH (1:5,000; Beyotime Institute of Biotechnology) monoclonal primary antibodies at 4°C overnight. After extensive washing with PBS with Tween-20 for 3 times of 15 min, the membranes were incubated with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5,000; Santa Cruz, Dallas, TX, USA) for 1 h at room temperature before washing with PBS with Tween-20 for 3 times of 15 min. Then, the membrane was developed with enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for imaging. Image lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to acquire and analyze imaging signals. The relative contents of target proteins were expressed against GAPDH.
Bioinformatics
On www.targetscan.org website, miR-378 was searched to analyze potential target genes. Then, the target genes were submitted on www.davidncifcrf.gov website for the analysis of potential signaling pathways that involved the target genes.
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). The data were expressed as means ± standard deviations. Data were tested for normality. Multigroup measurement data were analyzed using one-way ANOVA. In case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was used. Comparison between two groups was carried out using Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-378 in peripheral blood and lung tissues from asthmatic children is increased compared with that in healthy children
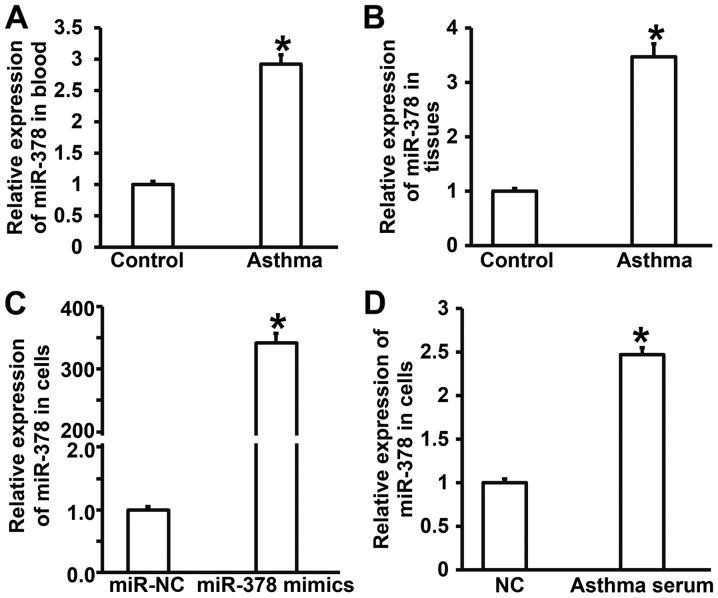
To measure the expression of miR-378 in peripheral blood, lung tissues and smooth muscle cells, RT-qPCR was performed. The data showed that the levels of miR-378 in peripheral blood and tissues from asthmatic children were significantly higher than those in control group (P<0.05) (Fig. 1A and B). Expression of miR-378 in smooth muscle cells transfected with miR-378 mimics was significantly higher than that in miR-NC group (P<0.05) (Fig. 1C). After incubating smooth muscle cells with serum from asthmatic children for 7 days, the level of miR-378 in smooth muscle cells was significantly higher than that of cells treated with serum from healthy children (NC group) (P<0.05) (Fig. 1D). The results suggest that expression of miR-378 in peripheral blood and lung tissues from asthmatic children is increased compared with that in healthy children.
Figure 1.
Expression of miR-378 in blood, lung tissues or airway smooth muscle cells. (A and B) Expression of miR-378 in (A) peripheral blood or (B) lung tissues from children with asthma measured by quantitative real-time polymerase chain reaction. *P<0.05 compared with control group. (C and D) Expression of miR-378 in airway smooth muscle cells (C) transfected with miR-378 mimics or (D) treated with serum from asthmatic children for 7 days measured by quantitative real-time polymerase chain reaction. *P<0.05 compared with miR-NC group or NC group. Student's t-test was used to compare differences between groups (n=3–5). Control, samples from healthy children; Asthma, samples from asthmatic children; miR, microRNA; miR-NC, cells transfected with miR-negative control; miR-378 mimics, cells transfected with miR-378 mimics; NC, smooth muscle cells treated with serum from healthy children; Asthma serum, smooth muscle cells treated with serum from asthmatic children.
Serum from asthmatic children promotes the proliferation of smooth muscle cells in vitro by affecting cell cycle
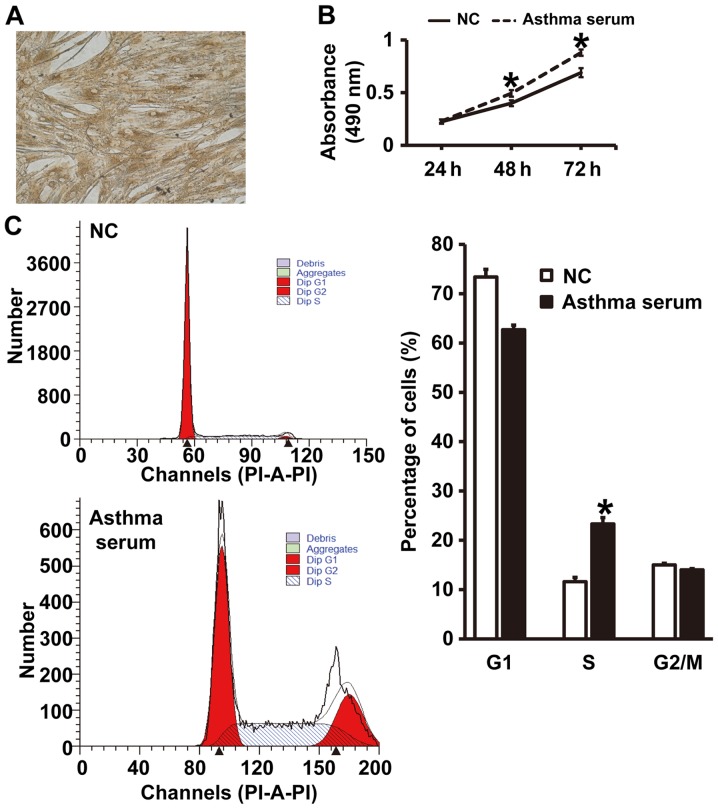
To test the effect of serum from asthmatic children on the proliferation and cell cycle of smooth muscle cells, CCK-8 assay and flow cytometry were carried out. The expression of α-SMA in smooth muscle cells was identified by immunohistochemical analysis (Fig. 2A), suggesting that primary airway smooth muscle cells were successfully isolated. CCK-8 assay showed that the absorbance of smooth muscle cells treated with serum from asthmatic children at 48 and 72 h was significantly higher than that of cells in NC group (P<0.05) (Fig. 2B). In addition, the percentage of S-phase cells among all smooth muscle cells treated with asthma serum was significantly higher than that in NC group (P<0.05) (Fig. 2C). The results indicate that serum from asthmatic children promotes the proliferation of smooth muscle cells in vitro by affecting cell cycle.
Figure 2.
Culture of primary airway smooth muscle cells and effect of serum from asthmatic children on their biological functions. (A) Identification of the expression of α-smooth muscle actin in smooth muscle cells by immunohistochemistry. Magnification, ×100. (B) Absorbance (490 nm) of airway smooth muscle cells at 24, 48 and 72 h. Proliferation was determined by CCK-8 assay. *P<0.05 compared with NC group. (C) Percentage of smooth muscle cells in G1, S and G2/M phases. Cell cycles were identified by flow cytometry. *P<0.05 compared with NC group. Student's t-test was used to compare differences between groups (n=3–5). NC, smooth muscle cells treated with serum from healthy children; Asthma serum, smooth muscle cells treated with serum from asthmatic children. PI-A-PI, phosphatidylserine-Annexin V-phosphatidylserine.
Serum from asthmatic children enhances apoptosis resistance of smooth muscle cells
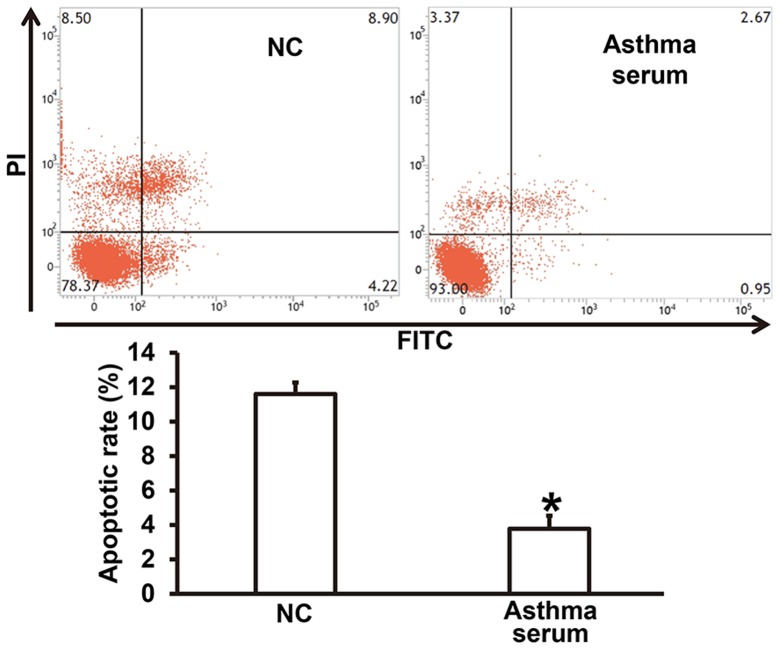
To examine how serum from asthmatic children affects the apoptosis of smooth muscle cells, flow cytometry was used. The data showed that treatment with serum from asthmatic children for 7 days reduced the apoptotic rate of smooth muscle cells compared with NC group (P<0.05) (Fig. 3). The result suggests that serum from asthmatic children enhances apoptosis resistance of smooth muscle cells.
Figure 3.
Effect of serum from asthmatic children on the apoptosis of airway smooth muscle cells. Flow cytometry was performed to detect apoptosis. *P<0.05 compared with NC group. Student's t-test was used to compare differences between groups (n=3–5). NC, smooth muscle cells treated with serum from healthy children; Asthma serum, smooth muscle cells treated with serum from asthmatic children; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Overexpression of miR-378 increases the proliferation of smooth muscle cells by affecting cell cycle
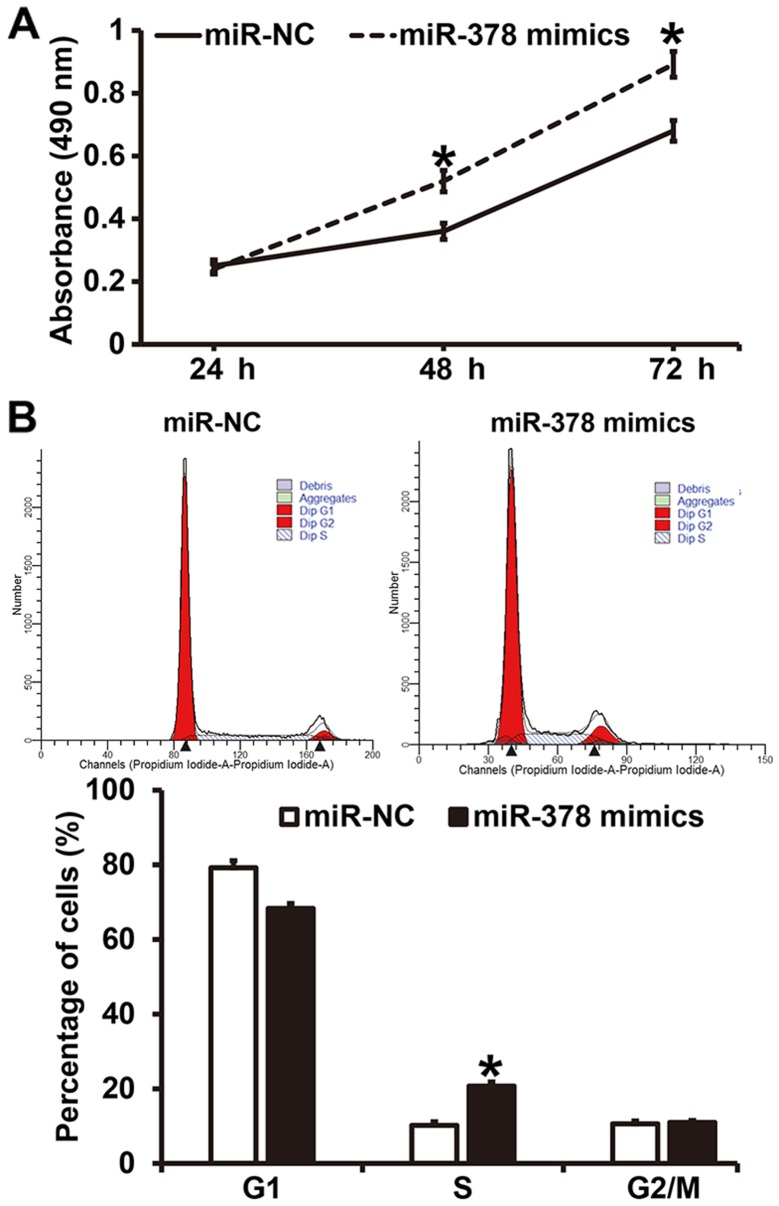
To investigate how miR-378 affects the proliferation and cell cycle of smooth muscle cells, CCK-8 assay and flow cytometry were employed. CCK-8 assay showed that the absorbance of smooth muscle cells transfected with miR-378 mimics at 48 and 72 h was significantly higher than that of cells in miR-NC group (P<0.05) (Fig. 4A). Similarly, the percentage of S-phase cells among all smooth muscle cells transfected with miR-378 mimics was significantly higher than that in miR-NC group (P<0.05) (Fig. 4B). The results indicate that overexpression of miR-378 increases the proliferation of smooth muscle cells by affecting cell cycle.
Figure 4.
Effect of miR-378 on the proliferation and cell cycle of airway smooth muscle cells. (A) Determination of the proliferation of cells by CCK-8 assay. *P<0.05 compared with miR-NC group. (B) Detection of cell cycle of the cells by flow cytometry. *P<0.05 compared with miR-NC group. Student's t-test was used to compare differences between groups (n=3–5). miR, microRNA; miR-NC, cells transfected with miR-negative control; miR-378 mimics, cells transfected with miR-378 mimics.
Overexpression of miR-378 upregulates apoptosis resistance of smooth muscle cells
To test how miR-378 overexpression affects the apoptosis of smooth muscle cells, flow cytometry was performed. The data showed that the apoptotic rate of smooth muscle cells transfected with miR-378 was significantly lower than that in miR-NC group (P<0.05) (Fig. 5). The result suggests that overexpression of miR-378 upregulatesapoptosis resistance of smooth muscle cells.
Figure 5.
Effect of miR-378 on the apoptosis of airway smooth muscle cells. Flow cytometry was performed to detect apoptosis. *P<0.05 compared with miR-NC group. Student's t-test was used to compare differences between groups (n=3–5). miR, microRNA; miR-NC, cells transfected with miR-negative control; miR-378 mimics, cells transfected with miR-378 mimics; FITC, fluorescein isothiocyanate; PI, propidium iodide.
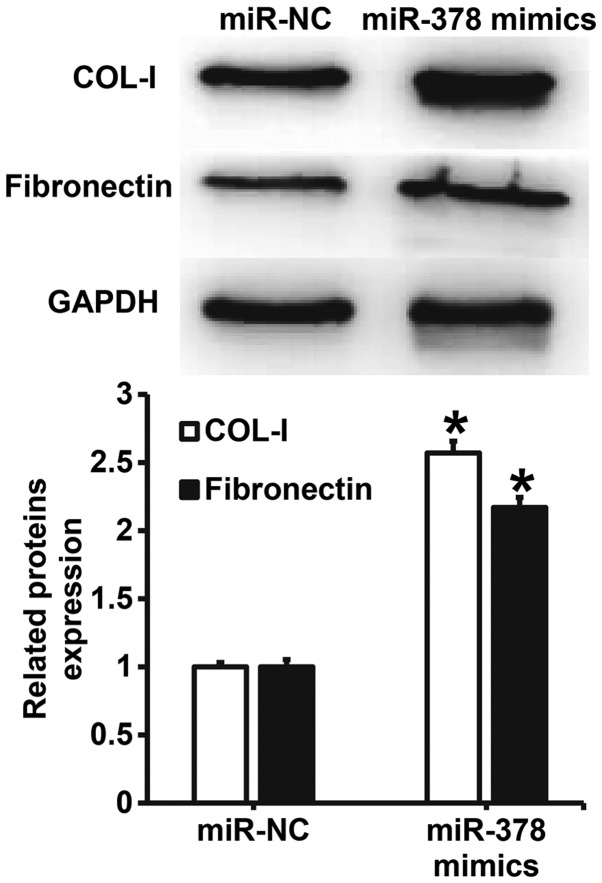
Overexpression of miR-378 increases the expression of extracellular matrix-related proteins in smooth muscle cells
To understand the effect of miR-378 overexpression on the secretion of extracellular matrix-related proteins by smooth muscle cells, western blotting was carried out. The data showed that the expression of COL-I and FN proteins in smooth muscle cells transfected with miR-378 was significantly higher than that in miR-NC group (P<0.05) (Fig. 6). The result indicates that overexpression of miR-378 increases the expression of extracellular matrix-related proteins in smooth muscle cells.
Figure 6.
Effect of miR-378 on the expression of extracellular matrix-related proteins in airway smooth muscle cells. Expression of COL-I and fibronectin was measured by western blotting. *P<0.05 compared with miR-NC group. Student's t-test was used to compare differences between groups (n=3–5). miR, microRNA; miR-NC, cells transfected with miR-negative control; miR-378 mimics, cells transfected with miR-378 mimics; COL-I, type I collagen.
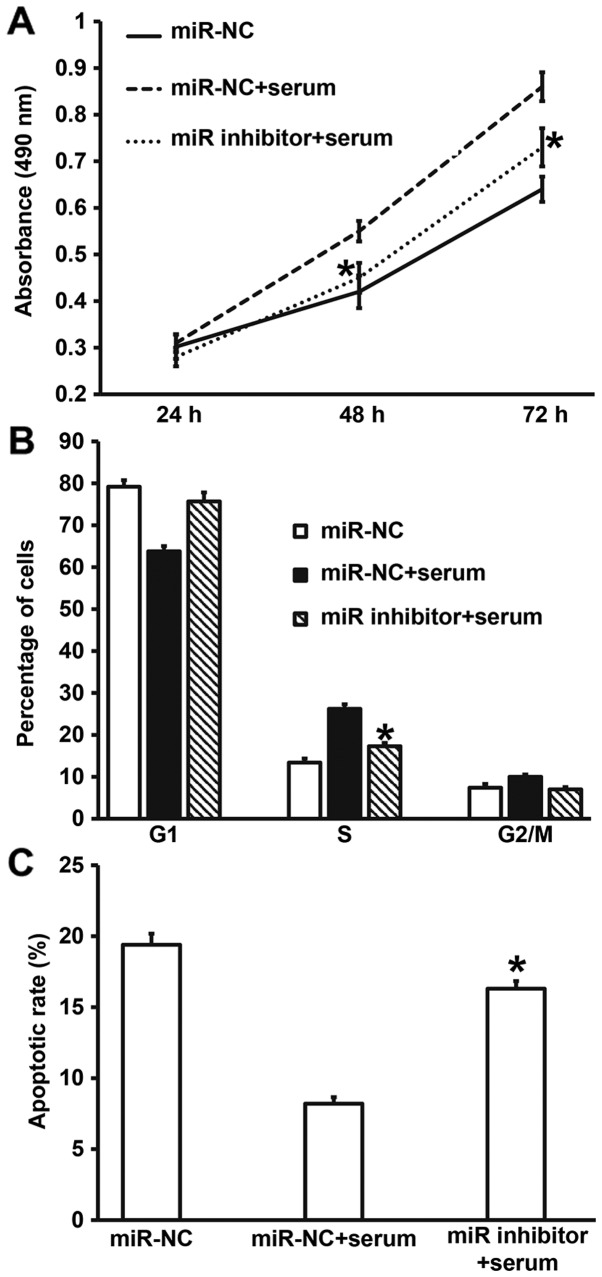
Downregulation of miR-378 expression reverses the promoting effect of asthma serum on the proliferation and apoptosis resistance of smooth muscle cells
To examine whether miR-378 was the key factor by which asthma serum promoted the proliferation of smooth muscle cells, we cultured smooth muscle cells transfected with miR-NC or miR-378 inhibitor together with serum from asthmatic children for 7 days. CCK-8 assay data showed that the absorbance of smooth muscle cells transfected with miR-378 inhibitor and treated with serum from asthmatic children at 48 and 72 h was significantly lower than that of cells transfected with miR-NC and treated with asthma serum (P<0.05), reaching a level similar to that of miR-NC group (Fig. 7A). In addition, the percentage of S-phase cells among all smooth muscle cells transfected with miR-378 inhibitor and treated with asthma serum was significantly lower than that in cells transfected with miR-NC and treated with asthma serum (P<0.05), reaching a level similar to that of miR-NC group (Fig. 7B). Apoptosis analysis showed that the apoptotic rate of smooth muscle cells transfected with miR-378 inhibitor and treated with asthma serum was significantly higher than that of cells transfected with miR-NC and treated with asthma serum (P<0.05), reaching a level similar to that of miR-NC group (Fig. 7C). These results suggest that downregulation of miR-378 expression reverses the promoting effect of asthma serum on the proliferation and apoptosis resistance of smooth muscle cells.
Figure 7.
Effect of miR-378 inhibition on the biological functions of airway smooth muscle cells treated with serum from asthmatic children. (A) Proliferation of smooth muscle cells evaluated by CCK-8 assay. *P<0.05 compared with miR-NC+serum group. (B) Cell cycles of smooth muscle cells determined by flow cytometry. *P<0.05 compared with miR-NC+serum group. (C) Apoptosis of smooth muscle cells detected by flow cytometry. Multigroup measurement data were analyzed using one-way ANOVA. In case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was used. *P<0.05 compared with miR-NC+serum group. Student's t-test was used to compare differences between groups (n=3–5). miR, microRNA; miR-NC, cells transfected with miR-negative control; miR-NC+serum, cells transfected with miR-NC and treated with serum from asthmatic children; miR-378 inhibitor+serum, cells transfected with miR-378 inhibitor and treated with serum from asthmatic children.
miR-378 may affect the proliferation, apoptosis and motility of airway smooth muscle cells via its downstream signaling pathways
To predict the downstream signaling pathways of miR-378, bioinformatics was used.
KEGG PATHWAY Database analysis showed that miR-378 has 282 potential target genes, and it possibly participates in the regulation of ErbB signaling pathway, Ras signaling pathway, calcium signaling pathway, and MAPK signaling pathway via its target genes (Table I). The result indicates that miR-378 may affect the proliferation, apoptosis and motility of airway smooth muscle cells via its downstream signaling pathways.
Table I.
Bioinformatics analysis of downstream signaling pathways of miR-378.
| Ranks | Signaling pathways predicted by KEGG |
|---|---|
| 1 | ErbB signaling pathway |
| 2 | Ras signaling pathway |
| 3 | Calcium signaling pathway |
| 4 | Pancreatic secretion |
| 5 | Phosphatidylinositol signaling system |
| 6 | Estrogen signaling pathway |
| 7 | MAPK signaling pathway |
Discussion
In recent years, the incidence of asthma is increasing year by year in children (25). Asthma is mainly characterized by airway hyperresponsiveness, inflammation, and remodeling (26). Airway smooth muscle plays important roles in these three stages, especially airway remodeling (27). miRNA molecules participate in almost all pathophysiological processes of the body, but their roles and mechanisms of action in asthma are rarely reported. Airway inflammation is a key factor in the occurrence and development of asthma, and airway remodeling is an inevitable result of persistent airway inflammation (11). Under repeated stimulation by various inflammatory factors, the repair mechanism of airway structural cells is disordered, resulting in pathological changes such as epithelial metaplasia, smooth hypertrophy, and extracellular matrix precipitation. Furthermore, airway contractile dysfunction, small airway spasm or stenosis, or even irreversible constriction and airflow limitation occurs, finally resulting in decreased pulmonary function in patients (24). Under the stimulation by inflammatory factors, smooth muscle cells are transformed from contractile type to synthetic type, leading to significant functional changes (28). Therefore, it is necessary to use smooth muscle cells as target cells for the treatment of asthma. In the present study, we have isolated and cultured airway smooth muscle cells in vitro. Spindle-shape smooth muscle cells are observed under a light microscope, and immunohistochemistry shows positive expression of α-SMA, suggesting that the isolated smooth muscle cells are suitable for subsequent experiments.
miR-378 is a miRNA molecule that was initially found in tumors. It is reported that miR-378 not only promotes the occurrence and development of tumors, but also acts as a tumor-suppressor gene, depending on the type of tumor (29). In addition, miR-378 has also been found to have biological functions in the development of myocardium and muscle. For example, miR-378 can regulate the hypertrophy of cardiomyocytes through Ras signaling pathway (30). Moreover, miR-378 can inhibit the regeneration of muscle cells (31). These reports suggest that miR-378 can participate in the regulation of biological functions of muscle cells. In the present study, we discover that expression of miR-378 is significantly increased in both lung tissues and peripheral blood from asthmatic children. In addition, stimulation of primary smooth muscle cells by serum from asthmatic children elevates the level of miR-378, and promotes the proliferation and apoptosis resistance of the cells. Of note, inhibition of miR-378 expression reduces the promoting effect of asthma serum on the proliferation and apoptosis resistance of the cells. Further analysis shows that upregulation of miR-378 promotes the proliferation and apoptosis resistance of smooth muscle cells, and elevates the expression of COL-I and FN proteins. Bioinformatics analysis shows that there are more than 200 target genes of miR-378 that are involved in Ras, MAPK or calcium signaling pathways. These results suggest that the biological functions of miR-378 are associated with changes in these signaling pathways. However, the detailed mechanism still remains to be elucidated in our future studies. The limitation of the study is the low sample number, due to difficulties in collecting samples in asthmatic children and healthy children.
In conclusion, the present study demonstrates that the expression of miR-378 in children with asthma is elevated, aggravating airway remodeling by promoting the proliferation and apoptosis resistance of airway smooth muscle cells. Therefore, miR-378 could be a potential therapeutic target for asthma in children. In the future, we will screen miR-378 pathway through signaling pathway inhibitors or agonists, and then focus on the target genes in this pathway.
Acknowledgements
The authors wish to thank the Department of Pediatrics, Maternity and Child Health Care Hospital of Zibo City and research team for their help and dedication.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PL and SX collaborated to design the study. PL and XL were responsible for performing experiments. PL and SX analyzed the data. All authors collaborated to interpret results and develop the manuscript. The final version of the manuscript has been read and approved by all authors.
Ethics approval and consent to participate
All procedures performed in the present study were approved by the Ethics Committee of Maternity and Child Health Care Hospital of Zibo City. Written informed consent was obtained from all patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kothari PH, Qiu W, Croteau-Chonka DC, Martinez FD, Liu AH, Lemanske RF, Jr, Ober C, Krishnan JA, Nicolae DL, Barnes KC, et al. Role of local CpG DNA methylation in mediating the 17q21 asthma susceptibility gasdermin B (GSDMB)/ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) expression quantitative trait locus. J Allergy Clin Immunol. 2018;141:2282–2286.e6. doi: 10.1016/j.jaci.2017.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyland ME, Lanario JW, Pooler J, Masoli M, Jones RC. How patient participation was used to develop a questionnaire that is fit for purpose for assessing quality of life in severe asthma. Health Qual Life Outcomes. 2018;16:24. doi: 10.1186/s12955-018-0851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan W. Toward better management for asthma: From smart inhalers to injections to wearables, researchers are finding new ways to improve asthma treatment. IEEE Pulse. 2018;9:28–33. doi: 10.1109/MPUL.2017.2772398. [DOI] [PubMed] [Google Scholar]
- 4.Davies HM. Living with asthma in 19th-century France: The doctor, Armand Trousseau and the patient, Emile Pereire. J Med Biogr 967772017741763. 2018 doi: 10.1177/0967772017741763. [DOI] [PubMed] [Google Scholar]
- 5.Frey SM, Jones MR, Goldstein N, Riekert K, Fagnano M, Halterman JS. Knowledge of inhaled therapy and responsibility for asthma management among young teens with uncontrolled persistent asthma. Acad Pediatr. 2018;18:317–323. doi: 10.1016/j.acap.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian R, Zhang Y, Yang Y, Yin Y, Zhao X, Chen H, Yuan Y. White matter integrity disruptions correlate with cognitive impairments in asthma. J Magn Reson Imaging. 2018 Jan 21; doi: 10.1002/jmri.25946. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Zhu D, Zhang C, Shen H, Ying S. Breaking through restricting bottleneck for better asthma control. J Transl Int Med. 2017;5:192–193. doi: 10.1515/jtim-2017-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitamanni P, Chandrasekaran V, Rajendiran S. Serum total magnesium level and its correlation with symptom control in children with mild persistent asthma. Indian J Pediatr. 2018;85:420–425. doi: 10.1007/s12098-017-2599-3. [DOI] [PubMed] [Google Scholar]
- 9.Ménard S, Jbilou J, Lauzier S. Family caregivers' reported nonadherence to the controller medication of asthma in children in Casablanca (Morocco): Extent and associated factors. J Asthma. 2018:1–11. doi: 10.1080/02770903.2017.1414235. [DOI] [PubMed] [Google Scholar]
- 10.Burgess JK, Ketheson A, Faiz A, Limbert Rempel KA, Oliver BG, Ward JPT, Halayko AJ. Phenotype and functional features of human telomerase reverse transcriptase immortalized human airway smooth muscle cells from asthmatic and non-asthmatic donors. Sci Rep. 2018;8:805. doi: 10.1038/s41598-017-18429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panariti A, Baglole CJ, Sanchez V, Eidelman DH, Hussain S, Olivenstein R, Martin JG, Hamid Q. Interleukin-17A and vascular remodelling in severe asthma; lack of evidence for a direct role. Clin Exp Allergy. 2018;48:365–378. doi: 10.1111/cea.13093. [DOI] [PubMed] [Google Scholar]
- 12.An G, Zhang X, Wang W, Huang Q, Li Y, Shan S, Corrigan CJ, Wang W, Ying S. The effects of interleukin-33 on airways collagen deposition and matrix metalloproteinase expression in a murine surrogate of asthma. Immunology. 2018 Feb 18; doi: 10.1111/imm.12911. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Jiang Y, Zhou J, Liu S, Qin N, Du J, Jin G, Hu Z, Ma H, Shen H, Dai J. Evaluation of CpG-SNPs in miRNA promoters and risk of breast cancer. Gene. 2018;651:1–8. doi: 10.1016/j.gene.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Staedel C, Tran TPA, Giraud J, Darfeuille F, Di Giorgio A, Tourasse NJ, Salin F, Uriac P, Duca M. Modulation of oncogenic miRNA biogenesis using functionalized polyamines. Sci Rep. 2018;8:1667. doi: 10.1038/s41598-018-20053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long YJ, Liu XP, Chen SS, Zong DD, Chen Y, Chen P. miR-34a is involved in CSE-induced apoptosis of human pulmonary microvascular endothelial cells by targeting Notch-1 receptor protein. Respir Res. 2018;19:21. doi: 10.1186/s12931-018-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maemura T, Fukuyama S, Sugita Y, Lopes TJS, Nakao T, Noda T, Kawaoka Y. Lung-derived exosomal miR-483-3p regulates the innate immune response to influenza virus infection. J Infect Dis. 2018;217:1372–1382. doi: 10.1093/infdis/jiy035. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, He Q, Guo Z, Xiong F, Li Y, Pan Y, Gao C, Li L, He C. MicroRNA-425 facilitates pathogenic Th17 cell differentiation by targeting forkhead box O1 (Foxo1) and is associated with inflammatory bowel disease. Biochem Biophys Res Commun. 2018;496:352–358. doi: 10.1016/j.bbrc.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Yin D, Zhou B, Li T. MiR-135a promotes inflammatory responses of vascular smooth muscle cells from db/db mice via downregulation of FOXO1. Int Heart J. 2018;59:170–179. doi: 10.1536/ihj.17-040. [DOI] [PubMed] [Google Scholar]
- 19.Oglesby IK, Agrawal R, Mall MA, McElvaney NG, Greene CM. miRNA-221 is elevated in cystic fibrosis airway epithelial cells and regulates expression of ATF6. Mol Cell Pediatr. 2015;2:1. doi: 10.1186/s40348-014-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho CS, Noor SM, Nagoor NH. MiR-378 and MiR-1827 regulate tumor invasion, migration and angiogenesis in human lung adenocarcinoma by targeting RBX1 and CRKL, respectively. J Cancer. 2018;9:331–345. doi: 10.7150/jca.18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Liu Y, Yang W, Han X, Li S, Liu H, Gerweck LE, Fukumura D, Loeffler JS, Yang BB, et al. MicroRNA-378 enhances radiation response in ectopic and orthotopic implantation models of glioblastoma. J Neurooncol. 2018;136:63–71. doi: 10.1007/s11060-017-2646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proctor CJ, Goljanek-Whysall K. Using computer simulation models to investigate the most promising microRNAs to improve muscle regeneration during ageing. Sci Rep. 2017;7:12314. doi: 10.1038/s41598-017-12538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta AK, Doherty T, Broide D, Croft M. Tumor necrosis factor family member LIGHT acts with IL-1β and TGF-β to promote airway remodeling during rhinovirus infection. Allergy. 2018;73:1415–1424. doi: 10.1111/all.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao YH, Tseng CM, Su KC, Chen WC, Wu MT, Wu YC, Chang SC, Lee YC, Kou YR, Perng DW. Glycopyrronium bromide inhibits lung inflammation and small airway remodeling induced by subchronic cigarette smoke exposure in mice. Respir Physiol Neurobiol. 2018;249:16–22. doi: 10.1016/j.resp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths LJ, Lyons RA, Bandyopadhyay A, Tingay KS, Walton S, Cortina-Borja M, Akbari A, Bedford H, Dezateux C. Childhood asthma prevalence: Cross-sectional record linkage study comparing parent-reported wheeze with general practitioner-recorded asthma diagnoses from primary care electronic health records in Wales. BMJ Open Respir Res. 2018;5:e000260. doi: 10.1136/bmjresp-2017-000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanigan C, Sheikh A, DunnGalvin A, Brew BK, Almqvist C, Nwaru BI. Prenatal maternal psychosocial stress and offspring's asthma and allergic disease: A systematic review and meta-analysis. Clin Exp Allergy. 2018;48:403–414. doi: 10.1111/cea.13091. [DOI] [PubMed] [Google Scholar]
- 27.Lee YT, Wu CT, Sun HL, Ko JL, Lue KH. Fungal immunomodulatory protein-fve could modulate airway remodel through by affect IL17 cytokine. J Microbiol Immunol Infect. 2018;51:598–607. doi: 10.1016/j.jmii.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Huang N, Liu K, Liu J, Gao X, Zeng Z, Zhang Y, Chen J. Interleukin-37 alleviates airway inflammation and remodeling in asthma via inhibiting the activation of NF-κB and STAT3 signalings. Int Immunopharmacol. 2018;55:198–204. doi: 10.1016/j.intimp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Yang F, Wang M, Cao W, Yang Z. miR-378 functions as an onco-miRNA by targeting the ST7L/Wnt/β-catenin pathway in cervical cancer. Int J Mol Med. 2017;40:1047–1056. doi: 10.3892/ijmm.2017.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagalingam RS, Sundaresan NR, Gupta MP, Geenen DL, Solaro RJ, Gupta M. A cardiac-enriched microRNA, miR-378, blocks cardiac hypertrophy by targeting Ras signaling. J Biol Chem. 2017;292:5123. doi: 10.1074/jbc.A112.442384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng P, Han W, Li C, Li H, Zhu D, Zhang Y, Liu X. miR-378 attenuates muscle regeneration by delaying satellite cell activation and differentiation in mice. Acta Biochim Biophys Sin (Shanghai) 2016;48:833–839. doi: 10.1093/abbs/gmw077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.