Abstract
Diabetic neuropathic pain (DNP) has a huge impact on quality of life and can be difficult to treat. Oral treatment is the most frequently used method for DNP, but its use is often limited by systemic side effects. Topical use of drugs as an alternative option for DNP treatment is currently gaining interest. In the present review, a summary is provided of the available agents for topical use in patients with DNP, including lidocaine plasters or patches, capsaicin cream, gel or patches, amitriptyline cream, clonidine gel, ketamine cream, extracts from medicinal plants including nutmeg extracts and Citrullus colocynthis extract oil, and certain compounded topical analgesics. Furthermore, the potential efficacy of these treatments is addressed according to the available clinical research literature. It has been indicated that these topical drugs have the potential to be valuable additional options for the management of DNP, with adequate safety and continuous long-term treatment efficacy. Compounded topical agents are also effective and safe for patients with DNP and could be another area worthy of further investigation based on the strategy of using low-dose, complementary therapies for DNP. The findings indicate that developing topical drugs acting on different targets in the process of DNP is a valuable area of future research.
Keywords: topical treatments, diabetes, diabetic neuropathy, diabetic neuropathic pain
1. Introduction
According to the International Diabetes Federation, there are 425 million adults with diabetes worldwide (1). Diabetic neuropathy is the most common complication of diabetes mellitus, becoming symptomatic after 14.5 years of chronic prolonged high blood glucose in type 1 diabetes, and after only 8.1 years in type 2 diabetes (2). A population-based study indicated that up to 50% of patients with either type 1 or type 2 diabetes develop diabetic neuropathy (3), and 15–30% of those manifestations are painful (4). Diabetic neuropathic pain (DNP) is among the most common, expensive and disabling complications of diabetes, affecting approximately 30% of patients with diabetes who are hospitalized and 25% of those in the community (5,6).
DNP is characterized by sensations of numbness, burning pain and prickling or stinging around the hands and feet, and the pain is more severe at night (7). The more severe occurrences of DNP can be intractable (8,9). Neuropathic pain can be constant and accompanied by cutaneous allodynia, which significantly influences quality of life (QOL) and prevents patients from performing their daily activities and roles of employment. The pain may also be associated with depression and may be a reason for withdrawal from recreational and social life (7,10,11). Thus, treating this debilitating condition appropriately is important, particularly because of the impact on QOL and the associated depression and anxiety (12).
The management of DNP is challenging and can require a multi-modal approach involving early recognition, glycemic control and psychological therapy, as well as therapies for symptomatic pain relief (13–15). At present, the pharmacological treatments for DNP include oral and topical therapies (16). Oral agents, such as amitriptyline, duloxetine, pregabalin and gabapentin, are recommended as first-line drug treatments (17). However, oral therapy requires multiple medications taken at varying times of the day and is often associated with a significantly elevated risk of systemic side effects, which may be severe (18). Some patients do not tolerate these drugs and some find them ineffective, which may cause a significant drop in patient compliance. With the potential to provide the same level of analgesic efficacy provided by oral analgesics, but relatively fewer systemic adverse events and drug-drug interactions (19,20), topical use of drugs as another treatment option for DNP is currently gaining interest (16,21). A variety of agents are used in the topical treatments of DNP, including lidocaine, capsaicin, amitriptyline, clonidine, nutmeg extracts, Citrullus colocynthis extract oil and even a combination of various compounds, and so far offer promising results (22–28). Given the trend towards using topical medications to treat DNP, the present review provides an overview of the current knowledge regarding the physiopathology of DNP and the existing topical pharmacological treatments. A recent review, focusing on topical treatment for localized neuropathic pain, including post-herpetic neuralgia (PHN), complex regional pain syndrome, diabetic peripheral neuropathy and human immunodeficiency virus distal sensory polyneuropathy, has been published (29). In the current review, an overview is provided of the current understanding of the pathogenesis of DNP and the mechanism of drug action. Furthermore, the clinical data on available agents for topical use in patients with DNP is summarized, their potential efficacy addressed, and the potential for use of compounded topical agents in the future is highlighted.
2. Pathogenesis of DNP
The exact pathogenic mechanisms involved in the generation of DNP are not fully established (8). A variety of potential factors have been postulated to elicit the pain associated with diabetic neuropathy, including hyperglycemia, advanced glycation end-products (AGEs)/AGE receptor (RAGE) activity, oxidative stress, neuroinflammation and endoneural hypoxia (30,31). A schematic summarizing the potential mechanisms underlying the pathogenesis of DNP is presented in Fig. 1.
Figure 1.
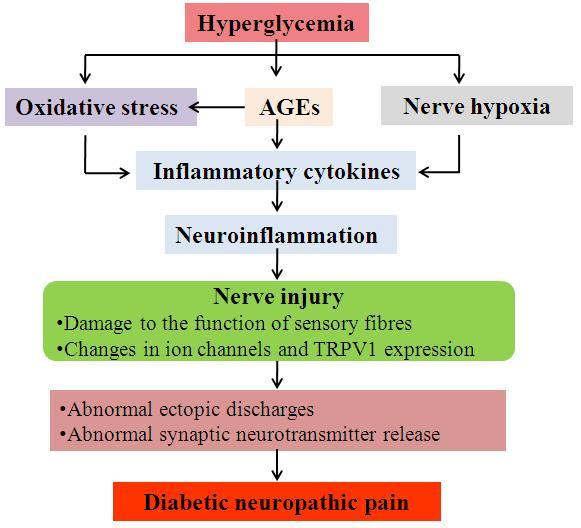
A summary of the pathogenesis of DNP. DNP, diabetic neuropathic pain; AGEs, advanced glycation end-products; TRPV1, transient receptor potential vanilloid 1.
There is a consensus that hyperglycemia serves a crucial role in the development of DNP (2,32–36). Studies in non-diabetic individuals and in animals have demonstrated that hyperglycemia can cause a decrease in pain thresholds (34,35). Hyperglycemia is correlated with the progression of neuropathy pain; approximately 50% of patients who have had diabetes for more than 25 years will develop neuropathy and the majority of symptomatic patients will complain of pain (2,36).
Other factors besides hyperglycemia may result in the generation of DNP (37). An increase in AGE production and a decrease in the regeneration of glutathione may be caused by hyperglycemia (38,39). Depletion of glutathione could be the primary cause of oxidative stress and related to the accumulation of toxic species (40). In addition, when the disposal of intracellular glucose is impaired, alternate pathways are activated, which may also lead to oxidative stress and nerve injury (41–43). Hyperglycemia serves a key role in oxidative stress in diabetic nerves (41). Nerve hypoxia may be evoked by hyperglycemia, particularly in sensory nerves, altering their function and electrical stability (44). AGEs, oxidative stress and hypoxia thereby cause the production of inflammatory cytokines and growth factors, which in turn cause neuroinflammation and nerve injury (45–47). Neuropathic pain has been associated with neurological damage to the function of sensory fibers, including fast-conducting myelinated Aδ fibers and slow-conducting unmyelinated C fibers, which conduct nerve impulses, and changes in voltage-gated ion channel distribution and expression (48,49). In addition, the upregulation of transient receptor potential vanilloid 1 (TRPV1) expression has been identified to be associated with neuropathic pain (50). Hyperglycemia, AGEs, hypoxia and oxidative stress-mediated damage in neurons and glial cells, as well as the subsequent activation of proinflammatory cascades and crosstalk between these disease processes, may ultimately result in abnormal ectopic discharges and abnormal synaptic neurotransmitter release, including of serotonin and norepinephrine (8), and thereby trigger development of neuropathic pain (51).
3. Topical pharmacological treatment of DNP
The analgesic effect and safety of various topical agents including lidocaine plasters and patches, capsaicin cream, capsaicin gel, capsaicin patches, amitriptyline cream, clonidine gel, ketamine cream, extracts from medicinal plants including nutmeg extracts and Citrullus colocynthis extract oil, and some compounded topical analgesics, have been assessed in patients affected by DNP. Topical agents evaluated in clinical trials are discussed in detail herein and their targets of action are presented in Fig. 2.
Figure 2.
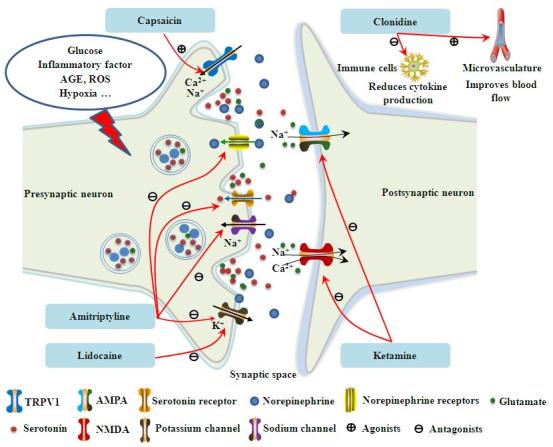
Therapeutic targets of topical agents for DNP. AGEs, advanced glycation end-products; ROS, reactive oxygen species; TRPV1, transient receptor potential vanilloid 1; AMPA, a-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid; NMDA, N-methyl-D-aspartate; DNP, diabetic neuropathic pain.
Topical lidocaine
Lidocaine, a blocker of voltage-gated sodium channels (particularly Nav 1.7 and 1.8), can stabilize the neuronal membrane potential on abnormally excitable Aδ and C fibers, resulting in a reduction of ectopic discharges (52,53). This raises the peripheral ectopic discharge threshold and reduces the pain transduction. Topical lidocaine has been demonstrated to be effective in the treatment of neuropathic pain conditions, including DNP (54–57). A network meta-analysis compared 5% lidocaine-medicated plaster for the relief of DPN with other relevant drugs, including amitriptyline (30, 75 and 125 mg/day), pregabalin (300 and 600 mg/day), capsaicin (0.075% cream, four times a day), gabapentin (≤900 and 1,200 mg/day) and placebo. It was indicated that all interventions were effective in comparisons with a placebo. Notably, lidocaine medicated plasters were comparable to all of the other interventions (58).
Here, five reports that assessed the effectiveness of topical lidocaine for DNP have been reviewed (Table I). A 4-week, randomized, open-label, multicenter, non-inferiority study, which compared lidocaine plaster with pregabalin in patients with DNP, was reported as an interim analysis (59) and published in full (52,60). The interim analysis revealed that patients administered with 5% lidocaine plasters experienced similar analgesic efficacy, fewer drug-related adverse events (DRAEs; 3.9 vs. 39.2%) and substantially fewer discontinuations due to DRAEs (1.3 vs. 20.3%) than pregabalin (59). In the full analysis the investigators also reported results for EuroQol-5D (EQ-5D) QOL evaluation. In line with the aforementioned results, 5% lidocaine-medicated plaster exerted comparable efficacy, greater improvements in the QOL based on EQ-5D, and fewer adverse events, DRAEs and related discontinuations compared with pregabalin (52). To our knowledge this was the only study that has assessed the lidocaine plaster as a monotherapy for DNP. An 8-week combination phase of this study also demonstrated additional decreases in recalled average pain intensity scores over the previous 3 days (numeric rating scale-3 scores) (60). Another open-label study on patients with DPN who experienced pain following a stable analgesic drug regimen and dosages for at least a week, and presented with an average daily pain diary rating of ≥4 on the brief pain inventory, exhibited significant improvements in pain and QOL outcome measures during a 3-week 5% lidocaine patch treatment period. These benefits were maintained during an additional 5-week period with a tapering of concomitant analgesics (56). A further two studies also included patients with PHN or lower back pain (55,61). The 5% lidocaine patch could effectively reduce the intensity of pain in patients with various pain-related conditions, including DNP. A reduction in pain intensity was also observed with topical 5% lidocaine in a double-blind, randomized, placebo-controlled crossover study including patients with DNP (62). These results of clinical trials indicated that administration with a topical lidocaine 5% patch or plaster could relieve pain symptoms, including in DNP.
Table I.
Summary of clinical studies on topical lidocaine for the treatment of DNP.
| Author, year | Topical agent(s) | Study design | No. of patients (% male) | Age, years (mean ± standard deviation) | Duration of pain | Outcomes | (Refs.) |
|---|---|---|---|---|---|---|---|
| Comparisons with oral pregabalin | |||||||
| Baron et al, 2009 | 5% Lidocaine plaster | Two-stage, adaptive, randomized, controlled, open-label, 4-week, multicenter trial that incorporated a drug wash-out phase of up to 2 weeks prior to the start of the comparative phase | Lidocaine: 47 (48.9) Pregabalin: 44 (54.5) | Lidocaine: 60.2±9.9 Pregabalin: 59.8±8.4 | ≥3 months | Showed similar analgesic efficacy, fewer DRAEs (3.9% vs. 39.2%) and fewer substantial discontinuations due to DRAEs (1.3 vs. 20.3%) than pregabalin. | (59) |
| Baron et al, 2009 | 5% Lidocaine plaster | Two-stage adaptive, randomized, open-label, 4-week, multicenter, non-inferiority study | Lidocaine: 105 (42.9) Pregabalin: 105 (46.7) | Lidocaine: 60.9±10.0 Pregabalin: 60.9±8.8 | ≥3 months | Showed comparable efficacy, greater improvements in QOL, and fewer AEs, DRAEs and related discontinuations compared with pregabalin. An 8-week combination phase of this study demonstrated additional decreases in NRS-3 scores. | (52,60) |
| Noncomparative studies | |||||||
| Barbano et al, 2004 | 5% Lidocaine patch | Open-label, flexible-dosing, 3-week study with a 5-week extension | 56 (NA) | NA | ≥3 months | Significantly reduced pain and improved QOL. These benefits were maintained during an additional 5 weeks with tapering of concomitant analgesics. | (56) |
| Argoff et al, 2004 | 5% Lidocaine patch | Open-label, non-randomized, prospective, 2-week study | 41 (41.5) | 56.7±12.6 | NA | Effectively reduced the intensity of pain in patients with DNP. Well tolerated in combination with other analgesic regimens. No serious AEs or adverse drug interactions. | (55) |
| White et al, 2003 | 5% Lidocaine patch | Open-label, non-randomized, 2-week multicenter pilot trial. | 49 (46.9) | 57.7±12.6 | NA | Improved pain intensity and pain relief scores. | (61) |
DNP, diabetic neuropathic pain; AE, adverse event; DRAE, drug-related adverse event; QOL, quality of life; NA, not available.
Topical capsaicin
Capsaicin, the active agent of the dried fruits or ground powder (paprika) of chili peppers, is a naturally occurring alkaloid (63). Capsaicin has been used with some success in the treatment of patients with DNP (6,64). These effects are thought to be caused by selective binding to TRPV1 expressed on Aδ and C fibers (65). Once capsaicin binds to the receptor and the TRPV1 channel is opened, sodium and calcium influx and substance P release occurs (66). Repeated TRPV1 exposure to capsaicin causes substance P depletion and TRPV1 desensitization and defunctionalization (7). Two forms of capsaicin are available for DNP, a low-dose cream and a high-dose patch, both of which should be used under specialist supervision (67). Capsaicin creams with concentrations of 0.025–0.250% must be applied multiple times per day for several weeks before analgesic effects become obvious (68). A single application of the Qutenza patch (capsaicin patch with an 8% concentration) following an appropriate local analgesia may provide up to 3 months of pain relief (23). Seven double-blind controlled studies and one open-label study of topical capsaicin in the treatment of DNP are summarized in Table II. No study specifically examined topical capsaicin as a monotherapy for DNP.
Table II.
Summary of clinical studies on topical capsaicin for the treatment of DNP.
| Author, year | Topical agent(s) | Study design | No. of patients (% male) | Age, years (mean ± standard deviation) | Duration of pain | Outcomes | (Refs.) |
|---|---|---|---|---|---|---|---|
| Comparisons with placebo or vehicle | |||||||
| Chad et al 1990 | 0.075% Capsaicin cream | Double-blind, 4-week, multicenter, vehicle-controlled, randomized study | Capsaicin: 24 (NA) Vehicle: 22 (NA) | NA | NA | No obvious effects on pain relief | (74) |
| The Capsaicin Study Group, 1991–1992 | 0.075% Capsaicin cream | Double-blind, 8-week, multicenter, vehicle-controlled parallel randomized study | Capsaicin: 138 (51.4) Vehicle: 139 (48.9) | Capsaicin: 60.1 (27–92)a Vehicle: 60.3 (22–81)a | NA | Well tolerated Effective for reducing pain Improved daily activities Enhanced QOL | (69,70) |
| Tandan et al, 1992 | 0.075% Capsaicin cream | Double-blind, 8-week, vehicle-controlled study with either 0.075% capsaicin cream or vehicle cream over the painful areas | Capsaicin: 11 (54.5) Vehicle: 11 (45.5) | Capsaicin: 55.1±7.6 Vehicle: 53.3±11.8 | NA | Decreased mean pain intensity Relieved mean pain scores No adverse effects on sensory thresholds Approximately 50% of subjects reported improved pain control or were cured in a follow-up open-label study | (72,73) |
| Kulkantrakorn et al, 2013 | 0.025% Capsaicin gel | Double-blind, 20-week, crossover, randomized, single-center study enrolling subjects with DNP | 33 (48.5) | 58.0 (35–76)a | ≥1 month | Safe and well tolerated Did not provide significant pain relief | (75) |
| Simpson et al, 2017 | 8% Capsaicin patch | Phase 3, randomized, double-blind, 12-week, placebo-controlled, multicenter trial | Capsaicin: 186 (61.3) Placebo: 183 (55.2) | Capsaicin: 63.9±10.6 Placebo: 62.0±10.8 | ≥1 year | Provided modest and statistically significant improvements in pain relief Improved sleep quality Well tolerated Did not cause any sensory deterioration or new safety concerns | (76) |
| Comparisons with oral amitriptyline | |||||||
| Biesbroeck et al, 1995 | 0.075% Capsaicin cream | Double-blind, 8-week, multicenter, parallel study compared the safety and efficacy of topical capsaicin and oral amitriptyline in patients with painful diabetic neuropathy | NA | NA | NA | Equally effective Considerably safer | (71) |
| Comparisons with SOC alone | |||||||
| Vinik et al, 2016 | 8% Capsaicin patch | Phase 3, multinational, open-label, randomized, controlled, 52-week safety study | Capsaicin 8% patch (30 min) + SOC: 156 (47.4) Capsaicin 8% patch (60 min) + SOC: 157 (50.3) SOC: 155 (45.8) | Capsaicin 8% patch (30 min) +SOC: 60.9±10.9 Capsaicin 8% patch (60 min) +SOC: 61.0±10.3 SOC: 59.1±10.3 | ≥1 year | In patients with PDPN, capsaicin 8% patch repeat treatment plus SOC over 52 weeks was well tolerated with no negative functional or neurological effects compared with SOC alone | (77) |
Mean age (range). SOC, standard of care; DNP, diabetic neuropathic pain; PDPN, painful diabetic peripheral neuropathy; QOL, quality of life; NA, not available.
The action of low concentration capsaicin (0.025 or 0.075%) in the treatment of DNP was reported in a series of the studies reviewed. The Capsaicin Study Group attempted to define the efficacy and safety of topical 0.075% capsaicin in patients with painful diabetic neuropathy. Improvement in pain relief, pain intensity and daily activities, including sleeping, walking and the ability to work and participate in recreational activities, were observed in significantly more patients treated with capsaicin than with a vehicle (69,70). Compared with oral amitriptyline, topical 0.075% capsaicin cream was equally effective but considerably safer according to the results of a comparative study in patients with painful diabetic neuropathy (71). A double-blind, 8-week vehicle-controlled study with either 0.075% capsaicin cream or a vehicle cream over the painful area further confirmed the value and safety of capsaicin for pain relief in subjects with DNP (72,73). By contrast, Chad et al (74) and Kulkantrakorn et al (75) observed that low concentration topical capsaicin had little effect in the treatment of DNP. After 4 weeks of 0.075% capsaicin cream treatment, there was no notable difference between capsaicin and the vehicle in terms of their beneficial effects on DNP (74). Meanwhile, 0.025% capsaicin gel was determined safe and well tolerated, but it did not provide significant pain relief in patients with DNP compared with a placebo (75). Due to the inconsistences between the aforementioned studies and their small sample sizes, it is difficult to conclude on the efficacy of low-concentration topical capsaicin in the treatment of DNP.
An 8% capsaicin patch, which is optimized for rapid delivery of a high concentration of capsaicin directly to the skin, contains 179 mg or 8% w/w capsaicin. One double-blind study that assessed the efficacy of an 8% capsaicin patch compared with a placebo and one open-label study that provided long-term safety and tolerability data on patients with painful diabetic peripheral neuropathy (PDPN) were available. In the double-blind trial that assessed efficacy, 369 patients were randomized to an 8% capsaicin patch (Qutenza) or a placebo patch regimen. The capsaicin patch provided modest but statistically significant improvements in pain relief and improved sleep quality compared with the placebo patch, was well tolerated, and was not associated with any sensory deterioration or new safety concerns (76). In the 52-week open safety study, 468 DNP patients were randomized to a capsaicin 8% patch repeat treatment for 30 or 60 min plus standard of care (SOC), or SOC alone. No worsening in sensory perception tested with sharp, cold, warm or vibration stimuli was observed with topical capsaicin. Compared with SOC therapy alone, SOC plus capsaicin 8% patch repeat treatment over 52 weeks was well tolerated, had no neurological or negative functional effects and raised no new safety concerns (77). An article from the Drug and Therapeutics Bulletin proposed that there is a limited role for topical capsaicin in the treatment of DNP due to the uncertain efficacy of low-concentration topical capsaicin and the considerably more expensive cost of the 8% capsaicin patch compared with oral therapies (67). However, topical 0.075% capsaicin was recommended as a likely effective treatment option for DNP in the American Academy of Neurology evidence-based guidelines (78). A network meta-analysis performed in patients with PDPN suggested that the 8% capsaicin patch was only as effective as oral centrally acting agents but offered superior systemic tolerability benefits (18). Either low concentration or high concentration capsaicin may provide a degree of pain relief to some patients with neuropathic conditions that cause different degrees of pain. Further developments in methods of application and formulation may lead to improved clinical efficacy.
Topical amitriptyline
Amitriptyline is a tricyclic anti-depressant that acts centrally by blocking Na+, K+, and Ca2+ voltage-gated ion channels (79–81), inhibiting neuronal reuptake of norepinephrine and serotonin. It is effective in treating various types of neuropathic pain (82). A topical form of this drug has been investigated in certain previous studies for the treatment of DNP (24,62,83) (Table III), however its adverse effects in oral administration have limited the higher doses needed to achieve adequate analgesia.
Table III.
Summary of clinical studies on other topical agents for the treatment of DNP.
| Author, year | Topical agent(s) | Study design | No. of patients (% male) | Age, years (mean ± standard deviation) | Duration of pain | Outcomes | (Refs.) |
|---|---|---|---|---|---|---|---|
| Comparisons with topical 5% lidocaine and placebo | |||||||
| Ho et al, 2008 | 5% Amitriptyline cream | Double-blind, randomized, placebo-controlled crossover study | 35 (45.7) | 57.4±13.8 | ≥6 months | No significant change in pain intensity was observed with topical amitriptyline or placebo Topical lidocaine and placebo each reduced pain more than topical amitriptyline | (62) |
| Comparisons with topical 0.75% capsaicin cream | |||||||
| Kiani et al, 2015 | 2% Amitriptyline cream | Double-blind, 12-week, randomized, and non-inferiority trial | Amitriptyline: 51 (33.3) Capsaicin: 51 (31.4) | Amitriptyline: 57.5±10.8 Capsaicin: 55.4±10.6 | ≥3 months | Both drugs significantly relieved pain in 12 weeks compared with baseline values. Well tolerated | (24) |
| Comparisons with placebo | |||||||
| Campbell et al, 2009 and Wrzosek et al, 2015 | 0.1 and 0.2% Clonidine gels | Double-blind, 8-week, randomized placebo-controlled trial | 0.1% clonidine: 54 (NA) 0.2% clonidine: 54 (NA) Placebo: 57 (NA) | NA | NA | The reduction in mean aggregate pain score from week 8 to baseline in subjects who received 0.1% gel was significantly greater compared with the placebo group. The reduction in pain for the 0.2% group was less impressive Adverse events were similar between the three groups. | (25,87) |
| Campbell et al, 2012 | 0.1% Clonidine gel | Double-blind, 12-week, randomized, placebo-controlled, parallel-group, multi-center trial | Clonidine: 89 (49) Placebo: 90 (47) | Clonidine: 59.4±9.9 Placebo: 57.6±9.5 | ≥6 months | Significantly reduced the level of pain Safe and without the problematic side effects typically associated with systemic therapies | (88) |
| Motilal and Maharaj, 2013 | Nutmeg extract | Double-blind, 4-week, randomized, placebo-controlled trial. | Nutmeg extract: 37 (32.4) Placebo: 37 (32.4) | Nutmeg extracts: 60.7±11.5 Placebo: 59.7±8.1 | NA | Reduced worst and mean pain scores Improved QOL No statistically significant differences between the groups for all outcome measures | (26) |
| Heydari et al, 2016 | Topical Citrullus colocynthis extract oil | Two-arm, double-blind, randomized, placebo-controlled, parallel study | Citrullus colocynthis extract oil: 30 (39.3) Placebo: 30 (55.6) | Citrullus colocynthis extract oil: 57.4±10.0 Placebo: 52.7±10.5 | ≥3 months | Decreased mean pain score Improved nerve function Improved physical domain of QOL | (27) |
| Mahoney et al, 2012 | Topical ketamine cream | Double-blind, randomized, placebo-controlled, study | Ketamine: 10 (40.0) Placebo: 7 (57.1) | Ketamine: 64.0±9.5 Placebo: 65.4±15.0 | NA | No more effective than placebo | (100) |
| ClinicalTrials. govNCT00476151, 2006 | Topical 4% amitriptyline and 2% ketamine | Phase 2, multicenter, randomized, placebo-controlled, parallel group study | Ami/Ket: 114 (57.0) Placebo: 112 (50.9) | Ami/Ket: 56.1±9.4 Placebo: 55.1±11.0 | ≥6 months | Showed a strong trend toward pain reduction in painful diabetic neuropathy | (28) |
DNP, diabetic neuropathic pain; QOL, quality of life; NA, not available.
In a double-blind, randomized, placebo-controlled crossover study, 35 patients with postsurgical neuropathic pain, PHN or DNP were included to examine the analgesic effect of topical 5% amitriptyline. This study failed to show efficacy of topical amitriptyline in alleviating neuropathic pain, though the results were based on a small sample size of DNP patients (62). Inconsistent with these results, a double-blind, 12-week, randomized and non-inferiority trial revealed that topical 2% amitriptyline was effective in managing DNP. The results were similar to 0.75% capsaicin cream, with fewer adverse effects and improved patient compliance. However, the study only measured the median pain score using the visual analogue scale (24). In addition, two cases treated with high doses of topical amitriptyline in neuropathic pain have been described to record the effect of topical amitriptyline (83). One of the cases reported a 39-year-old patient suffering from severe neuropathic pain in the feet and hands, due to type II diabetes mellitus (83). Following the use of 5% amitriptyline, the pain in the hands was markedly relieved, and following application of amitriptyline 10% to the feet, a total reduction of pain occurred within 20 min and lasted the whole day. These effects reportedly lasted for 7 months and the patient did not experience any amitriptyline-related side effects (83). Amitriptyline could also improve the quality of sleep for the patients by reducing severe pins and needles in the feet at night (83). Therefore, high dose topical amitriptyline at 5 and 10% may be a useful adjunct to treat severe and intractable DNP. However at present, the therapeutic effect of amtriptyline on DNP is still unclear. Given the conflicting results of the reports, more studies are required to confirm the efficacy and safety of this topical compound as a treatment for DNP.
Topical clonidine
Clonidine is used to treat hypertension and other conditions, including intraocular pressure rise (84) and attention deficit syndrome (85), but it also has actions on sensory neurons by reducing excitability, acts on the microvasculature by improving blood flow, and acts on immune cells by reducing cytokine production (86). It is a presynaptic α2-adrenergic receptor agonist; thus adverse events associated with systemic use of this drug have limited its application. As such, topical clonidine formulations have been investigated in clinical studies. Two randomized placebo-controlled studies for the treatment of DNP have been conducted in the USA by Wrzosek et al (25) and Campbell et al (87) (Table III). Both studies evaluated the efficacy and safety of topical clonidine gel in DPDN patients. In the first of the studies, 54 patients received 0.1% clonidine gel (650 µl) and another 54 patients were treated with 0.2% clonidine gel (500 µl). The control group (57 patients) was given a placebo gel. Participants remained on their existing pain management treatments and these gels were applied to both feet twice daily for 2 weeks, then 3 times daily to a total of 8 weeks (25). Over the 8-week study interval, the reduction in mean aggregate pain score when compared with baseline in subjects who received 0.1% gel was significantly greater compared with the placebo group, while the reduction in pain for the 0.2% group was less notable. Investigators suggested the possible reason leading to such difference was the amount applied to the skin (500 µl at 0.2% vs. 650 µl at 0.1%). Campbell et al (88) subsequently performed a 12-week study to examine further the efficacy of topical 0.1% clonidine in treating DNP. In the clonidine group, the decrease in average pain from baseline to week 12 was greater than in the placebo group. For safety, only a low concentration of clonidine (<10 pg/ml) was aimed for in the plasma during topical application (88), and it was considerably lower than the threshold for treating hypertension, suggesting that topical use will be associated with few of the adverse effects of clonidine. The results of these two clinical trials indicated that the topical use of clonidine may relieve DNP, but it is not known from these studies if clonidine is safe for long-term use. The use of active treatment rather than placebo may reveal more information about the comparative efficacy of topical clonidine vs. other drugs.
Topical nutmeg extracts
Nutmeg is the dried kernel of the broadly ovoid seed of Myristica fragrans. Certain animal studies support nutmeg as a potential analgesic for DNP (89,90). A double-blind, 4-week, randomized, placebo-controlled trial further tested the ability of topical nutmeg extracts to reduce pain or improve QOL in patients with DNP (26) (Table III). A total of 74 participants who met the criteria for painful diabetic neuropathy were recruited. Subjects were instructed to apply four sprays of either topical nutmeg extract or placebo to the affected area 3 times a day followed by gentle massage for 4 weeks. The topical nutmeg extract preparation significantly reduced the worst and mean pain scores, and improved QOL by the end of the 4-week study, but the effects were not superior to the placebo. Due to the limited clinical research data, it is not possible to conclude on the efficacy of topical nutmeg extracts for the treatment of painful diabetic neuropathy.
Topical Citrullus colocynthis (bitter apple) extract oil
Bitter apple, or Citrullus colocynthis, is a medicinal plant originating from Africa and Asia, where it has traditionally been used for various medicinal purposes, including pain relief (91). Citrullus colocynthis has been demonstrated to have anesthetic (92), anti-oxidant (93) and anti-ulcerogenic effects (94). These properties are considered important in the pathophysiology and/or progression of diabetic neuropathy. Heydari et al (27) examined its efficacy and safety in DNP patients (Table III): 60 patients with DNP were randomized to receive topical Citrullus colocynthis or placebo twice daily for 3 months. There was a significantly greater decrease in mean pain score after 3 months in the Citrullus colocynthis group than with the placebo. It also significantly improved nerve function and the mean score in the physical aspect of QOL (95). Therefore, topical Citrullus colocynthis may be a potential agent for use in the treatment of DNP and should be investigated further in studies with larger sample sizes performed over longer duration.
Topical ketamine
Ketamine is a non-barbiturate anesthetic agent that acts through blocking glutamate receptors, including peripheral N-methyl-D-aspartate receptors and α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (86,96–98), and also as an inhibitor of voltage-gated Na+ and K+ ion channels (99). A recent study evaluated the effects of topical ketamine in relieving DNP (Table III): 17 patients with DNP were randomly divided into a 5% ketamine cream group or a placebo group. The 5% topical ketamine cream was no more effective than the placebo in relieving pain caused by diabetic neuropathy (100). A limitation of this study was the sample size; therefore the clinical efficacy of ketamine needs to be further evaluated.
Compounded topical agents
Mixtures of two or more medicated forms of compounded topical agent have been developed into potentially valuable treatment options for DNP. The authors of the current study concluded that these may be effective due to the multiple complementary effects at lower doses of each individual medication. It is apparent that only one study with compounded topical agents has been performed in patients with DNP only (28) (Table III); 4 other related studies also selected patients with other types of neuropathic pain (96,101–103). In a pilot study and its follow-up studies of mixed neuropathic pain, including DNP, patients who were treated with topical low-dose combinations of amitriptyline (1–2%) and ketamine (0.5–1%) did not experience significant pain relief effect compared with those receiving placebo for 1–3 weeks (96,101). However, the treatment was associated with long-term perceived analgesic effectiveness for 12 months (102). As for higher concentrations of these combined agents (4% amitriptyline and 2% ketamine), a phase 2, multi-center, double-blind, randomized, placebo-controlled, parallel group study indicated a trend towards pain reduction in painful diabetic neuropathy (16,28). Somberg and Molnar (103) conducted a retrospective study on the analgesic activity of a topical (TT-CTAC) cream in patients with diabetic neuropathy and other chronic pain conditions. Two versions of TT-CTAC cream were evaluated: cream 6B and cream 7B. Both creams contain ketamine (10%), baclofen (2%), gabapentin (6%), amitriptyline (4%), bupivacaine (2%) and clonidine (0.2%). Additionally, one cream (7B) contained nifedipine (2%). Both creams provided considerable pain relief in the majority of the patients studied and were thus suggested to be useful modalities for pain therapy. Due to a lack of comparative studies between compounded topical agents and single drugs for DNP, it is not possible to conclude on the drug cost, efficacy and side effects of compounded agent compared with single-agent topical treatment of DNP. However, given the advantages of formula diversity, drug economy and safety, it may be that compounded topical agents have promising market prospects and clinical application value.
4. Conclusion and perspectives
DNP is a distressing consequence of diabetes that may be present in as many as one in five diabetic patients (104). The pain may be severe and management challenging. Oral treatment is the most frequently used and among the most convenient for pain medication, but is also associated with risk of adverse systematic effects (105,106), particularly in vulnerable patients with multiple comorbidities and altered pharmacokinetics and pharmacodynamics that may alter drug metabolism (107). This may decrease the patient's compliance. With this concern for the systematic side effects of oral agents, topical preparations for the treatment of DNP have gained increasing interest. Numerous clinical studies have evaluated the analgesic effect and safety of various topical agents, including topical anesthetic agent lidocaine, general anesthetic agent ketamine, antidepressant agent amitriptyline, α2 adrenergic agent clonidine, capsaicin, nutmeg extracts, Citrullus colocynthis extract oil, and some compounded topical analgesics. Overall topical agents are easy to administer, offer significant improvement in patient compliance and reduce the burden of multiple drug regimens.
For the topical treatments of DNP discussed here, a 5% lidocaine patch has been found to significantly reduce pain in DNP patients with 2–3 weeks of treatment and the benefits were maintained during an additional 5 weeks with tapering of concomitant analgesics. Furthermore, the 5% lidocaine plaster was reported to exhibit similar analgesic efficacy and fewer DRAEs compared with oral pregabalin. It is difficult to draw conclusions about the efficacy of low-concentration topical capsaicin in the treatment of DNP due to the inconsistencies among related research findings. The 8% capsaicin patch was as effective as oral centrally acting agents but offered systemic tolerability benefits, provided modest but statistically significant improvements in pain relief and was well tolerated. There is conflicting evidence regarding the effect of topical amitriptyline and it needs to be confirmed by further studies. Topical clonidine, nutmeg extract preparation, Citrullus colocynthis extract and some compounded topical agents may provide significant though less notable or only long-term improvements in pain relief. For instance, study demonstrated that 5% topical ketamine cream was no more effective than a placebo in relieving pain caused by diabetic neuropathy, but the study only included 17 patients with DNP.
Since published studies suggest that these drugs have the potential to be a valuable additional option for the management of DNP, with adequate safety and continuous long-term treatment efficacy, these topical drugs should be given further consideration for the treatment of DNP. Furthermore, as described in several studies, compounded agents are not only effective but also safe for patients with DNP. Therefore, the use of low-dose, complementary therapies with compounded agents is another area worthy of further investigation. Additionally, topical drugs that act on different targets in the process of DNP, including anti-inflammatory and abnormal ectopic discharge therapies, or anti-oxidant and AGE/RAGE therapies, should be studied in the future.
Acknowledgements
Not applicable.
Funding
The authors would like to thank the Natural Science Foundation of China (grant no. 81603171), the Natural Science Foundation of Hunan Province (grant no. 2018JJ3743) and the Open-End Fund for the Valuable and Precision Instruments of Central South University (grant no. CSUZC201734) for the financial support.
Availability of data and materials
Not applicable.
Authors' contributions
PFF, XDY and YYY designed the present study. XDY, PFF, DXX and YYY collected the literature and performed the literature review. XDY and YYY wrote the manuscript. YYY, PFF and XDY revised the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
References
- 1.International Diabetes Federation (IDF) IDF Diabetes Atlas. (7th) 2017 [Google Scholar]
- 2.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harati Y. Diabetic neuropathies: Unanswered questions. Neurol Clin. 2007;25:303–317. doi: 10.1016/j.ncl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Farmer KL, Li C, Dobrowsky RT. Diabetic peripheral neuropathy: Should a chaperone accompany our therapeutic approach? Pharmacol Rev. 2012;64:880–900. doi: 10.1124/pr.111.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spallone V, Lacerenza M, Rossi A, Sicuteri R, Marchettini P. Painful diabetic polyneuropathy: Approach to diagnosis and management. Clin J Pain. 2012;28:726–743. doi: 10.1097/AJP.0b013e318243075c. [DOI] [PubMed] [Google Scholar]
- 6.Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: An update. Am Fam Physician. 2016;94:227–234. [PubMed] [Google Scholar]
- 7.Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes. 2015;6:432–444. doi: 10.4239/wjd.v6.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJ. Toronto Expert Panel on Diabetic Neuropathy: Painful diabetic peripheral neuropathy: Consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27:629–638. doi: 10.1002/dmrr.1225. [DOI] [PubMed] [Google Scholar]
- 9.Jane SW, Lin MS, Chiu WN, Beaton RD, Chen MY. Prevalence, discomfort and self-relief behaviours of painful diabetic neuropathy in Taiwan: A cross-sectional study. BMJ Open. 2016;6:e11897. doi: 10.1136/bmjopen-2016-011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36:2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30:374–385. doi: 10.1016/j.jpainsymman.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: The association revisited. Diabetes Care. 2005;28:2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 13.Sadosky A, McDermott AM, Brandenburg NA, Strauss M. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008;8:45–56. doi: 10.1111/j.1533-2500.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler D. Painful diabetic neuropathy: Treatment and future aspects. Diabetes Metab Res Rev. 2008;24(Suppl 1):S52–S57. doi: 10.1002/dmrr.817. [DOI] [PubMed] [Google Scholar]
- 15.Kaku M, Vinik A, Simpson DM. Pathways in the diagnosis and management of diabetic polyneuropathy. Curr Diab Rep. 2015;15:609. doi: 10.1007/s11892-015-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawynok J, Zinger C. Topical amitriptyline and ketamine for post-herpetic neuralgia and other forms of neuropathic pain. Expert Opin Pharmacother. 2016;17:601–609. doi: 10.1517/14656566.2016.1146691. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler D, Fonseca V. From guideline to patient: A review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications. 2015;29:146–156. doi: 10.1016/j.jdiacomp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 18.van Nooten F, Treur M, Pantiri K, Stoker M, Charokopou M. Capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy: A systematic literature review and network meta-analysis. Clin Ther. 2017;39:787–803.e18. doi: 10.1016/j.clinthera.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013;88:195–205. doi: 10.1016/j.mayocp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Jorge LL, Feres CC, Teles VE. Topical preparations for pain relief: Efficacy and patient adherence. J Pain Res. 2010;4:11–24. doi: 10.2147/JPR.S9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derry S, Rice AS, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1:CD007393. doi: 10.1002/14651858.CD007393.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster-a review. Curr Med Res Opin. 2012;28:937–951. doi: 10.1185/03007995.2012.690339. [DOI] [PubMed] [Google Scholar]
- 23.Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP. Qutenza (capsaicin) 8% patch onset and duration of response and effects of multiple treatments in neuropathic pain patients. Clin J Pain. 2014;30:286–294. doi: 10.1097/AJP.0b013e31829a4ced. [DOI] [PubMed] [Google Scholar]
- 24.Kiani J, Ahmad Nasrollahi S, Esna-Ashari F, Fallah P, Sajedi F. Amitriptyline 2% cream vs. capsaicin 0.75% cream in the treatment of painful diabetic neuropathy (Double blind, randomized clinical trial of efficacy and safety) Iran J Pharm Res. 2015;14:1263–1268. [PMC free article] [PubMed] [Google Scholar]
- 25.Wrzosek A, Woron J, Dobrogowski J, Jakowicka-Wordliczek J, Wordliczek J. Topical clonidine for neuropathic pain. Cochrane Database Syst Rev. 2015;8:CD010967. doi: 10.1002/14651858.CD010967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motilal S, Maharaj RG. Nutmeg extracts for painful diabetic neuropathy: A randomized, double-blind, controlled study. J Altern Complement Med. 2013;19:347–352. doi: 10.1089/acm.2012.0016. [DOI] [PubMed] [Google Scholar]
- 27.Heydari M, Homayouni K, Hashempur MH, Shams M. Topical Citrullus colocynthis (bitter apple) extract oil in painful diabetic neuropathy: A double-blind randomized placebo-controlled clinical trial. J Diabetes. 2016;8:246–252. doi: 10.1111/1753-0407.12287. [DOI] [PubMed] [Google Scholar]
- 28.A study of the efficacy and safety of amitriptyline/ketamine topical cream in patients with diabetic peripheral neuropathy. ClinicalTrials.gov. 2006 NCT00476151.
- 29.Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21:15. doi: 10.1007/s11916-017-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan G, Shillo P, Selvarajah D, Wu J, Wilkinson ID, Tracey I, Anand P, Tesfaye S. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–191. doi: 10.1016/j.diabres.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, Kalita J, Manna P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 2018;833:472–523. doi: 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Dobretsov M, Hastings SL, Romanovsky D, Stimers JR, Zhang JM. Mechanical hyperalgesia in rat models of systemic and local hyperglycemia. Brain Res. 2003;960:174–183. doi: 10.1016/S0006-8993(02)03828-3. [DOI] [PubMed] [Google Scholar]
- 33.Oyibo SO, Prasad YD, Jackson NJ, Jude EB, Boulton AJ. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: A pilot study. Diabet Med. 2002;19:870–873. doi: 10.1046/j.1464-5491.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 34.Morley GK, Mooradian AD, Levine AS, Morley JE. Mechanism of pain in diabetic peripheral neuropathy. Effect of glucose on pain perception in humans. Am J Med. 1984;77:79–82. doi: 10.1016/0002-9343(84)90439-X. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Cox DJ, Mook DG, McCarty RC. Effect of hyperglycemia on pain threshold in alloxan-diabetic rats. Pain. 1990;40:105–107. doi: 10.1016/0304-3959(90)91057-P. [DOI] [PubMed] [Google Scholar]
- 36.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O'Brien PC. Variables influencing neuropathic endpoints: The rochester diabetic neuropathy study of healthy subjects. Neurology. 1995;45:1115–1121. doi: 10.1212/WNL.45.6.1115. [DOI] [PubMed] [Google Scholar]
- 37.Romanovsky D, Wang J, Al-Chaer ED, Stimers JR, Dobretsov M. Comparison of metabolic and neuropathy profiles of rats with streptozotocin-induced overt and moderate insulinopenia. Neuroscience. 2010;170:337–347. doi: 10.1016/j.neuroscience.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17:461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 39.Babizhayev MA, Strokov IA, Nosikov VV, Savel'Yeva EL, Sitnikov VF, Yegorov YE, Lankin VZ. The role of oxidative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type i diabetic patients. Cell Biochem Biophys. 2015;71:1425–1443. doi: 10.1007/s12013-014-0365-y. [DOI] [PubMed] [Google Scholar]
- 40.Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50:325–392. doi: 10.1016/S0074-7742(02)50082-9. [DOI] [PubMed] [Google Scholar]
- 41.Obrosova IG. How does glucose generate oxidative stress in peripheral nerve Int Rev Neurobiol. 2002;50:3–35. doi: 10.1016/s0074-7742(02)50071-4. [DOI] [PubMed] [Google Scholar]
- 42.Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Schemmel KE, Padiyara RS, D'Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J Diabetes Complications. 2010;24:354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs D, Birklein F, Reeh PW, Sauer SK. Sensitized peripheral nociception in experimental diabetes of the rat. Pain. 2010;151:496–505. doi: 10.1016/j.pain.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Bachewal P, Gundu C, Yerra VG, Kalvala AK, Areti A, Kumar A. Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. Biofactors. 2018;44:109–122. doi: 10.1002/biof.1397. [DOI] [PubMed] [Google Scholar]
- 46.Tobon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol Disord Drug Targets. 2014;13:1615–1626. doi: 10.2174/1871527313666140806144831. [DOI] [PubMed] [Google Scholar]
- 47.Gao X, Wu B, Fu Z, Zhang Z, Xu G. Carvedilol abrogates hypoxia-induced oxidative stress and neuroinflammation in microglial BV2 cells. Eur J Pharmacol. 2017;814:144–150. doi: 10.1016/j.ejphar.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Dworkin RH. An overview of neuropathic pain: Syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–349. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Djouhri L, Fang X, Koutsikou S, Lawson SN. Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias. Pain. 2012;153:1824–1836. doi: 10.1016/j.pain.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aslam A, Singh J, Rajbhandari S. Pathogenesis of painful diabetic neuropathy. Pain Res Treat. 2014;2014:412041. doi: 10.1155/2014/412041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahman MH, Jha MK, Suk K. Evolving insights into the pathophysiology of diabetic neuropathy: Implications of malfunctioning glia and discovery of novel therapeutic targets. Curr Pharm Des. 2016;22:738–757. doi: 10.2174/1381612822666151204001234. [DOI] [PubMed] [Google Scholar]
- 52.Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: An open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25:1663–1676. doi: 10.1185/03007990903047880. [DOI] [PubMed] [Google Scholar]
- 53.Krumova EK, Zeller M, Westermann A, Maier C. Lidocaine patch (5%) produces a selective, but incomplete block of Aδ and C fibers. Pain. 2012;153:273–280. doi: 10.1016/j.pain.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Herrmann DN, Barbano RL, Hart-Gouleau S, Pennella-Vaughan J, Dworkin RH. An open-label study of the lidocaine patch 5% in painful idiopathic sensory polyneuropathy. Pain Med. 2005;6:379–384. doi: 10.1111/j.1526-4637.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 55.Argoff CE, Galer BS, Jensen MP, Oleka N, Gammaitoni AR. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: Assessment with the neuropathic pain scale. Curr Med Res Opin. 2004;20(Suppl 2):S21–S28. doi: 10.1185/030079904X12979. [DOI] [PubMed] [Google Scholar]
- 56.Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61:914–918. doi: 10.1001/archneur.61.6.914. [DOI] [PubMed] [Google Scholar]
- 57.Devers A, Galer BS. Topical lidocaine patch relieves a variety of neuropathic pain conditions: An open-label study. Clin J Pain. 2000;16:205–208. doi: 10.1097/00002508-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): A systematic review. Swiss Med Wkly. 2010;140:297–306. doi: 10.4414/smw.2010.12995. [DOI] [PubMed] [Google Scholar]
- 59.Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: Interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig. 2009;29:231–241. doi: 10.2165/00044011-200929040-00002. [DOI] [PubMed] [Google Scholar]
- 60.Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of combination therapy with 5% lidocaine medicated plaster and pregabalin in post-herpetic neuralgia and diabetic polyneuropathy. Curr Med Res Opin. 2009;25:1677–1687. doi: 10.1185/03007990903047880. [DOI] [PubMed] [Google Scholar]
- 61.White WT, Patel N, Drass M, Nalamachu S. Lidocaine patch 5% with systemic analgesics such as gabapentin: A rational polypharmacy approach for the treatment of chronic pain. Pain Med. 2003;4:321–330. doi: 10.1111/j.1526-4637.2003.03045.x. [DOI] [PubMed] [Google Scholar]
- 62.Ho KY, Huh BK, White WD, Yeh CC, Miller EJ. Topical amitriptyline versus lidocaine in the treatment of neuropathic pain. Clin J Pain. 2008;24:51–55. doi: 10.1097/AJP.0b013e318156db26. [DOI] [PubMed] [Google Scholar]
- 63.Zheng J, Zheng S, Feng Q, Zhang Q, Xiao X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci Rep. 2017;37(pii) doi: 10.1042/BSR20170286. BSR20170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musharraf MU, Ahmad Z, Yaqub Z. Comparison of topical capsaicin and topical turpentine Oil for treatment of painful diabetic neuropathy. J Ayub Med Coll Abbottabad. 2017;29:384–387. [PubMed] [Google Scholar]
- 65.Mitchell K, Bates BD, Keller JM, Lopez M, Scholl L, Navarro J, Madian N, Haspel G, Nemenov MI, Iadarola MJ. Ablation of rat TRPV1-expressing Adelta/C-fibers with resiniferatoxin: Analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol Pain. 2010;6:94. doi: 10.1186/1744-8069-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fattori V, Hohmann MS, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21(pii):E844. doi: 10.3390/molecules21070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.What role for capsaicin in diabetic peripheral neuropathy? Drug Ther Bull. 2016;54:90–93. doi: 10.1136/dtb.2016.8.0417. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg CJ, Watson JC. Treatment of painful diabetic peripheral neuropathy. Prosthet Orthot Int. 2015;39:17–28. doi: 10.1177/0309364614542266. [DOI] [PubMed] [Google Scholar]
- 69.Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. The Capsaicin Study Group. Arch Intern Med. 1991;151:2225–2229. doi: 10.1001/archinte.1991.00400110079017. [DOI] [PubMed] [Google Scholar]
- 70.Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. The Capsaicin Study Group. Diabetes Care. 1992;15:159–165. doi: 10.2337/diacare.15.2.159. [DOI] [PubMed] [Google Scholar]
- 71.Biesbroeck R, Bril V, Hollander P, Kabadi U, Schwartz S, Singh SP, Ward WK, Bernstein JE. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Adv Ther. 1995;12:111–120. [PubMed] [Google Scholar]
- 72.Tandan R, Lewis GA, Badger GB, Fries TJ. Topical capsaicin in painful diabetic neuropathy. Effect on sensory function. Diabetes Care. 1992;15:15–18. doi: 10.2337/diacare.15.1.8. [DOI] [PubMed] [Google Scholar]
- 73.Tandan R, Lewis GA, Krusinski PB, Badger GB, Fries TJ. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care. 1992;15:8–14. doi: 10.2337/diacare.15.1.8. [DOI] [PubMed] [Google Scholar]
- 74.Chad DA, Aronin N, Lundstrom R, McKeon P, Ross D, Molitch M, Schipper HM, Stall G, Dyess E, Tarsy D. Does capsaicin relieve the pain of diabetic neuropathy? Pain. 1990;42:387–388. doi: 10.1016/0304-3959(90)91153-A. [DOI] [PubMed] [Google Scholar]
- 75.Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: A randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497–503. doi: 10.1111/papr.12013. [DOI] [PubMed] [Google Scholar]
- 76.Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, Schregardus DS, Long SK, Lambourg B, Katz N. Capsaicin 8% patch in painful diabetic peripheral neuropathy: A randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42–53. doi: 10.1016/j.jpain.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Vinik AI, Perrot S, Vinik EJ, Pazdera L, Jacobs H, Stoker M, Long SK, Snijder RJ, van der Stoep M, Ortega E, Katz N. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: A randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16:251. doi: 10.1186/s12883-016-0752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American academy of neurology, the American association of neuromuscular and electrodiagnostic medicine and the American academy of physical medicine and rehabilitation. PM R. 2011;3:345–352. doi: 10.1016/j.pmrj.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch CR III. Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998;284:208–214. [PubMed] [Google Scholar]
- 80.Nicholson GM, Blanche T, Mansfield K, Tran Y. Differential blockade of neuronal voltage-gated Na(+) and K(+) channels by antidepressant drugs. Eur J Pharmacol. 2002;452:35–48. doi: 10.1016/S0014-2999(02)02239-2. [DOI] [PubMed] [Google Scholar]
- 81.Joshi PG, Singh A, Ravichandra B. High concentrations of tricyclic antidepressants increase intracellular Ca2+ in cultured neural cells. Neurochem Res. 1999;24:391–398. doi: 10.1023/A:1020937717260. [DOI] [PubMed] [Google Scholar]
- 82.Watson CP. The treatment of neuropathic pain: Antidepressants and opioids. Clin J Pain. 2000;16(2 Suppl):S49–S55. doi: 10.1097/00002508-200006001-00009. [DOI] [PubMed] [Google Scholar]
- 83.Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: Two cases and literature review. Pain Pract. 2012;12:148–153. doi: 10.1111/j.1533-2500.2011.00477.x. [DOI] [PubMed] [Google Scholar]
- 84.Zeraatian S, Zakeri H, Boroojeny SB, Hourang MH, Ghaffarpasand F, Fard MM. Effect of oral clonidine on acute intraocular pressure rise after phacoemulsification: A prospective double-blind, randomized, clinical trial. J Ocul Pharmacol Ther. 2011;27:293–297. doi: 10.1089/jop.2010.0154. [DOI] [PubMed] [Google Scholar]
- 85.Otasowie J, Castells X, Ehimare UP, Smith CH. Tricyclic antidepressants for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2014;9:CD006997. doi: 10.1002/14651858.CD006997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sawynok J. Topical analgesics for neuropathic pain: Preclinical exploration, clinical validation, future development. Eur J Pain. 2014;18:465–481. doi: 10.1002/j.1532-2149.2013.00400.x. [DOI] [PubMed] [Google Scholar]
- 87.Campbell C, Campbell J, Schmidt W, Brady K, Stouch B. Topical clonidine gel reduces pain caused by diabetic neuropathy: Results of a multicenter, placebo-controlled clinical trial. J Pain. 2009;10(Suppl):S55. doi: 10.1016/j.jpain.2009.01.293. [DOI] [Google Scholar]
- 88.Campbell CM, Kipnes MS, Stouch BC, Brady KL, Kelly M, Schmidt WK, Petersen KL, Rowbotham MC, Campbell JN. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815–1823. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grover JK, Khandkar S, Vats V, Dhunnoo Y, Das D. Pharmacological studies on myristica fragrans-antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24:675–680. doi: 10.1358/mf.2002.24.10.802317. [DOI] [PubMed] [Google Scholar]
- 90.Hayfaa AA, Sahar AM, Awatif MA. Evaluation of analgesic activity and toxicity of alkaloids in myristica fragrans seeds in mice. J Pain Res. 2013;6:611–615. doi: 10.2147/JPR.S45591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forouzanfar F, Hosseinzadeh H. Medicinal herbs in the treatment of neuropathic pain: A review. Iran J Basic Med Sci. 2018;21:347–358. doi: 10.22038/IJBMS.2018.24026.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramanathan T, Gurudeeban S, Satyavani K. Local anesthetic effect of citrullus colocynthis on rana hexadactyla. Res J Med Plant. 2011;5:338–342. doi: 10.3923/rjmp.2011.338.342. [DOI] [Google Scholar]
- 93.Kumar S, Kumar D, Manjusha, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of citrullus colocynthis (L.) schrad. Methanolic fruit extract. Acta Pharm. 2008;58:215–220. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 94.Reddy VP, Sudheshna G, Shaik A, Saran SS, Kumar SN, Ram CR, Reddy KR. Evaluation of antiulcer activity of Citrullus colocynthis fruit against pylorus ligation induced ulcers in male Wistar rats. Int J Pharm Pharm Sci. 2012;4:446–451. [Google Scholar]
- 95.Nedjat S, Montazeri A, Holakouie K, Mohammad K, Majdzadeh R. Psychometric properties of the Iranian interview-administered version of the world health organization's quality of life questionnaire (WHOQOL-BREF): A population-based study. BMC Health Serv Res. 2008;8:61. doi: 10.1186/1472-6963-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: A randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005;103:140–146. doi: 10.1097/00000542-200507000-00021. [DOI] [PubMed] [Google Scholar]
- 97.Hocking G, Cousins MJ. Ketamine in chronic pain management: An evidence-based review. Anesth Analg. 2003;97:1730–1739. doi: 10.1213/01.ANE.0000086618.28845.9B. [DOI] [PubMed] [Google Scholar]
- 98.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: Risks and benefits. Br J Clin Pharmacol. 2014;77:357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mion G, Villevieille T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings) Cns Neurosci Ther. 2013;19:370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahoney JM, Vardaxis V, Moore JL, Hall AM, Haffner KE, Peterson MC. Topical ketamine cream in the treatment of painful diabetic neuropathy: A randomized, placebo-controlled, double-blind initial study. J Am Podiatr Med Assoc. 2012;102:178–183. doi: 10.7547/1020178. [DOI] [PubMed] [Google Scholar]
- 101.Lynch ME, Clark AJ, Sawynok J. A pilot study examining topical amitriptyline, ketamine, and a combination of both in the treatment of neuropathic pain. Clin J Pain. 2003;19:323–328. doi: 10.1097/00002508-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neuropathic pain syndromes: An open-label study. J Pain. 2005;6:644–649. doi: 10.1016/j.jpain.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Somberg JC, Molnar J. Retrospective study on the analgesic activity of a topical (TT-CTAC) cream in patients with diabetic neuropathy and other chronic pain conditions. Am J Ther. 2015;22:214–221. doi: 10.1097/MJT.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 104.Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6:15–28. doi: 10.1177/2040622314552071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med. 2013;125(4 Suppl):S25–S33. doi: 10.1080/00325481.2013.1110567111. [DOI] [PubMed] [Google Scholar]
- 106.Barkin RL. The pharmacology of topical analgesics. Postgrad Med. 2013;125(4 Suppl 1):S7–S18. doi: 10.1080/00325481.2013.1110566911. [DOI] [PubMed] [Google Scholar]
- 107.Pickering G, Martin E, Tiberghien F, Delorme C, Mick G. Localized neuropathic pain: An expert consensus on local treatments. Drug Des Devel Ther. 2017;11:2709–2718. doi: 10.2147/DDDT.S142630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


