Abstract
Correlation of the expression of inflammatory factors with expression of apoptosis-related genes, B-cell lymphoma 2 (Bcl-2) and Bcl-2 associated X protein (Bax), in burned rats was investigated. Forty healthy Sprague-Dawley rats were selected and randomly divided into SHAM group (n=10), I° burn group (n=10), II° burn group (n=10) and III° burn group (n=10). Changes in tumor necrosis factor-α (TNF-α), Bax messenger ribonucleic acid (mRNA), Bcl-2 mRNA, Bax protein and Bcl-2 protein expression levels were detected. The correlation of TNF-α, Bax and Bcl-2 with the degree of burn in rats was observed, and the correlation of TNF-α with Bax and Bcl-2 was also analyzed. Moreover, Bax mRNA and Bcl-2 mRNA were detected via reverse transcription-quantitative polymerase chain reaction, and TNF-α, Bax protein and Bcl-2 protein were detected via enzyme-linked immunosorbent assay. In burn groups, TNF-α, Bax mRNA and Bax protein levels were significantly increased at each time point compared with those at the previous time point (P<0.05), but Bcl-2 mRNA and protein levels were significantly decreased compared with those at the previous time point (P<0.05). At the same time point, TNF-α, Bax mRNA, Bcl-2 mRNA, Bax protein and Bcl-2 protein expression levels had statistically significant differences between any given two groups (P<0.05). The TNF-α expression level was positively correlated with Bax expression levels and negatively correlated with Bcl-2 expression levels. Additionally, TNF-α, Bax mRNA and Bax protein had positive correlations with the degree of burn and time after burn, while Bcl-2 mRNA and Bcl-2 protein had negative correlations with the degree of burn and time after burn. Continuous monitoring of changes in the TNF-α level can be used as a means to evaluate the degree of burn and apoptosis, and to prevent the deepening of burn wounds, thus facilitating the early clinical evaluation of prognosis.
Keywords: burn, TNF-α, Bax, Bcl-2
Introduction
Burn refers to a series of complex pathophysiological changes caused by such heat as high-temperature gas, liquid and solid, which lead to skin tissue damage, pain, loss of interstitial fluid and wound necrosis, and induce the systemic infection and even death (1,2). The treatment of burn wound is the key to improving the burn, but the burn wound often deepens, so it is extremely important to avoid the wound deepening (3,4).
In burned patients, a large number of inflammatory cells are activated, and a great number of inflammatory factors are secreted, among which the expression level of tumor necrosis factor-α (TNF-α) changes very significantly (5,6). Apoptosis is a normal physiological phenomenon in the body, and burn can lead to pathological apoptosis. Thus, apoptosis is also a kind of important pathological change in burned patients (7,8). B-cell lymphoma 2 (Bcl-2) is an anti-apoptotic gene, while Bcl-2 associated X protein (Bax) is a pro-apoptotic gene. The degree of apoptosis is closely related to the expression levels of Bax and Bcl-2 (9,10). Evidence suggests that the inflammatory factor TNF-α can induce apoptosis of nucleus pulposus cells in patients with protrusion of intervertebral disc (11). It has been reported that TNF-α can induce the apoptosis of type II alveolar epithelial cells in acute lung injury and hepatic cells in liver disease (12,13). Therefore, TNF-α is closely associated with apoptosis, but whether TNF-α is related to apoptosis in burned patients remains to be determined.
In this study, three rat models of burn of different degrees were constructed. Changes in TNF-α, Bax and Bcl-2 expression levels before and after burn were detected, and correlation of TNF-α, Bax and Bcl-2 with the degree of burn in rats and correlation of TNF-α with Bax and Bcl-2 were analyzed to offer some help to the clinical prevention of wound deepening.
Materials and methods
Objects of study
Forty Sprague-Dawley (SD) rats of clean grade were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China [production license SCXK (Shanghai) 2012-0002], and fed with rat chow purchased from Beijing Zhecheng Technology Co., Ltd., Beijing, China. SD rats were aged 49–56 days with an average of 55.3±4.5 days, and weight of 220–300 g with an average of 254.1±6.2 g. The animals were separately fed in feeding boxes at a temperature of 22±3°C and relative humidity of 45–60%. Noise was <85 decibels, the ammonia concentration was ≤20 ppm, and the air was exchanged 8–12 times/h. Light-dark cycle (12/12 h) was maintained using a fluorescent lamp, rats had free access to food and water, and feeding boxes were replaced 1–2 times every week. The humidity in the feeding box was ≤60%, and the water bottle was replaced once or twice every week. Finally, the rats were randomly divided into the SHAM group (n=10), I° burn group (n=10), II° burn group (n=10) and III° burn group (n=10).
SD rat modeling
After all 40 SD rats were anesthetized via intraperitoneal injection of 10% chloral hydrate (Wuhan Yuancheng Technology Development Co., Ltd., Wuhan, China) (300 µg/g), the hair on the back of rats was shaved off. The area of hair shaved accounted for approximately 30% of the total body surface area (TBSA) in SHAM group, approximately 5–10% in I° burn group, approximately 15–25% in II° burn group and approximately 30% in III° burn group. After cleaning and skin preparation, rats in the 4 groups were fixed in the supine position on the scald window horizontally. The exposed skin was soaked in boiling water at 100°C for 15 sec in the I°, II° and III° burn groups, while the exposed skin was soaked in warm water at 20°C for 15 sec in the SHAM group. After the rats were taken from water and cleaned, Ringer's lactate solution (Jiangsu Caiwei Biotechnology Co., Ltd., Jiangsu, China; NMPN H20067464) was injected intraperitoneally (4 ml/kg/TBSA). In addition, buprenorphine (Henan Tianfu Chemical Co., Ltd., Henan, China; NMPN H10970146) (0.25 mg/kg) was subcutaneously injected for analgesia after operation, followed by analgesia maintenance once every 12 h. The study was approved by the Ethics Committee of the Second People's Hospital of Liaocheng (Liaocheng, China).
Extraction of total messenger ribonucleic acid (mRNA) in serum
After the peripheral blood was drawn from the caudal vein of rats, the total RNA in serum was extracted using the TRIzol reagent (Shanghai Mingjin Biotechnology Co., Ltd., Shanghai, China) according to manufacturer's protocol. The concentration and purity of RNA extracted were analyzed using a micro-ultraviolet spectrophotometer MD1000 (Beijing THmorgan Biotechnology Co., Ltd., Beijing, China), and RNA integrity was analyzed via 3% agarose gel electrophoresis (the gel electrophoresis suite was purchased from Shanghai Jingke Chemical Technology Co., Ltd., Shanghai, China).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The total mRNA extracted was reverse transcribed into complementary deoxyribonucleic acid (cDNA) according to instructions of the SYBR-Green quantitative PCR kit (Thermo Fisher Scientific, Inc., Shanghai, China). PCR conditions were as follows: at 37°C for 45 min and 95°C for 5 min. The cDNA amplification system was 20 µl in total, and conditions were as follows: pre-denaturation at 95°C for 10 min, denaturation at 95°C for 10 sec, annealing at 60°C for 20 sec, extension at 72°C for 10 sec, a total of 40 cycles, extension at 72°C for 5 min. U6 was used as the internal reference. The operation was repeated in 3 wells for all samples, and results were analyzed using the 2−∆Cq method (14). RT-qPCR primers were synthesized by Suzhou Synbio Technologies Co., Ltd., Suzhou, China. Primer sequences are shown in Table I.
Table I.
Primer sequences.
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| Bax mRNA | 5′-ATCCAGAGACAAGACATGTAC-3′ | 5′-TTCAGATGTTCTAAGCCTACGG-3′ |
| Bcl-2 mRNA | 5′-TGGCGGTTTGCGGTGGAC-3′ | 5′-CCAGTGCAGGGTCCGAGGT-3′ |
| U6 | 5′-CGCTTCGGCAGCACATATAC-3′ | 5′-TTCACGAATTTGCGTGTCAT-3′ |
Enzyme-linked immunosorbent assay (ELISA)
TNF-α, Bax protein and Bcl-2 protein in the peripheral blood were detected via ELISA in accordance with instructions of the kit. TNF-α, Bax and Bcl-2 detection kits were purchased from Shanghai Jingkang Biological Engineering Co., Ltd., Shanghai, China.
Observation indexes
Changes in TNF-α, Bax and Bcl-2 levels before modeling and at 6, 12 and 18 h after operation were detected. Correlations of TNF-α, Bax and Bcl-2 with the degree of burn in rats were observed and analyzed.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 19.0 (AsiaAnalytics Formerly SPSS China) was used for the statistical analysis. Enumeration data are presented as rate, and the Chi-square test was used for comparison of the rate. Measurement data are presented as (mean ± SD). Analysis of variance was used for the comparison among groups, and the least significant difference (LSD) post hoc test was used for comparison between two or more groups after analysis of variance. Repeated measures analysis of variance was used for the comparison within the group at different time points. Pearson's correlation analysis was used for the correlation among TNF-α, Bax and Bcl-2. P<0.05 was considered to indicate a statistically significant difference.
Results
Bax mRNA and Bax protein detection results
Results of analysis of variance showed that Bax mRNA and Bax protein expression levels in the 4 groups of rats at 6 h, 12 h and 18 h after operation were different (P<0.05). There were no differences in Bax mRNA and Bax protein expression levels among the 4 groups of rats before modeling (P>0.05). In I°, II° and III° burn groups, Bax mRNA and Bax protein expression levels were increased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they were higher at 12 and 18 h after operation than those at 6 h after operation (P<0.05) and also higher at 18 h after operation than those at 12 h after operation (P<0.05). No significant differences were found in Bax mRNA and Bax protein expression levels at 4 time points in SHAM group (P>0.05). At the same time point, with the increased degree of burn, Bax mRNA and Bax protein expression levels were increased. Bax mRNA and Bax protein expression levels at each time point in I°, II° and III° burn groups were higher than those in SHAM group (P<0.05), and they were higher at each time point in II° and III° burn groups than those in I° burn group (P<0.05) and also higher at each time point in III° burn group than those in II° burn group (P<0.05) (Tables II and III, Figs. 1 and 2).
Table II.
RT-qPCR detection results of Bax mRNA.
| Variable | SHAM | I° | II° | III° | Statistical value | P-value |
|---|---|---|---|---|---|---|
| Before modeling | 0.84±0.03 | 0.82±0.02 | 0.83±0.02 | 0.85±0.04 | 2.020 | 0.128 |
| 6 h after operation | 0.86±0.03 | 1.17±0.02a,d | 1.45±0.01a,d,e | 1.62±0.03a,d,e,f | 10.46 | 0.015 |
| 12 h after operation | 0.90±0.02 | 1.48±0.03a,b,d | 1.66±0.05a,b,d,e | 1.85±0.06a,b,d,e,f | 11.05 | 0.011 |
| 18 h after operation | 0.91±0.04 | 1.60±0.03a–d | 1.88±0.04a–e | 2.01±0.08a–f | 10.06 | 0.018 |
| Statistical value | 3.887 | 18.743 | 17.782 | 18.451 | ||
| P-value | 0.274 | <0.001 | <0.001 | <0.001 |
P<0.05 increased compared with that before modeling in the same group.
P<0.05 increased compared with that at 6 h after operation in the same group.
P<0.05 increased compared with that at 12 h after operation in the same group.
P<0.05 increased compared with that at the same time point in SHAM group.
P<0.05 increased compared with that at the same time point in I° burn group.
P<0.05 increased compared with that at the same time point in II° burn group.
Table III.
ELISA detection results of Bax protein (ng/ml).
| Variable | SHAM | I° | II° | III° | Statistical value | P-value |
|---|---|---|---|---|---|---|
| Before modeling | 2.73±1.15 | 2.75±1.12 | 2.71±1.09 | 2.74±1.11 | 0.026 | 0.999 |
| 6 h after operation | 2.84±1.12 | 6.35±1.56a,d | 8.22±1.49a,d,e | 11.03±2.33a,d,e,f | 4.832 | 0.185 |
| 12 h after operation | 2.88±1.13 | 9.09±1.48a,b,d | 12.56±2.67a,b,d,e | 15.33±3.12a,b,d,e,f | 10.62 | 0.014 |
| 18 h after operation | 2.91±1.15 | 12.35±3.71a–d | 16.33±4.21a–e | 19.78±5.56a–f | 16.05 | 0.001 |
| Statistical value | 0.048 | 18.425 | 19.214 | 19.633 | ||
| P-value | 0.956 | <0.001 | <0.001 | <0.001 |
P<0.05 increased compared with that before modeling in the same group.
P<0.05 increased compared with that at 6 h after operation in the same group.
P<0.05 increased compared with that at 12 h after operation in the same group.
P<0.05 increased compared with that at the same time point in SHAM group.
P<0.05 increased compared with that at the same time point in I° burn group.
P<0.05 increased compared with that at the same time point in II° burn group.
Figure 1.
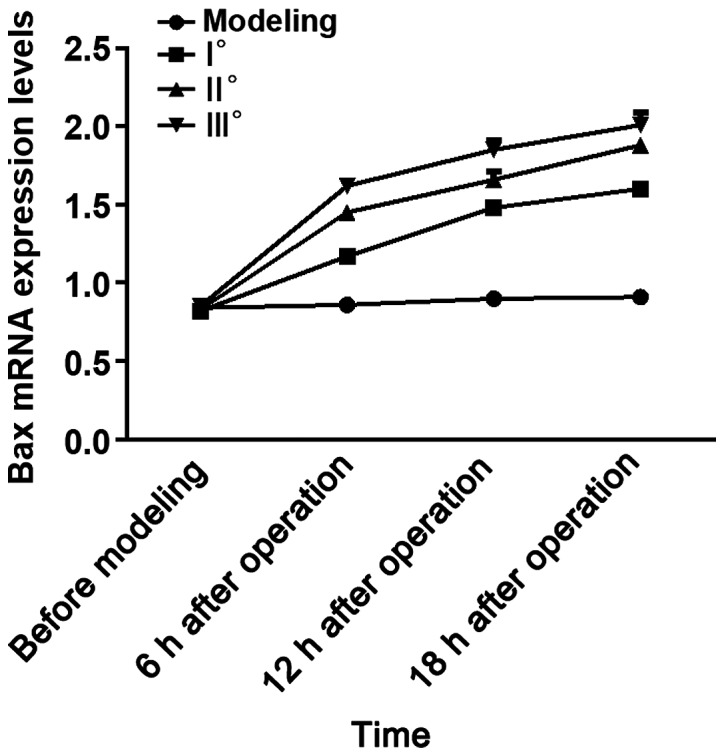
RT-qPCR detection results of Bax mRNA. Results of analysis of variance show that the Bax mRNA expression levels in the 4 groups of rats at 6, 12 and 18 h after operation are different (P<0.05). In I°, II° and III° burn groups, the Bax mRNA expression levels are increased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they are higher at 12 and 18 h after operation than those at 6 h after operation (P<0.05) and also higher at 18 h after operation than those at 12 h after operation (P<0.05). At the same time point, the Bax mRNA expression levels in I°, II° and III° burn groups are higher than that in the SHAM group (P<0.05), and they are higher at each time point in the II° and III° burn groups than that in I° burn group (P<0.05) and also higher at each time point in III° burn group than that in II° burn group (P<0.05).
Figure 2.
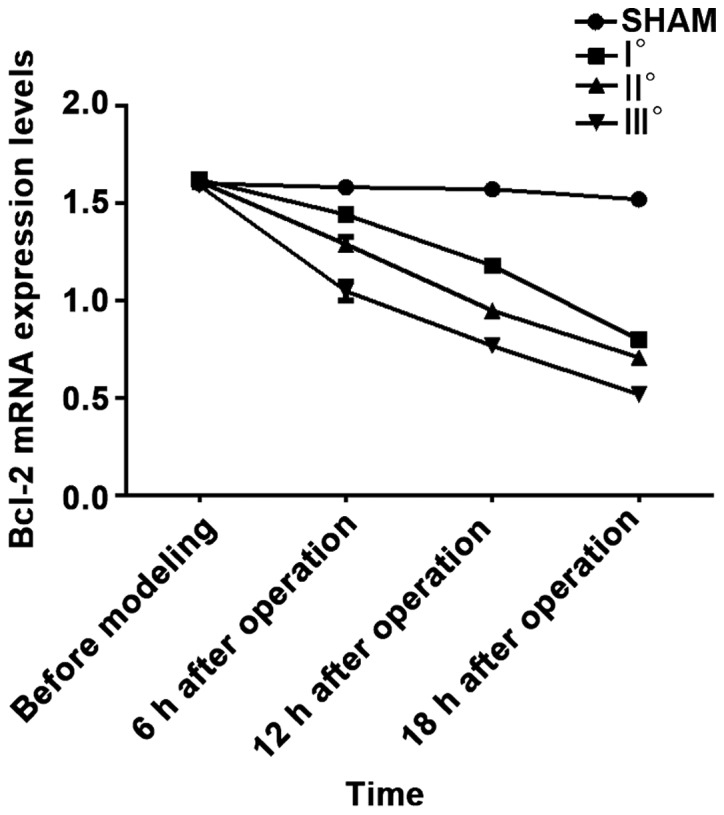
RT-qPCR detection results of Bcl-2 mRNA. Results of analysis of variance show that the Bcl-2 mRNA expression levels in the 4 groups of rats at 6, 12 and 18 h after operation are different (P<0.05). In I°, II° and III burn groups, the Bcl-2 mRNA expression levels are decreased at 6, 12 and 18 h after surgery compared with those before modeling (P<0.05), and they are decreased at 12 and 18 h after operation compared with those at 6 h after operation (P<0.05) and also decreased at 18 h after operation compared with those at 12 h after operation (P<0.05). At the same time point, the Bcl-2 mRNA expression levels in I°, II° and III° burn groups are decreased compared with that in the SHAM group (P<0.05), and they are decreased at each time point in II° and III° burn groups compared with that in I° burn group (P<0.05) and also decreased at each time point in III° burn group compared with that in II° burn group (P<0.05).
Bcl-2 mRNA and Bcl-2 protein detection results
Results of analysis of variance revealed that Bcl-2 mRNA and Bcl-2 protein expression levels in the 4 groups of rats at 6, 12 and 18 h after operation were different (P<0.05). There were no differences in Bcl-2 mRNA and Bcl-2 protein expression levels in the 4 groups of rats before modeling (P>0.05). In I°, II° and III° burn groups, Bcl-2 mRNA and Bcl-2 protein expression levels were decreased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they were decreased at 12 and 18 h after operation compared with those at 6 h after operation (P<0.05) and also decreased at 18 h after operation compared with those at 12 h after operation (P<0.05). No significant differences were found in Bcl-2 mRNA and Bcl-2 protein expression levels at 4 time points in SHAM group (P>0.05). At the same time point, with the increased degree of burn, Bcl-2 mRNA and Bcl-2 protein expression levels were decreased. Bcl-2 mRNA and Bcl-2 protein expression levels at each time point in I°, II° and III° burn groups were decreased compared with those in SHAM group (P<0.05), and they were decreased at each time point in II° and III° burn groups compared with those in I° burn group (P<0.05) and also decreased at each time point in III° burn group compared with those in II° burn group (P<0.05) (Tables IV and V, Figs. 3 and 4).
Table IV.
qRT-PCR detection results of Bcl-2 mRNA.
| SHAM | I° | II° | III° | Statistical value | P-value | |
|---|---|---|---|---|---|---|
| Before modeling | 1.60±0.03 | 1.62±0.02 | 1.61±0.03 | 1.59±0.03 | 1.830 | 0.608 |
| 6 h after operation | 1.58±0.02 | 1.44±0.01a,d | 1.29±0.04a,d,e | 1.05±0.05a,d,e,f | 2.037 | 0.126 |
| 12 h after operation | 1.57±0.03 | 1.18±0.02a,b,d | 0.95±0.02a,b,d,e | 0.77±0.02a,b,d,e,f | 2.381 | 0.049 |
| 18 h after operation | 1.52±0.02 | 0.80±0.01a–d | 0.71±0.03a–e | 0.52±0.02a–f | 20.57 | <0.001 |
| Statistical value | 2.754 | 19.73 | 16.23 | 19.51 | ||
| P-value | 0.431 | <0.001 | <0.001 | <0.001 |
P<0.05 decreased compared with that before modeling in the same group.
P<0.05 decreased compared with that at 6 h after operation in the same group.
P<0.05 decreased compared with that at 12 h after operation in the same group.
P<0.05 decreased compared with that at the same time point in SHAM group.
P<0.05 decreased compared with that at the same time point in I° burn group.
P<0.05 decreased compared with that at the same time point in II° burn group.
Table V.
ELISA detection results of Bcl-2 protein (ng/ml).
| SHAM | I° | II° | III° | Statistical value | P-value | |
|---|---|---|---|---|---|---|
| Before modeling | 13.09±0.81 | 13.25±4.36 | 13.27±4.88 | 13.18±5.87 | 0.006 | 0.999 |
| 6 h after operation | 13.02±0.77 | 11.49±1.37a,d | 10.18±3.52a,d,e | 9.33±3.98a,d,e,f | 5.268 | 0.153 |
| 12 h after operation | 13.01±0.78 | 9.12±1.02a,b,d | 7.92±1.14a,b,d,e | 6.34±1.67a,b,d,e,f | 23.79 | 0.001 |
| 18 h after operation | 12.91±0.77 | 7.92±0.77a–d | 5.91±0.78a–e | 3.93±0.76a–f | 22.75 | 0.001 |
| Statistical value | 0.090 | 17.698 | 18.446 | 18.010 | ||
| P-value | 0.965 | <0.001 | <0.001 | <0.001 |
P<0.05 decreased compared with that before modeling in the same group.
P<0.05 decreased compared with that at 6 h after operation in the same group.
P<0.05 decreased compared with that at 12 h after operation in the same group.
P<0.05 decreased compared with that at the same time point in SHAM group.
P<0.05 decreased compared with that at the same time point in I° burn group.
P<0.05 decreased compared with that at the same time point in II° burn group.
Figure 3.
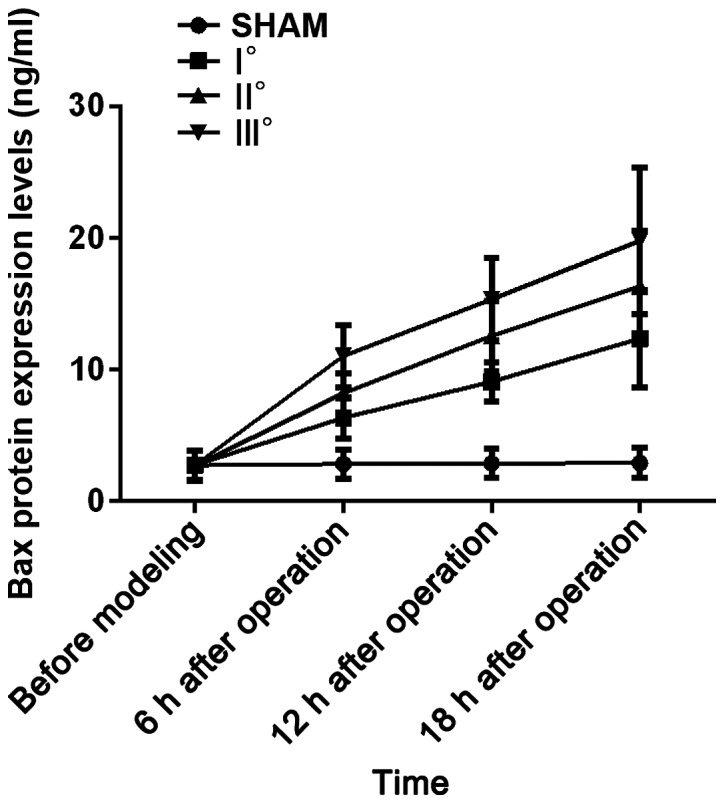
ELISA detection results of Bax protein. Results of analysis of variance display that the Bax protein expression levels in the 4 groups of rats at 6, 12 and 18 h after operation are different (P<0.05). In I°, II° and III° burn groups, the Bax protein expression levels are increased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they are higher at 12 and 18 h after operation than those at 6 h after operation (P<0.05) and also higher at 18 h after operation than those at 12 h after operation (P<0.05). At the same time point, the Bax protein expression levels in I°, II° and III° burn groups are higher than that in SHAM group (P<0.05), and they are higher at each time point in II° and III° burn groups than that in I° burn group (P<0.05) and also higher at each time point in III° burn group than that in II° burn group (P<0.05).
Figure 4.
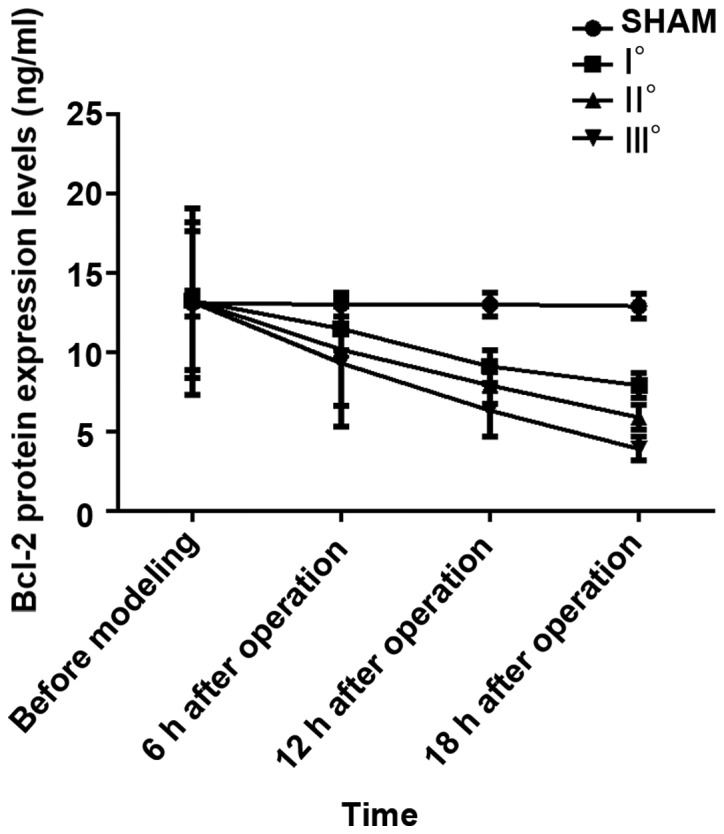
ELISA detection results of Bcl-2 protein. Results of analysis of variance display that the Bcl-2 protein expression levels in the 4 groups of rats at 6, 12 and 18 h after operation are different (P<0.05). In I°, II° and III° burn groups, the Bcl-2 protein expression levels are decreased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they are decreased at 12 and 18 h after operation compared with those at 6 h after operation (P<0.05) and also decreased at 18 h after operation compared with those at 12 h after operation (P<0.05). At the same time point, the Bcl-2 protein expression levels in I°, II° and III° burn groups are decreased compared with that in SHAM group (P<0.05), and they are decreased at each time point in II° and III° burn groups compared with that in I° burn group (P<0.05) and also decreased at each time point in III° burn group compared with that in II° burn group (P<0.05).
Results of analysis of variance revealed that TNF-α expression levels in the 4 groups of rats at 6, 12 and 18 h after operation were different (P<0.05). There was no difference in the TNF-α expression level among the 4 groups of rats before modeling (P>0.05). In I°, II° and III° burn groups, TNF-α expression levels were increased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they were higher at 12 and 18 h after operation than those at 6 h after operation (P<0.05) and also higher at 18 h after operation than those at 12 h after operation (P<0.05). No significant difference was found in the TNF-α expression level at 4 time points in the SHAM group (P>0.05). At the same time point, with the increased degree of burn, the TNF-α expression level was increased. TNF-α expression levels at each time point in I°, II° and III° burn groups were higher than that in SHAM group (P<0.05), and they were higher at each time point in II° and III° burn groups than that in I° burn group (P<0.05) and also higher at each time point in III° burn group than that in II° burn group (P<0.05) (Table VI and Fig. 5)
Table VI.
ELISA detection results of TNF-α (pg/ml).
| SHAM | I° | II° | III° | Statistical value | P-value | |
|---|---|---|---|---|---|---|
| Before modeling | 15.3±3.7 | 15.4±3.3 | 15.7±3.8 | 15.1±3.5 | 0.200 | 0.978 |
| 6 h after operation | 16.2±4.1 | 28.4±11.3a,d | 32.3±10.1a,d,e | 48.2±17.6a,d,e,f | 14.69 | 0.002 |
| 12 h after operation | 16.5±4.2 | 37.5±12.1a,b,d | 42.1±16.7a,b,d,e | 62.3±20.1a,b,d,e,f | 16.62 | 0.001 |
| 18 h after operation | 16.9±4.1 | 42.9±13.2a–d | 57.4±19.2a–e | 98.3±22.4a–f | 19.04 | 0.001 |
| Statistical value | 0.245 | 17.558 | 17.962 | 18.045 | ||
| P-value | 0.836 | <0.001 | <0.001 | <0.001 |
P<0.05 increased compared with that before modeling in the same group.
P<0.05 increased compared with that at 6 h after operation in the same group.
P<0.05 increased compared with that at 12 h after operation in the same group.
P<0.05 increased compared with that at the same time point in SHAM group.
P<0.05 increased compared with that at the same time point in I° burn group.
P<0.05 increased compared with that at the same time point in II° burn group.
Figure 5.
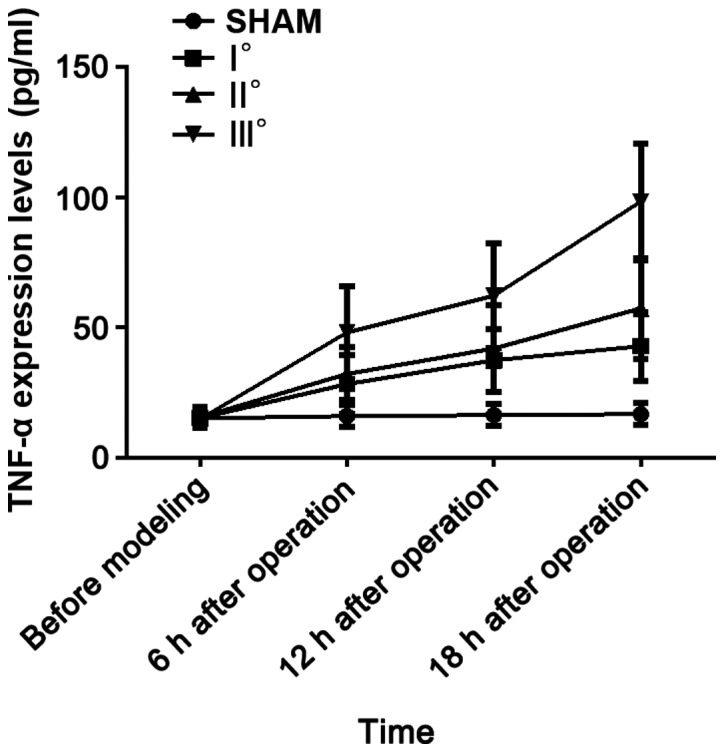
ELISA detection results of TNF-α. Results of analysis of variance manifest that the TNF-α expression levels in the 4 groups of rats at 6, 12 and 18 h after operation are different (P<0.05). In I°, II° and III° burn groups, the TNF-α expression levels are increased at 6, 12 and 18 h after operation compared with those before modeling (P<0.05), and they are higher at 12 and 18 h after operation than those at 6 h after operation (P<0.05) and also higher at 18 h after operation than those at 12 h after operation (P<0.05). At the same time point, the TNF-α expression levels in I°, II° and III° burn groups are higher than that in SHAM group (P<0.05), and they are higher at each time point in II° and III° burn groups than that in I° burn group (P<0.05) and also higher at each time point in III° burn group than that in II° burn group (P<0.05).
Correlation analyses among TNF-α, Bax and Bcl-2
According to the Pearson's correlation analysis, the TNF-α expression level was positively correlated with Bax mRNA and Bax protein expression levels (r=0.732, P=0.002) and negatively correlated with Bcl-2 mRNA and Bcl-2 protein expression levels (r= −0.685, P=0.009), and Bax mRNA and Bax protein expression levels were also negatively correlated with Bcl-2 mRNA and Bcl-2 protein expression levels (r= −0.704, P=0.005). Besides, TNF-α, Bax mRNA and Bax protein had positive correlation with the degree of burn (r=0.801, P=0.001, r=0.745, P=0.001) and time after burn (r=0.715, P=0.004, r=0.741, P=0.002), while Bcl-2 mRNA and Bcl-2 protein had negative correlation with the degree of burn (r=0.715, P=0.003) and time after burn (r=0.742, P=0.002).
Discussion
The early deepening of burn wound is one of the difficulties in clinical treatment of burn. According to previous studies, inflammatory response-induced tissue damage in burned patients is an important mechanism of the deepening of burn wound (15,16). After burn, the body defends against the burn and eliminates the necrotic tissues and pathogenic microorganisms through the inflammatory response, but the excessive inflammatory cascade may lead to tissue edema, ischemia and apoptosis, causing deepening of burn wound (17,18). In this study, serum TNF-α, Bax and Bcl-2 expression levels in burned rats were detected, and changes in TNF-α, Bax and Bcl-2 expression levels with the increase of burn degree and time after burn were analyzed, so as to offer help to the clinical prevention of deepening of burn wound.
In the current study, three rat models of burn in different degrees were constructed, and TNF-α, Bax and Bcl-2 expression levels prior to modeling and at 6, 12 and 18 h after operation were detected. Results of this study demonstrated that with the increased degree of burn in rats, TNF-α and Bax expression levels were increased gradually, while the Bcl-2 expression level was gradually decreased. It is speculated that with the increased degree of burn in rats, stronger inflammatory response may occur in the body and more TNF-α will be secreted, so TNF-α is possibly related to the degree of burn. With the increased degree of burn in rats, the degree of tissue damage is also increases and the pathological apoptosis signal pathway is activated, leading to the increased expression level of Bax, a pro-apoptotic gene, and decreased expression level of Bcl-2, an anti-apoptotic gene.
According to the Pearson's correlation analysis, TNF-α and Bax were positively correlated with the degree of burn, while Bcl-2 was negatively correlated with the degree of burn. TNF-α, as a key cytokine in the body's inflammatory response, will be rapidly activated and reach the peak within a short period of time after the body is stimulated by burn. If the body's inflammatory response is moderate, the changes in the TNF-α level will be limited, which is conducive to protecting the body and alleviating the body damage and infection. On the contrary, secondary damage may be caused to the tissue, promoting the development of disease (19–21). In this study, with the prolongation of time after burn in rats, TNF-α and Bax expression levels were also increased continuously, while the Bcl-2 expression level was decreased continuously, and the degree of apoptosis became increasingly higher, indicating that the inflammatory response in rats continued to be enhanced and the secondary damage of burn wound was deepened. Results of Pearson's correlation analysis also revealed that TNF-α and Bax had positive correlations with the after burn.
Cade et al (22) reported that reducing the TNF-α expression level can effectively improve the retinal and corneal injury induced by alkali burn. Barber et al (23) also reported that the increased level of TNF-α will increase the risk of sepsis in burned patients. According to the report of Maass et al (24), improving the TNF-α level can improve the cardiac systolic function of burned patients. The above conclusions are consistent with our speculation: Improving the TNF-α level will play a protective role in burned patients. Correlation analyses were performed among the expression levels of TNF-α, Bax and Bcl-2. Results showed that the TNF-α expression level was positively correlated with the Bax expression level, but negatively correlated with the Bcl-2 expression level, suggesting that TNF-α has a positive correlation with the degree of cell damage in burned rats. In other words, improving the TNF-α expression level in rats can improve the cell damage in burned rats. However, the burned rats were used as objects in this study, and there are differences between such burn and actual burn in patients. Despite of similarities between SD rats and human, the conclusion cannot represent the clinical experimental results. Therefore, the conclusion remains to be proved by more clinical experimental data.
In conclusion, continuous monitoring of changes in the TNF-α level can be used as a means to evaluate the degree of burn and apoptosis, prevent the deepening of burn wound and serve as a reference standard for the evaluation of clinical efficacy, thus facilitating the early clinical evaluation of prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZW conceived and designed this study. ZW and JF were responsible for model construction and PCR. JX wrote the manuscript. ZW and JX performed ELISA. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Second People's Hospital of Liaocheng (Liaocheng, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10:e0129996. doi: 10.1371/journal.pone.0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39:11–18. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Haren RM, Thorson CM, Valle EJ, Busko AM, Guarch GA, Andrews DM, Pizano LR, Schulman CI, Namias N, Proctor KG. Hypercoagulability after burn injury. J Trauma Acute Care Surg. 2013;75:37–43. doi: 10.1097/TA.0b013e3182984911. [DOI] [PubMed] [Google Scholar]
- 4.Peterson JR, De La Rosa S, Sun H, Eboda O, Cilwa KE, Donneys A, Morris M, Buchman SR, Cederna PS, Krebsbach PH, et al. Burn injury enhances bone formation in heterotopic ossification model. Ann Surg. 2014;259:993–998. doi: 10.1097/SLA.0b013e318291da85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraft R, Herndon DN, Finnerty CC, Cox RA, Song J, Jeschke MG. Predictive value of IL-8 for sepsis and severe infections after burn injury: A clinical study. Shock. 2015;43:222–227. doi: 10.1097/SHK.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang X, Liu W, Deng J, Lan L, Xue X, Zhang C, Cai G, Luo X, Liu J. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic Biol Med. 2013;60:292–299. doi: 10.1016/j.freeradbiomed.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Kubo H, Hayashi T, Ago K, Ago M, Kanekura T, Ogata M. Temporal expression of wound healing-related genes in skin burn injury. Leg Med (Tokyo) 2014;16:8–13. doi: 10.1016/j.legalmed.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Lootens L, Brusselaers N, Beele H, Monstrey S. Keratinocytes in the treatment of severe burn injury: An update. Int Wound J. 2013;10:6–12. doi: 10.1111/j.1742-481X.2012.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshyar R, Bathaie SZ, Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol. 2013;32:50–57. doi: 10.1089/dna.2012.1866. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Zhao PJ, Su D, Feng J, Ma SL. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro in vivo. Mol Med Rep. 2014;9:2265–2272. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Li P, Ma X, Tian P, Han C, Zang J, Kong J, Yan H. MicroRNA-494 inhibition protects nucleus pulposus cells from TNF-α-induced apoptosis by targeting JunD. Biochimie. 2015;115:1–7. doi: 10.1016/j.biochi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Wu W, Huang W, Hu G, Yuan W, Li W. NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm Res. 2013;62:387–397. doi: 10.1007/s00011-013-0590-7. [DOI] [PubMed] [Google Scholar]
- 13.Ceccarelli S, Panera N, Mina M, Gnani D, De Stefanis C, Crudele A, Rychlicki C, Petrini S, Bruscalupi G, Agostinelli L, et al. LPS-induced TNF-α factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget. 2015;6:41434–41452. doi: 10.18632/oncotarget.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Ryan CM, Schneider JC, Kazis LE, Lee A, Li NC, Hinson M, Bauk H, Peck M, Meyer WJ III, Palmieri T, et al. Multi-Center Benchmarking Study Group: Benchmarks for multidimensional recovery after burn injury in young adults: The development, validation, and testing of the American Burn Association/Shriners Hospitals for Children young adult burn outcome questionnaire. J Burn Care Res. 2013;34:e121–e142. doi: 10.1097/BCR.0b013e31827e7ecf. [DOI] [PubMed] [Google Scholar]
- 16.Duke JM, Rea S, Boyd JH, Randall SM, Wood FM. Mortality after burn injury in children: A 33-year population-based study. Pediatrics. 2015;135:e903–e910. doi: 10.1542/peds.2014-3140. [DOI] [PubMed] [Google Scholar]
- 17.Diego AM, Serghiou M, Padmanabha A, Porro LJ, Herndon DN, Suman OE. Exercise training after burn injury: A survey of practice. J Burn Care Res. 2013;34:e311–e317. doi: 10.1097/BCR.0b013e3182839ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Öster C, Willebrand M, Ekselius L. Burn-specific health 2 years to 7 years after burn injury. J Trauma Acute Care Surg. 2013;74:1119–1124. doi: 10.1097/TA.0b013e318283cca0. discussion 1124. [DOI] [PubMed] [Google Scholar]
- 19.Friedrich EE, Sun LT, Natesan S, Zamora DO, Christy RJ, Washburn NR. Effects of hyaluronic acid conjugation on anti-TNF-α inhibition of inflammation in burns. J Biomed Mater Res A. 2014;102:1527–1536. doi: 10.1002/jbm.a.34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Halloran E, Kular J, Xu J, Wood F, Fear M. Non-severe burn injury leads to depletion of bone volume that can be ameliorated by inhibiting TNF-α. Burns. 2015;41:558–564. doi: 10.1016/j.burns.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Abali AE, Karakayali H, Ozdemir BH, Bayraktar N, Abbas OL, Haberal M. Destructive pulmonary effects of smoke inhalation and simultaneous alterations in circulating IL-6, TNF-α, and IFN-γ levels at different burn depths: An experimental study on rats. J Burn Care Res. 2013;34:334–341. doi: 10.1097/BCR.0b013e3182644e9b. [DOI] [PubMed] [Google Scholar]
- 22.Cade F, Paschalis EI, Regatieri CV, Vavvas DG, Dana R, Dohlman CH. Alkali burn to the eye: Protection using TNF-α inhibition. Cornea. 2014;33:382–389. doi: 10.1097/ICO.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 23.Barber RC, Aragaki CC, Rivera-Chavez FA, Purdue GF, Hunt JL, Horton JW. TLR4 and TNF-alpha polymorphisms are associated with an increased risk for severe sepsis following burn injury. J Med Genet. 2004;41:808–813. doi: 10.1136/jmg.2004.021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maass DL, White J, Horton JW. IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock. 2002;18:360–366. doi: 10.1097/00024382-200210000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


