Abstract
Resveratrol, a natural polyphenolic phytoalexin, was reported to exert multiple anticancer effects as a traditional Chinese medicine. However, research regarding the anticancer mechanism of resveratrol for the treatment and prevention of gastric cancer has reported conflicting results. In the present study, it was determined that resveratrol inhibited cell viability in a dose-dependent manner in the human gastric cancer cell line BGC823. Cell migration and invasion were suppressed significantly following treatment with 200 µM resveratrol. Additionally, resveratrol inhibited metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) expression, which was overexpressed in gastric cancer cells. Further experiments revealed that MALAT1 knockdown suppressed cell viability, migration, invasion and epithelial-to-mesenchymal transition in BGC823 cells. The present study indicated that resveratrol inhibited migration and invasion in human gastric cancer cells via suppressing MALAT1-mediated epithelial-to-mesenchymal transition, providing novel evidence for understanding the anticancer mechanism of resveratrol.
Keywords: resveratrol, invasion, migration, metastasis-associated lung adenocarcinoma transcript 1, epithelial-to-mesenchymal transition, gastric cancer
Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a stilbenoid belonging to the stilbene family, was first reported by Takaoka in 1939 (1). It is a natural polyphenolic phytoalexin produced by a number of plants, including Arachis hypogaea Linn. and Vitis vinifera Linn., in response to fungal infection (2). Sources of resveratrol include food, such as the skin of grapes and red wine (3,4). Resveratrol is a natural compound indicated to have beneficial health effects, particularly anticancer effects in humans (5,6). In 1997, Jang et al (5) determined its chemopreventive properties, including antioxidant, antimutagen, anti-inflammatory and anti-progression activity in a number of disease models, including cancer. Subsequently, numerous studies revealed that resveratrol exhibits multiple anticancer effects, preventing tumor formation in different cancer types, including leukemia, breast cancer, skin tumor, colorectal cancer and liver cancer (6–12).
Gastric cancer was the fifth most common cancer globally, with almost 951,000 new cases occurring (6.8% of the total), causing an estimated 723,000 cancer-associated mortalities in 2012, becoming the third leading cause of cancer-associated mortality (13). Of gastric cancer cases, >70% were estimated to occur in developing countries and half of the total new cases occurred in China in 2012 (13). The estimated mortality rates are notably high in Eastern Asia (14.0/100,000 in males and 9.8/100,000 in females), but low in Northern America (2.8/100,000 in males and 1.5/100,000) (13). In the clinic, surgery, chemotherapy and radiotherapy are the primary treatment options for gastric cancer (14–16). Resveratrol is a polyphenol compound used in traditional Chinese medicine and has beneficial effects as a cancer chemopreventive agent in humans (5–7); however, there are limited studies focused on the action of resveratrol regarding the treatment and prevention of gastric cancer, and the anticancer mechanism of resveratrol remains unclear.
In the present study, the effects of resveratrol on gastric cancer cell line BGC823, the underlying mechanisms of the involvement of resveratrol and the role of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in epithelial-to-mesenchymal transition were investigated.
Materials and methods
Cell culture
Human gastric cancer cell lines SGC7901 and BGC823 were purchased from Cell-Land Biotech Co., Ltd. (Hangzhou, China; www.cell-land.com). Non-malignant gastric epithelium cell line GES1 was obtained from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). All cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and maintained at 37°C in an atmosphere containing 5% CO2 with saturated humidity in a humidified cell incubator (Thermo Fisher Scientific, Inc.). The cells were collected during their logarithmic phase and stored at −80°C for further study.
RNA interference
MALAT1 siRNA and negative control siRNA (siNC) were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The following sequences were used in the present study: siRNA-1, sense, 5′-GCAAAUGAAAGCUACCAAU-3′ and antisense 5′-AUUGGUAGCUUUCAUUUGC-3′; siRNA-2, sense, 5′-CUAGAAUCCUAAAGGCAAA-3′ and antisense, 5′-UUUGCCUUUAGGAUUCUAG-3′; and siNC, sense, 5′-UUCUCCGAACGUGUCACGU-3′ and anti-sense, 5′-ACGUGACACGUUCGGAGAA-3′. A total of 2 ml BGC823 cells (8×104 ells/ml) were plated onto 6 well plates and grown overnight at 37°C in an atmosphere containing 5% CO2 in a humidified cell incubator. Cell transfections were performed with the Lipofectamine RNAiMAX Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The final siRNA oligonucleotide concentration was 20 pM. Following 24, 48, 72 or 96 h of incubation, the transfected cells were harvested to be used in other experiments. Cell transfected with siNC were used as the control.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol® reagent and an RNA Fast Mini kit (cat. no. GK3016; Generay Biotech Co., Ltd., Shanghai, China). cDNA was synthesized using a RevertAid First Strand cDNA synthesis kit (cat. no. K1622; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. RT-qPCR was performed using a CFX connect Real-Time PCR system with SsoAdvance Universal SYBR® Green Supermix (cat. no. 172-5274; both Bio-Rad Laboratories, Inc., Hercules, CA, USA) in the following cycling conditions: 95°C denaturation for 3 min; 40 cycles of denaturation at 95°C for 15 sec, annealing at 59°C for 30 sec and extension at 72°C for 30 sec. GAPDH was used as a reference gene and all reactions were performed in triplicate. The following primers were used in the current study: Long non-coding RNA (lncRNA) MALAT1 (Gene ID, 378938; www.ncbi.nlm.nih.gov), forward, 5′-ATACCTAACCAGGCATAAC-3′, and reverse, 5′-GTAGACCAACTAAGCGAAT-3′; GAPDH (Gene ID: 2597), forward, 5′-CGGATTTGGTCGTATTG-3′; and reverse, 5′-GAAGATGGTGATGGGATT-3′; E-cadherin (Gene ID, 999), forward, 5′-CGCATTGCCACATACAC-3′, and reverse, 5′-CCTTCCATGACAGACCC-3′; and vimentin (Gene ID, 7431), forward, 5′-TTGAACGCAAAGTGGAATC-3′, and reverse, 5′-AGGTCAGGCTTGGAAACA-3′. The primers were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). Relative levels of each RNA were calculated using Bio-Rad CFX Manager software (version 3.1; Bio-Rad Laboratories, Inc.) (17).
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay was performed to determine the viability of the gastric cancer cells according to the manufacturer's protocol (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Firstly, 100 µl cell suspensions (3×104 cells/well) were seeded in a 96-well plate and pre-incubated for 24 h at 37°C in a humidified incubator. Secondly, a series of various concentrations of resveratrol (0, 5, 10, 25, 50, 100, 200 and 400 µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were added to the plate (100 µl/well) and were then pre-incubated for various periods of time (24, 48 and 72 h) at 37°C. In another experiment to assess the effect of small interfering (siRNA)-mediated MALAT1 knockdown on cell viability, cells were transfected with siRNA-1 for 24, 48, 72 and 96 h, respectively. Subsequently, 10 µl CCK-8 solution was added to each well followed by incubation at 37°C for 1 h with protection from light. All experiments were performed in triplicate. The absorbance was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.) and presented as the mean optical density (OD). The percentage of viable cells=OD of the treatment group/OD of the control group × 100%.
Cell invasion and migration assay
BGC823 cells (3×105 cells/well) were cultured in a humidified incubator in RPMI-1640 medium with 10% FBS at 37°C in an atmosphere containing 5% CO2 for 24 h. A total of 200 µM resveratrol was added to the cells, followed by incubation for 48 h at 37°C. The treated cells were digested with 0.02% EDTA supplemented with 0.25% trypsin and collected for trypan blue staining to count the cells at 37°C. For invasion analysis, 100 µl Matrigel (BD Biosciences, San Jose, CA, USA) was firstly added to the bottom of the Transwell chamber (8 µm) prior to BGC823 cells being seeded. RPMI-1640 medium with 10% FBS were plated in the lower chamber. Subsequently, 2×104 cells were placed into the upper chamber with RPMI-1640 medium and were allowed to invade for 24 h. Following this, the Transwell chambers were removed, and non-invading cells were removed from the upper chamber with a cotton swab. The upper chamber was then washed with PBS. Subsequently, cells of the upper chamber were fixed and stained with 0.1% crystal violet staining solution (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. The cells were counted and imaged under an ECLIPSE Ti-S microscope (Nikon Corporation, Tokyo, Japan) at a magnification of ×100.
For the cell migration assay, cells (5×104 cells/well) were plated in the upper chamber with RPMI-1640 medium, whereas the bottom chamber was filled with 600 µl RPMI-1640 medium supplemented with 10% FBS. Following incubation for 16 h, the cells were stained with crystal violet staining solution for 30 min at 37°C (Sigma-Aldrich; Merck KGaA), counted and imaged under an ECLIPSE Ti-S microscope. The cells without resveratrol (Mock) were used as the control group.
Western blot analysis
Cell lysates were prepared for western blot analysis as previously described (7). The samples were analyzed with a standard bicinchoninic acid (BCA) assay for protein concentration using a BCA Protein Assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Total protein (30 µg) was loaded per lane, separated by SDS-PAGE on a 12.5% gel and then all protein samples were transferred to 0.45-µm Immobilon® polyvinylidene difluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). For western blots, the PVDF membrane was blocked with TBS-T buffer containing 5% (w/v) nonfat dry milk, slowly agitated on a shaker and sealed at room temperature for 2 h. The primary antibodies [dilution, 1:1,000 (v/v)] used in the present study included E-cadherin (cat. no. 3195), vimentin (cat. no. 5741) and GAPDH (cat. no. 5174), which were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). A horseradish peroxidase-conjugated secondary antibody [goat anti-rabbit immunoglobulin G; cat. no. BL003A; 1:2,000 (v/v); Biosharp, Inc., Hefei, China] was incubated at room temperature for 2 h. GAPDH was used as the control. The signals were visualized with a Clarity™ Western ECL Substrate (cat. no. 170-5060; Bio-Rad Laboratories, Inc.) and detected with a ChemiDoc™ Touch Imaging system (version 5.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation. The mean values of the control and treatment groups were compared using one-way analysis of variance and Fisher's least significant difference tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Resveratrol inhibits viability of human gastric cancer cells
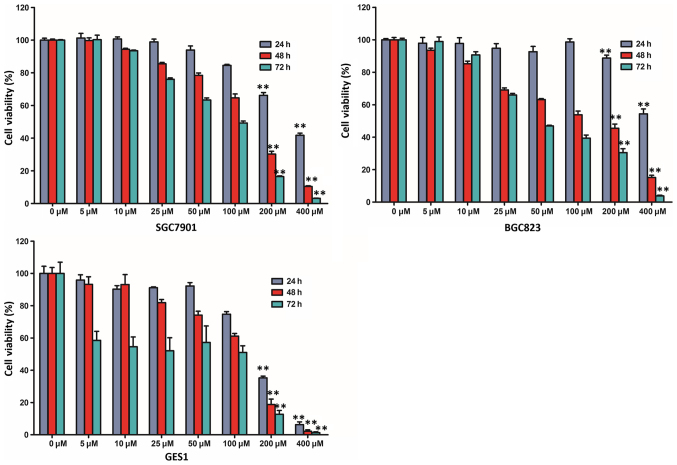
In order to assess the effect of resveratrol on cell viability, a cell viability assay was performed using two human gastric cancer cell lines (SGC7901 and BGC823) and a non-malignant gastric epithelium cell line (GES1). BGC823, SGC7901 and GES1 cells were treated with various doses of resveratrol (0, 5, 10, 25, 50, 100, 200 and 400 µM) and examined with a CCK-8 assay. The viability of the three cell lines was markedly reduced following treatment with resveratrol in a time- and dose-dependent manner (Fig. 1). The CCK-8 assay indicated that the viability of BGC823 and SGC7901 cells incubated with 200 or 400 µM resveratrol for 48 and 72 h was markedly lower than those incubated for 24 h. In addition, compared with the untreated control group, treatment with 200 µM resveratrol for 48 h reduced the viability of BGC823 cells by >50% (45.48±2.61%; P<0.01). The half maximal inhibitory concentration (IC50 value) was closest to 200 µM following treatment with resveratrol for 48 h, and, therefore, 200 µM resveratrol administration was selected for further study, according to the cell viability assay.
Figure 1.
Resveratrol inhibits the viability of gastric cancer cells in a dose- and time-dependent manner. BGC823, SGC7901 and GES1 cells were treated with various doses of resveratrol (0, 5, 10, 25, 50, 100, 200 and 400 µM) for differing treatment times (24, 48 and 72 h). The cell viability was subsequently examined with a Cell Counting Kit-8 assay. **P<0.01 compared with the control group treated with 0 µM resveratrol for different treatment times.
Resveratrol inhibits migration and invasion of BGC823 cells
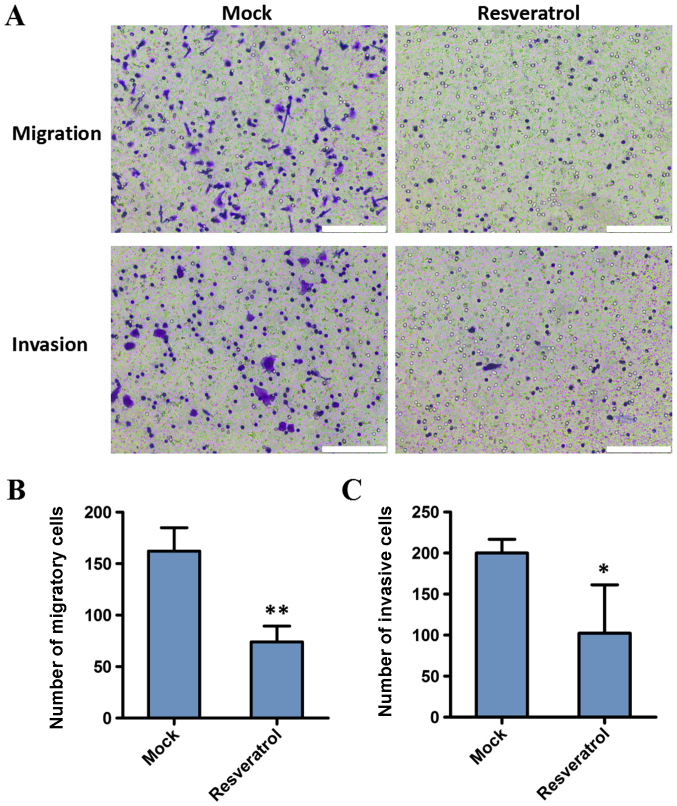
The present study investigated the effect of resveratrol on the migration and invasion capacities of BGC823 cells. Cell invasion and migration assays were conducted following treatment with 200 µM resveratrol for 48 h. The results demonstrated that the migration and invasion decreased in BGC823 cells following administration of resveratrol, compared with the control (162.25±22.60 vs. 74.00±15.38, P<0.01; and 200.00±16.70 vs. 102.33±58.97, P<0.05, respectively). These results indicated that the invasion and migration abilities of gastric cancer cells were significantly inhibited by resveratrol (Fig. 2).
Figure 2.
Resveratrol inhibits the migration and invasion of BGC823 cells, as demonstrated with Transwell and Matrigel assays. (A) Assessment of cell migration and invasion in BGC823 cells following treatment with or without resveratrol for 48 h. Scale bar=10 µm. The number of (B) migratory and (C) invasive cells was analyzed quantitatively. The values are presented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 compared with the mock group. Mock, group untreated with resveratrol.
Resveratrol suppresses epithelial-to-mesenchymal transition
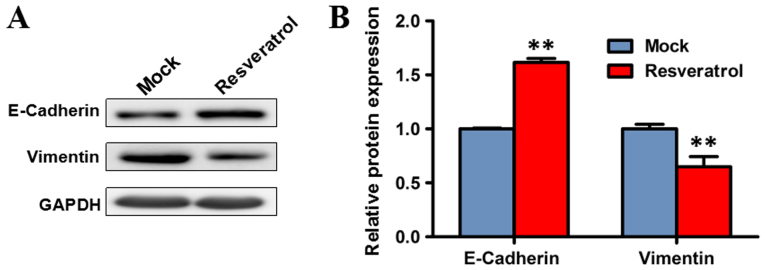
Since epithelial-to-mesenchymal transition is an important process facilitating tumor invasion and metastasis (18,19), the effect of resveratrol on epithelial-to-mesenchymal was investigated. Western blot analysis was performed to evaluate the expression levels of markers of epithelial-to-mesenchymal transition, including E-cadherin and vimentin, following treatment with resveratrol. The expression level of vimentin significantly decreased in BGC823 cells treated with 200 µM resveratrol for 48 h, compared with the mock group (P<0.01), while the protein expression of E-cadherin was significantly increased (P<0.01). These results indicated that resveratrol may suppress epithelial-to-mesenchymal transition (Fig. 3).
Figure 3.
Expression levels of epithelial-to-mesenchymal transition marker proteins, including E-cadherin and vimentin in BGC823 cells treated with resveratrol for 48 h. (A) Western blot analysis of protein expression. (B) Quantitative analysis of the relative grey value of western blot bands. Data are represented as the mean ± standard deviation of three independent experiments. GAPDH was used as the reference gene. **P<0.01 compared with the mock group. Mock, group untreated with resveratrol.
Resveratrol inhibits the expression of MALAT1
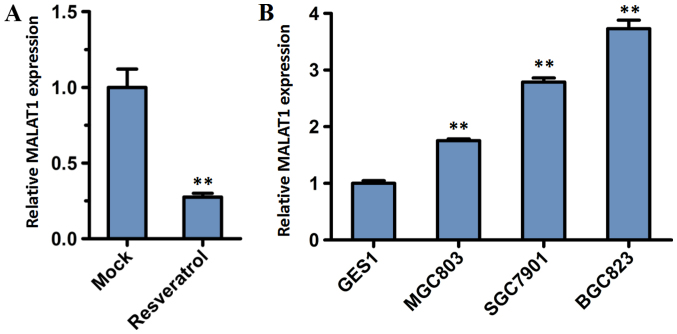
MALAT1 was reported as a prognostic biomarker of gastric cancer, and had significantly higher expression in gastric cancer tissues of patients with distant metastasis compared with adjacent normal tissues of those patients and gastric cancer patients without distant metastasis (20). To determine whether resveratrol exhibits any prognostic significance in gastric cancer cells through the role of MALAT1, the expression level of lncRNA MALAT1 was analyzed. BGC823 cells were incubated for 48 h with or without 200 µM resveratrol. RT-qPCR analysis demonstrated that the relative expression of lncRNA MALAT1 in BGC823 cells decreased significantly following treatment with resveratrol, compared with the mock group (P<0.01; Fig. 4A).
Figure 4.
Expression levels of MALAT1 were analyzed by reverse transcription-quantitative polymerase chain reaction. (A) The expression of MALAT1 in BGC823 cells treated with resveratrol for 48 h. (B) The expression of MALAT1 in gastric cancer cell lines and a non-malignant gastric epithelium cell line. Data are represented as the mean ± standard deviation of three independent experiments. GAPDH was used as the reference gene. **P<0.01 compared with the Mock group or GES1 cells. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; Mock, group untreated with resveratrol.
MALAT1 is overexpressed in gastric cancer cells
To investigate the lncRNA MALAT1 expression in tumor cells, a normal cell line (GES1) and three GC cell lines (BGC823, MGC803 and SGC7901) were subjected to RT-qPCR analysis. The expression level of MALAT1 increased in the BGC823, MGC803 and SGC7901 cell lines compared with the normal GES1 cell line (Fig. 4B). Furthermore, the expression level of MALAT1 was highest in BGC823 cells.
MALAT1 knockdown suppresses the viability of BGC823 cells
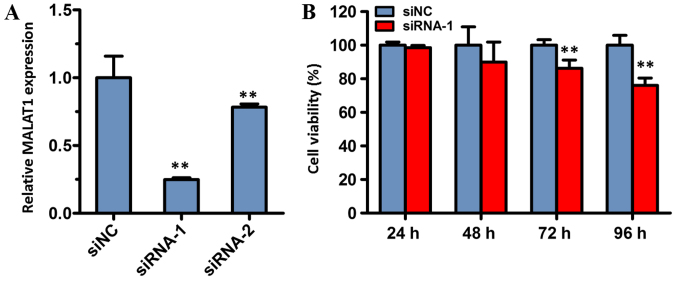
To determine the involvement of MALAT1 in the biological function of gastric cancer, MALAT1 was knocked down in the present study. Firstly, two individual siRNA oligonucleotides of MALAT1 (siRNA-1 and siRNA-2) were synthesized and transfected into BGC823 cells. The results demonstrated that the expression level of MALAT1 in cells transfected with siRNA-1 or siRNA-2 was significantly reduced compared with cells transfected with siNC (both P<0.01; Fig. 5A). The expression of MALAT1 was the lowest in the siRNA-1 group, and, therefore, this group was used in the subsequent experiments.
Figure 5.
Effect of MALAT1 knockdown on cell viability. (A) Efficiency of MALAT1 knockdown was evaluated with reverse transcription-quantitative polymerase chain reaction. (B) Cell viability following MALAT1 knockdown was measured with a Cell Counting Kit-8 assay in BGC823 cells. Cell transfection with siNC was used as the control. GAPDH was used as the reference gene. Data are represented as the mean ± standard deviation of three independent experiments. **P<0.01 compared with the siNC group. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; siNC, negative control small-interfering RNA; siRNA-1 and −2, small interfering RNA targeting MALAT1.
To assess the effect of siRNA-mediated MALAT1 knockdown on the viability of cancer cells, the viability of BGC823 cells was determined with a CCK-8 assay. The results of the CCK-8 assay revealed that cell viability of the siRNA-1 treatment group was significantly decreased compared with the siNC group, following 72 and 96 h of incubation. However, there was no significant difference between the siRNA-1 group and the control group following transfection for 24 or 48 h. These data indicated that MALAT1 may serve a role in cell viability.
Effect of MALAT1 knockdown on the migration and invasion of gastric cancer cells
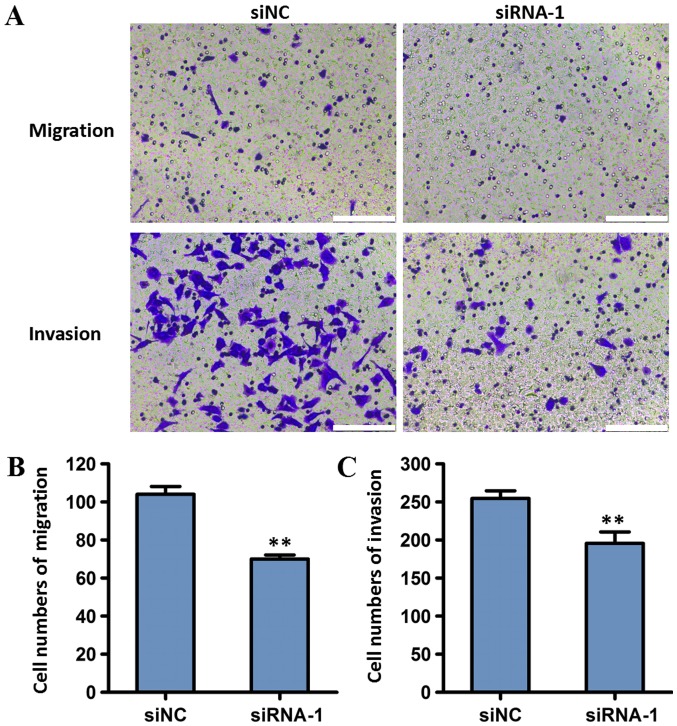
To investigate the effect of MALAT1 on the invasion and migration of tumor cells, BGC823 cells transfected with MALAT1 siRNA-1 were used in transwell migration and Matrigel invasion assays. Compared with the siNC group, cells transfected with siRNA-1 exhibited decreased invasion and migration (P<0.01; Fig. 6). These results indicated that MALAT1 knockdown may reduce the migration and invasion of gastric cancer cells.
Figure 6.
Effects of MALAT1 interference on the invasion and migration of BGC823 cells. (A) Transwell and Matrigel assays demonstrated that MALAT1 knockdown suppressed the migration and invasion of gastric cancer cells. Scale bar=10 µm. Quantitative analysis of the number of (B) migratory and (C) invasive cells. Cell transfection with siNC was used as the control. Data are represented as the mean ± standard deviation of three independent experiments. **P<0.01 compared with the siNC group. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; siNC, negative control small-interfering RNA; siRNA-1, small interfering RNA targeting MALAT1.
MALAT1 enhances epithelial-to-mesenchymal transition in BGC823 cells
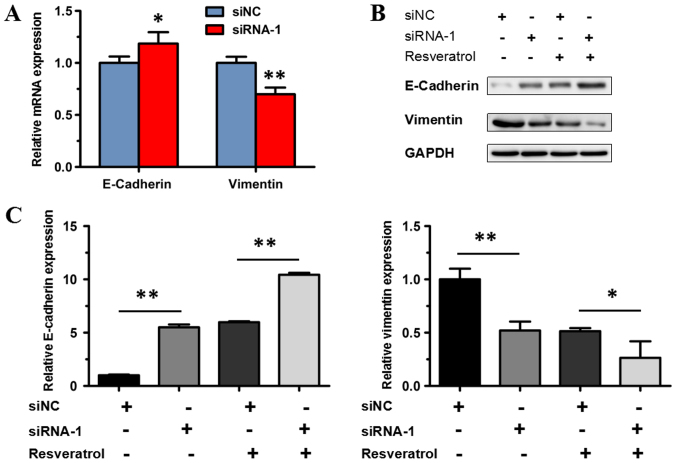
Previous studies indicated that epithelial-to-mesenchymal transition serves an important role in tumor invasion and metastasis (17,18). Therefore, the potential effect of MALAT1 on epithelial-to-mesenchymal transition was investigated in the present study. siRNA-1 was used to knock down the expression of MALAT1 in the transfected BGC823 cells. RT-qPCR and western blot analysis were used to detect E-cadherin and vimentin expression in BGC823 cells treated with siRNA-1. mRNA expression of E-cadherin increased significantly in BGC823 cells transfected with siRNA-1, compared with the siNC group (P<0.05); while the expression of vimentin significantly decreased (P<0.01; Fig. 7A). Western blot analysis revealed that protein expression of E-cadherin increased significantly in BGC823 cells transfected with siRNA-1 compared with the siNC group (P<0.01), while the expression of vimentin decreased significantly (P<0.01; Fig. 7B and C).
Figure 7.
Effects of MALAT1 knockdown on epithelial-to-mesenchymal transition of BGC823 cells. (A) Reverse transcription-quantitative polymerase chain reaction was performed to test the mRNA expression levels of E-cadherin and vimentin. Cell transfection with siNC was used as the control. GAPDH was used as the reference gene. Data are represented as the mean ± standard deviation of three independent experiments. *P<0.05 and **P<0.01 compared with the siNC group. (B) Expression of epithelial-to-mesenchymal transition-associated proteins, including E-cadherin and vimentin, in BGC823 cells transfected with siRNA-1/siNC and treated or untreated with resveratrol. (C) Relative grey value analysis of protein expression levels of E-cadherin and vimentin. GAPDH was used as the loading control. *P<0.05 and **P<0.01 as indicated. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; siNC, negative control small-interfering RNA; siRNA-1, small interfering RNA targeting MALAT1.
In order to investigate whether MALAT1 knockdown abolishes the effect of resveratrol on epithelial-to-mesenchymal transition, resveratrol was administered to BGC823 cells transfected with siRNA-1. The protein expression of vimentin decreased significantly (P<0.05), and the protein expression of E-cadherin increased significantly in BGC823 cells transfected with siRNA-1 following treatment with 200 µM resveratrol, compared with the control (the siNC group treated with resveratrol; P<0.01; Fig. 7B and C). The aforementioned result indicated that transfection with siRNA-1 could not abolish the effect of resveratrol, and, therefore, resveratrol may suppress MALAT1-mediated epithelial-to-mesenchymal transition. These results demonstrated that downregulated expression of MALAT1 inhibited epithelial-to-mesenchymal transition in BGC823 cells, indicating that MALAT1 may enhance epithelial-to-mesenchymal transition in gastric cancer cells.
Discussion
lncRNAs are >200 nucleotides in length and function in various aspects of cell biological processes, including proliferation, apoptosis, invasion, metastasis and epithelial-to-mesenchymal transition, by transcriptional and post-transcriptional regulation of gene expression or epigenetic regulation (21,22). A number of lncRNAs, including H19, HOX antisense intergenic RNA and gastric carcinoma high expressed transcript 1, were upregulated in different gastric cancer cell lines (23–26). Furthermore, MALAT1, also known as nuclear-enriched abundant transcript 2, is a frequently expressed lncRNA that has been associated with metastatic potential and poor prognosis in lung adenocarcinoma (19). Numerous studies have demonstrated that MALAT1 is associated with various tumor types, including lung cancer, hepatocellular carcinoma, prostate cancer, gallbladder cancer, bladder cancer, colorectal cancer, renal cell carcinoma and pancreatic cancer (27–36). Additionally, a number of reports also provided evidence for the biological and clinical significance of MALAT1 expression as a prognostic biomarker of gastric cancer (20,37–39). Xia et al (20) and Okugawa et al (37) revealed that the tissue MALAT1 expression level was significantly increased in gastric cancer tissues, compared with normal mucosa, and high expression of MALAT1 was independently associated with a poor prognosis for patients with gastric cancer. The study by Wang et al (38) demonstrated that MALAT1 expression was notably increased in gastric cancer cell lines, compared with the levels in GES1 cells (38). In the present study, it was determined that the expression level of MALAT1 increased in gastric cancer cells lines BGC823, MGC803 and SGC7901, compared with normal gastric epithelial cell line GES1, and the expression of MALAT1 was highest in BGC823 cells. The present results were supported by the studies by Wang et al (38) and Li et al (39), but partly conflicted with data reported by Xia et al (20), whose study indicated that the expression level of MALAT1 was increased in gastric cancer cell lines MKN45, CTC105 and CTC141, but lower in SGC7901 and BGC823, compared with GES1 cells (17). Therefore, further studies are necessary to investigate the expression of MALAT1 in various tumor cells.
MALAT1 is a potential biomarker of gastric cancer (19). Functional studies have demonstrated that the knockdown of MALAT1 could suppress cell viability, migration and invasion in gastric cancer cell lines MKN45, CTC141, SGC996 and NOZ (20,40). Another study indicated that mice administered with MALAT1-depleted BGC823 or NOZ cells exhibited a reduced degree of lung metastasis compared with mice treated with wild type BGC823 and NOZ cell lines (39). The current study revealed that MALAT1 knockdown notably suppressed the viability, migration and invasion of gastric cancer BGC823 cells, indicating the potential function of MALAT1 in promoting metastasis.
Epithelial-to-mesenchymal transition is an important process for human in wound healing, organ fibrosis and initiation of metastasis for cancer progression (18). When epithelial-to-mesenchymal transition occurs, epithelial cells lose polarity and relinquish cell-cell adhesion to gain migratory and invasive properties and become mesenchymal stem cells (40,41). MALAT1 induces epithelial-to-mesenchymal transition in multiple cancer types, including tongue cancer, cervical cancer, lung adenocarcinoma and oral squamous cell carcinoma (42–45). The present results also revealed that MALAT1 enhanced epithelial-to-mesenchymal transition in gastric cancer BGC823 cells. These results indicated that MALAT1 can promote tumor growth and metastasis by inducing epithelial-to-mesenchymal transition in various cancer types.
Previous studies have demonstrated beneficial effects of resveratrol in preventing and treating cancer (46–48). In the present study, it was determined that resveratrol inhibited cell viability in a dose-dependent manner in human gastric cancer BGC823 cells and suppressed cell migration and invasion. Notably, the IC50 value of resveratrol was high (~200 µM) in gastric cancer BGC823 cells after 48 h and resveratrol caused cytotoxicity to normal gastric GES1 cells. Additionally, this similar concentration of resveratrol in gastric cancer cells has been reported in other published articles. Using an MTT assay, Gao et al (49) determined that the viability of SGC7901 cells was inhibited by ~80% when they were exposed to 200 µM resveratrol for 48 h. Jing et al (50) obtained similar results using an MTT assay and trypan blue staining, with >80% of MGC803 cell growth being inhibited after 24 and 48 h of exposure to 200 µM resveratrol and the IC50 of resveratrol for MGC803 cells was 127 µM. Yang et al (51) determined that in three gastric cancer cell lines (AGS, BGC823 and SGC7901), 100 mM resveratrol was sufficient to induce >50% growth inhibition following treatment for 24 h (56.78% for AGS; 54.87% for BGC-823; and 52.16% for SGC7901) using an MTS assay. The present results demonstrated a similar growth inhibition rate of SGC7901 using a CCK-8 assay, as well as ~53.8% reduction in the cell viability of the gastric cancer cell line BGC823. The differences in doses of resveratrol in previous studies may be due to different methods and gastric cell lines used.
The underlying mechanism of resveratrol varies in different tumor types. In prostate cancer, the beneficial effect of resveratrol was due to inhibition of the protein kinase B (Akt)/microRNA-21 signaling pathway (52). The study of colorectal cancer indicated that resveratrol inhibited cell invasion and metastasis through regulating the MALAT1 and Wnt/β-catenin signaling pathways (34). Another report revealed that resveratrol inhibited the progression of the cell cycle by inhibiting the activity of phosphatase and tensin homolog, which suppresses the phosphoinositide 3-kinase/Akt pathway in MGC803 cells (50). Furthermore, resveratrol could suppress invasion and metastasis and inhibit the hedgehog signaling pathway in gastric cancer SGC7901 cells (49). Therefore, the anticancer mechanism of resveratrol remains unclear. In the present study, it was determined that resveratrol exerted an anticancer function by suppressing MALAT1-mediated epithelial-to-mesenchymal transition in human gastric cancer cells. Additionally, the present study provided novel evidence to understand the anticancer mechanism of resveratrol.
In conclusion, the present study determined that resveratrol decreased MALAT1 expression in two gastric cancer cell lines (BGC823 and SGC7901) and inhibited cell viability in a dose-dependent manner. Further experiments demonstrated that cell migration and invasion were inhibited by resveratrol via suppressing MALAT1-mediated epithelial epithelial-to-mesenchymal transition in gastric cancer cell line BGC823. These data provided novel evidence for understanding the anticancer mechanism in vitro and demonstrated the potential use of resveratrol as an anticancer drug.
Acknowledgements
The authors would like thank Dr Baoying Fei and Mr. Junxian Chen in the Department of Gastroenterology, Tongde Hospital of Zhejiang Province (Hangzhou, China), Mr. Guanping Chen in the Laboratory of Tumor Traditional Chinese Medicine Therapy in Institute of Cancer Research, Tongde Hospital of Zhejiang Province and Dr. Huwei Hou in the Laboratory of Cell Molecular Biology of Cell-Land Biological Technology Institute (Hangzhou, China) for the help in statistical analysis and technical assistance in this research.
Funding
This study was supported by grants from Zhejiang Provincial Natural Science Foundation (grant no. LY13H290012) and Zhejiang Provincial Science and Technology Program of Traditional Chinese Medicine (grant no. 2017ZA029).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZY and PC proposed the hypothesis and designed the majority of the experiments. ZY wrote the manuscript. ZY, QX and ZhaC performed the experiments and analyzed the results. HN, LX, QZ and ZhiC assisted in the execution of some experiments. QX, HN and LX were involved in revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Takaoka M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J Chem Society Japan. 1939;60:1090–1100. [Google Scholar]
- 2.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem. 2002;50:431–435. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- 4.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 5.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 6.Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 7.Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, Tsubura A. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol. 2001;127:258–264. doi: 10.1007/s004320000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82:348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 11.Alfaras I, Juan ME, Planas JM. Tans-resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. J Agric Food Chem. 2010;58:8104–8110. doi: 10.1021/jf100702x. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Chen YF, Hsu WH, Yang CW, Kao CH, Tsai TF. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev Res (Phila) 2012;5:952–962. doi: 10.1158/1940-6207.CAPR-12-0001. [DOI] [PubMed] [Google Scholar]
- 13.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365–5376. doi: 10.3748/wjg.v19.i32.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pretz JL, Wo JY, Mamon HJ, Kachnic LA, Hong TS. Chemoradiation therapy: Localized esophageal, gastric, and pancreatic cancer. Surg Oncol Clin N Am. 2011;22:511–524. doi: 10.1016/j.soc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 20.Xia H, Chen Q, Chen Y, Ge X, Leng W, Tang Q, Ren M, Chen L, Yuan D, Zhang Y, et al. The lncRNA MALAT1 is a novel biomarker for gastric cancer metastasis. Oncotarget. 2016;7:56209–56218. doi: 10.18632/oncotarget.10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Sun J, Wang J, Song Y, Gao P, Shi J, Chen P, Wang Z. Long noncoding RNAs in gastric cancer: Functions and clinical applications. Onco Targets Ther. 2016;9:681–697. doi: 10.2147/OTT.S95412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun W, Yang Y, Xu C, Xie Y, Guo J. Roles of long noncoding RNAs in gastric cancer and their clinical applications. J Cancer Res Clin Oncol. 2016;142:2231–2237. doi: 10.1007/s00432-016-2183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang EB, Han L, Yin DD, Kong R, De W, Chen J. c-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014;31:914. doi: 10.1007/s12032-014-0914-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Ye F, Yin C, Zhuang Y, Yue G, Zhang G. The interaction between MiR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell Physiol Biochem. 2015;36:1440–1452. doi: 10.1159/000430309. [DOI] [PubMed] [Google Scholar]
- 25.Zhang ZZ, Shen ZY, Shen YY, Zhao EH, Wang M, Wang CJ, Cao H, Xu J. HOTAIR long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(C)-binding protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162–1170. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi K, Gu Y, Fang G. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281:802–813. doi: 10.1111/febs.12625. [DOI] [PubMed] [Google Scholar]
- 27.Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, Sui H, Tang Y, Wang Y, Liu N, et al. Long non-coding RNA MALAT1 promotes tumor growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111:736–748. doi: 10.1038/bjc.2014.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY, Zhang F, Wu LM, Chen LM, Zheng SS. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29:1810–1816. doi: 10.1007/s12032-011-0004-z. [DOI] [PubMed] [Google Scholar]
- 30.Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, Wei M, Shen J, Hou J, Gao X, et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190:2278–2287. doi: 10.1016/j.juro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt LH, Spieker T, Koschmieder S, Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 32.Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding Q, Weng H, Shu YJ, Liu TY, Jiang L, et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol Ther. 2014;15:806–814. doi: 10.4161/cbt.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8:2289–2294. doi: 10.1039/c2mb25070e. [DOI] [PubMed] [Google Scholar]
- 34.Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, Sun J, Cai J, Qin J, Ren J, Li Q. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Zhang HM, Yang FQ, Chen SJ, Che J, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36:2947–2955. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 36.Pang EJ, Yang R, Fu XB, Liu YF. Overexpression of long non-coding RNA MALAT1 is correlated with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol. 2015;36:2403–2407. doi: 10.1007/s13277-014-2850-8. [DOI] [PubMed] [Google Scholar]
- 37.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, Goel A. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Su L, Chen X, Li P, Cai Q, Yu B, Liu B, Wu W, Zhu Z. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557–564. doi: 10.1016/j.biopha.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, Bin J, Liao Y, Liao W. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. doi: 10.1016/j.canlet.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Liang L, Ouyang K, Li Z, Yi X. MALAT1 induces tongue cancer cells' EMT and inhibits apoptosis through Wnt/β-catenin signaling pathway. J Oral Pathol Med. 2017;46:98–105. doi: 10.1111/jop.12466. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Song L, Yao H, Zhang L, Xu D, Gao F, Li Q. MiR-375 is epigenetically downregulated by HPV-16 E6 mediated DNMT1 upregulation and modulates EMT of cervical cancer cells by suppressing lncRNA MALAT1. PLoS One. 2016;11:e0163460. doi: 10.1371/journal.pone.0163460. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Li J, Wang J, Chen Y, Li S, Jin M, Wang H, Chen Z, Yu W. LncRNA MALAT1 exerts oncogenic functions in lung adenocarcinoma by targeting miR-204. Am J Cancer Res. 2016;6:1099–1107. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Liu S, Cai G, Kong L, Zhang T, Ren Y, Wu Y, Mei M, Zhang L, Wang X. Long non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. Sci Rep. 2015;5:15972. doi: 10.1038/srep15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 47.Qadri SM, Föller M, Lang F. Inhibition of suicidal erythrocyte death by resveratrol. Life Sci. 2009;85:33–38. doi: 10.1016/j.lfs.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278:88–100. doi: 10.1016/j.tox.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Gao Q, Yuan Y, Gan H, Peng Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol Lett. 2015;9:2381–2387. doi: 10.3892/ol.2015.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jing X, Cheng W, Wang S, Li P, He L. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway. Oncol Rep. 2016;35:472–478. doi: 10.3892/or.2015.4384. [DOI] [PubMed] [Google Scholar]
- 51.Yang Q, Wang B, Zang W, Wang X, Liu Z, Li W, Jia J. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS One. 2013;8:e70627. doi: 10.1371/journal.pone.0070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.