Abstract
Screening for diabetic kidney disease (DKD) remains a challenge; however, there has been an ongoing research to investigate the diagnostic value of different biomarkers to identify DKD. The aim of this study was to assess the diagnostic value of both N-acetyl-beta-d-glucosaminidase (NAG) and neutrophil gelatinase-associated lipocalin (NGAL) in the progression of DKD. This cross-sectional case–control study included 92 type 2 diabetic patients with or without DKD. Urinary NAG and NGAL were measured to evaluate their diagnostic values as biochemical markers related to DKD. Both urinary NAG and NGAL levels were significantly higher among patients with DKD. In multiple linear regression analysis, NAG showed a positive significant association with NGAL in the three different adjusted models, while no significant correlation with fasting blood glucose, glycated hemoglobin, serum creatinine, estimated glomerular filtration rate, and albumin creatinine ratio were observed. The area under the curve for NGAL was 0.659 (p = 0.01) and 0.564 (p = 0.297) for NAG in DKD patients. This study demonstrates the association between urinary NAG and NGAL as a tubular damage marker for DKD although longitudinal studies are needed to evaluate its diagnostic value.
Keywords: Diabetic kidney disease, N-acetyl-beta-d-glucosaminidase, Neutrophil gelatinase-associated lipocalin, Albuminuria, Tubular marker
Introduction
Chronic kidney disease (CKD), attributed to diabetes, is a leading cause of end stage renal disease (ESRD), which occurs in 20–40% of patients with diabetes (American Diabetes Association 2017). During the last three decades, microalbuminuria, which has been the only screening test for this complication, is not ideal for predicting or screening for patients who may develop diabetic kidney disease (DKD), although it indicates actual renal damage. There have been many studies recently evaluating different biochemical markers that may predict DKD using glomerular, tubular, and inflammatory markers that could identify glomerular or tubular injury.
The decline in renal function, commonly preceded by microalbuminuria that eventually leads to the clinical manifestations of renal insult, is followed by an increase in albumin creatinine ratio (ACR) and a decline in estimated glomerular filtration rate (eGFR) (American Diabetes Association 2013). Studies on the role of glomerular injury as a marker of early diabetic nephropathy have identified many biomarkers that appear to be related to tubular injury or inflammatory process, which eventually leads to DKD. N-acetyl-beta-D-glucosaminidase (NAG) has recently been recognized as a tubular injury marker related to DKD. This biomarker is known for its high molecular weight that cannot be filtered by the glomerulus; thus, it is released in the tubular lumen as a consequence of proximal tubular damage (Hong and Chia 1998). Since previous studies have reported that increase in urinary NAG excretion precedes albuminuria, and occurs in patients with normal to mildly increased albuminuria; therefore, among patients with type 2 diabetes mellitus (T2DM), this biomarker could be a good predictor of DKD before protein leak (Nauta et al. 2011; Fu et al. 2012).
The Saudi Diabetes Kidney Disease (SAUDI-DKD) study focused on the need to identify such a biomarker among Saudi population. The study will address this need and provide opportunities for testing of new biomarkers, which may help in predicting early changes for early diagnosis of DKD (Al-Rubeaan et al. 2017). Therefore, the aim of this study was to evaluate the urinary level of NAG along with NGAL as renal tubular biomarkers to identify cases with DKD.
Methods
This study is a case matched control cross-sectional study using the SAUDI-DKD for cases and controls subject selection. The full details of the SAUDI-DKD cohort has been published earlier (Al-Rubeaan et al. 2018). The study was approved by the institutional review board at the College of Medicine, King Saud University. Informed consent was obtained from each subject, and the study was conducted in accordance with the declarations of Helsinki (World Medical Association 2013).
A total of 92 patients with T2DM were selected from the SAUDI-DKD cohort, divided into 46 control subjects without DKD and 46 patients with DKD. Case definition was based on the Kidney Disease Improving Global Outcomes (KDIGO) guideline (Ketteler et al. 2018). All cases were matched for age, sex, and duration of diabetes with control subjects. The sample size was calculated using the Raosoft sample size calculator (Raosoft 2004). The studied subjects’ clinical data were retrieved from the patients’ clinical charts while anthropometric and vital signs including blood pressure were measured during recruitment visit.
Both cases and control subjects were asked to fast for 10 h overnight, and 5 ml of venous blood samples were collected from each subject using a plain tube. The serum was separated and stored immediately at − 20 °C for future analysis. Each sample was assessed for fasting blood glucose (FBG), glycated hemoglobin (HbA1c), serum creatinine, lipid profile [triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC)] and liver enzymes [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] using RX Daytona clinical chemistry analyzer (Randox, UK). Serum C-peptide and cystatin C were measured using Randox Evidence biochip analyzer (Randox, UK). A random spot urine sample, obtained from all subjects during their clinical visit, was stored at − 80 °C for future assessment. The urinary albumin and creatinine were analyzed using RX Daytona clinical chemistry analyzer (Randox, UK). The urinary albumin excretion was estimated by computing albumin-to-creatinine ratio in units of mg/g. The eGFR levels were calculated using the CKD-EPI creatinine equation (Levey et al. 2009) as follows:
eGFR = 141 × min (SCr/κ, 1)α × max(SCr /κ,1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black] (Levey et al. 2009).
The urinary NGAL levels were measured with solid phase enzyme-linked immunosorbent assay (ELISA), while NAG was measured using colorimetry, with commercially available standard kit designed for random spot urine samples (Abcam, Cambridge, USA).
Statistical analysis
All statistical analyses were performed using SPSS version 21.0 (SPSS Inc, Chicago, IL, USA). The data were presented as mean ± standard deviation for normally distributed variables and as median [interquartile range (IQR)] for variables with skewed data. Chi square test was used to analyze categorical data. We performed Student t test to compare group means. We performed Pearson or Spearman correlation analyses using ACR, NGAL, NAG as dependent variables; and clinical, biochemical, and urinary biomarkers as independent variables. In the multiple linear regression analysis, the biomarker, NAG, was considered as the dependent variable and other biochemical and urinary markers as independent variables. Different models were built to adjust for confounding factors such as age, sex, body mass index (BMI), duration of diabetes, systolic blood pressure (SBP), and diastolic blood pressure (DBP). We performed receiver operating characteristics (ROC) curves and evaluated the area under the curve (AUC) for NAG and NGAL. A p value of < 0.05 was considered statistically significant.
Results
This case matched control cross-sectional study demonstrated subjects with and without DKD, matched for all demographic and clinical data except higher SBP and percentage of retinopathy, among those with DKD. They were also matched for HbA1c level but with a significantly higher FBG level among patients with DKD. As expected, patients with DKD had significantly lower eGFR and higher ACR values. However, no significant differences were found for the rest of the biochemical markers including C-peptide level between the two groups. The cystatin C, NGAL, and NAG biomarkers were significantly higher in DKD subjects as shown in Table 1.
Table 1.
Demographic, clinical and biochemical characteristics of selected matched case -control cohort (without DKD (n = 46), with DKD (n = 46))
| Patient characteristics | Without DKD (n = 46) |
With DKD (n = 46) |
p value |
|---|---|---|---|
| Age (years) | 55.2 ± 4.9 | 55.0 ± 5.0 | 0.83 |
| Male, n (%) | 28 (60.9) | 28 (60.9) | 1.0 |
| BMI (kg/m2) | 33.4 ± 5.6 | 31.4 ± 5.1 | 0.07 |
| Systolic blood pressure (mmHg) | 130.7 ± 15.3 | 145.6 ± 26.2 | 0.001 |
| Diastolic blood pressure (mmHg) | 72.2 ± 9.8 | 75.4 ± 12.7 | 0.18 |
| Duration of diabetes (years) | 17.3 ± 4.3 | 17.2 ± 4.3 | 0.86 |
| Family history of diabetes (yes), n (%) | 44 (95.7%) | 43 (93.5%) | 0.64 |
| Family history of kidney disease (yes), n (%) | 6 (13.3%) | 6 (13.3%) | 1.0 |
| Diabetic retinopathy, n (%) | 15 (32.6) | 25 (54.3) | 0.035 |
| Diabetic neuropathy, n (%) | 20 (43.5) | 23 (50) | 0.53 |
| FBG (mg/dl) | 191.4 ± 75.8 | 226.3 ± 73.6 | 0.02 |
| HbA1c (%) | 10.0 ± 1.1 | 10.6 ± 2.0 | 0.089 |
| Serum creatinine (mg/dl) | 0.96 ± 0.16 | 1.2 ± 0.37 | < 0.001 |
| C-peptide (ng/ml) | 1.06 ± 0.86 | 0.89 ± 0.87 | 0.42 |
| eGFR (ml/min/1.73 m2) | 80.5 ± 14.5 | 61.2 ± 21.6 | < 0.001 |
| ACR (mg/g) | 11.2 ± 7.1 | 549.3 ± 747.5 | < 0.001 |
| ALT (U/l), median (IQR) | 13 (9–17) | 12 (9–17) | 0.74 |
| AST (U/l), median (IQR) | 20 (17–25) | 21 (19–28) | 0.13 |
| Cystatin C (ng/ml), median (IQR) | 316 (261–462) | 460 (325–651) | 0.001 |
| NGAL (µg/g), median (IQR) | 16.8 (8.9–38.9) | 34 (14–70) | 0.029 |
| NAG (U/g), median (IQR) | 17.3 (9.3–25.5) | 18.3 (9.8–34.2) | 0.028 |
Data represents in mean ± standard deviation, median (interquartile range) and in percentage
DKD diabetic kidney disease, BMI body mass index, FBG fasting blood glucose, eGFR estimated glomerular filtration rate, ACR albumin creatinine ratio, ALT alanine amino transferase, AST aspartate amino transferase, NGAL neutrophil gelatinase-associated lipocalin to creatinine ratio, NAG N-acetyl-beta-d-glucosaminidase to creatinine ratio
p value < 0.05 is statistically significant
Using Spearman correlation analysis for both NGAL and NAG, and Pearson correlation for ACR, with different clinical and biochemical variables as shown in Table 2, there was significantly positive correlation with increased SBP and increased level of NGAL biomarker, but not with NAG, among subjects with DKD. With increased DBP, NGAL biomarker was significantly increased in patients with DKD but not in those without DKD. This observation was not found with NAG that has shown no significant positive correlation. There was a significant positive correlation between ACR and NGAL in patients without DKD and the total subjects, but not with DKD patients; similar to that observed for NAG. The levels of NGAL and NAG showed a significant positive correlation in patients with DKD.
Table 2.
Spearman correlation of NGAL and NAG and Pearson correlation for ACR with different clinical and biochemical variables in studied subjects
| ACR (mg/g) | NGAL (µg/g) | NAG (U/g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without DKD | With DKD | Total subjects | Without DKD | With DKD | Total subjects | Without DKD | With DKD | Total subjects | ||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| Age (years) | 0.04 | 0.75 | 0.04 | 0.76 | 0.01 | 0.86 | − 0.15 | 0.31 | 0.00 | 1.0 | − 0.06 | 0.55 | 0.07 | 0.61 | 0.05 | 0.70 | 0.07 | 0.50 |
| BMI (kg/m2) | 0.05 | 0.70 | 0.02 | 0.88 | − 0.07 | 0.50 | 0.05 | 0.72 | − 0.24 | 0.10 | − 0.09 | 0.35 | 0.12 | 0.40 | − 0.10 | 0.49 | − 0.02 | 0.83 |
| Systolic blood pressure (mmHg) | 0.31 | 0.03 | 0.37 | 0.01 | 0.42 | < 0.001 | 0.32 | 0.02 | 0.39 | 0.006 | 0.39 | < 0.001 | 0.11 | 0.45 | 0.02 | 0.85 | 0.08 | 0.42 |
| Diastolic blood pressure (mmHg) | 0.18 | 0.22 | 0.12 | 0.42 | 0.15 | 0.15 | 0.24 | 0.10 | 0.33 | 0.02 | 0.27 | 0.008 | − 0.13 | 0.37 | 0.08 | 0.55 | 0.006 | 0.95 |
| Duration of diabetes (years) | 0.03 | 0.83 | 0.25 | 0.09 | 0.15 | 0.153 | 0.17 | 0.24 | 0.11 | 0.45 | 0.13 | 0.21 | 0.27 | 0.06 | 0.12 | 0.42 | 0.20 | 0.05 |
| HbA1c (%) | − 0.05 | 0.74 | 0.14 | 0.32 | 0.19 | 0.06 | 0.10 | 0.48 | 0.006 | 0.96 | 0.069 | 0.51 | − 0.007 | 0.96 | − 0.07 | 0.64 | − 0.02 | 0.82 |
| FBG (mg/dl) | 0.08 | 0.58 | − 0.08 | 0.56 | 0.05 | 0.61 | 0.12 | 0.40 | − 0.004 | 0.97 | 0.15 | 0.13 | − 0.10 | 0.50 | 0.004 | 0.97 | 0.005 | 0.96 |
| Serum creatinine (mg/dl) | − 0.13 | 0.37 | 0.27 | 0.06 | 0.39 | < 0.001 | − 0.05 | 0.73 | − 0.05 | 0.72 | 0.08 | 0.44 | − 0.21 | 0.16 | 0.02 | 0.86 | 0.02 | 0.84 |
| Cystatin C (ng/ml) | − 0.04 | 0.77 | 0.33 | 0.02 | 0.41 | < 0.001 | − 0.16 | 0.29 | − 0.10 | 0.50 | − 0.01 | 0.86 | 0.19 | 0.20 | − 0.18 | 0.22 | − 0.01 | 0.92 |
| eGFR (ml/min/1.73 m2) | 0.08 | 0.58 | − 0.33 | 0.03 | − 0.42 | < 0.001 | − 0.06 | 0.65 | − 0.05 | 0.73 | − 0.21 | 0.04 | 0.11 | 0.47 | − 0.17 | 0.28 | − 0.15 | 0.14 |
| ACR (mg/g) | 1 | 1 | 1 | 1 | 1 | 1 | 0.29 | 0.05 | 0.19 | 0.19 | 0.33 | 0.001 | 0.39 | 0.008 | − 0.05 | 0.74 | 0.17 | 0.10 |
| NGAL (µg/g) | 0.22 | 0.14 | 0.14 | 0.34 | 0.20 | 0.04 | 1 | 1 | 1 | 1 | 1 | 1 | 0.22 | 0.14 | 0.48 | 0.001 | 0.40 | < 0.001 |
| NAG (U/g) | 0.39 | 0.007 | − 0.02 | 0.88 | 0.09 | 0.39 | 0.22 | 0.14 | 0.48 | 0.001 | 0.40 | < 0.001 | 1 | 1 | 1 | 1 | 1 | 1 |
BMI body mass index, FBG fasting blood glucose, eGFR estimated glomerular filtration rate, ACR albumin creatinine ratio, NGAL neutrophil gelatinase-associated lipocalin to creatinine ratio, NAG N-acetyl-beta-D-glucosaminidase to creatinine ratio
p value <0.05 is statistically significant
In Table 3, NAG values are shown in three models, where Model 1 was adjusted for age, sex, BMI, and duration of diabetes. Model 2 was adjusted for the same factors as for Model 1 in addition to SBP, and Model 3 was adjusted for all factors in addition to DBP. All three models demonstrated no significant NAG value difference in FBG, HbA1c, serum creatinine, eGFR, and ACR in the multiple linear regression analysis. Although NAG demonstrated no significant correlation with FBG, HbA1c, serum creatinine, eGFR, and ACR in the three models built, however, there was positive significant correlation with NGAL in the three models.
Table 3.
Multiple linear regression analysis of NAG with different variable in total subjects
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| R 2 | p value | R 2 | p value | R 2 | p value | |
| FBG (mg/dl) | 0.026 | 0.685 | 0.026 | 0.811 | 0.047 | 0.659 |
| HbA1c (%) | 0.045 | 0.408 | 0.054 | 0.444 | 0.058 | 0.531 |
| Serum creatinine (mg/dl) | 0.041 | 0.463 | 0.048 | 0.515 | 0.053 | 0.586 |
| eGFR (ml/min/1.73 m2) | 0.051 | 0.356 | 0.253 | 0.362 | 0.069 | 0.436 |
| ACR (mg/g) | 0.022 | 0.752 | 0.022 | 0.854 | 0.023 | 0.923 |
| NGAL (µg/g) | 0.322 | 0.001 | 0.385 | 0.001 | 0.429 | 0.001 |
Model 1: age, sex, BMI, duration of diabetes; Model 2: age, gender, BMI, duration of diabetes, SBP; Model 3: age, gender, BMI, duration of diabetes, SBP and DBP
BMI body mass index, FBG fasting blood glucose, eGFR estimated glomerular filtration rate, ACR albumin creatinine ratio, NGAL neutrophil gelatinase-associated lipocalin to creatinine ratio, NAG N-acetyl-beta-d-glucosaminidase to creatinine ratio, SBP systolic blood pressure, DBP diastolic blood pressure
p value < 0.05 is statistically significant
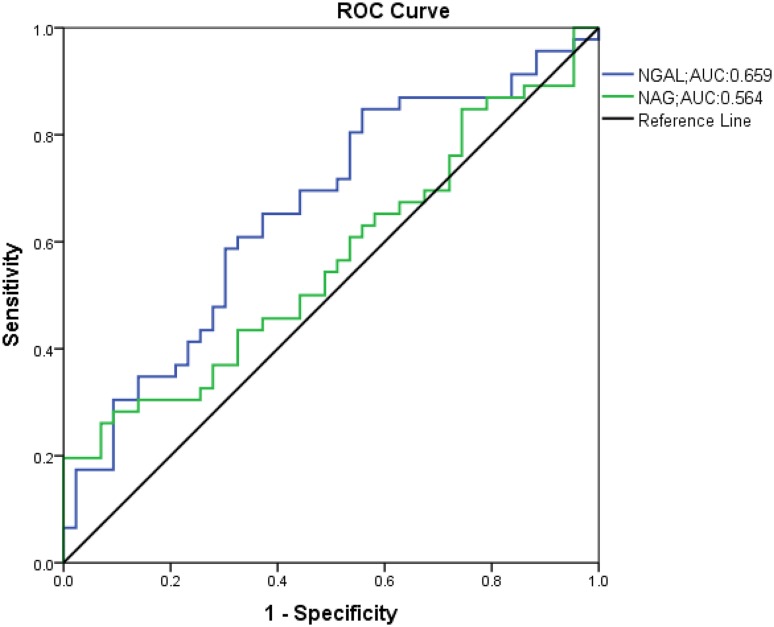
ROC curves for both NGAL and NAG values shown in Fig. 1 demonstrated significant AUC for NGAL (AUC: 0.659, p = 0.01), but was not significant for NAG (AUC: 0.564, p = 0.297).
Fig. 1.
ROC curve analysis of NAG and NGAL in DKD subjects. ROC receiver operating characteristics, NAG urinary N-acetyl-beta-d-glucosaminidase, NGAL neutrophil gelatinase-associated lipocalin, DKD diabetic kidney disease
Discussion
Many studies reported that NAG and NGAL biomarkers are important in the early detection and prediction of renal damage in DKD patients, and considered to be biomarkers for acute kidney injury (Fu et al. 2012; de Geus et al. 2012; Sheira et al. 2015; Sharifi et al. 2015). NAG is a lysosomal hydrolase predominantly located in the proximal tubules of the kidney, while NGAL is a protein that belongs to the lipocalin family (Bazzi et al. 2002; Mishra et al. 2006; Liangos et al. 2007). As reported in the “Results” section, we matched type 2 diabetic subjects with DKD and subjects without kidney disease to assess for the role of the two biomarkers in screening for DKD among T2DM patients. The two groups were matched perfectly except for the expected difference, in SBP, which was higher among DKD patients. We managed to match for HbA1c level, although FBG was significantly higher among DKD patients as expected. Since retinopathy has a strong association with DKD, DKD subjects in this study had a significantly higher prevalence.
From our previous study, we found a very good diagnostic accuracy for patients with DKD with cystatin C; therefore, cystatin C was assessed between the two groups and found to be significantly higher among patients with DKD (Al-Rubeaan et al. 2017). This would indicate that matching the two groups showed a significant value of such biomarkers. Both urinary NGAL and NAG tested in this study also demonstrated significantly higher median values among patients with DKD supporting the fact that those two biomarkers could be useful when screening for DKD.
The NAG is normally excreted through urine in very low quantities, and increased level of this enzyme in the urine is considered as a specific marker for functional tubular impairment. This enzyme, excreted from the renal tubular tissue is relatively stable in urine, has minimal diurnal variations and a high molecular weight that preclude its filtration by the glomerulus (Hong and Chia 1998; Skálová 2005). Consistent with the finding of a previous study, our observation demonstrated increased urinary NAG level among subjects with DKD, indicating renal tubular damage (Bouvet et al. 2014).
In the present study, urinary NGAL level was found to be twofold higher among subjects with DKD when compared with those without DKD, which is in line with a previous study reporting significantly higher levels of NGAL and NAG in albuminuria subjects when compared with normoalbuminuria subjects (Fu et al. 2012). Since NGAL is a protein released by the renal tubules and normally filtered by the glomerulus, this could be explained by the reduced absorption and increased production by the kidney tubules as a result of acute kidney injury (Mishra et al. 2006; Tramonti and Kanwar 2011).
There was a significantly positive correlation in this study between SBP and NGAL but not with NAG, in patients with DKD. The relationship between blood pressure and NGAL indicates the endothelial dysfunction in DKD (Dharmashankar and Widlansky 2010). We also found a significantly positive correlation between NAG and NGAL in patients with DKD. This was also observed by Fu et al. and Assal et al. in different ethnic populations (Fu et al. 2012; Assal et al. 2013). The multiple linear regression analysis for NAG in this study, support this significant association after adjusting for different risk factors related to DKD.
Although the diagnostic accuracy of NAG was previously reported, this is the first study to assess the diagnostic value of this marker in Saudi population. The AUC value reported for NAG in this study is not comparable with the previous study due to differences in diagnostic objectives and study cohort (Assal et al. 2013). ROC curve analysis of NGAL showed a diagnostic accuracy with significant AUC to detect DKD among diabetic patients. Diagnostic performance of NGAL was previously assessed in this population with a good accuracy (Mahfouz et al. 2016). The diagnostic accuracy of NGAL and tubular specificity as well as the prognostic accuracy of NAG (Patel and Kalia 2015) highlight the simultaneous analysis of these non-invasive biomarkers and provides more specific indication of tubular dysfunction in DKD.
The NAG values did not demonstrate any significant difference with FBG, HbA1c, serum creatinine, eGFR, and ACR while it showed a positive significant correlation with NGAL along with different risk factors, keeping in mind that the ROC curve demonstrated significant diagnostic value for NGAL, but not for NAG.
When comparing NAG value for different stages of DKD (i.e., moderately or severely increased albuminuria), this study demonstrated the highest NAG value among patients with DKD when compared with other ethnicities, as shown in Table 4. This could be attributed to the longer diabetes duration, which is supported by a previous study, whereby NAG activity was increased with diabetes duration and with the progression of DKD (Patel and Kalia 2015). Furthermore, NAG result in the current study is comparable with recently published studies that are found to be higher due to the longer diabetes duration of the study subjects (Hozo et al. 2005; Piwowar et al. 2006; Ambade et al. 2006; Mohammadi-Karakani et al. 2007; Fu et al. 2012; Bouvet et al. 2014; Wan et al. 2014; Asare-Anane et al. 2016, p. 2).
Table 4.
NAG values in different stages of DKD from recently published studies
| First author (years) | Age (years) | DM duration (years) | NAG (U/g) | ||
|---|---|---|---|---|---|
| DM without DKD | Albuminuria | ||||
| Moderately increased | Severely increased | ||||
| Current study | 55.1 ± 4.9 | 17.3 ± 4.2 | 17.9 | 25.6 | 25.3 |
| Asare-Anane et al. (2016) Bouvet et al. (2014) *** |
55.3 ± 11.7 66 ± 10 * |
8.2 ± 6.1 13 ± 8* |
11.83 4.87 |
26.7 15.8 |
NA NA |
| Fu et al. (2012)*** | 53.9 ± 13.8 | 2.7 ± 1.5 | 11.63 | 16.2 | 21.4 |
| Mohammadi-Karakani et al. (2007) | 56.7 ± 1.7 | NA | 4.96 | 7.6 | NA |
| Ambade et al. (2006) | 54 | NA | 9.49 | 15.99 | NA |
| Piwowar et al. (2006)*** | 62 ** | 11.5 ** | 3.5 | 7.09 | 12.8 |
DM duration (diabetes mellitus duration), DM without DKD (diabetes mellitus without diabetic kidney disease). NA (Not available). Mean NAG (N-acetyl-beta-D-glucosaminidase to creatinine ratio) values are given
*Calculated values
**Median values
***Mean NAG values calculated based on references (Hozo et al., Wan et al.)
This study derives its strength from being a case–control study with different stages of DKD with clear case definition, but was limited by the cross-sectional study design.
In conclusion, this study showed a strong association between urinary NAG and NGAL in T2DM patients with DKD. Simultaneous analysis of these two biomarkers provided additional value to assess tubular dysfunction in diabetic patients. However, longitudinal studies are needed to evaluate these markers in the progression of DKD. Early detection of tubular damage may have a positive impact on the quality of life of diabetic patients by preventing the progression of DKD.
Acknowledgements
The authors would like to acknowledge the research unit team, the hospitals involved in the study, and all staff nurses in different wards, for their participation in conducting the study. The authors would also like to acknowledge King Abdulaziz City for Science and Technology (KACST) for funding this project (grant for project no: A-T-34-194).
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Al-Rubeaan K, Siddiqui K, Al-Ghonaim MA, et al. Assessment of the diagnostic value of different biomarkers in relation to various stages of diabetic nephropathy in type 2 diabetic patients. Sci Rep. 2017;7:2684. doi: 10.1038/s41598-017-02421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rubeaan K, Siddiqui K, Alghonaim M, et al. The Saudi Diabetic Kidney Disease study (Saudi-DKD): clinical characteristics and biochemical parameters. Ann Saudi Med. 2018;38:46–56. doi: 10.5144/0256-4947.2018.03.01.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambade V, Sing P, Somani BL, Basanna D. Urinary N-acetyl beta glucosaminidase and gamma glutamyl transferase as early markers of diabetic nephropathy. Indian J Clin Biochem IJCB. 2006;21:142–148. doi: 10.1007/BF02912930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standards of Medical Care in Diabetes—2017. Diabetes Care. 2017;40:S1–S135. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- Asare-Anane H, Twum F, Kwaku Ofori E, et al. urinary lysosomal enzyme activities and albuminuria in ghanaian patients with type 2 diabetes mellitus. Dis Markers. 2016;2016:2810639. doi: 10.1155/2016/2810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assal HS, Tawfeek S, Rasheed EA, et al. Serum cystatin C and tubular urinary enzymes as biomarkers of renal dysfunction in type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes. 2013;6:7–13. doi: 10.4137/CMED.S12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi C, Petrini C, Rizza V, et al. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17:1890–1896. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- Bouvet BR, Paparella CV, Arriaga SMM, et al. Evaluation of urinary N-acetyl-beta-D-glucosaminidase as a marker of early renal damage in patients with type 2 diabetes mellitus. Arq Bras Endocrinol Metabol. 2014;58:798–801. doi: 10.1590/0004-2730000003010. [DOI] [PubMed] [Google Scholar]
- de Geus HRH, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–108. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W-J, Xiong S-L, Fang Y-G, et al. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2012;41:82–88. doi: 10.1007/s12020-011-9509-7. [DOI] [PubMed] [Google Scholar]
- Hong CY, Chia KS. Markers of diabetic nephropathy. J Diabetes Complications. 1998;12:43–60. doi: 10.1016/S1056-8727(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Ann Intern Med. 2018;168:422. doi: 10.7326/M17-2640. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- Mahfouz MH, Assiri AM, Mukhtar MH. Assessment of Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Retinol-Binding Protein 4 (RBP4) in Type 2 Diabetic Patients with Nephropathy. Biomark Insights. 2016;11:31–40. doi: 10.4137/BMI.S33191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Karakani A, Asgharzadeh-Haghighi S, Ghazi-Khansari M, Hosseini R. Determination of urinary enzymes as a marker of early renal damage in diabetic patients. J Clin Lab Anal. 2007;21:413–417. doi: 10.1002/jcla.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta FL, Bakker SJL, van Oeveren W, et al. Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis Off J Natl Kidney Found. 2011;57:733–743. doi: 10.1053/j.ajkd.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Patel DN, Kalia K. Efficacy of urinary N-acetyl-β-glucosaminidase to evaluate early renal tubular damage as a consequence of type 2 diabetes mellitus: a cross-sectional study. Int J Diabetes Dev Ctries. 2015;35:449–457. doi: 10.1007/s13410-015-0404-2. [DOI] [Google Scholar]
- Piwowar A, Knapik-Kordecka M, Fus I, Warwas M. Urinary activities of cathepsin B, N-acetyl-beta-D-glucosaminidase, and albuminuria in patients with type 2 diabetes mellitus. Med Sci Monit. 2006;12:CR210–C214. [PubMed] [Google Scholar]
- Raosoft (2004) Sample size calculator. In: Sample size calc. raosoft Inc. http://www.raosoft.com/samplesize.html. Accessed 30 Dec 2018
- Sharifi AM, Zare B, Keshavarz M, et al. Urinary N-acetyl-β-D-glucosaminidase (NAG) activity in the early detection of diabetic nephropathy. Int J Diabetes Dev Ctries. 2015;35:369–374. doi: 10.1007/s13410-015-0325-0. [DOI] [Google Scholar]
- Sheira G, Noreldin N, Tamer A, Saad M (2015) Urinary biomarker N-acetyl-β-D-glucosaminidase can predict severity of renal damage in diabetic nephropathy. J Diabetes Metab Disord 14:. 10.1186/s40200-015-0133-6 [DOI] [PMC free article] [PubMed]
- Skálová S. The diagnostic role of urinary N-acetyl-beta-D-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Medica. 2005;48:75–80. [PubMed] [Google Scholar]
- Tramonti G, Kanwar YS. Tubular biomarkers to assess progression of diabetic nephropathy. Kidney Int. 2011;79:1042–1044. doi: 10.1038/ki.2011.9. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]