Abstract
The aim of the present study was to explore whether ciprofloxacin-incorporated waterborne polyurethane (WBPU) polymers have the capacity to inhibit bacterial biofilm formation in vitro. WBPU polymers were incorporated with ciprofloxacin and were cultured with Escherichia coli (E. coli) or Staphylococcus aureus (S. aureus) in media for 2, 4 or 7 days. In another experiment, the WBPU membranes were cultured with Proteus mirabilis (P. mirabilis) in artificial urine for 2, 4 or 7 days. Colony counting, scanning electron microscopy and fluorescence confocal microscopy were utilized to examine bacterial biofilms on the surfaces of membranes. The membranes were further co-cultured with P. mirabilis in a simple model of an artificial catheterized bladder in order to evaluate their ability to control encrustation. The WBPU films with ciprofloxacin effectively inhibited bacterial biofilm formation in the culture medium and in artificial urine. In addition, in artificial urine, the films with ciprofloxacin reduced catheter obstruction. In conclusion, ciprofloxacin-incorporated WBPU polymers are able to effectively inhibit bacterial biofilm formation in vitro.
Keywords: bacterial biofilm, polyurethane, artificial urine, ciprofloxacin
Introduction
The global biomaterial market size has increased to 90 billion dollars in 2017 from 44.0 billion dollars in 2012 (1). Polymers, including polyethylene (PE), polytetrafluoroethylene and polypropylene, are high-molecular-weight materials used for several medical applications. However, the surfaces of these materials are frequently colonized by bacterial biofilms. It has been reported that the biofilm layers on urethral catheters emerge between 4 and 12 h following catheterization, and develop completely between 12 and 24 h (2). Bacterial biofilm consists of resident bacteria and extracellular matrix. The resident bacteria are protected from antibiotics, disinfectants and dynamic environments, e.g. urine flow (3). The extracellular polysaccharides in the biofilm matrix also trap nutrients that are required for cell growth (4). Biofilm transmission requires the detachment of bacterial cells from a mature biofilm and the attachment of dispersed cells to a new surface (5). Consequently, biofilms act as bacterial reservoirs that colonize new surfaces (5).
Modification of the surface of biomedical materials with various antibacterial agents inhibits the bacterial attachment to surfaces (6–9). It has been reported that local delivery of anti-microbial substances from simultaneous drug carriers may be an efficient way of inhibiting bacterial attachment. Biodegradable polyurethanes, including waterborne polyurethanes (WBPUs), have attracted considerable scientific interest due to their limited toxicity, high biocompatibility and excellent self-assembly properties (10–12). According to Sivak et al (13,14), biodegradable polyurethane polymers are ideal drug carriers, and further studies indicated the potential role of WBPUs in several clinical applications, including catheter materials (15–17).
It has remained elusive whether biodegradable polyurethane materials with incorporated anti-microbials are able to prevent bacterial attachment and biofilm formation on the surface of the implant. In the present study, the ability of a ciprofloxacin-incorporated WBPU polymer to inhibit biofilm formation in vitro was assessed.
Materials and methods
Reagents and equipment
Ciprofloxacin and streptomycin were purchased from Sheng Technology Co., Ltd (Chengdu, China). The remaining materials and equipment included trypticase soy broth (TSB) and trypticase soy agar (each, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), Live/Dead® Baclight Bacterial Viability Kit (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA), a fluorescence confocal microscope (Carl Zeiss AG, Oberkochen, Germany), an ultrasonic vibration device (Shanghai Kudos Ultrasonic Instrument Co., Ltd., Shanghai, China) and a scanning electron microscope (SEM, Hitachi, Ltd., Tokyo, Japan).
Polyurethane emulsion
Emulsion of WBPU polymers was performed using a two-step polymerization process as reported previously (11). Isophorone diisocyanate (IPDI) was used as the hard segment and the soft segment was composed of polyethylene glycol (PEG) and polycaprolactone (PCL) in a molar ratio of 1:2. 1,4-butandiol (BDO) and l-lysine were used as chain extender in a molar ratio of 1:1. The molar ratio of hard segment, soft segment and chain extender was 3:1:1.7.
Bacterial strains
The Escherichia coli (E. coli) strain ATCC25922, the Staphylococcus aureus (S. aureus) strain ATCC25923 and the Proteus mirabilis (P. mirabilis) strain ATCC12453 were purchased from Nanjing Diagnostic Biotechnology Co., Ltd. (Nanjing, China) and were used for testing the attachment and biofilm formation on the implant surface.
Artificial urine
The artificial urine used in the present study was prepared as described by Griffith et al (18). The following reagents were mixed: NaCl (4.6 g/l), CaCl2 (0.49 g/l), Na2SO4 (2.3 g/l), MgCl2x6H2O (0.65 g/l), sodium citrate (0.65 g/l), KCl (1.6 g/l), sodium oxalate (0.02 g/l), NH4Cl (1.0 g/l), monopotassium phosphate (2.8 g/l), gelatine powder (5.0 g/l), urea (25 g/l) and TSB (1.0 g/l). The final solution was adjusted to pH 6.1 by addition of NaOH. The artificial urine was sterilized by membrane filtration.
Artificial bladder
The model of the bladder was generated as previously described (19). The artificial bladder was a fermentation flask sustained in a 37°C incubator. Artificial urine was stored in a sealed glass container and pumped into the bladder at a rate of 0.5 ml/min. A 14 Fr all-silicone catheter was attached to the lower outlet of the flask to mimic the urethra of the bladder. Subsequently, the urine flowed out via the catheter. The pH of residual urine was recorded.
Prevention of bacterial biofilm formation on WBPU films incorporated with antibiotic in liquid media
WBPU emulsions were mixed with ciprofloxacin (0.1 mg) or streptomycin (1 mg) and prepared into films (1×1 cm; thickness, 0.5 cm) by heating the emulsions in an oven at 60°C for 48 h. The blank WBPU film was made in the same way and was used as a control. The membranes were transferred to 24-well plates and each well contained 1.8 ml TSB (TSB+1.25% glucose) and 0.2 ml bacterial solution [107 colony-forming units (CFU)/ml of E. coli strain ATCC25922 or S. aureus strain ATCC25923]. The plates were placed in a thermostatic incubator at 37°C for 2, 4 or 7 days. Colony counting was performed to assess the bacteriostatic ability of the different films. The films were placed in 10 ml normal saline (NS) under ultrasonic shockwave (40 Hz, 7 min, 22°C, 160 W) to ensure the total stripping of bacteria that had adhered on the surface. Subsequently, the NS bacterial solution was continuously diluted by a 10−1-fold-gradient to a final dilution of 10−6 fold for colony counting. The experiments were performed three times. SEM was employed to characterize the bacterial biofilm on day 7. However, only ciprofloxacin-incorporated membranes and controls were examined as they exhibited the most significant effect.
Prevention of bacterial biofilm formation on WBPU films incorporated with antibiotics in artificial urine and artificial bladder matrices
Blank WBPU films and WBPU films incorporated with antibiotics (as described above) were transferred to 24-well plates. Each well contained 1.8 ml artificial urine and 0.2 ml bacterial solution (107 CFU/ml of P. mirabilis strain ATCC12453). The plates were placed in a thermostatic incubator at 37°C for 2, 4 or 7 days. Colony counting was performed to assess the potential inhibition of bacterial biofilm formation on the membranes. Furthermore, immunofluorescence staining with propidium iodide (PI)/cyto9 fluorescence dye (Live/Dead® Baclight Bacterial Viability Kit) was performed to detect bacterial viability on the ciprofloxacin-incorporated film by confocal microscopy.
Following co-culture with P. mirabilis solution (107 CFU/ml, 10 ml) for 4 h, the WBPU films incorporated with ciprofloxacin (or blank WBPU films as the control group) were transferred to artificial bladder and incubated in a thermostatic water tank under constant conditions at 37°C. Artificial urine in a sealed glass container was flowed into the simulated bladder at a rate of 0.5 ml/min and then flowed out through the catheter attached to the lower outlet of the bladder. 30 ml of urine was retained in the artificial bladder during the experiment. The end-points of the test were the incidence of catheter obstruction or a testing time period of >3 days. The volume of artificial urine used in this experiment depended on the time of end points, but no more than 2160 ml was utilized as the maximal observation time was 72 h. The pH of the residual urine at the end-point was recorded.
Statistical analysis
The data were statistically evaluated by using GraphPad Prism5 (GraphPad Software Inc., La Jolla, CA, USA). Colony numbers of bacterial strains in the blank, ciprofloxacin and streptomycin groups at 7 days were analyzed by one-way analysis of variance, and Bonferroni's post-hoc tests were used for comparisons between any two groups in these data sets. The pH of the artificial urine was compared between the ciprofloxacin and control groups by using the Mann-Whitney U-test. With a 95% confidence interval, P<0.05 was considered to indicate a statistically significant difference.
Results
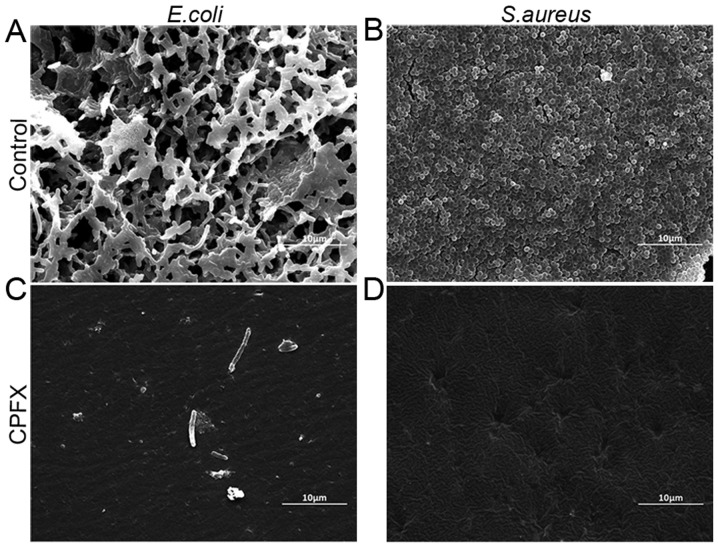
Ciprofloxacin and streptomycin-incorporated WBPU membranes and blank membranes were co-cultured with E. coli strain ATCC25922 and S. aureus strain ATCC25923 in liquid media for 2, 4 or 7 days. The results suggested that these drug-incorporated membranes effectively inhibit bacterial biofilm formation of the two bacterial strains tested (Fig. 1). Furthermore, ciprofloxacin-incorporated membranes exhibited a greater capacity to prevent biofilm formation than streptomycin-incorporated membranes. The ciprofloxacin-incorporated membranes were further analyzed by SEM to confirm their inhibitory effect on the formation of the biofilm (Fig. 2). The results demonstrated that ciprofloxacin-incorporated WBPU membranes effectively inhibited biofilm formation by E. coli (Fig. 2A and C) and S. aureus (Fig. 2B and D) compared with the corresponding formation of the biofilm on the blank membranes.
Figure 1.
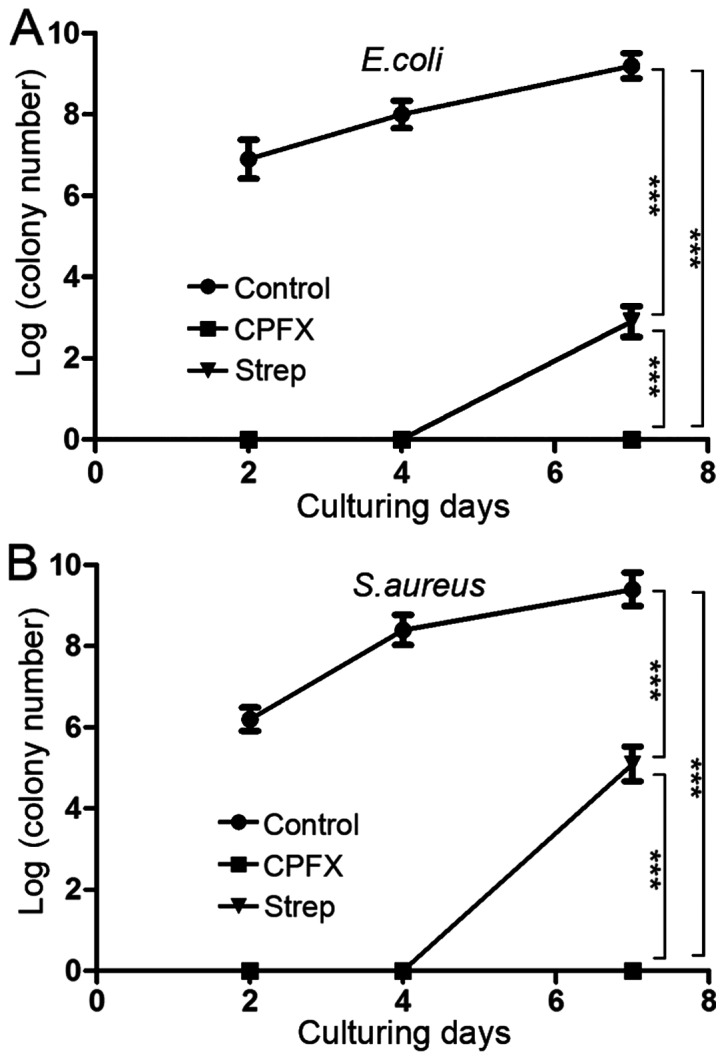
Following incubation with (A) E. coli or (B) S. aureus for 2, 4 or 7 days, the apparent bacterial colonies on blank membranes, CPFX membranes and streptomycin membranes were quantified and displayed in logarithmic form. ***P<0.001. CPFX, ciprofloxacin; Strep, streptomycin.
Figure 2.
CPFX -incorporated waterborne polyurethane films with inhibited bacterial biofilm formation on the membrane surfaces. The films were examined under a scanning electron microscope (magnification, ×5,000; scale bar, 10 µm). (A) Control membrane incubated with E. coli for 7 days. (B) Control membrane incubated with S. aureus for 7 days. (C) CPFX membrane incubated with E. coli for 7 days. (D) CPFX membrane incubated with S. aureus for 7 days. CPFX, ciprofloxacin.
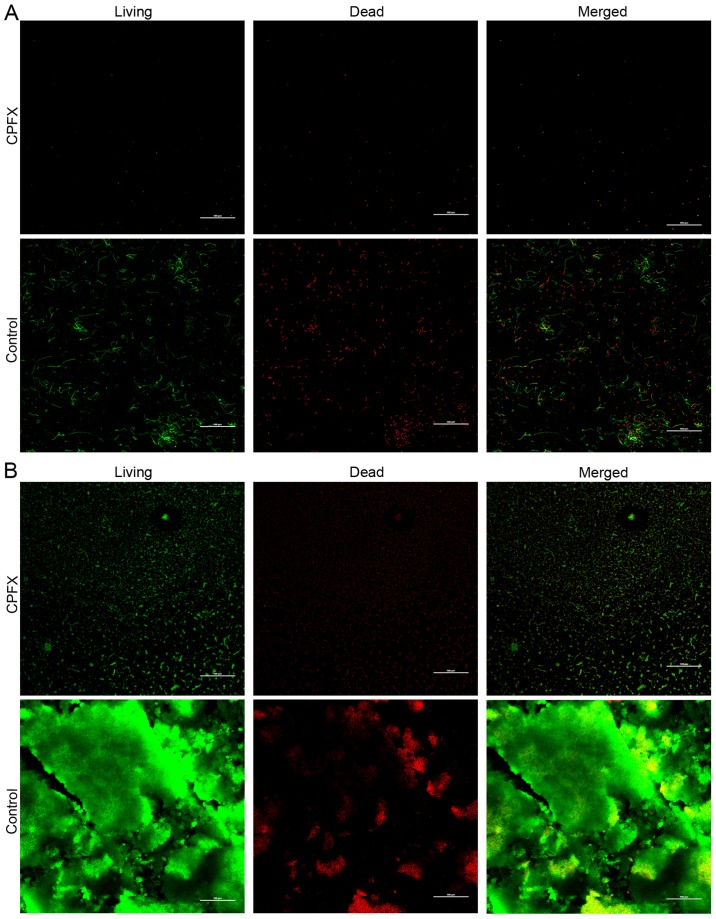
In addition to E. coli and S. aureus, which represent commonly encountered Gram-negative and -positive bacteria, respectively, the urease-producing P. mirabilis was considered to be a potential causative factor for the encrustation and blockage of urinary catheters. Consequently, WBPU membranes were co-cultured with P. mirabilis in artificial urine and an artificial bladder model. Ciprofloxacin- or streptomycin-incorporated WBPU membranes exhibited significantly lower colony counts following co-culture with P. mirabilis in artificial urine compared with those in the group using a control membrane (Fig. 3). At 7 days after co-culturing membranes with P. mirabilis in artificial urine, the log colony count in the ciprofloxacin and streptomycin group was 2.4±0.41 and 4.0±0.39, respectively. At the same time, the log number of colonies in the control group was 8.9±0.61 (P<0.001 between each group). In addition, the number of viable bacteria on the ciprofloxacin-incorporated membrane was lower compared with that on the streptomycin-incorporated membrane (P<0.001). To investigate the effect of the ciprofloxacin membrane on bacterial viability, ciprofloxacin-incorporated films were stained with PI/cyto9 fluorescence dye and analyzed by fluorescence confocal microscopy. The non-viable bacteria were stained red, while the viable bacteria were stained green. The results indicated that ciprofloxacin-incorporated WBPU polymers effectively inhibited P. mirabilis biofilm formation compared to the blank membranes (Fig. 4).
Figure 3.
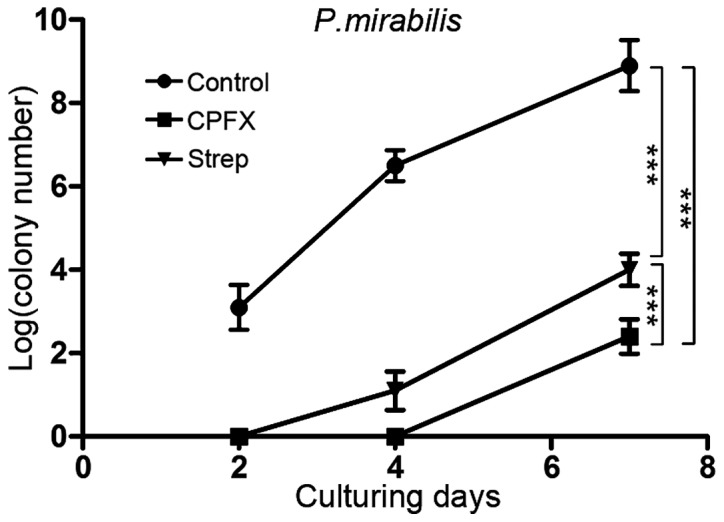
Bacterial colony numbers of P. mirabilis were quantified after 2, 4 or 7 days of co-culture with blank films, CPFX films and streptomycin films with P. mirabilis in artificial urine, and displayed in logarithmic form. ***P<0.001. CPFX, ciprofloxacin; Strep, streptomycin.
Figure 4.
CPFX-incorporated and blank waterborne polyurethane films following co-culture with P. mirabilis in artificial urine. Following staining with propidium iodide/cyto9, CPFX films and control films were examined by confocal microscopy (magnification, ×200; scale bar, 100 µm). Viable bacteria were stained green, while non-viable bacteria presented with a red stain. (A) CPFX film (upper panel) and control film (lower panel) cultured with P. mirabilis for 2 days. (B) CPFX film (upper panel) and control film (lower panel) cultured with P. mirabilis for 7 days. CPFX, ciprofloxacin.
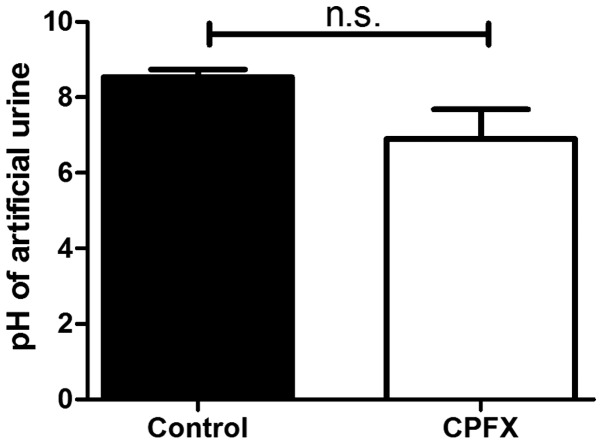
The effect of ciprofloxacin-incorporated membranes on urine pH and catheter obstruction was assessed by co-culturing WBPU membranes with P. mirabilis in flowing artificial urine from an artificial bladder. A pH increase was noted in the residual urine of the blank WBPU polymer group compared with that in the ciprofloxacin-incorporated group (8.5±0.21 vs. 6.9±0.78), but the difference was not statistically significant (P=0.1; Fig. 5). However, complete catheter obstruction occurred in 2 of 3 independent experiments in the control group, while no obstruction was observed in the ciprofloxacin group.
Figure 5.
CPFX and blank waterborne polyurethane films cultured in an artificial bladder following co-culture with P. mirabilis. The pH of the artificial urine in the CPFX and control groups was recorded at the end-point, which was total obstruction and/or an outflow time of >3 days. n.s., not significant; CPFX, ciprofloxacin.
Discussion
Biofilms are responsible for 80% of chronic microbial infections in humans and is associated with high mortality, morbidity, hospitalization rates and healthcare costs (20). The bacteria embedded in the biofilm exhibit a ~100-fold increased drug resistance compared with that of free-floating bacteria (21), which results from reduced diffusion of anti-microbial drugs, exchange of genetic material that encodes resistance elements and the presence of dormant cells (22). These parameters cause a high rate of treatment failure and persistence of infection. Consequently, prevention of biofilm formation is critical for the reduction of bacterial infections. Biofilm formation may be inhibited by prevention of bacterial adhesion, inactivation of adherent bacteria and/or their combination. A high number of studies on the prevention and control of medical material-associated infections have investigated the integration of anti-microbial agents within medical materials, including the physical entrapment of drugs into polymer matrices and the coating of materials with anti-microbial substances (23,24).
WBPU polymers exhibit hydrophilic features in water, which may reduce the adhesion of bacteria on its surface (9). In addition, biodegradable features of WBPU make it a promising method of drug delivery for anti-bacterial substances (14). The results of the present study indicated that ciprofloxacin and streptomycin-incorporated WBPU membranes feature a significantly reduced bacterial biofilm formation by Gram-negative and -positive bacteria compared with that on the blank WBPU. Furthermore, biodegradable hydrophilic polyurethane polymers possess excellent self-assembly properties and do not cause toxicity following their degradation (10,11,25). In addition, the degradation speed of these WBPU polymers may be tuned via adjustment of the molar ratio of PEG or PCL according to different experimental or clinical requirements (11,26). Consequently, WBPU polymers incorporated with anti-microbial substances may be used to produced different medical appliances (i.e. double-J stents) or applied as a coating on the material surface (i.e. on the surface or cavity of the catheter).
The standard of care for patients undergoing long-term indwelling catheterization is reduced by the encrustation and blockage of the catheters (27). Certain studies have indicated that an elevation of the urinary pH to ~8.2 causes P. mirabilis biofilm development in a crystal form on catheter surfaces and the intra-cavity regions that normally inhibit bacterial attachment (27,28). Urease has a key role in encrustation due to its ability to increase the urine pH via urea hydrolysis, which enhances the absorption of ammonia and carbon dioxide on the biofilm. Morris et al (29) and Stickler et al (30) proposed that this effect was caused by infection from urease-producing P. mirabilis, which was also inhibited by ciprofloxacin-incorporated WBPU membranes, as demonstrated in the present study. Suller et al (31) reported that a high velocity of artificial urine was able to dilute the urine in the artificial bladder and lower its pH, thus preventing crystallization. In the present study, the application of only a small amount of antibiotic substance reduced the obstruction at a low velocity of urine (0.5 ml/min) pumped into the simulated bladder. Consequently, ciprofloxacin-incorporated WBPU polymers inhibited encrustation of biological material surfaces. The combination of the antibiotics with a carrier material may prolong the prevention of infection and encrustation. However, the generation of antibiotic-resistant strains should be taken into serious consideration.
One limitation of the present study is that the bacterial strains used in the experiments were standard strains instead of clinical isolates, which may not accurately represent specific conditions encountered in the clinic, e.g. drug resistance. In addition, the present study did not include any in vivo experiment, and the present results do not sufficiently reflect bacterial biofilm formation in patients. However, the present study still indicates that antibiotic-incorporated WBPU effectively inhibits biofilm formation by drug-sensitive bacteria. Furthermore, antibacterial effects and the application of WBPU/antibiotic-coated catheters requires further investigation.
Acknowledgements
None declared.
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 81500521 to JW) and the Natural Science Foundation of Anhui Province (1608085QH203 to JW).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YX and JW designed the study, performed the experiments, analyzed the data and wrote the manuscript. SW contributed to data analysis. ZH and CL contributed to study design and manuscript editing.
Ethical approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Orthopedic implants-a global market overview. Indus Exp. 2011 [Google Scholar]
- 2.Koseoglu H, Aslan G, Esen N, Sen BH, Coban H. Ultrastructural stages of biofilm development of Escherichia coli on urethral catheters and effects of antibiotics on biofilm formation. Urology. 2006;68:942–946. doi: 10.1016/j.urology.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Veerachamy S, Yarlagadda T, Manivasagam G, Yarlagadda PK. Bacterial adherence and biofilm formation on medical implants: A review. Proc Inst Mech Eng H. 2014;228:1083–1099. doi: 10.1177/0954411914556137. [DOI] [PubMed] [Google Scholar]
- 4.Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials. 2007;28:4192–4199. doi: 10.1016/j.biomaterials.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magana M, Sereti C, Ioannidis A, Mitchell CA, Ball AR, Magiorkinis E, Chatzipanagiotou S, Hamblin MR, Hadjifrangiskou M, Tegos GP. Options and limitations in clinical investigation of bacterial biofilms. Clin Microbiol Rev. 2018;31(pii):e00084–16. doi: 10.1128/CMR.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai Z, Ma H, Liu B, Yan X, Xue Q. Facile synthesis of Ag/GNS-g-PAA nanohybrids for antimicrobial applications. Colloids Surf B Biointerfaces. 2012;89:147–151. doi: 10.1016/j.colsurfb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Sivakumar PM, Iyer G, Natesan L, Doble M. 3′-Hydroxy-4-methoxychalcone as a potential antibacterial coating on polymeric biomaterials. Appl Surf Sci. 2010;256:6018–6024. doi: 10.1016/j.apsusc.2010.03.112. [DOI] [Google Scholar]
- 8.Kowalczuk D, Ginalska G, Golus J. Characterization of the developed antimicrobial urological catheters. Int J Pharm. 2010;402:175–183. doi: 10.1016/j.ijpharm.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Kara F, Aksoy EA, Yuksekdag Z, Hasirci N, Aksoy S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr Polym. 2014;112:39–47. doi: 10.1016/j.carbpol.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Yu L, Ding M, Tan H, Li J, Fu Q. Preparation and rapid degradation of nontoxic biodegradable polyurethanes based on poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) and l -lysine diisocyanate. Polym Chem. 2011;2-3:601. doi: 10.1039/C0PY00235F. [DOI] [Google Scholar]
- 11.Jiang X, Yu F, Wang Z, Li J, Tan H, Ding M, Fu Q. Fabrication and characterization of waterborne biodegradable polyurethanes 3-dimensional porous scaffolds for vascular tissue engineering. J Biomater Sci Polym Ed. 2010;21:1637–1652. doi: 10.1163/092050609X12525750021270. [DOI] [PubMed] [Google Scholar]
- 12.Kang SY, Ji Z, Tseng LF, Turner SA, Villanueva DA, Johnson R, Albano A, Langer R. Design and synthesis of waterborne polyurethanes. Adv Mater. 2018;30:e1706237. doi: 10.1002/adma.201706237. [DOI] [PubMed] [Google Scholar]
- 13.Sivak WN. Synthesis and characterization of novel polyurethane drug delivery systems. Dissertations & Theses-Gradworks. 2007 [Google Scholar]
- 14.Sivak WN, Zhang J, Petoud S, Beckman EJ. Simultaneous drug release at different rates from biodegradable polyurethane foams. Acta Biomater. 2009;5:2398–2408. doi: 10.1016/j.actbio.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Li J, Ding M, Tan H, Ling Q, Zhong Y, Fu Q. Synthesis and degradation of nontoxic biodegradable waterborne polyurethanes elastomer with poly(ε-caprolactone) and poly(ethylene glycol) as soft segment. Eur Polym J. 2007;43:1838–1846. doi: 10.1016/j.eurpolymj.2007.02.029. [DOI] [Google Scholar]
- 16.Wang J, Liu Q, Tian Y, Jian Z, Li H, Wang K. Biodegradable hydrophilic polyurethane PEGU25 loading antimicrobial peptide Bmap-28: A sustained-release membrane able to inhibit bacterial biofilm formation in vitro. Sci Rep. 2015;5:8634. doi: 10.1038/srep08634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye T, Jian Z, Wang J, He W, Liu Q, Wang K, Li H, Tan H. Antimicrobial activity study of triclosan-loaded WBPU on Proteus mirabilis in vitro. Int Urol Nephrol. 2017;49:563–571. doi: 10.1007/s11255-017-1532-z. [DOI] [PubMed] [Google Scholar]
- 18.Griffith DP, Musher DM, Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 19.Morris NS, Stickler DJ, Winters C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br J Urol. 1997;80:58–63. doi: 10.1046/j.1464-410X.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 20.Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 21.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 22.Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med. 1999;340:1–8. doi: 10.1056/NEJM199901073400101. [DOI] [PubMed] [Google Scholar]
- 24.Ruggeri V, Francolini I, Donelli G, Piozzi A. Synthesis, characterization, and in vitro activity of antibiotic releasing polyurethanes to prevent bacterial resistance. J Biomed Mater Res A. 2007;81:287–298. doi: 10.1002/jbm.a.30984. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Yu L, Ding M, Li J, Tan H, Wang Z, Fu Q. Synthesis and characterization of pH-sensitive biodegradable polyurethane for potential drug delivery applications. Macromolecules. 2011;44:857–864. doi: 10.1021/ma102346a. [DOI] [Google Scholar]
- 26.Cakić SM, Ristić IS, Krakovský I, Stojiljković DT, Bělský P, Kollová L. Crystallization and thermal properties in waterborne polyurethane elastomers: Influence of mixed soft segment block. Mater Chem Phys. 2014;144:31–40. doi: 10.1016/j.matchemphys.2013.12.008. [DOI] [Google Scholar]
- 27.Williams GJ, Stickler DJ. Some observations on the diffusion of antimicrobial agents through the retention balloons of foley catheters. J Urol. 2007;178:697–701. doi: 10.1016/j.juro.2007.03.091. [DOI] [PubMed] [Google Scholar]
- 28.Stickler DJ, Lear JC, Morris NS, Macleod SM, Downer A, Cadd DH, Feast WJ. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J Appl Microbiol. 2006;100:1028–1033. doi: 10.1111/j.1365-2672.2006.02840.x. [DOI] [PubMed] [Google Scholar]
- 29.Morris NS, Stickler DJ, McLean RJ. The development of bacterial biofilms on indwelling urethral catheters. World J Urol. 1999;17:345–350. doi: 10.1007/s003450050159. [DOI] [PubMed] [Google Scholar]
- 30.Stickler D, Ganderton L, King J, Nettleton J, Winters C. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol Res. 1993;21:407–411. doi: 10.1007/BF00300077. [DOI] [PubMed] [Google Scholar]
- 31.Suller MT, Anthony VJ, Mathur S, Feneley RC, Greenman J, Stickler DJ. Factors modulating the pH at which calcium and magnesium phosphates precipitate from human urine. Urol Res. 2005;33:254–260. doi: 10.1007/s00240-004-0458-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.