Abstract
The aim of the present study was to determine the influence of stress on the colonic mucosa and immune system and to further investigate the association between stress and development and pathogenesis of ulcerative colitis (UC). Mice were treated with 2,4,6-trinitrobenzenesulfonic acid to induce an animal model of UC, and stress was induced by water immersion and restraint. Subsequently, the disease activity index (DAI), secretory immunoglobulin A (sIgA), IgA, interleukin (IL)-6 and −8, tumor necrosis factor-α (TNF-α), complement component (C)3 and C4, and alterations in the colonic mucosa were observed. The DAI scores and the expression levels of IL-6, IL-8 and TNF-α significantly increased in the experimental UC mice compared with the control mice, while the expression levels of IgA and sIgA decreased (all P<0.01). DAI and colonic mucosa damage scores increased in the stress-treated mouse models of UC compared with the untreated mouse models of UC (P<0.05). Expression levels of IgA and sIgA decreased, while IL-6, IL-8 and TNF-α further increased in the stress-treated UC mice (P<0.05). The expression levels of C3 and C4 were not affected by stress or UC (P>0.05). These results indicated that UC may be associated with an immune disorder and that stress can aggravate colonic mucosa injury and alter the immune response. Furthermore, stress and immunity may serve roles in the pathogenesis of UC.
Keywords: ulcerative colitis, mice, stress, mucosa, immune response, injury
Introduction
Ulcerative colitis (UC), an inflammatory bowel disease (IBD) affecting the colon, is characterized by a chronic, relapsing, organ-specific inflammatory state of the mucosa (1). The incidence and prevalence of UC are higher in countries with greater economic development, particularly in the northern countries of Western Europe, Canada, USA, Australia and New Zealand (2). The incidence of UC has recently increased (3). The pathogenesis of UC is associated with various factors, including genetic predisposition (4), environmental risk factors (5) and the state of the immune system (6). Furthermore, the disorder of the immune system is considered an important factor (7), and psychological stress is considered one of the risk factors for UC (8).
The recent increase in the pace of human life, competition, pollution and the number of stress stimuli contributed to greater susceptibility to disease. Research into the influence of stress on disease status has received increasing attention from clinicians. Stress causes alterations in psychological and physiological functions due to recognition and evaluation of the stimuli from a stress source (9). The mechanism by which psychological factors influence the body has not been extensively studied. It is challenging to design experiments to quantitatively measure psychological factors and evaluate psychosomatic associations. However, animal models of stress have been previously established and used to study physiological responses (10). Therefore, the present study aimed to observe the effects of stress on the general condition, characteristics of the colonic mucosa and immunity-associated indicators of normal control mice and mouse models of UC mice. In addition, the present study investigated the effect of stress on the occurrence, development, prognosis and possible pathogenesis of UC.
Materials and methods
Materials
Mice were weighed 1 h before the initiation of the model, and a calculation was made to obtain a 5% 2,4,6-trinitrobenzenesulfonic acid solution (TNBS; 150 mg/kg; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The final solution was mixed to achieve a solution of 5% TNBS and 380 g/l ethanol using a 4:1 volume ratio of ethanol to TNBS.
The laboratory equipment used in the present study included a LabSystems Multiskan MS plate reader (Thermo Labsystems, Helsinki, Finland), anesthesia machine (Beijing Yiren Hengye Technology Co. Ltd, Beijing, China), optical microscope (Olympus Corporation, Tokyo, Japan), water jacketed incubator (Shanghai Yiheng Scientific Instruments, Co., Ltd., Shanghai, China) and gavage needles (Changzhou Jinliyuan Medical Instrument Co. Ltd, Changzhou, China). Enzyme-linked immunosorbent assay (ELISA) kits for secretory (s) immunoglobulin (Ig)A (SBJ-R0054), interleukin (IL)-6 (SBJ-H0465), IL-8 (SBJ-R0033), tumor necrosis factor (TNF)-α (SBJ-R0040), complement component (C)3 (SBJ-H0199) and C4 (SBJ-R0368) were obtained from SBJBio (Nanjing, China), and the ELISA kit for IgA (FU-D1033) was obtained from Beijing Xin Fangcheng Biotechnology Co., Ltd. (Beijing, China).
Animals
A total of 80 experimental specific pathogen-free BALB/c mice (age, 6–8 weeks; weight, 18–22 g) were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China). Mice were maintained at a specific pathogen-free facility with 40–70% humidity and 24–26°C temperature, with a 12-h light/dark cycle and free access to food and water. The research program was approved by the Ethics Committee of Tianjin Nankai Hospital. The mice were allowed to acclimate to laboratory conditions for 7 days and then fasted for 36 h with ad libitum access to water. The mice were subsequently deprived of water 1 h before the experiment and randomly divided into 4 groups (n=20/group): i) Control group (A); ii) stress group (B), iii) UC + stress group (C); and UC model alone (D). Mice in groups C and D were treated with TNBS-ethanol solution to induce UC. After 5 days, the mice in groups B and C were restrained in water, as described below.
Establishment of the animal model
Establishment of the animal model of UC
The present study used a modified TNBS protocol combined with an addition of an alcohol complex to establish the animal model of UC (11). Mice were fasted for 36 h with free access to water. Animals were subsequently weighed 1 h before the experiment in order to calculate the drug dose to be administered and were anaesthetized by isoflurane inhalation, as previously described (anesthesia machine oxygen flow rate, 0.6 nl/min; isoflurane concentration, 4–6%; maintenance anesthesia, isoflurane 1–2% for 1 min using an anesthesia tube and plastic funnel to cover the nose and mouth of the mice) (12). Anesthesia was considered successful when the mice were calm, supine and breathing smoothly. For administration of the TNBS-ethanol solution, a gavage needle was connected to a 1-ml syringe. The needle was lubricated with liquid paraffin and slowly inserted into the anus of each mouse to a depth of ~1–2 cm. The solution was slowly injected with the gavage needle continuously pushed. The solution was completely injected at a depth of 4–5 cm into the anus. The tail of the mouse was slowly lifted to clench the anus to prevent the outflow of liquid and in order to ensure that the TNBS solution was dispersed in the colon. In addition, the anesthesia table and the mice were maintained at angle of 45–60°C and the position of the mice was rotated for 5 min to ensure that the TNBS solution was in contact with the wall of the colon. The mice naturally woke and became alert, and were subsequently released into the cages and provided free access to food and water. Measurements were taken 5 days following induction of the model of UC.
Establishment of the animal model of stress
Groups B and C were subjected to stress via the water immersion restraint method. Mice were fasted for 24 h with ad libitum access to water. Mice were anaesthetized in a cage and immersed in water continuously for 4 h (water temperature, 25±1°C; water level, cartilago ensiformis) as previously described (13).
General condition of the mice
The following characteristics of mice were observed and recorded: Mental state (via duration of sleep, time of waking and response to external stimuli), physical activity (14), food consumption, body weight, fur gloss (15), stool type, fecal occult blood and mortality rate. The disease activity index (DAI) was calculated as previously described by Murthy et al (16) (Table I), using the following formula: DAI=[weight loss (%) + characterization of feces + hematochezia)/3.
Table I.
Disease activity index scores.
| Score | Weight loss (%) | Characterization of feces | Hematochezia |
|---|---|---|---|
| 0 | 0 | Normal | Negative |
| 1 | 1–5 | – | – |
| 2 | 6–10 | Semi-loose | Occult blood (+) |
| 3 | 11–15 | – | – |
| 4 | >15 | Loose | Bloody visible by naked eye |
Macroscopic scores
Mice were restrained and blood samples were collected via orbital puncture following anesthesia as described above. Mice were euthanized by cervical dislocation and dissected to open the abdominal cavity and access the colon. Colonic lavage was performed. Following removal of the colon lavage fluid and brushing of intestinal tissue, the colon was cut into sections along the mesenteric side and fixed with a pin. The colon was subsequently observed by the naked eye and scored according to the criteria described in Table II (17,18).
Table II.
Macroscopic scores of the colonic mucosa.
| Score | Alterations in colonic mucosa |
|---|---|
| 0 | No damage |
| 1 | Mucosal hyperemia, edema, no ulcer |
| 2 | Mucosal hyperemia, edema, mild erosion, no ulcer |
| 3 | Mucosal hyperemia, edema, moderate erosion, single ulcer |
| 4 | Mucosal hyperemia, edema, severe erosion, multiple ulcers |
| 5 | Mucosal hyperemia, edema, severe erosion, ulcer >1 cm |
Microscopic scores
Distal colon sections (0.5 cm from the anus; dimensions, lx0.5 cm) were obtained for analysis, fixed with 4% formalin at room temperature for 24 h, and embedded in paraffin. Subsequently, 4-µm-thick sections were prepared and hematoxylin-eosin staining was performed at room temperature. For each slice, 3 fields of view were randomly selected for analysis and scored according to the parameters previously described by Gaudio et al (19) (Table III).
Table III.
Ulcerative colitis pathological scoring standard.
| Pathological morphology | Score |
|---|---|
| Destruction of epithelium and glands | |
| Morphologically normal | 0 |
| Focal destruction of the epithelial surface and/or focal crypt dropout | 1 |
| Zonal destruction of the epithelial surface and/or zonal crypt loss | 2 |
| Diffuse and/or mucosal ulceration involving submucosa and/or diffuse crypt losses | 3 |
| Dilatation of glandular crypts | |
| Normal | 0 |
| Focal dilatation | 1 |
| Zonal dilatation | 2 |
| Dilated crypts | 3 |
| Depletion and loss of goblet cells | |
| Normal | 0 |
| Slightly depleted goblet cells | 1 |
| Zonal or moderately depleted goblet cells | 2 |
| Diffusely or completely depleted goblet cells | 3 |
| Inflammatory cell infiltration | |
| Absence of infiltrate | 0 |
| Infiltrate at the subepithelial and lamina propria level or crypt bases | 1 |
| Infiltrate reaching muscularis mucosae | 2 |
| Severe and extensive infiltrate reaching submucosa and/or involving muscularis propria | 3 |
| Edema | |
| Absent | 0 |
| Focal | 1 |
| Zonal and/or moderately diffuse | 2 |
| Extensive or severe | 3 |
| Vascular congestion | |
| Absent | 0 |
| Focal | 1 |
| Zonal | 2 |
| Diffuse | 3 |
| Crypt abscesses | |
| Absent | 0 |
| Focal | 1 |
| Zonal | 2 |
| Diffuse | 3 |
| Atrophia | |
| Absent | 0 |
| Focal | 1 |
| Zonal | 2 |
| Diffuse | 3 |
Detection of immune factors
Following the induction of the animal model of stress, mice were dried off to prevent water from contaminating the microcentrifuge tubes and causing hemolysis. Following blood collection for detection of immunoglobulin (Ig)A, interleukin (IL)-6, IL-8, TNF-α, C3 and C4 by orbital puncture, mice were sacrificed, and the abdominal cavity was dissected to isolate the colon, as detailed above, which was subsequently washed thrice with 2 ml physiological saline. The saline was removed, and the solution was centrifuged for 20 min at 4°C (1,369.5 × g). The supernatant was removed to detect soluble (s)IgA. All samples were processed using ELISA kits, according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation. Differences between any 2 groups were determined by one-way analysis of variance with the Student-Newman-Keuls post hoc test. SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used for all calculations. P<0.05 was considered to indicate a statistically significant difference.
Results
General condition of the mice
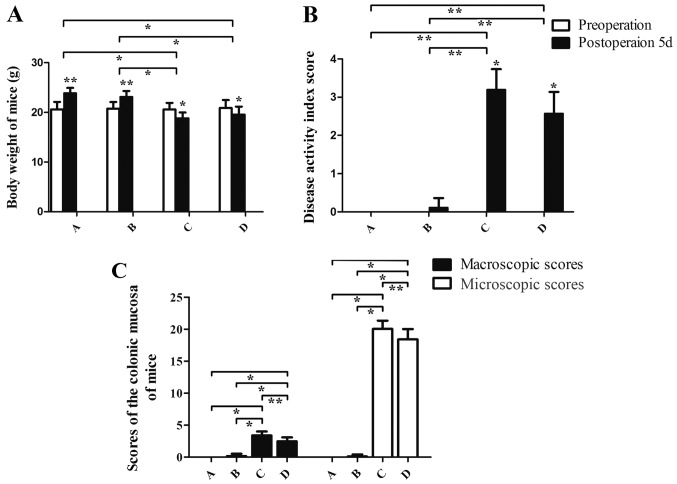
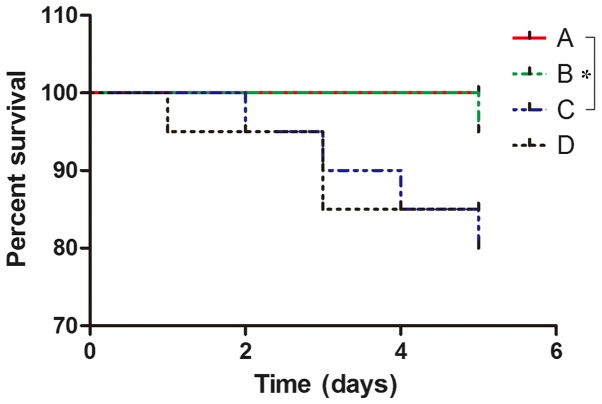
Following 36 h of fasting with ad libitum access to water, the maximum body weight loss of mice was 1.06 g. The rage of weight loss was 0.85±0.21 g. The following observations were made regarding the general condition of group A mice: Glossy fur, responsive to stimuli, physically active and flexible, healthy weight and granular stool. In comparison with group A, mice in groups C and D exhibited slow activity, increased duration of sleeping, loose and dull skin, decreased appetite, bloody stool and weight loss (Fig. 1). Mice in group B exhibited excitability, and increased breathing and heart rate in the early stage of the stress experiment. These mice gradually became subdued with smooth breathing and reduced responses to external stimuli. Group C mice exhibited similar characteristics to group B mice but their excitement (physical activity, propensity to cry and struggle when being handled) intensity was weaker and more rapidly suppressed. Preoperative DAI values for the mice in each group exhibited no significant differences (P>0.05). The DAI values of each group at 5 days post-administration of TNBS-ethanol solution were in the following order from high to low: C>D>B>A. There was a statistically significant difference between group A and both groups C and D (P<0.01). Furthermore, the DAI index in group B was different compared with both groups C and D (P<0.01). DAI scores were not significantly different between groups C and D (P>0.05; Fig. 1). No mortality was observed in group A. Mortality was observed in one mouse in group B due to asphyxia; 4 in group C, including 2 from intestinal necrosis, 1 from mechanical intestinal obstruction and 1 from asphyxia; and 3 in group D, including 1 case of mortality due to intestinal necrosis and 2 due to mechanical intestinal obstruction (Fig. 2). The survival of mice in group A was better than that ingroup C, and the difference was statistical significant (P=0.0455). However, there was no significant difference in survival among groups B, C and D at day 5.
Figure 1.
Body weight, disease activity index score and mucosa macroscopic and microscopic scores of mice. (A) The body weight of mice. (B) The disease activity index score score in group A, B, C and D. TNBS-ethanol solution was only administered in groups C and D (Establishment of the animal model of UC with TNBS-ethanol solution). (C) The macroscopic and microscopic scores of the colonic mucosa of mice. A (control group), B (stress group), C (UC and stress group), D (UC model alone). *P,0.05, **P<0.01. UC, ulcerative colitis.
Figure 2.
Survival conditions of mice. A, control group; B, stress group; C, UC and stress group; D, UC model alone. The results demonstrated that the survival of mice after administration of TNBS-ethanol solution in group C was lower than group A (P=0.0455). However, there were no significant differences among groups B, C and D. UC, ulcerative colitis.
Macroscopic and microscopic characteristics of the colonic mucosa
Macroscopic characteristics were evaluated by the naked eye. Normal colonic mucosa should appear red and smooth with no injury, as in group A. However, following induction of the UC model, colonic mucosa became extensively congested and edema and ulcers were observed along with the development of rough mucus membrane with mucosal erosion, which was obvious in group C. Group B had a less marked manifestation than group C (Fig. 3).
Figure 3.
Macroscopic appearance of the colonic mucosa. (A) The external appearance of colonic tissue after excision in group A. (B) Section mucosal appearance of colonic tissue after incision in group A. (C) External appearance of colonic tissue after excision in group B. (D) Section mucosal appearance of colonic tissue after incision in group B. (E) External appearance of colonic tissue after excision in group C. (F) The section mucosal appearance of colonic tissue after incision in group C. (G) The external appearance of colonic tissue after excision in group D. (H) Section mucosal appearance of colonic tissue after incision in group D.
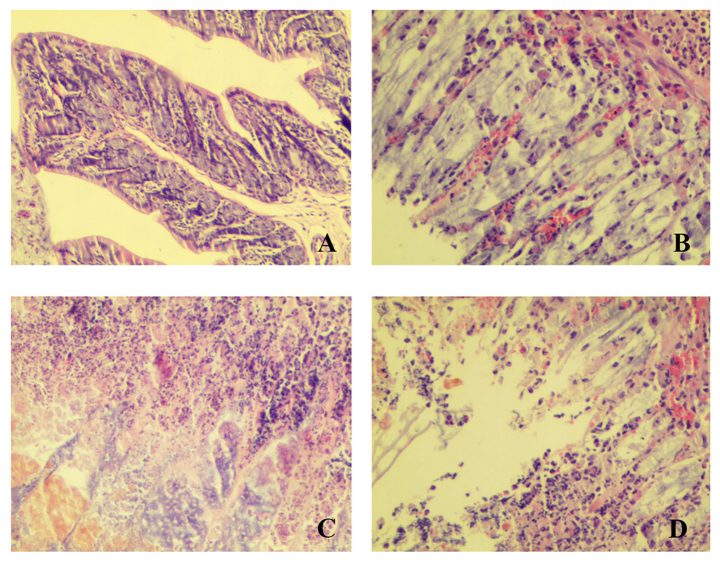
Pathological analysis using microscopy indicated inflammatory cell infiltration, diffuse mucosal hyperaemia, extensive edema, epithelial and glandular damage, decreased number of goblet cells, and crypt abscesses (Fig. 4). Groups B, C and D had different degrees of injury. Among them, group C was the most severe. The above results indicated that stress can aggravate injury of the colonic mucosa (Figs. 1, 3 and 4).
Figure 4.
The microscopic appearance of the colonic mucosa. (A) control group; (B) stress group; (C) UC and stress group; (D) UC model alone. Observed under inverted microscopy at ×200 magnification. UC, ulcerative colitis.
Serum levels of IgA and secretory sIgA in colonic lavage fluid
The levels of serum IgA and sIgA in the colonic lavage fluid of each group 5 days post-administration were in the following order from the highest to the lowest: A>B>D>C. Statistically significant differences were identified between groups A and C (P<0.01), and groups B and C (P<0.01). In comparison with group D, the level of group C was also declined (P<0.05; Fig. 5).
Figure 5.
The serum expression levels of IL-6, IL-8, TNF-α, C3, C4, IgA and sIgA in different groups. A, control group; B, stress group; C, UC and stress group; D, UC model alone. *P,0.05, **P<0.01. IL, interleukin; TNF, tumor necrosis factor; Ig, immunoglobulin; s, soluble; C, complement component; ns, not significant.
Serum levels of IL6, IL8, TNF-α, C3 and C4
The serum levels of IL6, IL8, TNF-α, C3 and C4 in each group 5 days post-administration were in the following order from the highest to the lowest: C>D>B>A. Significant differences were observed in IL-6, IL-8 and TNF-α expression between groups A and C, and between groups B and C (P<0.01). Group C exhibited higher levels than group D (P<0.05; Fig. 5). However, there was no significant difference in the levels of C3 and C4 between groups.
Discussion
UC is an immune-mediated chronic inflammatory disease with symptoms including abdominal pain, bloody diarrhea and fatigue. UC is associated with numerous complications involving all systems of the body and its chronic nature follows an unpredictable course with instances of exacerbation and remission (20). In recent years, it has become an increasingly common disease of the digestive system. With increasing incidence, UC can severely affect the physical and mental health, and the quality of life of patients (21). Previous studies indicated that UC may be associated with a complex interaction of genetic, environmental, immune and psychological factors in susceptible individuals. The interaction between these factors results in severe intestinal inflammation (22). Psychological effects from UC and UC medications are experienced by many patients with UC (23).
Adaptation to environmental stimuli is necessary to maintain homeostasis. Stress can be defined as a threat to an organism's homeostasis (8). Psychological stress is defined as a psychological alteration resulting from patients' feelings of unease, worry, and/or fear. Psychological stress induces gastrointestinal symptoms, including dyspepsia and abdominal pain (24,25), and increased colonic motility (26). Stress is a risk factor, trigger and perpetuating factor in UC (27). Mechanisms by which stress affects the nervous system to alter the immune function at both the systemic and gut mucosal level are currently under investigation (28,29).
The results of the present study indicated that stress can aggravate the injury of the colonic mucosa and immune system. The effects of stress are complex and depend on the duration and intensity of the stimulus. A small amount of stress has been hypothesized to enhance immune function; however, increased intensity of stress can be detrimental due to excessive production of neuroendocrine-derived mediators that weaken immune responses to invasive pathogens (30). By acting on the catecholamine receptors of immune cells, stress may reduce the capacity of the sympathetic nervous system to release large amounts of catecholamines, thereby weakening the immune response (31). In addition, stress can cause intestinal motility dysfunction and intestinal flora imbalance, and induce spasms of intestinal smooth muscles and vessels, thus leading to a lack of blood and oxygen supply and an increase in mucosal barrier dysfunction (32).
Stress may be classified into 4 types, including physical, social, cultural and psychological (33). Among these, psychological stress is one of the most ubiquitous and notable (34). A number of experimental methods have been previously used to induce animal models of stress, including restraint, water immersion, thermal stress and fatigue (35,36). Among these methods, the former two lead to pronounced stress and exhibited numerous advantages, including simplicity and low variability among different groups (10,37). Furthermore a combination of restraint and water immersion can successfully induce experimental anxiety to study the underlying psychological or emotional factors (38).
The mouse models of UC established in the present study consumed less food, exhibited decreased activity, weight loss, diarrhea, bloody stool, a decrease in fur glossiness, edema in colonic mucosa, congestion and ulcer formation, as observed by the naked eye. Furthermore, these mice exhibited infiltration of inflammatory cells, loss of goblet cells and ulcer formation, as exhibited by pathological analysis. All of the above parameters suggested that the model of UC was successfully induced. In addition, stress induced a decline in the general condition (mental state, activity, food consumption, body weight and fur gloss), an increase in DAI scores of mice with UC and aggravated macroscopic and microscopic damage of the colonic mucosa. These results indicated that stress may be a pathogenic factor for patients with UC and can result in disease exacerbation. Stress treatment, such as anti-anxiety therapy, may be a part of a comprehensive treatment strategy for patients with UC.
Healthy intestinal mucosa is protected against external stimuli by a complex mucosal defense barrier (39). In mouse models of UC, the colon mucosa was thin and extensively congested with edema and ulcers, which may be due to the defects of the intestinal barrier function and immune mechanisms (40).
Compared with the control group, the level of serum IgA and colonic lavage fluid sIgA in the mouse models of UC with stress decreased significantly, demonstrating that stress produced the downward trend. Previous studies have reported a reduction in sIgA levels in patients with IBD, which is supported by the results of the present study (41). IgA and sIgA are the most abundant Igs in humans and the first line of specific immunological defense against environmental antigens (42). Possible reasons for the decreased levels of IgA in mouse models of UC with stress are that the stress acted on the neuroendocrine immune regulation network, which decreased the release of large amounts of catecholamines by the sympathetic nerves, whereas catecholamines acted on catecholamine receptors of immune cells to weaken the body's immunity (43). Alternatively, stress may act through stimulating the release of glucocorticoids by the pituitary adrenal cortex system of the brain to induce immune suppression (44). Immune suppression in addition to the weakened colonic mucosal barrier function, may cause reduced resistance to external stimulation and aggravated severity of UC (45). This abnormal intestinal immune response may be involved in the pathogenesis of UC.
TNF-α is an important proinflammatory cytokine associated with cell apoptosis, inflammation, metabolism and thrombosis. TNF-α stimulates the secretion of IL-6 and −8 to promote the activation of nuclear factor (NF)-κB, which induces proinflammatory and immunomodulatory gene expression (46). Active NF-κB can further stimulate the expression of TNF-α and increase the intensity of inflammation (47). The development of neuroendocrine immunology introduces a new perspective for understanding the mechanisms underlying stress (48). Stress can activate the peripheral and central immune system responses and induce the release of inflammatory mediators, including TNF-α, IL-6 and IL-8 (49). Activated immune system mediates the process of psychological disorder through the interaction with neuronal and neuroendocrine systems (50). An animal model of UC used in the present study exhibited significantly increased serum IL-6, IL-8 and TNF-α expression levels. It has been previously demonstrated that stress may lead to neuroendocrine immune system abnormalities that increase the secretion of IL-6, IL-8 and TNF-α. Therefore, it may be hypothesized that in the present study, stress exacerbated UC due to increased secretion of IL-6, IL-8 and TNF-α via the neuroendocrine-immune system.
Serum C3 and C4 serve important roles in immune defense, immune regulation, and immune pathology. Decreased expression levels of these factors may cause a decreased ability to resist external pathogens and increased susceptibility to illness (51). In the present study, induction of the UC model and stress exhibited no significant effects on serum levels of C3 and C4, and the reason may be associated with insufficient sample size or the type of stress and the duration of stimulation. Alternatively, stress may have no effect on C3 and C4; however, further studies are required to test this hypothesis.
Using the water immersion with restraint method of stress induction, the present study concluded that stress may increase colonic mucosa damage in mice with UC and interfere with the immune regulation involving TNF-α, IgA, sIgA, C3 and C4, thereby increasing the severity of UC in mice. The above data suggested that, at least in some UC mice, stress may reflect primary disease activity via immune damage, and that it is possible that stress treatment rather than etiological and medical treatment would be more effective. Psychological stress is common among patients with UC and can lead to reduced quality of life (52). Given the association between UC and stress, clinicians may consider the treatment of stress is an integral part of therapies for patients with UC.
The present study has certain limitations. First, an animal model was used for exposure to a stress-inducing environment. The experiment should be continued following the elimination of the stress environment to evaluate the change of colonic mucosa and serum levels of IgA, IL-6, IL-8, TNF-α, C3 and C4. Furthermore, the underlying mechanism of action of stress in the development of UC remains to be elucidated. The mechanism of stress in the progression of UC requires further investigation. Future studies should investigate the association between stress and the nervous, endocrine, and immune systems in the context of the pathogenesis of UC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Tianjin Foundation of Science and Technology (grant no. 2013KY25).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YG and YT were responsible for the conception and design of the study. WN, XW, QZ and YX assisted in animal experiments. YG, SL and XL assisted in the ELISA and immunohistochemistry assays. YG, YT and QZ wrote, reviewed and revised the manuscript. All authors participated in final approval of the manuscript.
Ethics approval and consent to participate
The research program was approved by the Ethics Committee of Tianjin Nankai Hospital (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DeRoche TC, Xiao SY, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep. 2014;2:178–192. doi: 10.1093/gastro/gou031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parente JM, Coy CS, Campelo V, Parente MP, Costa LA, da Silva RM, Stephan C, Zeitune JM. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World J Gastroenterol. 2015;21:1197–1206. doi: 10.3748/wjg.v21.i4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV., Jr Incidence and prevalence of crohn's disease and ulcerative colitis in olmsted county, minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15:857–863. doi: 10.1016/j.cgh.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabi A, Nickerson KP, Homer CR, McDonald C. Digesting the genetics of inflammatory bowel disease: Insights from studies of autophagy risk genes. Inflamm Bowel Dis. 2012;18:782–792. doi: 10.1002/ibd.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo AY, Stevens BW, Wilson RG, Russell CN, Cohen MA, Sturgeon HC, Thornton A, Giallourakis C, Khalili H, Nguyen DD, et al. Early life environment and natural history of inflammatory bowel diseases. BMC Gastroenterol. 2014;14:216. doi: 10.1186/s12876-014-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vadasz Z, Rainis T, Nakhleh A, Haj T, Bejar J, Halasz K, Toubi E. The involvement of immune semaphorins in the pathogenesis of inflammatory bowel diseases (IBDs) PLoS One. 2015;10:e0125860. doi: 10.1371/journal.pone.0125860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cătană CS, Berindan Neagoe I, Cozma V, Magdaş C, Tăbarăn F, Dumitraşcu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823–5830. doi: 10.3748/wjg.v21.i19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mawdsley JE, Rampton DS. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J, Mrug S, Knight DC. Emotion socialization as a predictor of physiological and psychological responses to stress. Physiol Behav. 2017;175:119–129. doi: 10.1016/j.physbeh.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: Animal models, intestinal pathophysiology, and translational implication. Am J Physiol Gastrointest Liver Physiol. 2015;309:G927–G941. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majewska-Szczepanik M, Góralska M, Marcińska K, Zemelka-Wiącek M, Strzępa A, Dorożyńska I, Szczepanik M. Epicutaneous immunization with protein antigen TNP-Ig alleviates TNBS-induced colitis in mice. Pharmacol Rep. 2012;64:1497–1504. doi: 10.1016/S1734-1140(12)70947-7. [DOI] [PubMed] [Google Scholar]
- 12.Hohlbaum K, Bert B, Dietze S, Palme R, Fink H, Thöne-Reineke C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice - Assessing the degree of distress. PLoS One. 2017;12:e0179588. doi: 10.1371/journal.pone.0179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YM, Lu GM, Zou XP, Li ZS, Peng GY, Fang DC. Dynamic functional and ultrastructural changes of gastric parietal cells induced by water immersion-restraint stress in rats. World J Gastroenterol. 2006;12:3368–3372. doi: 10.3748/wjg.v12.i21.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath G, Kekesi G, Petrovszki Z, Benedek G. Abnormal motor activity and thermoregulation in a schizophrenia rat model for translational science. PLoS One. 2015;10:e0143751. doi: 10.1371/journal.pone.0143751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharpley CF, McFarlane JR, Slominski A. Stress-linked cortisol concentrations in hair: What we know and what we need to know. Rev Neurosci. 2011;23:111–121. doi: 10.1515/RNS.2011.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 17.Souza MM, Aguilar-Nascimento JE, Gomes-da-Silva MH, Carlos Junior R. Effects of budesonide and probiotics enemas on the colonic mucosa of rats with experimental colitis. Acta Cir Bras. 2007;22:34–38. doi: 10.1590/S0102-86502007000100006. [DOI] [PubMed] [Google Scholar]
- 18.Araki Y, Andoh A, Fujiyama Y, Bamba T. Development of dextran sulphate sodium-induced experimental colitis is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin Exp Immunol. 2000;119:264–269. doi: 10.1046/j.1365-2249.2000.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R. Dextran sulfate sodium (DSS) colitis in rats: Clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–1475. doi: 10.1023/A:1026620322859. [DOI] [PubMed] [Google Scholar]
- 20.Bürger M, Schmidt C, Teich N, Stallmach A. Medical therapy of active ulcerative colitis. Viszeralmedizin. 2015;31:236–245. doi: 10.1159/000436959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cawthorpe D, Davidson M. Temporal comorbidity of mental disorder and ulcerative colitis. Perm J. 2015;19:52–57. doi: 10.7812/TPP/14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehkamp J, Götz M, Herrlinger K, Steurer W, Stange EF. Inflammatory bowel disease. Dtsch Arztebl Int. 2016;113:72–82. doi: 10.3238/arztebl.2016.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bannaga AS, Selinger CP. Inflammatory bowel disease and anxiety: Links, risks, and challenges faced. Clin Exp Gastroenterol. 2015;8:111–117. doi: 10.2147/CEG.S57982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Sousa Rodrigues ME, Bekhbat M, Houser MC, Chang J, Walker DI, Jones DP, Oller do Nascimento CM, Barnum CJ, Tansey MG. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav Immun. 2017;59:158–172. doi: 10.1016/j.bbi.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moloney RD, O'Mahony SM, Dinan TG, Cryan JF. Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front Psychiatry. 2015;6:15. doi: 10.3389/fpsyt.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SX, Wu WC. Effects of psychological stress on small intestinal motility and bacteria and mucosa in mice. World J Gastroenterol. 2005;11:2016–2021. doi: 10.3748/wjg.v11.i13.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keefer L, Keshavarzian A, Mutlu E. Reconsidering the methodology of ‘stress’ research in inflammatory bowel disease. J Crohns Colitis. 2008;2:193–201. doi: 10.1016/j.crohns.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. Am J Physiol Heart Circ Physiol. 2015;308:H1476–H1498. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 29.Shah E, Rezaie A, Riddle M, Pimentel M. Psychological disorders in gastrointestinal disease: Epiphenomenon, cause or consequence? Ann Gastroenterol. 2014;27:224–230. [PMC free article] [PubMed] [Google Scholar]
- 30.Radek KA. Antimicrobial anxiety: The impact of stress on antimicrobial immunity. J Leukoc Biol. 2010;88:263–277. doi: 10.1189/jlb.1109740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priyadarshini S, Aich P. Effects of psychological stress on innate immunity and metabolism in humans: A systematic analysis. PLoS One. 2012;7:e43232. doi: 10.1371/journal.pone.0043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rho SG, Kim YS, Choi SC, Lee MY. Sweet food improves chronic stress-induced irritable bowel syndrome-like symptoms in rats. World J Gastroenterol. 2014;20:2365–2373. doi: 10.3748/wjg.v20.i9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol. 2018;9:2013. doi: 10.3389/fmicb.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports Medl. 2014;44:81–121. doi: 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nirmal J, Babu CS, Harisudhan T, Ramanathan M. Evaluation of behavioural and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Complement Altern Med. 2008;8:15. doi: 10.1186/1472-6882-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim TK, Park JY, Han PL. Physiological parameters in the blood of a murine stress-induced depression model before and after repeated passive exercise. Endocrinol Metab. 2015;30:371–380. doi: 10.3803/EnM.2015.30.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo S, Gao Q, Jiao Q, Hao W, Gao X, Cao JM. Gastric mucosal damage in water immersion stress: Mechanism and prevention with GHRP-6. World J Gastroenterol. 2012;18:3145–3155. doi: 10.3748/wjg.v18.i24.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 39.Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim Biophys Acta Mol Cell Res. 2017;1864:1183–1194. doi: 10.1016/j.bbamcr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antoni L, Nuding S, Wehkamp J, Stange EF. Intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2014;20:1165–1179. doi: 10.3748/wjg.v20.i5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marteau P, Colombel JF, Nemeth J, Vaerman JP, Dive JC, Rambaud JC. Immunological study of histologically non-involved jejunum during Crohns disease: Evidence for reduced in vivo secretion of secretory IgA. Clin Exp Immunol. 1990;80:196–201. doi: 10.1111/j.1365-2249.1990.tb05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyna-Garfias H, Miliar A, Jarillo-Luna A, Rivera-Aguilar V, Pacheco-Yepez J, Baeza I, Campos-Rodríguez R. Repeated restraint stress increases IgA concentration in rat small intestine. Brain Behav Immun. 2010;24:110–118. doi: 10.1016/j.bbi.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Heffner KL. Neuroendocrine effects of stress on immunity in the elderly: Implications for inflammatory disease. Immunol Allergy Clin North Am. 2011;31:95–108. doi: 10.1016/j.iac.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer JS, Hamel AF. Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. ILAR J. 2014;55:347–360. doi: 10.1093/ilar/ilu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med. 2012;271:421–428. doi: 10.1111/j.1365-2796.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 46.Aggarwal BB, Gupta SC, Sung B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo SY, Le Z, Lv XH, Zhong ZG. Study on effect of total flavonoids of Oldenlendia difflusa on ulcerative colitis and its immunological mechanism Zhongguo Zhong Yao Za Zhi. 2014;39:896–900. (In Chinese) [PubMed] [Google Scholar]
- 48.Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng YL, Wang WY, Jiang CL, Wang YX. Roles of cytokines in stress-induced depression. Sheng Li Xue Bao. 2013;65:229–236. (In Chinese) [PubMed] [Google Scholar]
- 51.Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3-The ‘Swiss Army Knife’ of innate immunity and host defense. Immunol Rev. 2016;274:33–38. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sajadinejad MS, Asgari K, Molavi H, Kalantari M, Adibi P. Psychological issues in inflammatory bowel disease: An overview. Gastroenterol Res Pract. 2012;2012:106502. doi: 10.1155/2012/106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.